Abstract
The nuclear lamina is increasingly being appreciated for its epigenetic role in regulating gene expression. The nuclear lamina underlies the inner nuclear membrane and, in post mitotic cells, is composed of a latticework primarily formed by the intermediate filament protein, lamin A/C. Although not well defined, lamin-associated domains have been described, and these domains are determined by DNA sequence and chromatin conformation. Lamin-associated domains are positioned to mediate the interaction with the nuclear membrane, where they contribute to transcriptional regulation. Although lamin-associated domains are primarily considered to be repressive in nature, those nearer to nuclear pores may actually promote transcription. Mutations in LMNA, the gene encoding lamins A and C, are a relatively common cause of inherited cardiomyopathy. As substantial data supports a role for the lamina in its interaction with chromatin and gene regulation, we examined the role of a genetically disrupted lamina and the consequences thereof. A dominant LMNA mutation, E161K, that causes inherited cardiomyopathy was studied. Gene expression changes were profiled in a human cardiomyopathic E161K heart, and it was found that chromosome 13 had a high percentage of misexpressed genes. Chromosome 13 was also found to be less tightly associated with the nuclear membrane in E161K mutant cells, thereby linking abnormal gene expression and intranuclear position. These and other studies support a role for the nuclear membrane as an active regulator of gene expression and provide additional support that disrupting this regulation is a mechanism of human disease.
Key words: chromosome territory, gene expression, LMNA, LINC complex
Individual chromosomes are organized in interphase nuclei into chromosome territories. The organization of chromatin in the nucleus is thought to participate in the epigenetic regulation of gene expression with whole chromosome territories or regions of chromosomes shifting within the three dimensional space of the nucleus concomitant with changes in gene expression.1,2 Chromosome territories change in response to differentiation. Chromosome territories also relocalize in proliferating cells with quiescence or senescence.3,4 The structural support for chromatin territories is not well understood but one scaffold is the nuclear lamina. The nuclear lamina is a fibrous structure composed of the A- and B-types lamins and lamin-associated proteins. The B-type lamins are expressed in all somatic cells, while the A-type lamins, lamin A and C, are characteristically expressed in terminally differentiated cells. Lamins A and C are alternatively spliced isoforms encoded by the LMNA gene. Mutations in LMNA lead to cardiac and skeletal myopathies, lipodystrophies, neuropathies and premature aging disorders. The majority of LMNA mutations are dominant and presumed to act through a gain of function effect. Herein, we discuss intranuclear chromosome position and propose a model of how mutations in lamin A/C and associated proteins may disrupt intranuclear positioning leading to aberrant gene expression that may contribute to disease pathogenesis.
The Lamina as a Regulator of Gene Expression
In most cells, a dense heterochromatin network is visualized as electron dense material adjacent to the inner nuclear membrane and the nuclear lamina. The dense network is typically considered to reflect the association of transcriptionally inactive genes with the nuclear lamina. Specifically, this heterochromatin is thought to reflect a repressed gene state and suggests that the nuclear membrane and lamina may play an active role in gene repression.5 Lamin A/C and its binding partners can interact with DNA, histones, transcription factors and chromatin.6 Lamin-associated domains (LADs) are cis-acting sequences thought to range in size from ∼0.1–10 Mb, and these domains are thought to regulate the interaction of chromatin with the nuclear lamina; generally LADs are considered to be transcriptionally repressive.7 The precise sequences that define LADs have been elusive but nonetheless the LAD concept implies that the lamina and the genome interact in a specific manner. Therefore, it follows that one mechanism by which LMNA mutations may confer pathology is by altering the interaction between the lamina and chromatin. Consistent with this, LMNA mutant cells have altered chromosome territories.4 Meaburn and colleagues first examined chromosome localization in proliferating LMNA mutant fibroblasts and found that it resembled the chromosome organization associated with quiescent or senescent fibroblasts. They suggested that mutations in LMNA produced structural disruption to the lamina inducing a chromatin organization that may resemble an inappropriate cell cycle state. The presence of LADs and the fact that mutations in LMNA associate with altered nuclear architecture suggest that the nuclear lamina plays a vital role in both scaffolding chromatin and regulating chromatin organization. In this model, it is possible that distinct LMNA mutations may alter distinct regions of the genome leading to mutation specific profiles of altered gene expression.
Lamina-Chromosome Reorganization during Differentiation
During differentiation, gene programs are under tight regulation. Higher order chromatin organization contributes to the fine control of this transcriptional regulation. Whole chromosome movement has been noted during erythroid differentiation, adipogenesis, T-cell differentiation and keratinocyte differentiation.8–11 Recent work has shown that there are dramatic changes in the interactions between chromatin and the nuclear lamina during terminal differentiation.12 Regions of the genome that associate with the nuclear lamina can be marked using DNA adenine methylase (Dam) referred to as DamID.13–15 With this method, a nuclear membrane protein is fused to Dam methylase and then expressed in cells resulting in methylation of those sequences in close proximity to the nuclear membrane protein. The methylated sequences are commonly determined using tiling arrays. DamID using lamin B1 was used to map genome-nuclear lamina interactions in progressive stages of the neural differentiation pathway, embryonic stem cells, neural precursor cells and astrocytes. Lamin-associated domains (LADs) were found to cover approximately 40% of the genome, and the results were similar between human and mouse cells.7 Features of LADs included comparatively low gene density and those genes that were present within LADs were expressed at very low levels. Promoters found within LADs generally carried repressive histone marks. Together, these data suggest that LADs are repressive.
The LAD maps for multiple cell types were found to share many genomic regions in common, and large scale changes in the LAD maps were not seen with differentiation. Rather, the LAD maps underwent smaller scale refinement during differentiation.12 Many genes associated with pluripotency or “stemness” became associated with the nuclear lamina, concomitant with reduced expression levels, as differentiation progressed. Closer analysis revealed that single transcription units were released from the lamina with differentiation. With larger transcriptional units that contain multiple genes, intragenic regions were also released from the lamina. Together, these data support a model where the lamina participates in differentiation by contributing to repression of stemness genes and release and expression of maturation genes.12 The authors also noted that while 4,052 genes of the 13,798 tested had decreased expression, only 215 had enhanced association with the nuclear lamina. Therefore, association with the nuclear lamina is not a consequence of loss of transcriptional activity, but a more directed and specific process for certain genes. There were also a number of loci that were released from the lamina during differentiation without upregulation of expression. The authors provide a model whereby loci are released from the lamina in one stage of differentiation and while not transcriptionally active are “unlocked”. Their “unlocked” status leaves them available for transcription at later stages of differentiation. The authors propose that this “unlocked” status is important for cell type identity.12 These data provide evidence that the nuclear lamina acts to regulate gene expression in a cell-type specific manner.
LMNA Mutations Alter Gene Expression and Chromosome Territories
We recently sought to determine large-scale genomic changes in response to mutations in the nuclear lamina.16 Mutations in the gene encoding lamin A/C cause a variety of diseases including cardiac and skeletal muscle disease. Because LMNA mutations are a common cause of inherited dilated cardiomyopathy, we examined gene expression changes in an LMNA mutant heart and fibroblasts. Specifically, we studied the E161K mutation because this mutation has been associated with inherited cardiomyopathy.17 We hypothesized that the lamin A/C mutation would disrupt genomic interactions with the nuclear lamina. We expected that these interactions would be larger than single genes and would mimic the size of LADs. To test this hypothesis we searched for genomic clusters of misexpressed genes. Ten regions containing two misexpressed genes each were identified, and two misexpressed gene clusters were further analyzed because they each harbor genes linked to striated muscle disease. Three dimensional fluorescence in situ hybridization (3D FISH) using probes for each of the two clusters revealed that both clusters were mislocalized in LMNA mutant cells. Interestingly, both loci were displaced toward the nuclear interior and away from the periphery compared to control fibroblasts. Notably, both genes in each cluster were downregulated in the mutant heart compared to control, providing further evidence that localization to the periphery does not automatically result in gene repression.
With this analysis, it was also found that misexpressed genes were not randomly distributed across the genome, as might be expected. The misexpressed genes in LMNA mutant versus LMNA normal hearts or fibroblasts were evaluated. Three percent of the genes were misregulated in the heart and 6% were misregulated in the fibroblasts. Surprisingly, chromosome 13 had the greatest percentage of misexpressed genes and the two clusters that were studied using 3D FISH were both found on chromosome 13. Whole chromosome analysis of the intranuclear position of chromosome 13 revealed that the entire chromosome 13 territory was internally displaced compared to control cells (Fig. 1). Chromosome 7 did not have a greater than expected percentage of misexpressed genes and we hypothesized that it would not be displaced in the LMNA mutant fibroblasts. Whole chromosome analysis of chromosome 7 revealed that the chromosome 7 territory is not significantly displaced in mutant cells, indicating that territory mislocalization is not a general defect of the LMNA mutant cells. The chromosome 13 territory in two other lamin A/C mutant fibroblast lines was also examined. One mutation p.Ser303Cysfs27* is a frameshifting dominant mutation associated with inherited cardiomyopathy.18 The other, D596N, is associated with both cardiomyopathy and muscle disease.19 Gross mislocalization of chromosome 13 was also seen in both these LMNA mutants; however, in these two lines chromosome 13 was found completely abutted against the nuclear periphery. These data provide evidence that LMNA mutations not only associate with altered chromosome territories but that each mutation may impart unique effects. Given the broad range of clinical findings with LMNA mutations, this mechanism may explain some of the variable but allele-specific phenotypes. We provide a model whereby changes in gene expression result from large scale chromatin rearrangements because chromatin is displaced either towards or away from transcriptional complexes (Fig. 2).
Figure 1.
LMNA mutations disrupt the nuclear lamina and alter chromosome territories. To the left is shown a normal nucleus with chromosome territories indicated in gray. Chromosome 13 territory is schematized in pink. The nucleolus is in black and nuclear speckles are in orange. With the LMNA E161K mutation (center), chromosome 13 shows the highest percentage of misexpresed genes and the entire chromosome was displaced to the nuclear interior.16 With the LMNA D596N mutation, the chromosome 13 territory was shown abutted against the nuclear membrane. With LMNA mutations, the nuclei may be unusually shaped and the nuclear lamina may be disorganized disrupting its interaction with chromatin.
Figure 2.
Model for nuclear membrane disruption from LMNA mutations. The normal nuclear lamina is shown on the left and an LMNA mutant is depicted on the right. The proteins of the LINC (LInks the Nucleus to the Cytoplasm) complex are indicated and include the nesprins (light blue), SUN proteins (pink), emerin (green) and lamins (maroon). Chromatin (black) interacts with the lamina and active transcription complexes (orange) in normal cells, but is relocalized to the interior in the LMNA mutant cells and therefore cannot interact with transcription complexes.
Transcriptional Complexes and the Nuclear Lamina
Gene expression is partially regulated by bringing chromatin in contact with compartments of the nucleus that either upregulate or downregulate transcription. During differentiation, this nuclear reorganization may be particularly relevant. For example, during adipogenesis the nuclear position of several genes is internalized correlating with the upregulation of gene expression.20 These small-scale changes mirror the large-scale movement of whole chromosome territories of the respective genes. The relationship and regulation between chromosome territory and gene expression is not well understood. One possibility is that territories move to position specific loci in areas compatible with active gene expression. SC-35 domains, also known as splicing domains or nuclear speckles, are structures enriched for splicing components, poly (A) RNA and mRNA metabolic factors. SC-35 domains are consistently found in somatic nuclei and are associated with active genes. Szczerbal and colleagues examined genes that are activated during porcine adipogenesis, with little or no expression in mesenchymal stem cells. The location of these loci in the nucleus was determined with respect to SC-35 domains.21 Genes expressed during differentiation were more often associated with nuclear speckles in mature adipocytes compared to mesenchymal stem cells. There were no differences in the number or distribution of SC-35 domains between the two cell types. These results are comparable to earlier studies in muscle. Muscle-specific genes were localized to SC-35 domains in terminally differentiated muscle, but not in myoblasts. Genes necessary for differentiation are moved into SC-35 domains to allow for fast and efficient transcription.22 Together, these data suggest that genes involved in differentiation may move to SC-35 domains for efficient transcription. These changes in chromatin localization and gene expression may help confer tissue identity to an undifferentiated tissue.
Recent work has shown that undifferentiated human embryonic stem cells lack defined SC-35 domains. SC-35 domains form as pluripotent stem cells commit to specific lineages.23 LMNA mutations have been hypothesized to disrupt differentiation, particularly in muscle and fat, and therefore, altered nuclear architecture from LMNA mutations may impair differentiation or maintenance of maturation directly.24,25 Inappropriate chromatin association with the nuclear lamina, as is thought to occur from LMNA mutations, may create a cascade effect that disrupts normal chromatin relocalization important for differentiation. Consistent with this, several studies have suggested that differentiation is impaired in LMNA mutant cells.26–28 This differentiation defect may be the result of impaired lamina-chromatin interaction resulting in aberrant gene expression.
Transcription Near the Nuclear Lamina
Although the nuclear lamina is thought to be associated with gene repression, one notable exception to this generalization is the nuclear pore complex, which is associated with open chromatin configuration and gene transcription.29,30 The nuclear pore complex (NPC) is a multiprotein complex that spans the outer and inner nuclear membrane. The NPC is composed of approximately 30 nucleoporins (NUPs) and serves as a gatekeeper between the nucleus and cytoplasm. The NPC also serves as an attachment site for chromatin.31 The mechanisms that control gene activation at the nuclear pore are not well understood. In Drosophila, nucleoporin-chromatin binding is important for chromosomal organization and gene expression.11 Histone deacetylases (HDACs) can modify NUPs and alter their association with chromatin.32 Post-translational histone modifications contribute to chromatin configuration and transcriptional regulation. Histone acetyltransferases (HATs) acetylate conserved histone residues and create an open chromatin conformation that is favorable for transcriptional activity while HDACs promote a closed chromatin conformation and repress gene expression. HATs and HDACs can bind to non-histone nuclear proteins and together with these proteins can activate or repress specific target genes. Class II HDACs contain a regulatory domain that when phosphorylated causes the HDACs to exit the nucleus resulting in the expression of HDAC gene targets.33–35
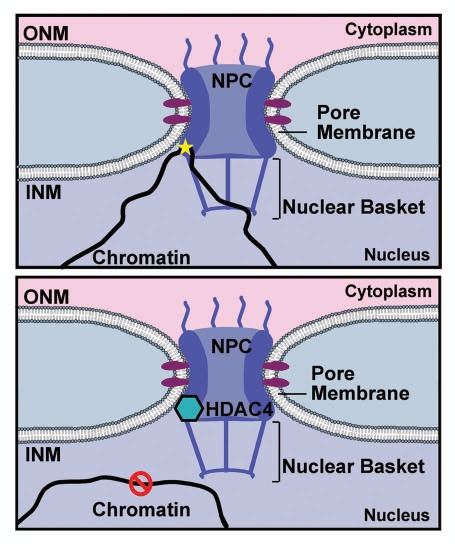
Recently, Kehat and colleagues have shown that HDAC4, a class II HDAC, interacts with nucleoporin 155 (Nup155) and that this interaction affects both chromatin position and gene expression.36 Class II HDACs are repressors of cardiomyocyte growth, so the authors identified specific gene loci that are targets of HDAC4 in myocytes. In order to investigate the mechanism that confers gene specificity to HDAC4 action, the authors performed a modified yeast two-hybrid with HDAC4. HDAC4 was shown to bind Nup155 and this association occurred with other NUPs at the nuclear pore complex. Notably, this interaction prevented Nup155 from binding chromatin. This data indicates that HDAC4 may act on NUPs to alter association of specific genomic loci with the nuclear pore complexes. Disrupting the Nup155/HDAC4 interaction by either inhibiting HDAC4 or using a non-HDAC4 binding NUP155 mutant, resulted in association of NUPs with several target gene loci, indicating that HDAC4 is important for preventing the interaction between specific loci and the nuclear pore complex. This interaction also impacts large-scale chromatin organization. Loci that are more highly expressed when at the nuclear periphery relocate to the interior when HDAC4 is overexpressed, suggesting that specific genomic loci are active when linked to the nuclear pore complex and can be deactivated by displacement to the nuclear interior (Fig. 3). HDAC4 binding to Nup155 may serve as a general mechanism to regulate gene expression indicating that chromatin movement is a highly regulated process.
Figure 3.
HDAC4 prevents chromatin/NUP155 binding. Chromatin (black) binds to Nup155, one of the nucleoporins that compose the nuclear pore complex (purple). When bound, these loci are transcriptionally active (star). The lower part depicts the binding of HDAC4 (turquoise) to Nup155 which then disrupts chromatin binding.36 This interaction displaces chromatin to the nuclear interior and results in transcriptional repression.
The Nucleoskeleton and Chromosome Movement
Mehta and colleagues recently demonstrated that chromosome territories relocate in human fibroblasts made quiescent through serum withdrawal.37 In proliferating human dermal fibroblasts, gene-poor chromosomes were consistently found at the nuclear periphery which correlates with fewer expressed genes and a greater number of LADs. Upon serum withdrawal, a number of chromosomes were observed to change position in the interphase nucleus. The chromosome repositioning was rapid, occurring in approximately 15 minutes. This rapid response indicates that chromosome territory movement is an active process. Inhibition of actin and myosin polymerization prevented the movement of chromosome territories after serum withdrawal. Inhibiting ATPase and/or GTPase in proliferating cultures also prevented chromosome territory movement upon serum withdrawal. These data suggest that chromosome movement is an energy-dependent process. RNA interference was used to reduce the expression of MYO1C that encodes both a cytoplasmic myosin isoform and the nuclear isoform of myosin, myosin 1β. Reduction of myosin 1β stifled chromosome movement after serum withdrawal. These data are supported by an earlier study preventing chromosome relocalization using actin and myosin mutants.38
These studies implicate nuclear actin and myosin in chromosome territory movement. Actin can actively shuttle into the nucleus and functions in a number of nuclear pathways including chromatin remodeling, transcription and RNA export.39,40 Lamin tails bind F-actin directly, while their interaction with G-actin is unknown.41 Simon and colleagues tested binding of mutant lamin A/C tails to F-actin and found that the mutant tails bound less well than wildtype. While F-actin is not detectable in the nucleus by phalloidin-staining, some actin in the nucleus exists in an altered polymeric state.39,42 Emerin has also been shown to bind the pointed end of actin filaments at the nuclear lamina.43 Taken together, these data suggest that actin filaments at the nuclear periphery are available to bind emerin and lamin. This binding may provide structural support or may be important for the mechanotransduction properties of the nucleus.
The LMNA null mouse (LMNA-/-) lacks lamin A/C and develops severe dilated cardiomyopathy by 4–6 weeks of age.44 LMNA-/- cardiomyocytes have altered nuclear envelope morphology, disorganization of nesprin-1 and heterogeneity in the distribution of both cytoskeletal and nuclear actin.32 Nikolova-Krstevski and colleagues demonstrated functional interaction between nesprin-1 isoforms and actin in the nucleus. The authors hypothesize that changes in the LINC complex in the LMNA-/- cardiomyocytes alter the actin cyto- and nucleo-skeleton and result in disease. The LINC complex LInks the Nucleus to the Cytoplasm and is composed of actin-bound giant nesprins at the outer nuclear membrane and smaller nesprin isoforms at the inner nuclear membrane interacting with lamin A/C (Fig. 2). SUN proteins span the luminal domain between the outer and inner nuclear membranes and link the nesprins through binding of their carboxy-termini. The SUN proteins in turn bind lamin A/C in the nucleus which in turn can bind chromatin.45
Mutations in both lamin A/C and nesprin-1 that cause striated muscle disease do not result in gross mislocalization of components of the LINC complex.16,46,47 The LINC complex is hypothesized to serve as a mechanosensor between the nucleus and cytoplasm and impaired mechanosensing is responsible for the striated muscle disease caused by these mutations. We propose that mechanosensing defects from an abnormal LINC complex may also include aberrant chromosome territories and as well as gene dysregulation. The LINC complex may have direct interaction with an actin-myosin motor through interactions with an undefined nuclear actin-binding nesprin isoform. It is also feasible that perturbations in the mechanosensing of the LINC complex lead to altered lamin-actin interactions resulting in aberrant chromatin movement. Further studies need to be done to determine chromatin relocalization patterns and gene expression changes in lamin A/C and nesprin mutant models to elucidate the role of the LINC complex in striated muscle disease.
Acknowledgments
Supported by NIH HL 0942443 and the Doris Duke Charitable Foundation (to E.M.M.) and NIH T32 007237 (MRP).
Abbreviations
- LAD
lamin-associated domain
- HATs
histone acetyltransferases
- HDACs
histone deacetylases
- NPC
nuclear pore complex
- Nup
nucleoporin
Extra View to: Mewborn SK, Puckelwartz MJ, Abuisneineh F, Fahrenbach JP, Zhang Y, MacLeod H, et al. Altered chromosomal positioning, compaction and gene expression with a lamin A/C gene mutation. PLoS One. 2010;5:14342. doi: 10.1371/journal.pone.0014342. and Kehat I, Accornero F, Aronow B, Molkentin J. Modulation of chromatin position and gene expression by HDAC4 interaction with nucleoporins. J Cell Biol. 2011;193:21–29. doi: 10.1083/jcb.201101046.
References
- 1.Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007;447:413–417. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- 2.Parada L, Misteli T. Chromosome positioning in the interphase nucleus. Trends Cell Biol. 2002;12:425–432. doi: 10.1016/s0962-8924(02)02351-6. [DOI] [PubMed] [Google Scholar]
- 3.Bridger JM, Boyle S, Kill IR, Bickmore WA. Re-modelling of nuclear architecture in quiescent and senescent human fibroblasts. Curr Biol. 2000;10:149–152. doi: 10.1016/s0960-9822(00)00312-2. [DOI] [PubMed] [Google Scholar]
- 4.Meaburn KJ, Cabuy E, Bonne G, Levy N, Morris GE, Novelli G, et al. Primary laminopathy fibroblasts display altered genome organization and apoptosis. Aging Cell. 2007;6:139–153. doi: 10.1111/j.1474-9726.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 5.Shaklai S, Amariglio N, Rechavi G, Simon AJ. Gene silencing at the nuclear periphery. FEBS J. 2007;274:1383–1392. doi: 10.1111/j.1742-4658.2007.05697.x. [DOI] [PubMed] [Google Scholar]
- 6.Prokocimer M, Davidovich M, Nissim-Rafinia M, Wiesel-Motiuk N, Bar D, Barkan R, et al. Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 8.Galiova G, Bartova E, Kozubek S. Nuclear topography of beta-like globin gene cluster in IL-3-stimulated human leukemic K-562 cells. Blood Cells Mol Dis. 2004;33:4–14. doi: 10.1016/j.bcmd.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Kuroda M, Tanabe H, Yoshida K, Oikawa K, Saito A, Kiyuna T, et al. Alteration of chromosome positioning during adipocyte differentiation. J Cell Sci. 2004;117:5897–5903. doi: 10.1242/jcs.01508. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, McQueen PG, Lichtman MK, Shevach EM, Parada LA, Misteli T. Spatial genome organization during T-cell differentiation. Cytogenet Genome Res. 2004;105:292–301. doi: 10.1159/000078201. [DOI] [PubMed] [Google Scholar]
- 11.Vaquerizas JM, Suyama R, Kind J, Miura K, Luscombe NM, Akhtar A. Nuclear pore proteins nup153 and megator define transcriptionally active regions in the Drosophila genome. PLoS Genet. 2010;6:1000846. doi: 10.1371/journal.pgen.1000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Steensel B, Dekker J. Genomics tools for unraveling chromosome architecture. Nat Biotechnol. 2010;28:1089–1095. doi: 10.1038/nbt.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel MJ, Peric-Hupkes D, van Steensel B. Detection of in vivo protein-DNA interactions using DamID in mammalian cells. Nat Protoc. 2007;2:1467–1478. doi: 10.1038/nprot.2007.148. [DOI] [PubMed] [Google Scholar]
- 15.Graf S, Nielsen FG, Kurtz S, Huynen MA, Birney E, Stunnenberg H, et al. Optimized design and assessment of whole genome tiling arrays. Bioinformatics. 2007;23:195–204. doi: 10.1093/bioinformatics/btm200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mewborn SK, Puckelwartz MJ, Abuisneineh F, Fahrenbach JP, Zhang Y, MacLeod H, et al. Altered chromosomal positioning, compaction and gene expression with a lamin A/C gene mutation. PloS one. 2010;5:14342. doi: 10.1371/journal.pone.0014342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebillon P, Bouchier C, Bidot LD, Bonne G, Ahamed K, Charron P, et al. Expanding the phenotype of LMNA mutations in dilated cardiomyopathy and functional consequences of these mutations. J Med Genet. 2003;40:560–567. doi: 10.1136/jmg.40.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLeod HM, Culley MR, Huber JM, McNally EM. Lamin A/C truncation in dilated cardiomyopathy with conduction disease. BMC Med Genet. 2003;4:4. doi: 10.1186/1471-2350-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correa M, Gomez CG. A novel mutation in limb girdle muscular dystrophy. P R Health Sci J. 2007;26:229–232. [PubMed] [Google Scholar]
- 20.Szczerbal I, Foster HA, Bridger JM. The spatial repositioning of adipogenesis genes is correlated with their expression status in a porcine mesenchymal stem cell adipogenesis model system. Chromosoma. 2009;118:647–663. doi: 10.1007/s00412-009-0225-5. [DOI] [PubMed] [Google Scholar]
- 21.Szczerbal I, Bridger JM. Association of adipogenic genes with SC-35 domains during porcine adipogenesis. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2010;18:887–895. doi: 10.1007/s10577-010-9176-1. [DOI] [PubMed] [Google Scholar]
- 22.Moen PT, Jr, Johnson CV, Byron M, Shopland LS, de la Serna IL, Imbalzano AN, et al. Repositioning of muscle-specific genes relative to the periphery of SC-35 domains during skeletal myogenesis. Mol Biol Cell. 2004;15:197–206. doi: 10.1091/mbc.E03-06-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler JT, Hall LL, Smith KP, Lawrence JB. Changing nuclear landscape and unique PML structures during early epigenetic transitions of human embryonic stem cells. J Cell Biochem. 2009;107:609–621. doi: 10.1002/jcb.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, et al. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci USA. 2004;101:9677–9682. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11:769–777. doi: 10.1093/hmg/11.7.769. [DOI] [PubMed] [Google Scholar]
- 26.Kandert S, Wehnert M, Muller CR, Buendia B, Dabauvalle MC. Impaired nuclear functions lead to increased senescence and inefficient differentiation in human myoblasts with a dominant p.R545C mutation in the LMNA gene. Eur J Cell Biol. 2009;88:593–608. doi: 10.1016/j.ejcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Park YE, Hayashi YK, Goto K, Komaki H, Hayashi Y, Inuzuka T, et al. Nuclear changes in skeletal muscle extend to satellite cells in autosomal dominant Emery-Dreifuss muscular dystrophy/limb-girdle muscular dystrophy 1B. Neuromuscul Disord. 2009;19:29–36. doi: 10.1016/j.nmd.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Favreau C, Delbarre E, Courvalin JC, Buendia B. Differentiation of C2C12 myoblasts expressing lamin A mutated at a site responsible for Emery-Dreifuss muscular dystrophy is improved by inhibition of the MEK-ERK pathway and stimulation of the PI3-kinase pathway. Exp Cell Res. 2008;314:1392–1405. doi: 10.1016/j.yexcr.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Brickner JH, Walter P. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol. 2004;2:342. doi: 10.1371/journal.pbio.0020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taddei A. Active genes at the nuclear pore complex. Curr Opin Cell Biol. 2007;19:305–310. doi: 10.1016/j.ceb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Lim RY, Fahrenkrog B, Koser J, Schwarz-Herion K, Deng J, Aebi U. Nanomechanical basis of selective gating by the nuclear pore complex. Science. 2007;318:640–643. doi: 10.1126/science.1145980. [DOI] [PubMed] [Google Scholar]
- 32.Brown CR, Kennedy CJ, Delmar VA, Forbes DJ, Silver PA. Global histone acetylation induces functional genomic reorganization at mammalian nuclear pore complexes. Genes Dev. 2008;22:627–639. doi: 10.1101/gad.1632708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet. 2003;19:286–293. doi: 10.1016/S0168-9525(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 34.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 35.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kehat I, Accornero F, Aronow BJ, Molkentin JD. Modulation of chromatin position and gene expression by HDAC4 interaction with nucleoporins. J Cell Biol. 2011;193:21–29. doi: 10.1083/jcb.201101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta IS, Amira M, Harvey AJ, Bridger JM. Rapid chromosome territory relocation by nuclear motor activity in response to serum removal in primary human fibroblasts. Genome Biol. 2010;11:5. doi: 10.1186/gb-2010-11-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang CH, Carpenter AE, Fuchsova B, Johnson T, de Lanerolle P, Belmont AS. Long-range directional movement of an interphase chromosome site. Curr Biol. 2006;16:825–831. doi: 10.1016/j.cub.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 39.Gieni RS, Hendzel MJ. Actin dynamics and functions in the interphase nucleus: moving toward an understanding of nuclear polymeric actin. Biochem Cell Biol. 2009;87:283–306. doi: 10.1139/O08-133. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann WA. Cell and molecular biology of nuclear actin. Int Rev Cell Mol Biol. 2009;273:219–263. doi: 10.1016/S1937-6448(08)01806-6. [DOI] [PubMed] [Google Scholar]
- 41.Simon DN, Zastrow MS, Wilson KL. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus. 2010;1:264–272. doi: 10.4161/nucl.1.3.11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald D, Carrero G, Andrin C, de Vries G, Hendzel MJ. Nucleoplasmic beta-actin exists in a dynamic equilibrium between low-mobility polymeric species and rapidly diffusing populations. J Cell Biol. 2006;172:541–552. doi: 10.1083/jcb.200507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holaska JM, Kowalski AK, Wilson KL. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2004;2:231. doi: 10.1371/journal.pbio.0020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest. 2004;113:357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, et al. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, Morris G, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puckelwartz MJ, Kessler EJ, Kim G, Dewitt MM, Zhang Y, Earley JU, et al. Nesprin-1 mutations in human and murine cardiomyopathy. J Mol Cell Cardiol. 2010;48:600–608. doi: 10.1016/j.yjmcc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]