Abstract
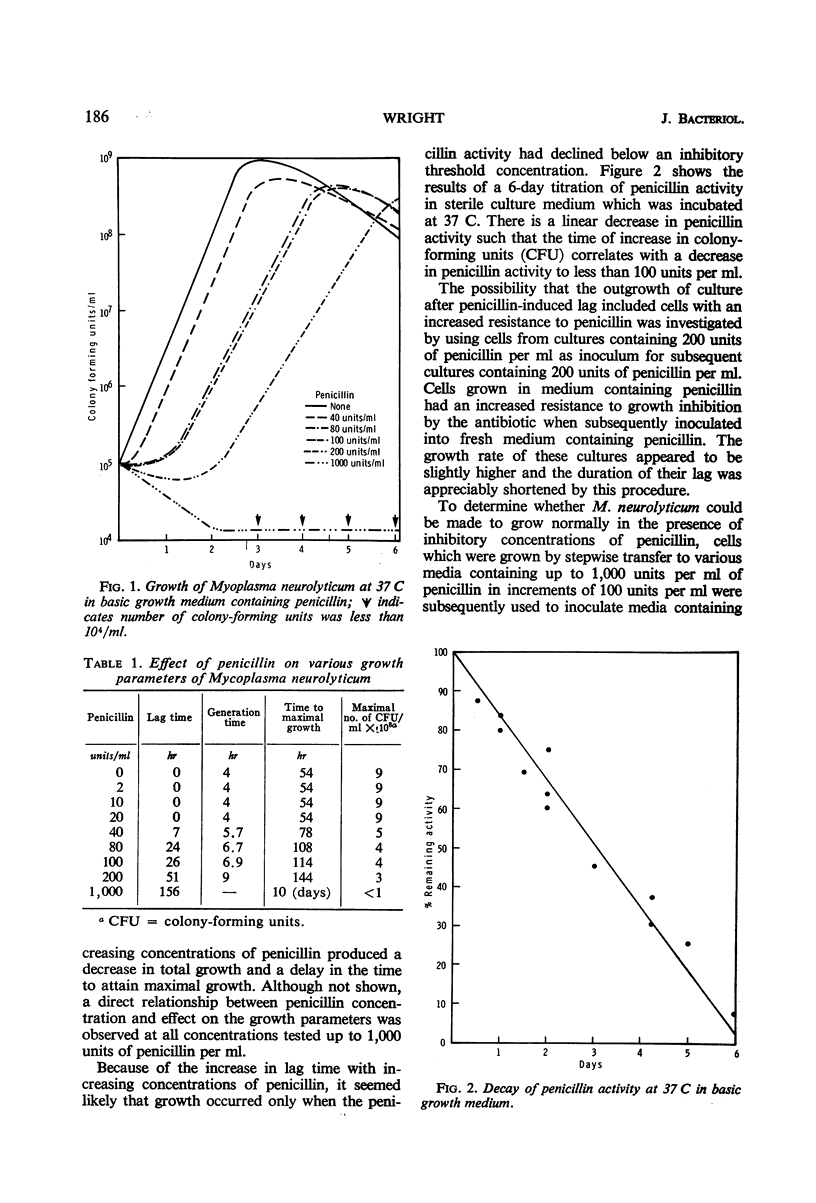
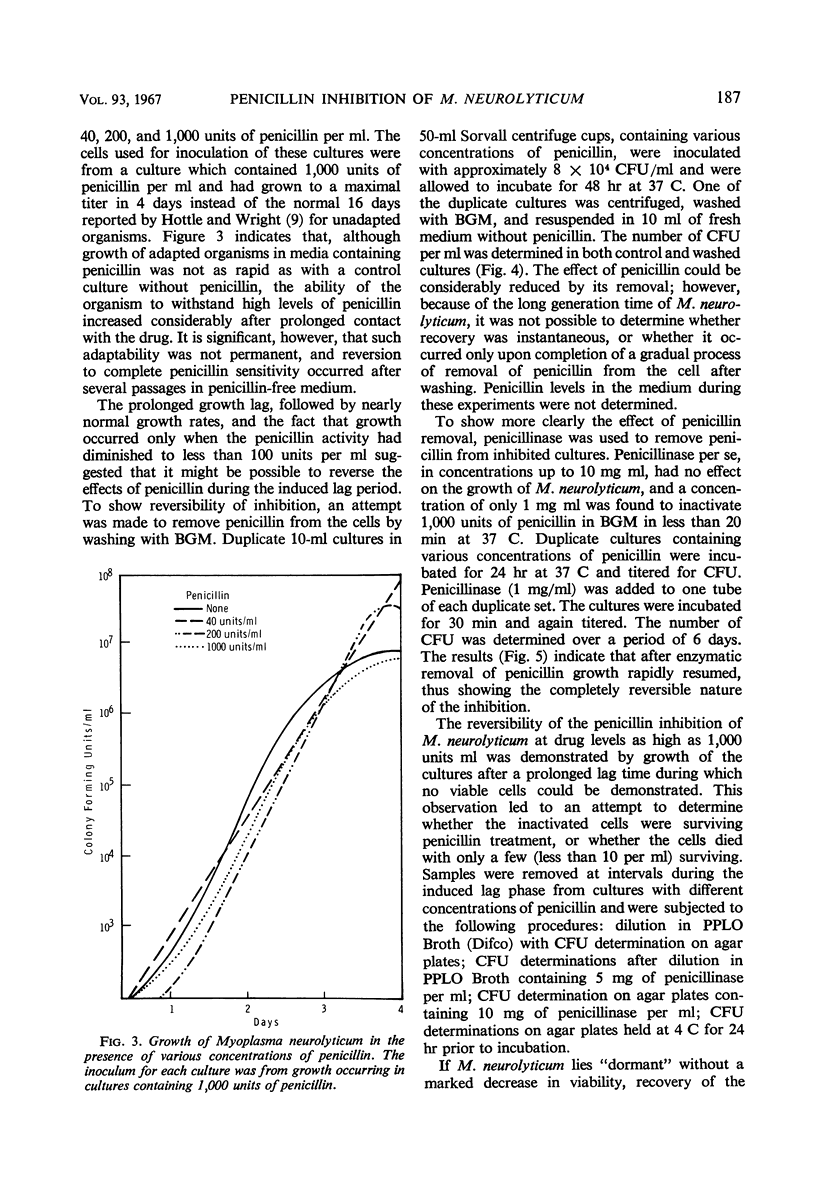
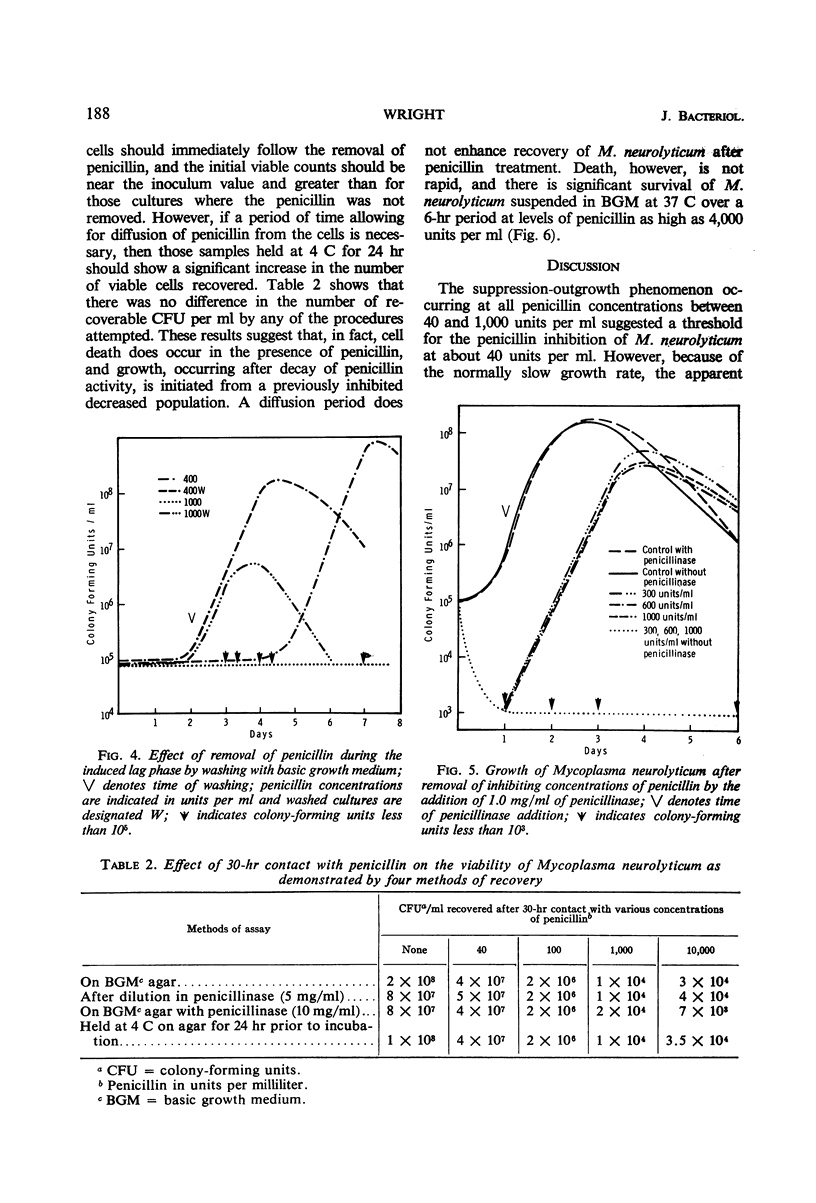
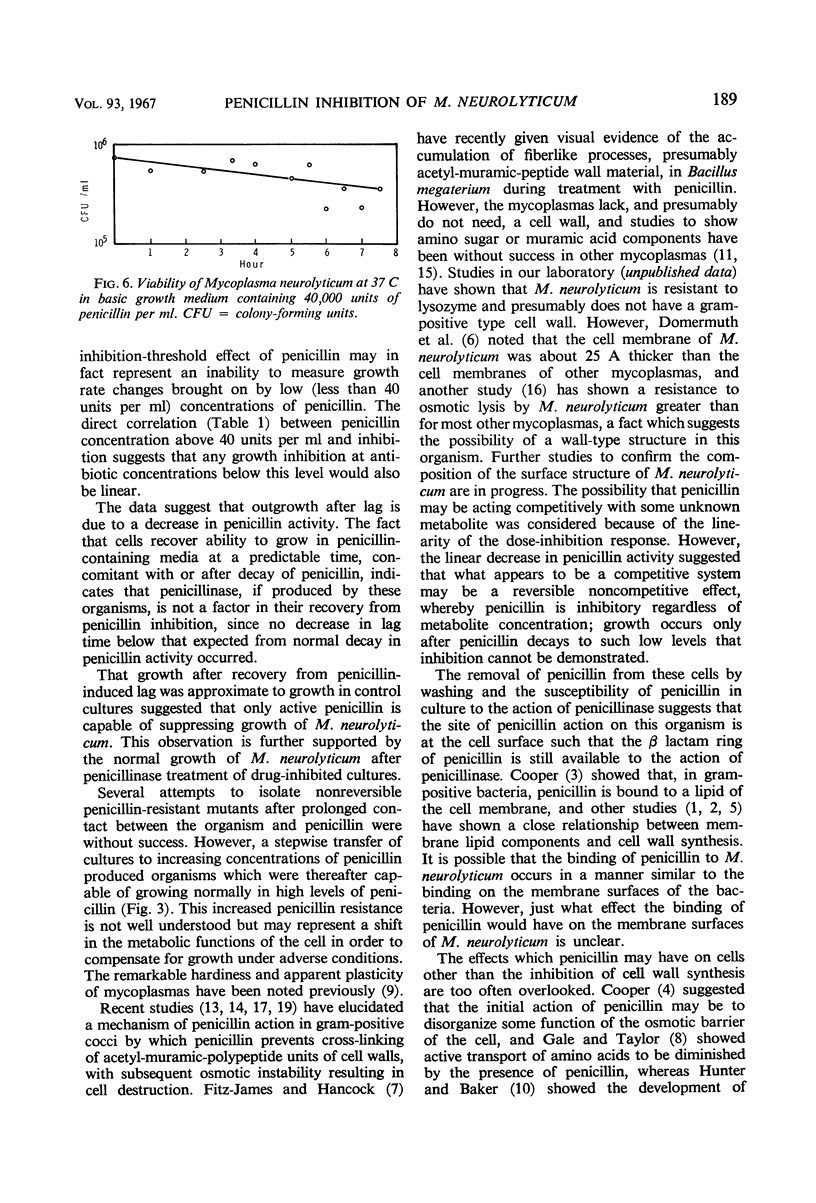
When penicillin was added to cultures of Mycoplasma neurolyticum in amounts up to 1,000 units per ml, lag time and generation time were increased and the total population was reduced in proportion to the antibiotic concentration. Although growth suppression by penicillin was complete, the death rate was slow and linear over periods up to 12 days. Growth after induced lag was due to a decay in penicillin activity and was not the result of mutant selection. However, repeated transfer in media which contained increasing concentrations of penicillin resulted in normal growth of M. neurolyticum at penicillin levels as high as 1,000 units per ml. Penicillinase did not play a role in recovery from penicillin-induced lag, and the inactive penicillin molecule did not prevent normal growth of M. neurolyticum. Removal of penicillin from the medium by washing or penicillinase during the induced lag was immediately followed by normal growth of the organism. These results suggest a reversible antibiotic function for penicillin which prevents multiplication of the organism by means unrelated to cell wall formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. S., Strominger J. L. Isolation and utilization of phospholipid intermediates in cell wall glycopeptide synthesis. Biochem Biophys Res Commun. 1965 Dec 9;21(5):516–521. doi: 10.1016/0006-291x(65)90414-6. [DOI] [PubMed] [Google Scholar]
- COOPER P. D. The association of the penicillin-binding component of Staphylococcus aureus with a lipid fraction. J Gen Microbiol. 1954 Apr;10(2):236–245. doi: 10.1099/00221287-10-2-236. [DOI] [PubMed] [Google Scholar]
- COOPER P. D. The site of action of penicillin: some changes in Staphylococcus aureus during the first two hours growth in penicillin media. J Gen Microbiol. 1955 Aug;13(1):22–38. doi: 10.1099/00221287-13-1-22. [DOI] [PubMed] [Google Scholar]
- DOMERMUTH C. H., NIELSEN M. H., FREUNDT E. A., BIRCH-ANDERSEN A. ULTRASTRUCTURE OF MYCOPLASMA SPECIES. J Bacteriol. 1964 Sep;88:727–744. doi: 10.1128/jb.88.3.727-744.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. P., Matsuhashi M., Strominger J. L. Glycerol diphosphate disaccharide-pentapeptide: a functional group of the lipid intermediate in cell wall glycopeptide synthesis. Biochem Biophys Res Commun. 1965 Dec 21;21(6):619–623. doi: 10.1016/0006-291x(65)90531-0. [DOI] [PubMed] [Google Scholar]
- Fitz-James P., Hancock R. The initial structural lesion of penicillin action in Bacillus megaterium. J Cell Biol. 1965 Aug;26(2):657–667. doi: 10.1083/jcb.26.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottle G. A., Wright D. N. Growth and survival of Mycoplasma neurolyticum in liquid media. J Bacteriol. 1966 May;91(5):1834–1839. doi: 10.1128/jb.91.5.1834-1839.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. H., Baker K. T. The Action of Penicillin on Bacillus subtilis Growing in the Absence of Amino Acids. Science. 1949 Oct 21;110(2860):423–425. doi: 10.1126/science.110.2860.423. [DOI] [PubMed] [Google Scholar]
- MARTIN H. H. COMPOSITION OF THE MUCOPOLYMER IN CELL WALLS OF THE UNSTABLE AND STABLE L-FORM OF PROTEUS MIRABILIS. J Gen Microbiol. 1964 Sep;36:441–450. doi: 10.1099/00221287-36-3-441. [DOI] [PubMed] [Google Scholar]
- Meadow P. M., Anderson J. S., Strominger J. L. Enzymatic polymerization of UDP-acetylmuramyl.L-ala.D-glu.L-lys.D-ala.D-ala and UDP-acetylglucosamine by a particulate enzyme from Staphylococcus aureus and its inhibition by antibiotics. Biochem Biophys Res Commun. 1964;14:382–387. doi: 10.1016/s0006-291x(64)80014-0. [DOI] [PubMed] [Google Scholar]
- PLACKETT P. On the probable absence of "mucocomplex" from Mycoplasma mycoides. Biochim Biophys Acta. 1959 Sep;35:260–262. doi: 10.1016/0006-3002(59)90362-2. [DOI] [PubMed] [Google Scholar]
- RAZIN S., ARGAMAN M. Lysis of Mycoplasma, bacterial protoplasts, spheroplasts and L-forms by various agents. J Gen Microbiol. 1963 Jan;30:155–172. doi: 10.1099/00221287-30-1-155. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- VAICHULIS E. M., DOLL J. P. Instability of penicillin in Dubos media. Am Rev Respir Dis. 1959 Aug;80:262–263. doi: 10.1164/arrd.1959.80.2.262. [DOI] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]



