Summary
Src family kinase- (SFK-) targeting agents are currently undergoing clinical investigation for treatment of solid malignancies. EGFR-independent phosphorylation of STAT3 (P-STAT3) has been identified as a mechanism of tumor resistance to agents targeting SFK. Tumor P-STAT3 levels may be an important indicator of EGFR- and SKF-targeted anti-tumor treatment efficacy.
In this issue of Clinical Cancer Research (CCR), Nagaraj and colleagues use preclinical models of pancreatic cancer to evaluate the anti-tumor efficacy of combining dasatinib with an FDA-approved targeted therapy treatment for unresectable or metastatic pancreatic cancer, erlotinib plus gemcitabine (1). Epidermal growth factor receptor- (EGFR-) targeted therapies have been approved for the treatment of several cancers including erlotinib (Genetech and OSI Pharmaceuticals), a small molecule tyrosine kinase inhibitor (TKI) of EGFR, as a monotherapy for non-small cell lung cancer (NSCLC) and in combination with gemcitabine (Eli Lilly and Company), a deoxycytidine analog, for pancreatic cancer. Gefinitib (Astrazeneca), also an EGFR TKI, was FDA-approved for treatment of NSCLC with revised labeling restricting indications for use. The EGFR-targeted chimeric monoclonal antibody cetuximab (Imclone and Bristol-Myers Squibb) has been FDA-approved for treatment of head and neck squamous cell carcinoma (HNSCC). Cetuximab and the fully human anti-EGFR antibody panitumumab (Amgen) are both FDA-approved for the treatment of colorectal cancers. Except for the subpopulation of NSCLC tumors harboring EGFR activating mutations where tumor response to EGFR kinase inhibitors has been impressive, the clinical response rates to EGFR-targeting have in general been modest.
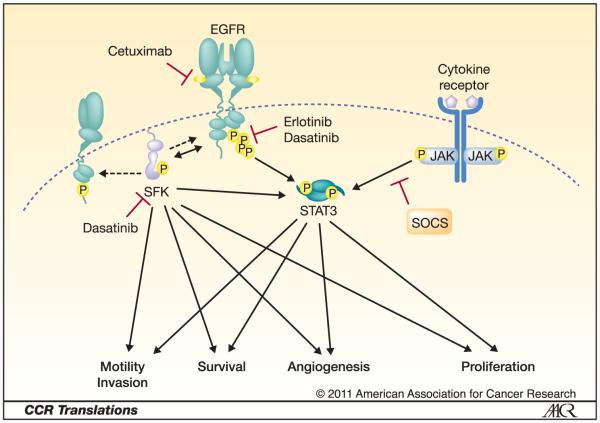
Dasatinib (Bristol-Myers Squibb) is a spectrum kinase inhibitor of Src family kinases (SFK), EGFR and several other kinases (2). SFK are overexpressed and activated in many cancers, augment EGFR signaling and mediate the activation of many pathways important in tumorigenesis and progression (Figure 1). In addition to activation by EGFR, SFK mediate signaling pathway activation by other receptor tyrosine kinases, G-protein-coupled receptors, and integrins. Because SFK are central to cancer and SFK inhibitors with acceptable toxicities are currently in clinical development, studies evaluating the anti-tumor efficacy of SFK inhibitors alone and in combination with other targeted therapies are ongoing. Of the SFK-targeting agents studied to date for treatment of solid malignancies, dasatinib, which has been FDA-approved for treatment of chronic myelogenous leukemia and acute lymphoblastic leukemia, is the most developed.
Fig. 1.
EGFR- and SFK-independent activation of STAT3 provides a mechanism for Overcoming EGFR- and SFK-targeted therapies. Solid arrows indicate activation of signaling components or pathways. Dashed arrow indicates phosphorylation independent of activation. Inhibition is represented by a red T.
Sustained inhibition of SFK by dasatinib or specific knock-down of c-Src has been shown to result in JAK-dependent phosphorylation of STAT3 (P-STAT3) in NSCLC and HNSCC preclinical models (3-4). In these cell lines the EGFR- and SFK-independent, JAK-dependent phosphorylation of STAT3 provided a mechanism for the activation of signaling pathways important to cancer progression even in the presence of EGFR and SFK blockade (Figure 1). Similarly, Nagaraj and colleagues previously demonstrated that STAT3 phosphorylation in the presence of dasatinib was associated with resistance to dasatinib in pancreatic cancer models though the mechanism of sustained STAT3 phosphorylation was not defined (5).
Importantly, in this issue of CCR Nagaraj et al. demonstrate that the triple combination of dasatinib, erlotinib and gemcitabine reduced levels of P-STAT3 in pancreatic cancer xenograft models. From a panel of nine pancreas cancer cell lines, in vitro and in vivo data were presented primarily for two cell lines: the PANC1 cell line, which had the highest erlotinib and gemcitabine IC-50s (1118 nM and 100.2 nM, respectively) and second highest dasatinib IC-50 (45.7 nM) and the BxPC3 cell line, which was most sensitive to erlotinib and dasatinib with IC-50 values of 99.7 nM and 2.8 nM, respectively, and intermediately sensitive to gemcitabine (IC-50 30.2 nM). Though these cell lines represented the extremes of the panel, both exhibited reduced in vitro cell migration in the presence of dasatinib, erlotinib and gemcitabine compared to any single agent or combination of two agents. Both cell lines also exhibited significant reduction in xenograft tumor growth with the triple agent combination compared to single or dual agent treatment and a corresponding reduction in P-STAT3 tumor levels. However, in vitro cell viability assays following single or combined agent treatments demonstrated no statistically significant reduction in cell viability with the triple combination compared to dual agent treatment. These data are an example of the incompletely understood discordance between in vitro cell viability and in vivo xenograft tumor growth, suggesting the limitations of preclinical models.
Though the combination of erlotinib, dasatinib and gemcitabine resulted in reduced xenograft tumor volumes for both the sensitive and insensitive cell lines, the mechanism of action of was largely undefined. Image analysis of the xenograft tumors indicated that pAKT, pSFK, Ki67 and Caspase 3 levels in both xenograft tumor types were not altered from dasatinib-erlotinib treatment levels by the addition of gemcitabine, yet P-STAT3 levels in both tumor types were markedly reduced following the triple agent treatment compared to single or any dual agent treatment. Given then pleiotropic actions of gemcitabine, it is difficult to speculate about the precise mechanism of P-STAT3 abrogation. However, STAT3 is activated and hence tyrosine phosphorylated downstream of both EGFR and Src family kinases. Thus, dual inhibition of EGFR and SFK provides more potent blockade of STAT3 activation. In HNSCC, the activation of STAT3 following knockdown of c-Src with small interfering RNA (siRNA) has been reported to be JAK-dependent and result from reduced suppressor of cytokine signaling 2 (SOCS2) expression (6). Whether the activation of STAT3 following dasatinib treatment in pancreatic cancer cells results from the same alterations in JAK and SOCS2 activities is unknown. Like many malignancies, pancreatic cancers have been reported to be genetically heterogeneous (7). The mechanisms contributing to STAT3 activation and the ability of combined treatment with erlotinib, dasatinib and gemcitabine to reduce P-STAT3 may contribute to the success of this therapeutic strategy across heterogeneous pancreatic cancers. It is possible that the addition of gemcitabine to EGFR and SFK co-targeting may have utility for treatment of other cancers where treatments combining EGFR- and SFK-targeting agents are being clinically evaluated, including NSCLC, HNSCC and colorectal cancers. More generally, STAT3 phosphorylation has been identified as a mechanism of resistance to SFK-targeted therapies, but the precise contribution of STAT3 phosphorylation/activation to cancer therapy resistance remains unknown.
As has often been the case with targeted therapies, there was heterogeneity of sensitivities to the three agents observed among the panel of cell lines evaluated by Nagaraj et al. but no identified correlation with baseline levels of c-Src, EGFR or their phosphorylated forms was observed. In clinical trials to date combining erlotinib with gemcitabine for the treatment of pancreatic cancer, neither tumor EGFR gene amplification nor KRAS mutation status was found to be associated with treatment response to combined erlotinib-gemcitabine treatment (8-9), and pancreatic cancers have been reported to very rarely harbor erlotinib-sensitizing EGFR-activating mutations(8). Though tumor biomarkers associated with response/resistance to EGFR-targeted therapies have been identified for NSCLC and colorectal cancers (10), no biomarker has yet been identified to be associated with response to SFK-targeting agents in patients with any solid tumor (11).
Clinical trials evaluating combined EGFR- and SFK-targeting agents are currently in Phase I/II. One Phase I/II clinical study evaluating dasatinib with erlotinib for treatment of 34 NSCLC patients reported 2 partial responses where this regimen was tolerated (12). Two Phase I/II trials combining SFK- and EGFR-targeting agents are currently ongoing: (1) combining dasatinib with erlotinib for NSCLC (ClinicalTrials.gov NCT00826449) and (2) combining dasatinib with cetuximab and radiation with or without cisplatin for patients with locally advanced HNSCC (ClinicalTrials.gov NCT00882583). However, there is a need to prospectively define responsive patient subpopulations. P-STAT3 levels in early post-treatment tumor biopsies has potential as a predictive biomarker, but these correlative studies may be difficult even for accessible tumors given the short biological half-life of dasatinib (12). More importantly, the response rates with combined EGFR- and SFK-targeted treatment in the NSCLC Phase I/II study were modest. Identifying agents that abrogate P-STAT3 following combined EGFR- and SFK-targeted agents such as gemcitabine in pancreatic preclinical models may improve upon these response rates.
References
- 1.Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Can Res. 2010:17. doi: 10.1158/1078-0432.CCR-10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Rix U, Fang B, Bai Y, Edwards A, Colinge J, et al. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat Chem Biol. 2010;6:291–9. doi: 10.1038/nchembio.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byers LA, Sen B, Saigal B, Diao L, Wang J, Nanjundan M, et al. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin Cancer Res. 2009;15:6852–61. doi: 10.1158/1078-0432.CCR-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res. 2009;69:1958–65. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagaraj NS, Smith JJ, Revetta F, Washington MK, Merchant NB. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol Cancer Ther. 2010;9:2322–32. doi: 10.1158/1535-7163.MCT-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen B, Saigal B, Parikh N, Gallick G, Johnson F. Sustained Src inhibition results in JAK activation and cancer cell survival via altered SOCS expression. Proc Am Assoc Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-08-2944. Abstract nr 4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Jang KT, Ki CS, Lim T, Park YS, Lim HY, et al. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer. 2007;109:1561–9. doi: 10.1002/cncr.22559. [DOI] [PubMed] [Google Scholar]
- 9.da Cunha Santos G, Dhani N, Tu D, Chin K, Ludkovski O, Kamel-Reid S, et al. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: national Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer. 2010 doi: 10.1002/cncr.25393. [DOI] [PubMed] [Google Scholar]
- 10.Egloff AM, Grandis JR. Improving Response Rates to EGFR-Targeted Therapies for Head and Neck Squamous Cell Carcinoma: Candidate Predictive Biomarkers and Combination Treatment with Src Inhibitors. J Oncol. 2009;2009:896407. doi: 10.1155/2009/896407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson FM, Bekele BN, Feng L, Wistuba I, Tang XM, Tran HT, et al. Phase II study of dasatinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:4609–15. doi: 10.1200/JCO.2010.30.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haura EB, Tanvetyanon T, Chiappori A, Williams C, Simon G, Antonia S, et al. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1387–94. doi: 10.1200/JCO.2009.25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]