Abstract
Nuclear position is actively controlled and can be adjusted according to the needs of a cell by nuclear movement. Microtubules mediate the majority of nuclear movements studied to date, although examples of nuclear movements mediated by the actin cytoskeleton have been described. One such actin-dependent nuclear movement occurs during centrosome orientation in fibroblasts polarizing for migration. Here, the centrosome is maintained at the cell center while the nucleus is moved to the cell rear by actin retrograde flow thus positioning the centrosome between the nucleus and the leading edge of the cell. We have explored the molecular mechanism for actin dependent movement of the nucleus during centrosome centration. We found that a novel linear array of nuclear envelope membrane proteins composed of nesprin-2G and SUN2, called transmembrane actin-associated nuclear (TAN) lines, couple the nucleus to moving actin cables resulting in the nucleus being positioned toward the cell rear. TAN lines are anchored by A-type lamins and this allows the forces generated by the actin cytoskeleton to be transmitted across the nuclear envelope to move the nucleus. Here we review the data supporting this mechanism for nuclear movement, discuss questions remaining to be addressed and consider how this new mechanism of nuclear movement may shed light on human disease.
Key words: actin, nesprin, SUN, lamina, centrosome, migration, LINC complex, TAN lines
Introduction
Contrary to the impression one gets from diagrams and images in cell biology textbooks, the position of nuclei in cells is not static. Nuclei move within the cytoplasm to specific locations in response to the needs of the cell especially during cell polarization and movement associated with tissue reorganization and organ development. Nuclear movements have been best documented during cell division and fertilization, where nuclei are positioned to ensure equal distribution between daughter cells and karyogamy, respectively.1–3 Recently, nuclear movement has been shown to play important roles during various developmental processes including meiosis, cell migration and differentiation, and the formation of proper tissue architecture.4–6 How nuclear movement and positioning contributes to these processes remains unclear.
Nuclear movement, like the movement of other organelles, requires forces generated by the cytoskeleton. Forces from microtubule polymerization or motor proteins drive the majority of nuclear movements studied to date (reviewed in refs. 2 and 3). However, a growing list of actin-dependent nuclear movements have been described including the movement of root tip nuclei during root growth in Arabidopsis,7 axial expansion of nuclei in the Drosophila melanogastar syncytial blastoderm,8 nuclear centration in the Caenorhabditis elegans zygote,9 and rearward movement of nuclei to orient the centrosome for migration in fibroblasts10 (Table 1). Interestingly, there are types of nuclear movement which use forces generated by both microtubules and actin, such as nucleokinesis during neuronal cell migration11–13 and interkinetic nuclear movement in pseudostratified epithelia14,15 (Table 1). Moreover, the nuclei of the C. elegans embryonic hypodermis use both actin and microtubule cytoskeletons but at different times and for different purposes. These nuclei specifically engage microtubule motor proteins to move within the hypoderm while they interact with actin filaments for anchorage to specific subcellular localizations suggesting that regulatory mechanisms dictate when and where these nuclei will interact with a specific cytoskeletal system.5,6,16 While mechanisms for Microtubule dependent nuclear movement have been proposed,3,17,18 mechanisms for actin-dependent nuclear movement are unknown and it is unclear how actin forces may be coupled to the nucleus.
Table 1.
Actin-dependent nuclear movements
| Nuclear positioning event | System | References | |
| Actin-dependent | Nuclear movement in mature hyphae | Neurospora crassa | 67 |
| Nuclear Movement during Root hair development in primary roots | Arabidopsis thaliana | 7, 68 | |
| Phototropin-dependent positioning of nuclei in leaf cells | Arabidopsis thaliana | 69 | |
| Pre-mitotic nuclear migration in subsidiary mother cells | Tradescantia virginiana | 70 | |
| Nuclear centration in the zygote | Caenorhabditis elegans | 9 | |
| Axial expansion of nuclei in pre-syncytial blastoderm | Drosophila melanogastar | 71 | |
| Nuclear movement during fibroblast polarization for migration | Mus musculus | 10, 30 | |
| Actin- and microtubule-dependent | Nuclear movement into the bud during mitosis | Saccharomyces cerevisiae | 72–74 |
| Post-mitotic nuclear migration | Micrasterias denticulate | 75, 76 | |
| Nuclear centering | Spirogyra crassa | 77 | |
| Interkinetic nuclear movement during retinal development | Danio rerio, Oryzias latipes | 78–81 | |
| Nucleokinesis during neuronal migration | Mus musculus, Rattus rattus | 11–13, 82–85 | |
| Interkinetic nuclear movement during neocortex development | Mus musculus, Rattus rattus | 85–91 |
To determine the functions of nuclear positioning, a better understanding of the molecular mechanisms for nuclear movement is required. To this end, much progress has been made in the past few years with the identification of the linker of nucleoskeleton and cytoskeleton (LINC) complex, a molecular bridge that allows the transmit of forces generated by bytoplasmic cytoskeletal elements into the nucleoplasm.5,6,19 The LINC complex consists of the outer nuclear membrane nesprin* proteins and the inner nuclear membrane SUN proteins.19 Nesprins posses divergent N-termini but contain a conserved, nuclear envelope targeting, KASH domain at their C-termini and are sometimes referred to as “KASH proteins.”6,20 The KASH domain consists of a transmembrane domain and a short tail that projects into the perinuclear space and interacts with the C-terminus of SUN proteins which contains the conserved SUN domain.6 The divergent N-termini of SUN proteins are found in the nucleoplasm where they interact with the nuclear lamina and/or chromatin binding proteins.6,20,21 The cytoplasmic N-termini of nesprins differ and this allows for nesprin family members to interact with all three filament systems of the cytoskeleton: actin filaments, microtubules and intermediate filaments.6,22 The LINC complex has been primarily implicated in microtubule-driven nuclear movement, lending support to the nuclear envelope bridge model of nuclear movement.5
A number of years ago, we modified the wounded fibroblast monolayer system to explore the initial polarization of cells in preparation for cell migration.23,24 NIH3T3 fibroblasts grown to confluence are serum starved for two days and then scratched to create a wound. In the absence of serum, wounding alone does not stimulate polarization or migration into the wound. Subsequent addition of serum or the specific serum factor, lysophosphatidic acid (LPA), triggers rapid polarization of the wound-edge cells. LPA triggers only polarization while serum triggers both polarization and migration.24,25
A classic marker for cell polarity in wound edge cells is the reorientation of the centrosome to a position between the nucleus and the leading edge of a cell.26,27 Centrosome reorientation is thought to be important for directed cell migration due to the fact that the centrosome is intimately associated with the Golgi complex, resulting in the polarization of the secretory system towards the front of the cell.28 Several years ago we found that centrosome reorientation in wounded monolayers of NIH3T3 fibroblasts did not involve movement of the centrosome, as had been expected, but resulted from movement of the nucleus past a stationary centrosome.10 Our initial findings indicated that this nuclear movement was required to make protrusive activity at the leading edge productive.10 Myosin II driven retrograde actin flow was necessary for this nuclear movement, yet it was unclear whether there was a direct connection between actin and the nucleus and if so, what might mediate such an interaction to allow transduction of force to move the nucleus. Below, we describe recent results that have begun to suggest a model for how the linkage between actin filaments and the nuclear envelope is established and how this linkage is anchored by SUN proteins and the nuclear lamina.
TAN Lines: Transmembrane Actin-Associated Nuclear Lines
The data described below are from Luxton et al. 2010.30 Based on the requirement of actin and myosin II for nuclear movement during centrosome reorientation and the ability of specific mammalian nesprins (nesprin-1 and nesprin-2) to interact with actin filaments,6,10 we hypothesized that the nucleus might be coupled to moving (retrograde flowing) actin filaments via the LINC complex during centrosome reorientation. To test this hypothesis, we expressed the KASH domain of nesprin-2, which disrupts the nuclear localization of all nesprins by competing for binding to SUN proteins, in serum-starved wound-edge NIH3T3 fibroblasts. Addition of LPA normally triggers nuclear movement, but in KASH expressing cells, nuclear movement failed to occur (Fig. 1). This result suggested that nesprins, and by association SUNs, were involved in the rearward movement of the nucleus in LPA stimulated fibroblasts. There are two mammalian nesprins that encode actin binding calponin homology (CH) domains at their N-termini, nesprin-1G and nesprin-2G (“G” refers to the giant splice form; there are smaller splice variants that do not contain CH domains31), but we found only nesprin-2G was expressed in NIH3T3 cells. Knockdown of nesprin-2G by siRNA inhibited nuclear movement and re-expression of a green fluorescent protein (GFP)-mini-nesprin-2G chimeric construct comprising the N- and C-termini of nesprin-2G rescued nuclear movement showing the specificity of the nesprin-2G knockdown. As GFP-mini-nesprin-2G lacks most of the spectrin repeats between the N-terminus and C-terminus, this result also suggests that most of the spectrin repeats are dispensable for nuclear movement. Additional experiments revealed that GFP-mini-nesprin-2G lacking CH domains or encoding mutated CH domains was unable to rescue nuclear movement in nesprin-2G depleted cells. Therefore, nesprin-2G and its ability to interact with actin filaments are required for nuclear movement during centrosome reorientation in fibroblasts polarizing for migration.
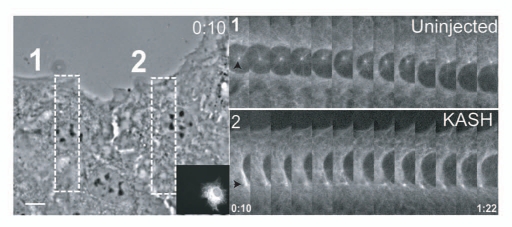
Figure 1.
Nuclear movement during centrosome orientation involves nesprins. Nuclear movement in RFP-KASH-expressing (insert), GFP-α-tubulin NIH3T3 fibroblasts. The left panel shows a phase contrast image from the begining of the movie. Boxes indicate the regions used to generate the GFP-α-tubulin fluorescence kymographs on the right. Arrowheads indicate centrosome position. Time is in h:min. Bar: 10 µm. From Luxton et al. Science 2010; 329:956.
In cells rescued with GFP-mini-nesprin-2G, we were surprised to find that GFP-mini-nesprin-2G accumulated in linear arrays, mostly parallel to the leading edge and on the dorsal surface of nuclei (Fig. 2A). Endogenous nesprin-2G also formed linear arrays as revealed by immunofluorescence with a nesprin-2G specific antibody. These linear arrays of GFP-mini-nesprin-2G co-localized with actin cables near the dorsal surface, were sensitive to drugs that perturbed actin or myosin-II and required the actin binding CH domains of GFP-mini-nesprin-2G (Fig. 2A). Fluorescence recovery after photobleaching demonstrated that GFP-mini-nesprin-2G within the linear arrays was immobilized relative to bulk GFP-mini-nesprin-2G in the nuclear membrane. Endogenous SUN2, but not SUN1 nor a number of other nuclear envelope proteins, co-localized with these nesprin-2G arrays, indicating the arrays had a specific molecular composition and were not the result of nuclear envelope deformations (Fig. 2B). Depletion of SUN2 also prevented nuclear movement in response to LPA. Based on these findings, we concluded that the linear arrays of nesprin-2G and SUN2 represent a novel structure on the nuclear envelope and named them trans-membrane actin-associated nuclear (TAN) lines. The co-localization of TAN lines with dorsal actin cables suggested a possible mechanism for moving the nucleus rearward during centrosome orientation. Previous studies in the 1980s identified “transverse arcs” of actin filaments beneath the dorsal surface of migrating fibroblasts (and other cells) that moved rearward toward the nucleus.32,33 These transverse arcs resemble the dorsal actin cables in our study, although the actin cables in NIH3T3 fibroblasts are not typically curved, perhaps because of constraints to cell spreading imposed by adjacent cells in the wounded monolayer.
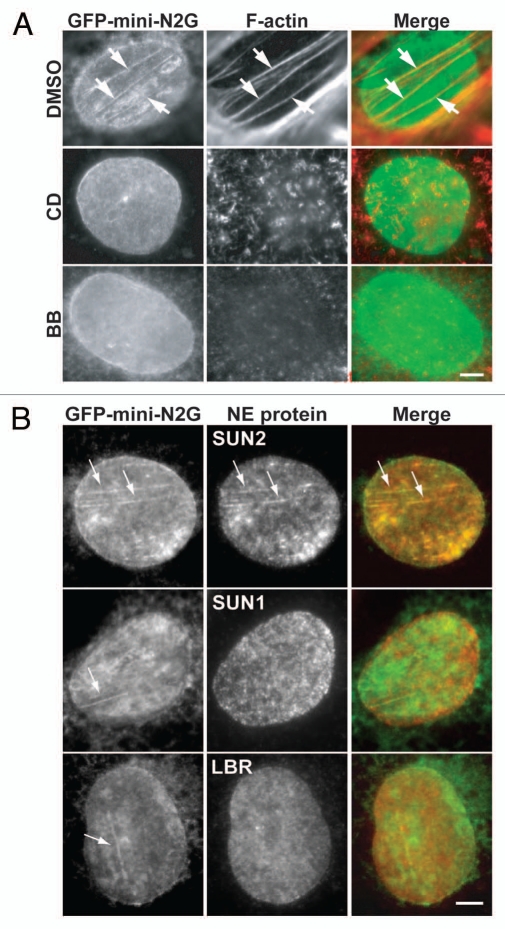
Figure 2.
TAN lines are actomyosin-dependent structures composed of nesprin-2G and SUN2. (A) Fluorescence images of nuclei in nesprin-2G-depleted cells expressing GFP-mini-nesprin-2G (GFP-mini-N2G). Staining: GFP antibody (GFP-mini-N2G) and rhodamine-phalloidin (F-actin). Cells were treated with vehicle (DMSO), 0.5 µM cytochalasin D (CD) or 50 µM blebbistatin (BB) for 1 hr before and during LPA treatment. The wound edge is towards the top left in all parts. (B) Fluorescence images of nuclei in nesprin-2G-depleted cells expressing GFP-mini-nesprin-2G (GFP-mini-N2G). Staining: SUN1, SUN2, LBR and GFP (GFP-mini-N2G) antibodies. Arrows indicate GFP-mini-nesprin-2G colocalizing with SUN2, but not SUN1 or LBR. The wound edge is towards the top (SUN2, SUN1) and toward the right (LBR). All images are of the dorsal nuclear surface. Bars in (A and B): 5 µm. From Luxton et al. Science 2010; 329:956.
To test whether the dorsal actin cables moved rearward, we used Lifeact-mCherry, which detects filamentous actin,34 to monitor the dynamics of the dorsal actin cables during nuclear movement. These studies showed that several dorsal actin cables were associated with the nucleus during its movement and moved at the same rate as the nucleus. Ventral stress fibers, generally oriented perpendicular to the dorsal cables, did not move with the nucleus. In LPA stimulated cells, actin initially formed from an isotropic meshwork of filaments that resolved into dorsal actin cables and began moving rearward just before nuclear movement was initiated. Depletion of nesprin-2G did not alter the formation of dorsal actin cables nor their rearward movement. These results strongly suggested that the dorsal actin cables were responsible for moving the nucleus rearward. Questions that remain to be addressed are how the isotropic actin meshwork forms dorsal actin cables with near uniform orientation parallel to the leading edge and what forces drive their retrograde movement. Recent work from the Lappalainen lab has identified stress fibers that project from the ventral towards the dorsal surface of the cell.35 It is possible that the contraction of these fibers provides the motive force for the retrograde movement of the dorsal actin cables.
There was also a strong correlation between the rate of movement of the nucleus and the rate of movement of the TAN lines. Direct imaging of TAN lines on individual moving nuclei showed that the TAN lines moved at the same rate as the leading or lagging edge of the nucleus. To confirm that all three structures (TAN lines, actin cables and nuclei) moved coordinately, we prepared live cell recordings of cells expressing both GFP-mini-nesprin-2G and Lifeact-mCherry. TAN lines were observed to form on a subset of the dorsal actin cables above the nucleus and then moved coincidently with the nucleus. These results provide strong evidence for the direct coupling of the nucleus through TAN lines to moving dorsal actin cables.
Previous studies in developmental systems (C. elegans and mouse) have implicated nesprin and SUN proteins in cell migration,36,37 raising the possibility that nuclear position was important for cell migration. Yet, these studies were unable to assess whether the effects of interfering with nesprins or SUNs were cell autonomous or whether alteration of nesprins or SUNs in these systems significantly altered actin organization. In the fibroblast system, we could directly ask whether nuclear positioning was important for cell migration since our studies showed that interfering with nesprin-2G or SUN2 blocked TAN line formation and nuclear movement, but not actin filament organization or movement. Importantly, we found that expression of the KASH domain or siRNA-mediated depletion of either nesprin-2G or SUN2 inhibited efficient migration into the wound. Combined with the earlier results from model organisms, these data strengthen the idea that nuclear position within a migrating cell is an important component of cell migration.
TAN Lines are Anchored by SUN2 and the Nuclear Lamina
The data described below are from Folker, et al. 2011.38 The TAN lines we have described are important for coupling the nucleus to moving actin filaments. Yet, to move the nucleus productively, TAN lines must be anchored to a nuclear structure capable of resisting the force exerted on the nucleus by moving actin cables. We hypothesized that the nuclear lamina that lies just under the inner nuclear membrane may provide such an anchoring site. The nuclear lamina is a fibrillar network composed of type V intermediate filaments that are either A-type (lamin A and lamin C) or B-type (lamin B1 and lamin B2).39 Lamin A/C has been shown to interact directly with SUN proteins in C. elegans and in mammalian cells.19,40,41 A type lamins have been implicated in nuclear-cytoplasmic interactions because lamin A/C deficient fibroblasts fail to orient their centrosome toward the leading edge, although it was unclear whether this was due to an effect on the centrosome or on the nucleus (or both).42,43
To explore the role of lamins in nuclear movement, we first tested whether lamins might be found in TAN lines and did not detect accumulation of either endogenous lamin A/C or lamin B1 in TAN lines. Nonetheless, in fibroblasts derived from lamin A/C knockout mice or NIH3T3 fibroblasts acutely depleted of lamin A/C, we observed that the lack of centrosome orientation was due to a defect in nuclear movement. Similar to cells depleted of nesprin-2G, the overall organization of actin filaments and their retrograde movement was unperturbed in the absence of lamin A/C. These results suggested that the absence of lamin A/C might affect either the formation or dynamics of TAN lines.
To probe for TAN lines, we expressed GFP-mini-nesprin-2G in cells lacking lamin A/C. TAN lines composed of nesprin-2G and SUN2 still formed yet they appeared discontinuous and their stability was reduced compared to lamin A/C expressing cells: only 30–40% of the TAN lines persisted for 20 min in cells lacking lamin A/C compared to 70% of them in wild type controls. In movies of TAN lines and nuclei, nuclei did not move in the lamin A/C deficient cells and yet the TAN lines still moved, showing that the TAN lines slipped over the nucleus rather than coupling to the actin cables for movement. A similar TAN line “slippage” phenotype was observed in cells depleted of SUN2. Therefore, nesprin-2G TAN lines form in the absence of either SUN2 or lamin A/C, but they are less stable and are unable to couple the nuclear envelope to the actin cytoskeleton in order to move the nucleus. Together, these data suggest that lamin A/C and thus the nuclear lamina functions to anchor TAN lines for productive force transmission across the nuclear envelope during nuclear movement.
There are two possible scenarios leading to TAN line assembly following LPA-stimulated actin cable formation on the dorsal nuclear surface. In the first scenario, actin cables recruit freely diffusing nesprin-2G molecules in the outer nuclear envelope along the length of the cable. The high, local concentration of nesprin-2G along the actin cable then acts to recruit SUN2 in the inner nuclear membrane. In the second scenario, actin cables recruit pre-assembled nesprin-2G/SUN2 LINC complexes along the length of the actin cable. Consistent with the first scenario, nesprin-2G accumulates along a perinuclear actin cable in the absence of SUN2. Additional experiments, such as simultaneous imaging of nesprin-2G and SUN2 during TAN line assembly, are necessary to differentiate between these two models.
TAN Lines and Laminopathies
Mutations in LMNA are associated with a variety of tissue-specific diseases, commonly referred to as laminopathies, which include diseases of striated muscle (Emery-Dreifuss muscular dystrophy [EDMD]), dilated cardiomyopathy [DCM] and limb girdle muscular dystrophy 1B, adipose tissue (Dunnigan-type familial partial lipodystrophy [FPLD]), peripheral nerve (Charcot-Marie-Tooth type 2B1), as well as progeroid syndromes characterized by symptoms of premature aging.44,45 Currently, there are two favored hypotheses used to explain the pathogenesis of these diseases. The mechanical stress hypothesis posits that the nuclear lamina is important for nuclear integrity, especially in tissues subjected to high levels of stress such as striated muscle. This is supported by the findings that A-type lamin deficiency disrupts nuclear integrity in model systems.46,47 The gene expression hypothesis posits that since the lamina interacts with chromatin, alterations in the nuclear lamina may result in aberrant gene expression. This is supported by the finding that lamin A is necessary to repress inhibitors of MyoD expression.48 A third possibility is that compromised interactions between the nucleus and cytoskeleton may lead to nuclear positioning defects that contribute to the pathogenesis of laminopathies. This third hypothesis is supported by the recent identification of mutations in nesprin-1 and nesprin-2 which are associated with EDMD49 as well as the finding that nesprin-2G appears to protect LMNA mutant cells associated with progeria from developing dysmorphic nuclei.50 In addition, skeletal muscle nuclei in EDMD and other muscle disorders including Duchenne muscular dystrophy and central nuclear myopathy are observed in the center of the myofiber, rather than at their normal location at the periphery.51,52 Although the presence of central nuclei is indicative of a damaged myofiber undergoing a repair response,53 it is possible that the abundance of central nuclei in muscle disorders reflects an inability of nuclei to move towards the periphery of the myofiber due to defects in nuclear-cytoskeletal connections. Such a defect in proper nuclear positioning could compromise proper sarcomere alignment, which in turn could contribute to disease.
Based upon these considerations, we explored whether the wounded fibroblast monolayer system could be used to determine if disease-associated variants of LMNA were defective in nuclear movement or centrosome centration. Given that the lamin A/C null phenotype was defective nuclear movement, this analysis would also reveal whether individual disease variants resulted in a loss of lamin A/C function. We screened a panel of lamin A variants by expressing them in NIH3T3 fibroblasts and then stimulating nuclear movement and centrosome orientation with LPA. The panel contained variants associated with striated muscle diseases including EDMD and DCM as well as variants associated with lipodystrophy including FPLD. While expression of wild type lamin A had no effect on centrosome orientation, expression of almost all of the disease associated variants inhibited centrosome reorientation. Interestingly, there was a segregation of phenotypes based on whether the variant caused disease affecting striated muscle or adipose tissue. Nuclear movement was inhibited by almost all of the striated muscle disease variants (11/12), while centrosome centration, but not nuclear movement, was inhibited by the adipose tissue disease variants (3/4). Similar results were observed following expression of the disease variants in fibroblasts lacking lamin A/C suggesting that the defects observed were intrinsic properties of the variants. Fibroblasts from a patient with EDMD also showed a defect in nuclear movement. Consistent with their affects on nuclear movement, expression of striated muscle disease variants in lamin A/C knockout cells expressing GFP-mini-nesprin-2G resulted in TAN line “slippage” while adipose tissue disease variants did not. These results point to the possiblity that defective nuclear movement may be contributing factor in the pathology of muscle diseases caused by lamin A variants.
A Model for TAN Lines
We have described an actin-dependent mechanism for nuclear movement during centrosome reorientation in fibroblasts polarizing for migration. This mechanism involves the coupling of the nucleus to actin cables through a novel structure within the nuclear envelope, which we refer to as TAN lines (Fig. 3A). Composed of linear arrays of nesprin-2G/SUN2, TAN lines form on the dorsal surface of the nuclear envelope along actin cables that move towards the rear of the cell through the action of non-muscle myosin II. This coupling of the nuclear envelope to the actin cytoskeleton results in nuclear movement because TAN lines are anchored in the nuclear lamina by lamin A/C and this anchoring allows force generated by the actin cytoskeleton to be transferred across the nuclear envelope and into the nuclear lamina. Interfering with nesprin-2G, SUN2 or lamin A/C all block nuclear movement, but with distinct TAN line phenotypes (Fig. 3B). The identification of TAN lines and their role in nuclear movement raises several new questions that we consider below.
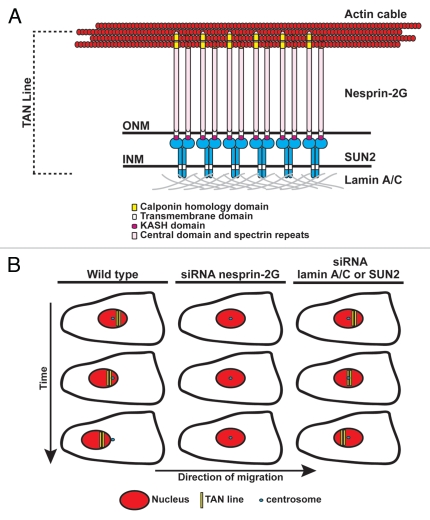
Figure 3.
Working model of TAN line structure and function. (A) Illustration of the molecular composition of a TAN line. INM, inner nuclear membrane; ONM, outer nuclear membrane. (B) Depiction of TAN line behavior during centrosome orientation under different conditions. TAN lines in wild type cells (left) form on the dorsal surface of the nucleus and function to harness the forces generated by the actin cytoskeleton to move the nucleus rearward while the centrosome remains stationary resulting in centrosome orientation towards the leading edge. In nesprin-2G-depleted cells (middle) TAN lines do not form resulting in the lack of nuclear movement and centrosome orientation. In lamin A/C- or SUN2-depleted cells (right), nesprin-2G TAN lines form but are unanchored causing them to slip over the surface of the nucleus with the rearward moving actin cables. Neither nuclear movement nor centrosome orientation occurs in this situation.
Questions for the Future
Our studies highlight a number of questions about the proper positioning of the nucleus in migrating cells and how this positioning impacts overall cellular function. Perhaps the most intriguing question is how nuclear positioning affects cell migration. Does the position of the nucleus affect cytoskeletal organization or dynamics? Or could it affect gene expression? We did not observe striking alterations in actin filament arrays when nuclear positioning was disrupted, but perhaps more subtle effects or changes in dynamics result from improper nuclear position. Alternatively, perhaps positioning the nucleus toward the back of a migrating cell simply gets it out of the way so that the cell can create a thin leading edge and lamella that concentrate molecules necessary for efficient migration?
Additional questions raised by our work concern how TAN lines are formed. It will be important to determine the steps in the assembly of TAN lines. GFP-mini-nesprin-2G has relatively high diffusional mobility in the outer nuclear membrane suggesting that it may randomly encounter actin cables that closely approach the nucleus.56 However, it is not yet clear whether the full length endogenous nesprin-2G might have to be activated in some way to interact with actin cables. SUN2 also accumulates in TAN lines above its concentration in the bulk nuclear membrane. How this occurs is somewhat of a puzzle, because it requires that SUN2 differentiate between nesprin-2G that is engaged by actin cables from the remainder of nesprin-2G in the nuclear envelope that is not. Given that the latter is more abundant, it is possible that there is a recognition signal in the actin-engaged nesprin-2G. Perhaps, as in focal adhesions,54,55 it will turn out that there is “outside-in” signaling (a change in nesprin-2G conformation or recruitment of other proteins) that may help the SUN2 recognize actin-associated nesprin-2G from unengaged molecules. Additionally, it remains unclear why SUN2 is recruited to TAN lines while SUN1 is not. Both molecules interact with the KASH domain of nesprin-2G with nearly equal affinities,56 yet only SUN2 appears in TAN lines. There is increasing evidence that SUN1 and SUN2 associate with the nuclear envelope through different mechanisms. SUN1 appears more closely associated with the nuclear lamina than SUN2 as measured by fluorescence resonance energy transfer experiments56 and SUN1 interacts with the nuclear pore complex.57 Furthermore, nesprin-2G mobility in the nuclear envelope appears to depend on SUN2 not SUN1.56 Further studies of TAN lines may lead to insights into how specific LINC complexes are established.
To better understand how TAN lines form, it would be helpful to define more precisely the structure of a TAN line. An obvious first step would be to determine the ultrastructure of TAN lines using either electron microscopy or super-resolution light microscopy. Recent work has beautifully demonstrated the utility of super-resolution microscopy with another actin-dependent structure, the focal adhesion.58 Given the similarities of TAN lines to focal adhesions (both are actin-dependent, trans-membrane and force transmitting structures required for cell migration), it will be interesting to see if nanoscale architectural features of TAN lines resemble those of focal adhesions.
Another important question is whether there are other proteins that accumulate with nesprin-2G and SUN2 in TAN lines. By analogy to focal adhesions, which are composed of dozens of cytoplasmic proteins important for linking transmembrane integrins to the actin cytoskeleton and signaling pathways,59 it is likely that TAN lines are composed of additional cytoplasmic and nucleoplasmic proteins. The identification of additional TAN line proteins will not only extend our knowledge of how TAN lines form and transmit force across the nuclear envelope, but may also shed light on other functions TAN lines perform in the cell. For instance, work in the fission yeast, Schizosaccharomyces pombe, has elegantly shown that focal accumulations of LINC complexes in association with heterochromatin harness the forces of microtubule polymerization to position the nucleus in the cell center.60 The inner nuclear membrane protein, Ima1, interacts with centromeric heterochromatin and is thought to help distribute the forces generated by microtubules through the LINC complex throughout the nuclear envelope allowing for productive force transduction. It will be interesting to see if the mammalian homologue of Ima1 (NET-5/Samp1),61 or other heterochromatin interacting proteins are important for TAN line formation and function.
A final question that needs to be addressed is how widespread the use of TAN lines is during nuclear positioning events. Are TAN lines restricted to fibroblasts polarizing for 2D migration or do they occur in other cellular contexts, such as 3D migration where the movement of the nucleus through narrow channels in the extracellular matrix can be rate-limiting?62 Exploring the possibility that TAN lines mediate other processes dependent on LINC complexes such as meiosis (particularly in budding yeast, given its dependence on actin63), nuclear movement during neuronal migration as well as movement and anchorage of nuclei in myotubes would be informative.
LINC complexes are becoming increasingly implicated in developmental processes in such tissues as the retina, brain, muscle, skin and testes.6 It would be interesting to examine these processes more carefully to see whether TAN line-like structures are involved. How defective TAN line structure and function might contribute to disease pathogenesis is an avenue of research, which is sure to be informative and may present new avenues for disease treatment. An increasing number of human diseases are being linked to mutated and dysfunctional nesprins including EDMD,49 autosomal recessive cerebellar ataxia type 1,64 as well as colorectal and breast cancer.65,66 The use of the simple wounded fibroblast monolayer system to explore the mechanism of nuclear movement and its deficiency in disease has opened a new line of research that promises to uncover exciting new biology as well as increase our understanding of disease pathogenesis.
Acknowledgments
This work was supported by a Dystonia Medical Research Foundation Fellowship (G.W.G.L.), a Muscular Dystrophy Association grant (E.R.G.), an Association pour la Recherche sur le Cancer grant (E.R.G.), a Ligue Nacionale contre le Cancer grant (E.R.G.), an American Heart Association Fellowship (E.S.F.), a Dystonia Medical Research Foundation grant (G.G.G.) and NIH grants (GM06929) (G.G.G.) and NS059352 (H.J.W.).
Extra View to: Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. and Folker ES, Ostlund C, Luxton GW, Worman HJ, Gundersen GG. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci U S A. 2011;108:131–136. doi: 10.1073/pnas.1000824108.
Footnotes
The original name for mammalian nesprins was Syne, for synaptic nuclei enriched.22 Lately, the field appears to be adopting the name “nesprin”, which we use here.
References
- 1.Burke B, Roux KJ. Nuclei take a position: managing nuclear location. Dev Cell. 2009;17:587–597. doi: 10.1016/j.devcel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Morris NR. Nuclear migration. from fungi to the mammalian brain. J Cell Biol. 2000;148:1097–1101. doi: 10.1083/jcb.148.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinsch S, Gonczy P. Mechanisms of nuclear positioning. J Cell Sci. 1998;111:2283–2295. doi: 10.1242/jcs.111.16.2283. [DOI] [PubMed] [Google Scholar]
- 4.Starr DA. Communication between the cytoskeleton and the nuclear envelope to position the nucleus. Mol Biosyst. 2007;3:583–589. doi: 10.1039/b703878j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starr DA. A nuclear-envelope bridge positions nuclei and moves chromosomes. J Cell Sci. 2009;122:577–586. doi: 10.1242/jcs.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ketelaar T, Faivre-Moskalenko C, Esseling JJ, de Ruijter NC, Grierson CS, Dogterom M, et al. Positioning of nuclei in Arabidopsis root hairs: an actin-regulated process of tip growth. Plant Cell. 2002;14:2941–2955. doi: 10.1105/tpc.005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamb MM, Laird CD. Increase in nuclear poly(A)-containing RNA at syncytial blastoderm in Drosophila melanogaster embryos. Dev Biol. 1976;52:31–42. doi: 10.1016/0012-1606(76)90004-x. [DOI] [PubMed] [Google Scholar]
- 9.Goulding MB, Canman JC, Senning EN, Marcus AH, Bowerman B. Control of nuclear centration in the C. elegans zygote by receptor-independent Galpha signaling and myosin II. J Cell Biol. 2007;178:1177–1191. doi: 10.1083/jcb.200703159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Martini FJ, Valdeolmillos M. Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. J Neurosci. 2010;30:8660–8670. doi: 10.1523/JNEUROSCI.1962-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- 13.Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron. 2009;63:63–80. doi: 10.1016/j.neuron.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai JW, Lian WN, Kemal S, Kriegstein AR, Vallee RB. Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat Neurosci. 2010;13:1463–1471. doi: 10.1038/nn.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer EJ, Ikmi A, Gibson MC. Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia. Curr Biol. 2011 doi: 10.1016/j.cub.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Starr DA, Han M. ANChors away: an actin based mechanism of nuclear positioning. J Cell Sci. 2003;116:211–216. doi: 10.1242/jcs.00248. [DOI] [PubMed] [Google Scholar]
- 17.Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46:383–388. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Vallee RB, Seale GE, Tsai JW. Emerging roles for myosin II and cytoplasmic dynein in migrating neurons and growth cones. Trends Cell Biol. 2009;19:347–355. doi: 10.1016/j.tcb.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starr DA, Fischer JA. KASH 'n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. Bioessays. 2005;27:1136–1146. doi: 10.1002/bies.20312. [DOI] [PubMed] [Google Scholar]
- 21.Lee KK, Starr D, Cohen M, Liu J, Han M, Wilson KL, et al. Lamin-dependent localization of UNC-84, a protein required for nuclear migration in Caenorhabditis elegans. Mol Biol Cell. 2002;13:892–901. doi: 10.1091/mbc.01-06-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellad JA, Warren DT, Shanahan CM. Nesprins LINC the nucleus and cytoskeleton. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Gundersen GG, Kim I, Chapin CJ. Induction of stable microtubules in 3T3 fibroblasts by TGFbeta and serum. J Cell Sci. 1994;107:645–659. doi: 10.1242/jcs.107.3.645. [DOI] [PubMed] [Google Scholar]
- 24.Cook TA, Nagasaki T, Gundersen GG. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J Cell Biol. 1998;141:175–185. doi: 10.1083/jcb.141.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, et al. Cdc42, dynein and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol. 2001;11:1536–1541. doi: 10.1016/s0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 26.Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9:860–873. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 27.Bornens M. Organelle positioning and cell polarity. Nat Rev Mol Cell Biol. 2008;9:874–886. doi: 10.1038/nrm2524. [DOI] [PubMed] [Google Scholar]
- 28.Sutterlin C, Colanzi A. The Golgi and the centrosome: building a functional partnership. J Cell Biol. 2010;188:621–628. doi: 10.1083/jcb.200910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apel ED, Lewis RM, Grady RM, Sanes JR. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J Biol Chem. 2000;275:31986–31995. doi: 10.1074/jbc.M004775200. [DOI] [PubMed] [Google Scholar]
- 30.Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Ragnauth C, Greener MJ, Shanahan CM, Roberts RG. The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics. 2002;80:473–481. [PubMed] [Google Scholar]
- 32.Heath JP. Behaviour and structure of the leading lamella in moving fibroblasts. I. Occurrence and centripetal movement of arc-shaped microfilament bundles beneath the dorsal cell surface. J Cell Sci. 1983;60:331–354. doi: 10.1242/jcs.60.1.331. [DOI] [PubMed] [Google Scholar]
- 33.Heath JP. Arcs: curved microfilament bundles beneath the dorsal surface of the leading lamellae of moving chick embryo fibroblasts. Cell Biol Int Rep. 1981;5:975–980. doi: 10.1016/0309-1651(81)90214-9. [DOI] [PubMed] [Google Scholar]
- 34.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malone CJ, Fixsen WD, Horvitz HR, Han M. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development. 1999;126:3171–3181. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, et al. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folker ES, Ostlund C, Luxton GW, Worman HJ, Gundersen GG. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci USA. 2011;108:131–136. doi: 10.1073/pnas.1000824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell. 2009;17:626–638. doi: 10.1016/j.devcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, et al. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem. 2010;285:3487–3498. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridkin A, Mills E, Margalit A, Neufeld E, Lee KK, Feinstein N, et al. Matefin, a Caenorhabditis elegans germ line-specific SUN-domain nuclear membrane protein, is essential for early embryonic and germ cell development. Proc Natl Acad Sci USA. 2004;101:6987–6992. doi: 10.1073/pnas.0307880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houben F, Willems CH, Declercq IL, Hochstenbach K, Kamps MA, Snoeckx LH, et al. Disturbed nuclear orientation and cellular migration in A-type lamin deficient cells. Biochim Biophys Acta. 2009;1793:312–324. doi: 10.1016/j.bbamcr.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, et al. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization and migration. Biophys J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, et al. Decreased mechanical stiffness in LMNA-/- cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 47.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain. 2006;129:996–1013. doi: 10.1093/brain/awl023. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 50.Kandert S, Luke Y, Kleinhenz T, Neumann S, Lu W, Jaeger VM, et al. Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum Mol Genet. 2007;16:2944–2959. doi: 10.1093/hmg/ddm255. [DOI] [PubMed] [Google Scholar]
- 51.Engel AGM, Franzini-Armstrong G, editors. Myology. New York: McGraw-Hill; 1994. [Google Scholar]
- 52.Romero NB. Centronuclear myopathies: a widening concept. Neuromuscul Disord. 2010;20:223–228. doi: 10.1016/j.nmd.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Walton JN. On the classification and natural history of the myopathies. Trans Am Neurol Assoc. 1954;13:19–21. [PubMed] [Google Scholar]
- 54.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 55.Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 56.Ostlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J Cell Sci. 2009;122:4099–4108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Q, Pante N, Misteli T, Elsagga M, Crisp M, Hodzic D, et al. Functional association of Sun1 with nuclear pore complexes. J Cell Biol. 2007;178:785–798. doi: 10.1083/jcb.200704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King MC, Drivas TG, Blobel G. A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell. 2008;134:427–438. doi: 10.1016/j.cell.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buch C, Lindberg R, Figueroa R, Gudise S, Onischenko E, Hallberg E. An integral protein of the inner nuclear membrane localizes to the mitotic spindle in mammalian cells. J Cell Sci. 2009;122:2100–2107. doi: 10.1242/jcs.047373. [DOI] [PubMed] [Google Scholar]
- 62.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koszul R, Kim KP, Prentiss M, Kleckner N, Kameoka S. Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell. 2008;133:1188–1201. doi: 10.1016/j.cell.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gros-Louis F, Dupre N, Dion P, Fox MA, Laurent S, Verreault S, et al. Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet. 2007;39:80–85. doi: 10.1038/ng1927. [DOI] [PubMed] [Google Scholar]
- 65.Doherty JA, Rossing MA, Cushing-Haugen KL, Chen C, Van Den Berg DJ, Wu AH, et al. ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: an Ovarian Cancer Association Consortium study. Cancer Epidemiol Biomarkers Prev. 2010;19:245–250. doi: 10.1158/1055-9965.EPI-09-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 67.Ramos-Garcia SL, Roberson RW, Freitag M, Bartnicki-Garcia S, Mourino-Perez RR. Cytoplasmic bulk flow propels nuclei in mature hyphae of Neurospora crassa. Eukaryot Cell. 2009;8:1880–1890. doi: 10.1128/EC.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chytilova E, Macas J, Sliwinska E, Rafelski SM, Lambert GM, Galbraith DW. Nuclear dynamics in Arabidopsis thaliana. Mol Biol Cell. 2000;11:2733–2741. doi: 10.1091/mbc.11.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iwabuchi K, Minamino R, Takagi S. Actin reorganization underlies phototropin-dependent positioning of nuclei in Arabidopsis leaf cells. Plant Physiol. 2010;152:1309–1319. doi: 10.1104/pp.109.149526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kennard JL, Cleary AL. Pre-mitotic nuclear migration in subsidiary mother cells of Tradescantia occurs in G1 of the cell cycle and requires F-actin. Cell Motil Cytoskeleton. 1997;36:55–67. doi: 10.1002/(SICI)1097-0169(1997)36:1<55::AID-CM5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 71.von Dassow G, Schubiger G. How an actin network might cause fountain streaming and nuclear migration in the syncytial Drosophila embryo. J Cell Biol. 1994;127:1637–1653. doi: 10.1083/jcb.127.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maddox P, Chin E, Mallavarapu A, Yeh E, Salmon ED, Bloom K. Microtubule dynamics from mating through the first zygotic division in the budding yeast Saccharomyces cerevisiae. J Cell Biol. 1999;144:977–987. doi: 10.1083/jcb.144.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shaw SL, Yeh E, Maddox P, Salmon ED, Bloom K. Astral microtubule dynamics in yeast: a microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud. J Cell Biol. 1997;139:985–994. doi: 10.1083/jcb.139.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carminati JL, Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oertel A, Holzinger A, Lutz-Meindl U. Involvement of myosin in intracellular motility and cytomorphogenesis in Micrasterias. Cell Biol Int. 2003;27:977–986. doi: 10.1016/j.cellbi.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Meindl U, Zhang D, Hepler PK. Actin microfilaments are associated with the migrating nucleus and the cell cortex in the green alga Micrasterias. Studies on living cells. J Cell Sci. 1994;107:1929–1934. doi: 10.1242/jcs.107.7.1929. [DOI] [PubMed] [Google Scholar]
- 77.Grolig F. Nuclear centering in Spirogyra: force integration by microfilaments along microtubules. Planta. 1998;204:54–63. doi: 10.1007/s004250050229. [DOI] [PubMed] [Google Scholar]
- 78.Tsuda S, Kitagawa T, Takashima S, Asakawa S, Shimizu N, Mitani H, et al. FAK-mediated extracellular signals are essential for interkinetic nuclear migration and planar divisions in the neuroepithelium. J Cell Sci. 2010;123:484–496. doi: 10.1242/jcs.057851. [DOI] [PubMed] [Google Scholar]
- 79.Norden C, Young S, Link BA, Harris WA. Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell. 2009;138:1195–1208. doi: 10.1016/j.cell.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–1065. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsujikawa M, Omori Y, Biyanwila J, Malicki J. Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc Natl Acad Sci USA. 2007;104:14819–14824. doi: 10.1073/pnas.0700178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- 83.Bellion A, Baudoin JP, Alvarez C, Bornens M, Metin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci. 2005;25:5691–5699. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci USA. 2005;102:13652–13657. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis and motility at multiple stages. J Cell Biol. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gambello MJ, Darling DL, Yingling J, Tanaka T, Gleeson JG, Wynshaw-Boris A. Multiple dose-dependent effects of Lis1 on cerebral cortical development. J Neurosci. 2003;23:1719–1729. doi: 10.1523/JNEUROSCI.23-05-01719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie Z, Moy LY, Sanada K, Zhou Y, Buchman JJ, Tsai LH. Cep120 and TACCs control interkinetic nuclear migration and the neural progenitor pool. Neuron. 2007;56:79–93. doi: 10.1016/j.neuron.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, et al. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007;134:901–908. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- 89.Minobe S, Sakakibara A, Ohdachi T, Kanda R, Kimura M, Nakatani S, et al. Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci Res. 2009;63:294–301. doi: 10.1016/j.neures.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Schenk J, Wilsch-Brauninger M, Calegari F, Huttner WB. Myosin II is required for interkinetic nuclear migration of neural progenitors. Proc Natl Acad Sci USA. 2009;106:16487–16492. doi: 10.1073/pnas.0908928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ge X, Frank CL, Calderon de Anda F, Tsai LH. Hook3 interacts with PCM1 to regulate pericentriolar material assembly and the timing of neurogenesis. Neuron. 2010;65:191–203. doi: 10.1016/j.neuron.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]