Abstract
Neurotoxic doses of methamphetamine (METH) are known to cause depletions in striatal dopamine (DA) tissue content. However, the effects of METH-induced insults on dopaminergic neurotransmission are not fully understood. Here, we employed fast-scan cyclic voltammetry at a carbon-fiber microelectrode in the anesthetized rat striatum to assess the effects of a neurotoxic regimen of METH on phasic and tonic modes of dopaminergic signaling and underlying mechanisms of DA release and uptake. Extracellular DA was electrically evoked by stimulation of the medial forebrain bundle mimicking tonic and phasic firing patterns for dopaminergic cells and was monitored simultaneously in both the dorsomedial and dorsolateral striatum. Kinetic analysis of evoked recordings determined parameters describing DA release and uptake. Striatal DA tissue content was quantified by high performance liquid chromatography with electrochemical detection. METH-pretreatment (four doses of 7.5 or 10.0 mg/kg s.c.) induced DA depletions of ~40% on average, which are reported in both striatal subregions. METH pretreatment significantly decreased the amplitude of signals evoked by phasic, but not tonic, stimulation. Parameters for DA release and uptake were also similarly reduced by ~40%, consistent with effects on evoked phasic-like responses and DA tissue content. Taken together, these results suggest that METH-pretreatment selectively diminishes phasic, but not tonic, dopaminergic signaling in the dorsal striatum.
Keywords: dopamine, methamphetamine, neurotoxicity, voltammetry, phasic, tonic
Introduction
Methamphetamine (METH) is a highly addictive psychostimulant that has been shown to cause forebrain dopamine (DA) depletion when administered at high doses (Seiden et al. 1976; Ricaurte et al. 1980; Hotchkiss and Gibb 1980). While extensive work has investigated the cellular mechanisms of METH-induced dopaminergic (DAergic) neuronal injury (Davidson et al. 2001; Cadet et al. 2007), much less is known about the impact of this insult on DAergic neurotransmission. A neurotoxic regimen of METH has been shown to decrease dialysate DA collected in the striatum initially, but this concentration normalizes within one month (Cass and Manning 1999). Microdialysis is thought to assess DAergic tone, a steady-state, basal level of extracellular DA generated by low-frequency (~5 Hz) firing and integral to motor functioning (Schultz 2007a; Schultz 2007b). A similar compensatory maintenance of tonic DA signaling has been reported in the 6-hydroxydopamine (6-OHDA)-lesioned rat model of Parkinson’s disease (PD) (Zigmond et al. 1990). In this case, DAergic tone is maintained at normal levels across a wide range of striatal DA depletion (0 to ~80%) (Abercrombie et al. 1990; Robinson et al. 1994).
Another important mode of DAergic neurotransmission is phasic DA signaling, where bursts of high-frequency (>15 Hz) firing produce subsecond spikes in extracellular DA concentration (Carelli and Wightman 2004). These so-called DA transients can be recorded by real-time microsensors (Robinson et al. 2002; Robinson et al. 2008). The switch from tonic to phasic firing in DAergic neurons may be caused by auditory, visual, or other sensory input (Chiodo 1988; Overton and Clark 1997), including unpredicted rewards such as food, or cues (a tone or light, for example) that are paired to a reward (Schultz 1998). Interestingly, partial (<80%) DA lesions of the rat striatum generated by 6-OHDA and mimicking the preclinical phase of PD selectively reduce electrically evoked phasic-like DA signals, while leaving electrically evoked tonic-like DA signals intact (Garris et al. 1997; Bergstrom and Garris 2003). The effects of METH-induced neurotoxicity have not been examined on phasic DA signaling.
As neurotoxic regimens of METH produce DA depletions similar in degree to the 6-OHDA-lesioned rat model of preclinical parkinsonism (Bergstrom and Garris 2003), we speculate that METH-induced DAergic terminal degradation similarly causes a selective deficit in phasic DA signals. The purpose of the present study thus is to test this hypothesis by examining the effects of METH pretreatment on extracellular DA levels elicited using electrical stimulation at frequencies corresponding to phasic and tonic DAergic cell firing. Kinetic analysis of these evoked DA responses, monitored by fast-scan cyclic voltammetry (FSCV) at a carbon-fiber microelectrode (CFM), was used to assess the associated METH-induced changes in DA uptake and release mechanisms.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (250–450g) were purchased from Harlan (Indianapolis, IN, USA) and were housed in a light- and temperature-controlled vivarium. Access to food and water was provided ad libtum. All procedures conform to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Illinois State University.
Drugs and Chemicals
(±)-Methamphetamine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD, USA). METH doses were calculated as the free base. All other reagents were purchased from Sigma (St. Louis, MO, USA).
METH Pretreatment
The neurotoxic METH regimen is previously described (Daberkow et al. 2005). Briefly, rats were placed in plastic housing tubs (50 cm length x 40 cm width x 20 cm height: 4 rats/tub). METH was dissolved in 0.9% saline solution, and all injections were made subcutaneously. Injections of saline or METH (7.5 or 10 mg/kg) occurred every two hours until a total of four injections were administered. Directly after injections were made, and between injections, core body temperature was monitored rectally with a Thermalert TH-5 (Phystemp, Clifton, NJ, USA) every hour and two additional hours after the final injection.
Voltammetry
Rats were anesthetized with urethane (1.5 g/kg i.p.) and placed in a stereotactic apparatus (David Kopf Instruments, Tajunga, CA, USA). Skin and fascia were removed and four holes were drilled to allow lowering of two recording electrodes, a reference electrode, and a stimulating electrode. The Ag/AgCl reference electrode was placed in the contralateral cortex, anterior to both working electrodes. Evoked DA levels were recorded using FSCV at a CFM (Cahill et al. 1996) by applying a triangular waveform (-0.4 to 1.3 V and back, 400 V/s) every 100 ms. A CFM was implanted into both the dorsomedial (DM) and dorsolateral (DL) striatum (+1.2 AP; +1.0 and +4.0 ML, respectively; and − ~4.5 DV; Paxinos and and Watson 1986) at a 6° angle, to allow side-by-side ipsilateral placement. DA was recorded at a total of five depths (at 0.2 mm increments) to account for heterogeneity of evoked DA signals recorded voltametrically in the striatum (Garris et al. 1994) and the average of these five responses in each animal was compared. An EI400 bipotentiostat (Ensman Instruments, Bloomington, IN, USA) was used to perform FSCV, which was computer controlled using TH-1 software (ESA, Chelmsford, MA, USA). Current recorded through FSCV was converted to concentration by postcalibration of each electrode (Logman et al. 2000; Wu et al. 2001).
Electrical Stimulation
A twisted bipolar stimulating electrode (Plastics One, Roanoke, VA, USA), with tips separated by ~1 mm, was placed above the medial forebrain bundle (MFB) (−4.6 AP; +1.4 ML; and -8.0 DV) and then incrementally lowered until robust DA levels were evoked in the striatum. Once optimized, this electrode was not moved for the remainder of the experiment. Electrical stimulation was optically isolated, constant current (NL 800, Neurolog, Medical Systems, Great Neck, NY, USA), and synchronized with FSCV. Biphasic pulses (2 ms and 300 μA each phase) were applied as trains mimicking firing rates of DAergic neurons in either a phasic (30 Hz, 6 pulses) or tonic (5 Hz, 50 pulses) manner (Hyland et al. 2002). In addition, a high-frequency (60 Hz), long-train (60 pulses) stimulation was also applied. The high DA concentrations achieved by this stimulation, upwards of 1.5 μM, greatly exceed Km for DA uptake (0.2 μM), which makes these responses valuable for determining Vmax for DA uptake (Wu et al. 2001).
Kinetic Analysis of DA Release and Uptake
DA release and uptake were assessed using a mathematical model describing the rate of change in electrically evoked DA concentrations as the counteracting balance of these two mechanisms (Wightman et al. 1988):
| (1) |
where [DA]p refers to the extracellular DA concentration evoked per each stimulus pulse, Vmax represents the Michaelis-Menten term for maximal uptake rate, Km is the Michaelis-Menten term that is inversely proportional to the affinity of DA to the DA transporter, and f is the frequency of the stimulus train. Used in combination with curve fitting software incorporating a simplex minimization algorithm (Wu et al. 2001), values for both Vmax and [DA]p were determined, while Km was fixed at a value of 0.2 μM, which is the reported value in the anesthetized rat striatum (Wu et al. 2001). Responses evoked by all three stimulation parameter sets (5 Hz, 50 pulses; 30 Hz, 6 pulses; 60 Hz, 60 pulese) were simultaneously fit to determine one set of values for [DA]p and Vmax for each region in each animal. In addition to kinetic analysis of experimental data, simulated DA responses were generated and analyzed for maximal DA concentration. Simulated curves were produced using the average Vmax and [DA]p values determined from kinetic analysis of experimental data, in addition to a fixed Km of 0.2 μM, as is previously described by Bergstrom and Garris (2003).
DA Tissue Content
After voltammetric recordings, brains were extracted and the right hemisphere, where voltammetric signals were recorded, was submerged directly into ice cold saline and then placed in a −20°C freezer for ~20 min to firm the tissue (Bergstrom et al. 2001). Brains were then placed into an ice cold brain block and sliced into 1-mm sections using razor blades. Striatal portions, corresponding to recording locations from both the DM and DL striatum (~1 mm3), were dissected, wet weighed, and placed in a −80°C freezer until analysis of tissue DA content. Within two weeks, tissues were homogenized in 0.1 N perchloric acid and DA content was determined by high performance liquid chromatography using electrochemical detection (HPLC-EC) (BAS 200B, Bioanalytical Systems, West Lafayette, IN, USA) as previously described (Bergstrom and Garris 2003). The mobile phase was comprised of 0.5 g EDTA, 0.4 g octane sulfonic acid, 24.56 g monochloroacetic acid, 1.16 g sodium chloride, and 50 mL acetonitrile dissolved in 2 L of water (pH 2.8). To adjust for variability in the size of tissue samples, DA content was expressed per milligram protein, which was determined using a protein content kit (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
Statistical analysis was performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Data in Table 1 were analyzed using a factorial ANOVA with a Ryan-Einot-Gabriel-Welsch multiple range test to indicate significant differences. All other data was analyzed using a student’s t-test assuming equal variances. Significance was set at p < 0.05. Where applicable, data are expressed as mean ± SEM.
Table 1.
Effects of METH pretreatment on striatal tissue DA content.
| Dorsomedial Striatum | Dorsolateral Striatum | |||||
|---|---|---|---|---|---|---|
| METH Treatment (mg/Kg) | Saline | 7.5 | 10 | Saline | 7.5 | 10 |
| Tissue DA Content (ng/mg protein) | 132.28 ± 11.75 | 91.64 ± 11.57 * | 52.19 ± 12.02 *† | 147.17 ± 15.28 | 101.96 ± 16.19* | 64.52 ± 15.28 *† |
significantly (p < 0.05) different from saline
significantly (p < 0.05) different from 7.5 mg/Kg METH
Results
METH-induced Striatal DA Tissue Loss
METH caused a dose-dependent decrease in striatal tissue DA content. There was a significant main effect of dose (F2,40= 14.07, p < 0.0001) but not striatal region (F1,40= 1.19, p = 0.2822) or interaction (F2,40= 0.04, p = 0.9562). Both 7.5 and 10 mg/kg METH were significantly different from saline and each other (p < 0.05). Overall, dorsal striatal DA depletion was ~31 and ~58% for 7.5 and 10 mg/kg METH, respectively. These METH-induced denervation levels are consistent with previous reports (Ricaurte et al. 1980; Daberkow et al. 2005; Yuan et al. 2006). However, no significant dose effects of METH pretreatment were observed either on evoked DA concentrations or on parameters for DA release and uptake (data not shown). Therefore, all data for the two METH groups were subsequently pooled for further analysis. When combined, METH-pretreatment caused a significant loss of tissue DA content in the DM (75.39 ± 9.49 ng/mg protein or 43%; p = 0.0013) and DL (87.66 ± 9.49 ng/mg protein or 37%; p = 0.0115) striatum.
METH Pretreatment Alters Phasic-like, but not Tonic-like, DA Dynamics
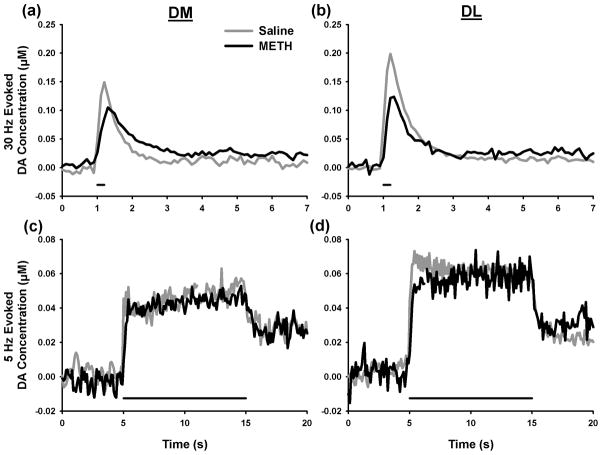
Figure 1 shows representative traces of evoked DA levels measured by FSCV at a CFM. Electrical stimulation mimicking phasic DAergic signaling (30 Hz, 6 pulses) generated transient, peak-shape changes in extracellular DA levels. Dynamics (~1 s) and amplitudes (~0.15 μM) of these evoked responses recorded in both the DM and DL striatum of saline-treated animals (grey lines, Figs. 1a and b, respectively) are consistent with naturally occurring DA transients monitored under awake, freely behaving conditions (Robinson et al. 2002), demonstrating the suitability of the selected phasic-stimulation pattern (see Discussion). In both the DM and DL striatum, METH pretreatment decreased the amplitude of evoked responses (black lines, Figs. 1a and b, respectively).
Figure 1.
Representative dopamine (DA) traces evoked by phasic (30 Hz, 6 pulses, top) or tonic (5 Hz, 50 pulses, bottom) stimulation. DA signals were recorded in the dorsomedial (DM, left) and dorsolateral (DL, right) striatal subregions. Horizontal bars under traces indicate stimulation initiation and duration.
Electrical stimulation mimicking tonic DAergic signaling (5 Hz, 50 pulses) generated steady-state responses in extracellular DA levels that reached a plateau shortly after commencement of the pulse-train application. In these representative traces collected in both the DM and DL striatum of saline-treated animals (grey lines, Figs. 1c and d, respectively), maximal DA levels were ~3-fold lower than those evoked by the phasic stimulation pattern. Evoked DA dynamics and concentrations are therefore consistent with dopaminergic tone (Bergstrom and Garris 2003). In contrast to evoked phasic-like DA responses, METH pretreatment exhibited no appreciable effect on evoked tonic-like DA responses recorded in either the DM or DL striatum (black lines, Figs. 1c and d, respectively).
METH Pretreatment Decreases the Amplitude of Phasic-like, but not Tonic-like, DA Responses
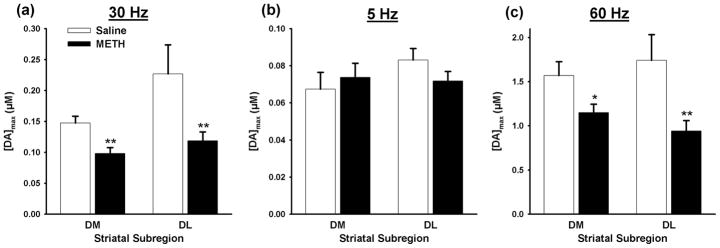
Figure 2 shows the effects of METH pretreatment on the amplitude of evoked DA responses. In both the DM and DL striatum, METH pretreatment significantly (p = 0.0032 and p = 0.0057, respectively) reduced maximal DA concentration evoked by stimulation mimicking phasic DAergic signaling (Fig. 2a). In contrast, METH pretreatment did not significantly (p = 0.311 and p = 0.0967, respectively) alter maximal DA concentration evoked by stimulation mimicking tonic DAergic signaling in either the DM or DL striatum (Fig. 2b). On average, the amplitude of phasic-like responses was ~3-fold greater than tonic-like responses in saline-treated animals.
Figure 2.
Maximum concentration of dopamine ([DA]max) evoked in the dorsomedial (DM) and dorsolateral (DL) striatal subregions of saline- and METH-pretreated rats. (a) phasic stimulation (30 Hz; 6 pulses), (b) tonic stimulation (5 Hz; 50 pulses) and (c) 60 Hz, 60 pulse stimulation. Data are expressed as means (± SEM). * p value < 0.05; **p < 0.01.
By comparison, the averaged amplitude of DA responses evoked by 60-Hz, 60-pulse stimulus trains was ten-fold greater than phasic-like signals in saline-treated animals (Fig. 2c). This high-frequency, long-train stimulation was used to evoke DA levels sufficiently above Km for DA uptake to improve the accuracy of the kinetic analysis (Wu et al. 2001). Similar to phasic-like DA traces, responses evoked by high-frequency, long-train stimulation are peak shaped (data not shown; Bergstrom and Garris, 2003), and METH pretreatment significantly (p = 0.0130 and p = 0.0033, respectively) decreases their maximal amplitude in both the DM and DL striatum. Taken together, these data indicate that denervation caused by METH pretreatment diminishes phasic-like, peak-shaped DA signals, but not tonic-like, steady-state DA signals in the dorsal striatum.
METH Pretreatment Decreases Parameters for DA Release and Uptake
Kinetic analysis of evoked DA traces was used to assess changes in DA release and uptake mechanisms associated with the effects of METH observed on phasic- and tonic-like DA responses. METH pretreatment significantly reduced Vmax for DA uptake (p = 0.0006 and p = 0.0003, respectively; Fig. 3a) and the DA release parameter, [DA]p (p = 0.0035 and p = 0.0097, respectively; Fig. 3b), in both the DM and DL striatum. Overall in both striatal subregions, the degree to which METH decreased DA uptake and release (47 and 40%, respectively), and phasic-like DA responses (41%) was similar to tissue DA content (41%), indicating a straightforward, proportional relationship between these indices of DAergic neurotransmission and DA depletion. On the contrary, tonic-like DAergic responses, which are negligibly altered by METH pretreatment, do not conform to this tendency.
Figure 3.
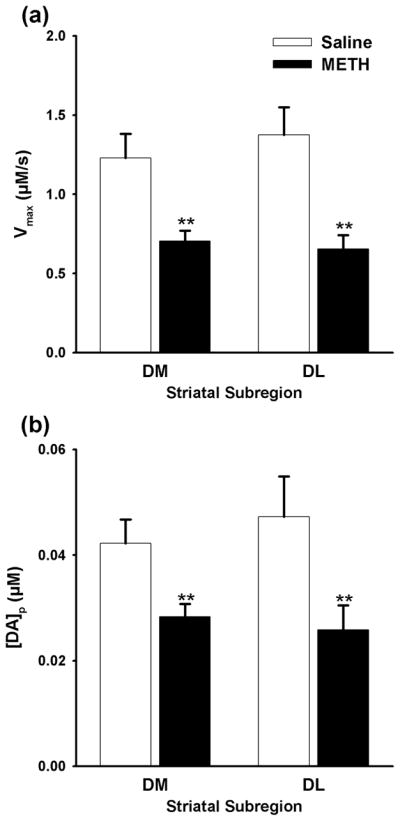
Dopamine (DA) uptake and release parameters in the dorsomedial (DM) and dorsolateral (DL) striatal subregions of METH- and saline-pretreated rats. (a) Vmax and (b) [DA]p. Data are expressed as means (± SEM). **, p < 0.01.
Simulated DA Release Supports Experimental Results
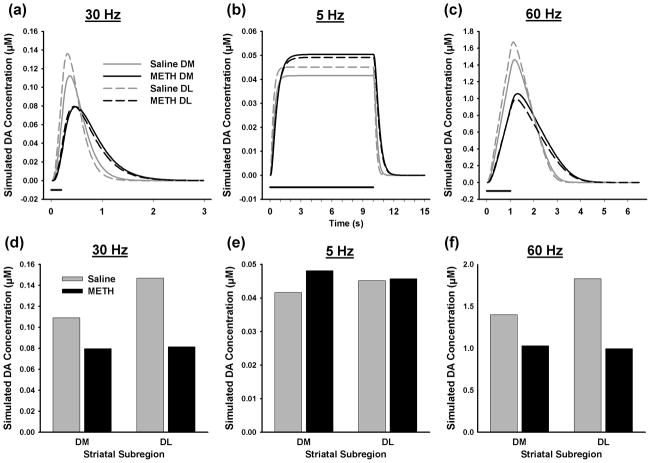
Coupling real-time voltammetric monitoring and kinetic analysis provides a unique opportunity to assign underlying mechanisms of DA release and uptake to recorded dynamic changes in extracellular DA levels generated by electrical stimulation (Wu et al. 2001). To assess the veracity of this approach for investigating the effects of a neurotoxic regimen of METH on DAergic neurotransmission, evoked DA responses were calculated from averaged parameters determined by the kinetic analysis procedure for each subregion. DA response curves are shown in Figure 4 along with the maximal amplitude of these simulated responses for phasic (Figs. 4a and d), tonic (Figs. 4b and e), and high-frequency, long-train (Fig. 4c and f) stimulation. Overall, there is good agreement between theoretical and experimental (Fig. 2) results in terms of extracellular DA dynamics and absolute DA concentrations evoked by the different stimulation patterns and the effects of METH pretreatment in both DM and DL striatum, suggesting that the kinetic analysis was veracious.
Figure 4.
Simulated tonic- and phasic-like DA responses. Calculations were based on average values for Vmax and [DA]p shown in Figure 3 and assumed a Km of 0.2 μM. Individual curves are shown in (a), (b) and (c), and maximal DA concentrations are shown in (d), (e) and (f) for phasic, tonic and 60-Hz, 60-pulse stimulations, respectively.
Discussion
Combined Chemical Microsensor, Kinetic Analysis and Simulation Approach
To study the effects of a neurotoxic regiment of METH on DAergic neurotransmission, the present study employed real-time microsensors for monitoring electrically evoked DA levels, kinetic analysis for determination of DA release and uptake mechanisms, and calculations using these best-fit kinetic parameters to simulate measured responses. We have previously used this same combined approach successfully for characterizing, for example, the psychostimulant cocaine (Wu et al. 2001), dopamine autoreceptors (Wu et al. 2002), 6-OHDA lesions mimicking PD (Bergstrom and Garris 2003), and mitochondrial impairment mimicking Huntington’s disease (Kraft et al. 2009). The primary advantage is that perturbations of extracellular DA can be assigned fundamentally to changes in the two primary mechanisms governing these concentrations, DA release and uptake. Moreover, simulations of measured responses serve to verify the veracity of the kinetic analysis. To our knowledge, this is the first report applying this approach to a METH neurotoxicity model.
The major weakness of the experimental approach used in the present study is that the tonic and phasic DA signals under investigation are artificial. However, we argue that they are physiologically relevant based on several important criteria. First, physiological firing rates were selected to evoke the tonic- and phasic-like DA responses. Second, measured DA dynamics are consistent with tonic (steady state) and phasic (transient and peak-shaped) DA signals. Third, the amplitude of phasic-like DA responses mirrors behaviorally associated DA transients recorded in awake animals (Robinson et al. 2002) and are greater than those of tonic-like DA responses, as expected based on theoretical calculations (Venton et al. 2003). Fourth, in the 6-OHDA-lesioned model of PD, evoked tonic-like DA responses (Garris et al. 1997; Bergstrom and Garris 2003) demonstrate the same denervation profile across the preclinical spectrum as dialysate DA (Abercrombie et al. 1990; Robinson et al. 1994), a more established index of DAergic tone. Taken together, these characteristic suggest that the tonic- and phasic-like DA signals used in the present study are valid indices for these two modes of DAergic neurotransmission.
Compensatory Maintenance of DAergic Tone after Partial DA Depletion
A hallmark of PD is the extended preclinical phase, during which cardinal symptoms do not present until striatal tissue DA loss is quite severe (>80%; Hornykiewicz and Kish 1987). Parallel microdialysis measurements in the 6-OHDA-lesioned rat model of PD suggest that motor behavior is preserved by the normalization of DAergic tone across the presymptomatic denervation range (Abercrombie et al. 1990; Robinson et al. 1994). A similar phenomenon appears to occur following the partial DA depletions induced by a neurotoxic METH regimen, which are typically below 80% (Ricaurte et al. 1980; Daberkow et al. 2005; Yuan et al. 2006), with a re-instatement of dialysate DA to control levels by one month after METH pretreatment (Cass and Manning 1999). Maintenance of dialysate DA in both 6-OHDA-lesioned and neurotoxic-METH rats could indicate a similar compensatory adaptation to striatal DA depletion.
Whether DA release is up-regulated in the setting of partial DA depletion in parkinsonian animals (Zigmond et al. 1990; Zigmond 1997) is controversial, with some studies supporting this view (Stachowiak et al. 1987; Snyder et al. 1990; McCallum et al. 2006; Perez et al. 2008), but others finding no evidence for this compensatory adaptation (Garris et al. 1997; Bergstrom and Garris 2003; Bezard et al. 2003). In the present study with a different DAergic neurodegenerative model, a neurotoxic regimen of METH, we also fail to demonstrate activated DA release. Moreover, we additionally established that tonic-like DA responses are maintained at control levels, and could be simulated as such with fidelity, without up-regulated DA release. Although these results are in excellent agreement with our previous work in the 6-OHDA-lesioned rat model of PD (Garris et al. 1997; Bergstrom and Garris 2003), it should be stated with caution that a similar experimental approach combining voltammetric microsensors and kinetic analysis (Wu et al. 2001) was used (see above). Interestingly, the amplitude of DA levels evoked by a depolarizing K+ solution when measured by in vivo voltammetry and evoked by amphetamine when measured by microdialysis are both reduced by neurotoxic doses of METH (Cass 1997; Cass and Manning 1999), in apparent agreement with non-compensated DA release.
Compromised Phasic DAergic Signaling after Partial DA Depletion
Much less is known about the status of phasic DAergic signaling after DA depletion. Surviving nigrostriatal DAergic neurons in the human parkinsonian brain still retain their ability to respond with phasic bursts of action potentials to an unexpected reward (Zaghloul et al. 2009). However, procedural learning is altered in PD, which can be theoretically modeled by diminished phasic DAergic signaling (Frank et al. 2004). In 6-OHDA-lesioned rats, there is a compensatory increase in burst firing of DAergic cells in the substantia nigra, but this adaptation requires striatal DA depletion that is nearly complete (~95%; Hollerman and Grace 1990). Phasic-like DA responses evoked by stimulation and recorded by microsensors are also decreased in both rat (Garris et al. 1997; Bergstrom and Garris 2003) and mice (Bezard et al. 2000) parkinsonian models. Taken together, these results suggest that while the capability for burst firing by nigrostriatal DAergic neurons is conserved in PD, phasic DA signals may be compromised in the striatum.
In excellent agreement but in a different neurodegenerative model, we show here using a physiological firing pattern that phasic-like DA responses recorded in the striata of rats with partial DA loss induced by a neurotoxic regimen of METH are similarly diminished in amplitude. Moreover, this decrement is associated with proportional decreases in DA release and uptake, and tissue DA content. We have previously postulated the “Passive Stabilization” hypothesis (Bergstrom and Garris 2003) to account for all of these relationships, as well as the compensatory maintenance of tonic-like DA signals. In contrast to the tonic-like DA responses, which are steady state and governed by the balance of DA release and uptake, phasic-like DA responses are peak shaped and dominated by DA release (Venton et al. 2003). Thus, while decreased DA release is offset by decreased DA uptake for tonic-like DA responses, resulting in no change in the steady-state level, it reduces the amplitude of phasic-like DA signals, because DA uptake contributes less.
Implications of METH-induced Decreases in Phasic DAergic Signaling
Diminished phasic DAergic signaling may contribute to cognitive deficits reported for METH addicts (Simon et al. 2000; Volkow et al. 2001; Scott et al. 2007). In addition to decline in fine and gross motor coordination and decreased performance on auditory verbal memory tasks (Volkow et al. 2001), METH abuse has been shown to deleteriously affect individuals' ability to recall words and pictures, as well as sort information (Simon et al. 2000). However, the relation between diminished phasic DAergic signaling and these cognitive impairments is not known. On the one hand, not all cognitive deficits observed in animal models of METH exposure are associated with significant loss of striatal DA tissue content DA loss, as an extended-access METH self-administration model is associated with impaired novel object recognition, but not tissue DA loss in dorsal striatum (Rogers et al. 2008; Schwendt et al. 2009). Thus, it is likely that not all cognitive deficits observed in human METH abusers will be secondary to altered phasic DA transmission in the dorsal striatum.
On the other hand, a METH-induced reduction in the amplitude of phasic DA signals in the dorsal striatum, as we propose here, may compromise behaviors requiring this important DAergic signaling mode. In general, phasic DA-related behaviors have been established to include the acquisition of conditioned responses and cue-based reward learning (Zweifel et al. 2009). Specifically, subregion-localized and DA-associated behaviors in the striatum, such as action-outcome learning in the medial portion and stimulus-response learning in the lateral portion (Yin et al. 2004; Yin and Knowlton 2006), may be affected, as suggested by preliminary data from our laboratory showing impaired formation of stimulus-response associations underlying instrumental learning in METH-pretreated rats (Son et al. 2010). After METH pretreatment, deficits in reversal learning also have been observed (Izquierdo et al. 2010), as have deficits in the relationship between expression of the effector immediate early gene Arc (activity regulated, cytoskeleton-associated) in DM striatum and reversal learning (Daberkow et al. 2008). Preliminary data from our laboratory further suggest that rats with such partial striatal DA loss induced by METH no longer rely on DM striatal function to solve a reversal learning task (Pastuzyn and Keefe 2010). Finally, METH-pretreated rats show impairments on a basal ganglia-mediated, sequential motor-learning task (Chapman et al. 2001; Daberkow et al. 2005). These findings clearly indicate impairment of basal ganglia-mediated learning and memory functions. The extent to which it is impairment in phasic DAergic signaling as reported herein per se that underlies these deficits in basal ganglia-mediated learning and memory functions remains to be determined, as does the extent to which specific basal ganglia-mediated learning and memory functions are disrupted in humans with a history of METH abuse.
Acknowledgments
This research was supported by National Institute on Drug Abuse grant DA 024036. CDH was sponsored by the Mockford Fellowship and Weigel Grant through Phi Sigma at Illinois State University. DPD was supported by POENB at Illinois State University. We thank Kim Garris, Kendra Bunner, Stephen Kraniotis, and John Robinson for skilled technical assistance.
Abbreviations
- DA
dopamine
- DAergic
dopaminergic
- METH
methamphetamine
- 6-OHDA
6-hydroxydopamine
- PD
Parkinson’s disease
- DAT
dopamine transporter
- DM
dorsomedial
- DL
dorsolateral
- FSCV
fast-scan cyclic voltammetry
- CFM
carbon fiber microelectrode
- MFB
medial forebrain bundle
Reference List
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Bergstrom BP, Garris PA. "Passive stabilization" of striatal extracellular dopamine across the lesion spectrum encompassing the presymptomatic phase of Parkinson's disease: a voltammetric study in the 6-OHDA-lesioned rat. J Neurochem. 2003;87:1224–1236. doi: 10.1046/j.1471-4159.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom BP, Schertz KE, Weirick T, Nafziger B, Takacs SA, Lopes KO, Massa KJ, Walker QD, Garris PA. Partial, graded losses of dopamine terminals in the rat caudate-putamen: an animal model for the study of compensatory adaptation in preclinical parkinsonism. J Neurosci Methods. 2001;106:15–28. doi: 10.1016/s0165-0270(00)00372-1. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci. 2003;26:215–221. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Bezard E, Jaber M, Gonon F, Boireau A, Bloch B, Gross CE. Adaptive changes in the nigrostriatal pathway in response to increased 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration in the mouse. Eur J Neurosci. 2000;12:2892–2900. doi: 10.1046/j.1460-9568.2000.00180.x. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal Chem. 1996;68:3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280:105–113. [PubMed] [Google Scholar]
- Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Chiodo LA. Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology. Neurosci Biobehav Rev. 1988;12:49–91. doi: 10.1016/s0149-7634(88)80073-3. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons. Neurotox Res. 2008;14:307–315. doi: 10.1007/BF03033855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Wightman RM. Heterogeneity of evoked dopamine overflow within the striatal and striatoamygdaloid regions. Neuroscience. 1994;59:417–427. doi: 10.1016/0306-4522(94)90606-8. [DOI] [PubMed] [Google Scholar]
- Garris PA, Walker QD, Wightman RM. Dopamine release and uptake rates both decrease in the partially denervated striatum in proportion to the loss of dopamine terminals. Brain Res. 1997;753:225–234. doi: 10.1016/s0006-8993(97)00003-6. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Grace AA. The effects of dopamine-depleting brain lesions on the electrophysiological activity of rat substantia nigra dopamine neurons. Brain Res. 1990;533:203–212. doi: 10.1016/0006-8993(90)91341-d. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson's disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O'Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft JC, Osterhaus GL, Ortiz AN, Garris PA, Johnson MA. In vivo dopamine release and uptake impairments in rats treated with 3-nitropropionic acid. Neuroscience. 2009;161:940–949. doi: 10.1016/j.neuroscience.2009.03.083. [DOI] [PubMed] [Google Scholar]
- Logman MJ, Budygin EA, Gainetdinov RR, Wightman RM. Quantitation of in vivo measurements with carbon fiber microelectrodes. J Neurosci Methods. 2000;95:95–102. doi: 10.1016/s0165-0270(99)00155-7. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Perez XA, Bao S, McIntosh JM, Grady SR, Quik M. Compensation in pre-synaptic dopaminergic function following nigrostriatal damage in primates. J Neurochem. 2006;96:960–972. doi: 10.1111/j.1471-4159.2005.03610.x. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Pastuzyn ED, Keefe KA. Impact of partial dopamine loss induced by methamphetamine on Arc-mediated, striatally-based learning. Soc Neurosci Abstr. 2010:577.16. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Perez XA, Parameswaran N, Huang LZ, O'Leary KT, Quik M. Pre-synaptic dopaminergic compensation after moderate nigrostriatal damage in non-human primates. J Neurochem. 2008;105:1861–1872. doi: 10.1111/j.1471-4159.2008.05268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Hermans A, Seipel AT, Wightman RM. Monitoring rapid chemical communication in the brain. Chem Rev. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Mocsary Z, Camp DM, Whishaw IQ. Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. J Neurosci. 1994;14:2687–2696. doi: 10.1523/JNEUROSCI.14-05-02687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007a;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007b;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Keller RW, Jr, Zigmond MJ. Dopamine efflux from striatal slices after intracerebral 6-hydroxydopamine: evidence for compensatory hyperactivity of residual terminals. J Pharmacol Exp Ther. 1990;253:867–876. [PubMed] [Google Scholar]
- Son J-H, Garcia C, Keefe KA. Impaired formation of stimulus-response, but not action-outcome associations in rats with partial dopamine loss induced by methamphetamine neurotoxicity. Soc Neurosci Abstr. 2010:577.14. [Google Scholar]
- Stachowiak MK, Keller RW, Jr, Stricker EM, Zigmond MJ. Increased dopamine efflux from striatal slices during development and after nigrostriatal bundle damage. J Neurosci. 1987;7:1648–1654. doi: 10.1523/JNEUROSCI.07-06-01648.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem. 2003;87:1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Hatzidimitriou G, Suthar P, Mueller M, McCann U, Ricaurte G. Relationship between temperature, dopaminergic neurotoxicity, and plasma drug concentrations in methamphetamine-treated squirrel monkeys. J Pharmacol Exp Ther. 2006;316:1210–1218. doi: 10.1124/jpet.105.096503. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Blanco JA, Weidemann CT, McGill K, Jaggi JL, Baltuch GH, Kahana MJ. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323:1496–1499. doi: 10.1126/science.1167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ. Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol Dis. 1997;4:247–253. doi: 10.1006/nbdi.1997.0157. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Stricker EM. Partial damage to nigrostriatal bundle: compensatory changes and the action of L-dopa. J Neural Transm Suppl. 1990;29:217–232. doi: 10.1007/978-3-7091-9050-0_21. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]