Abstract
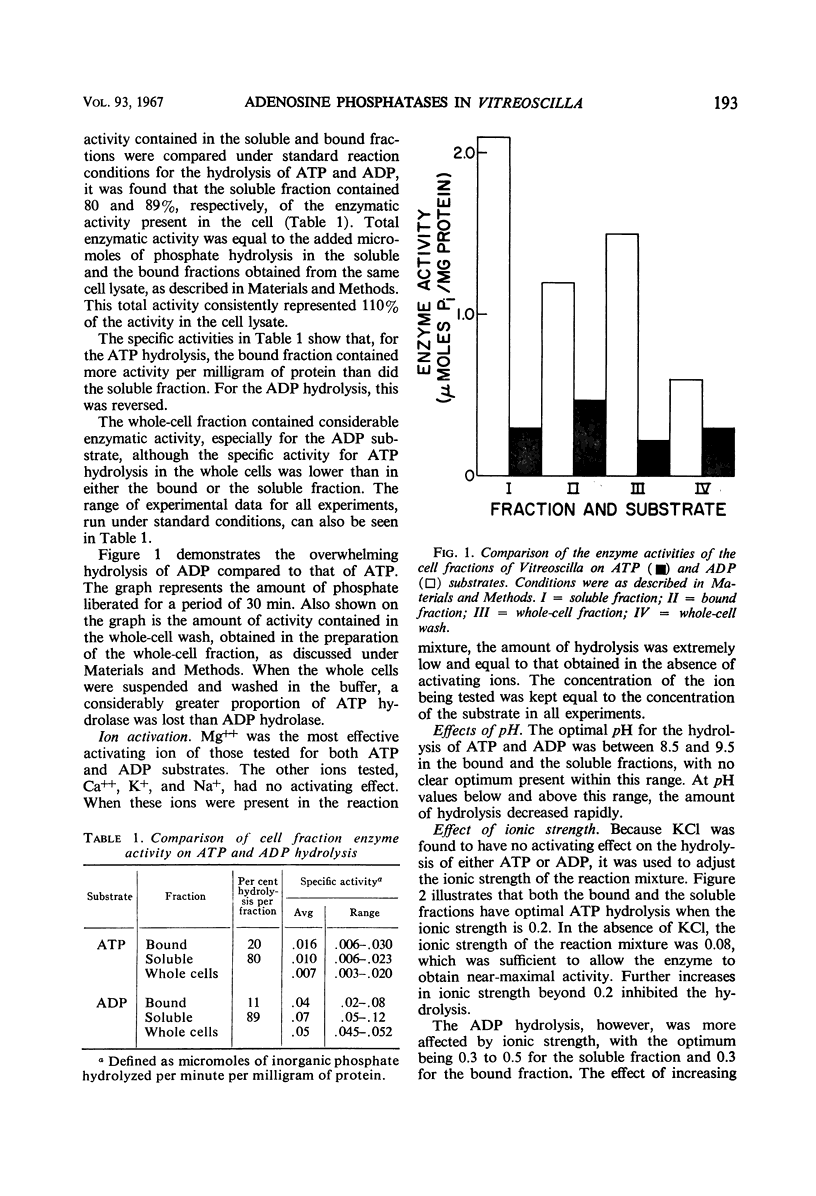
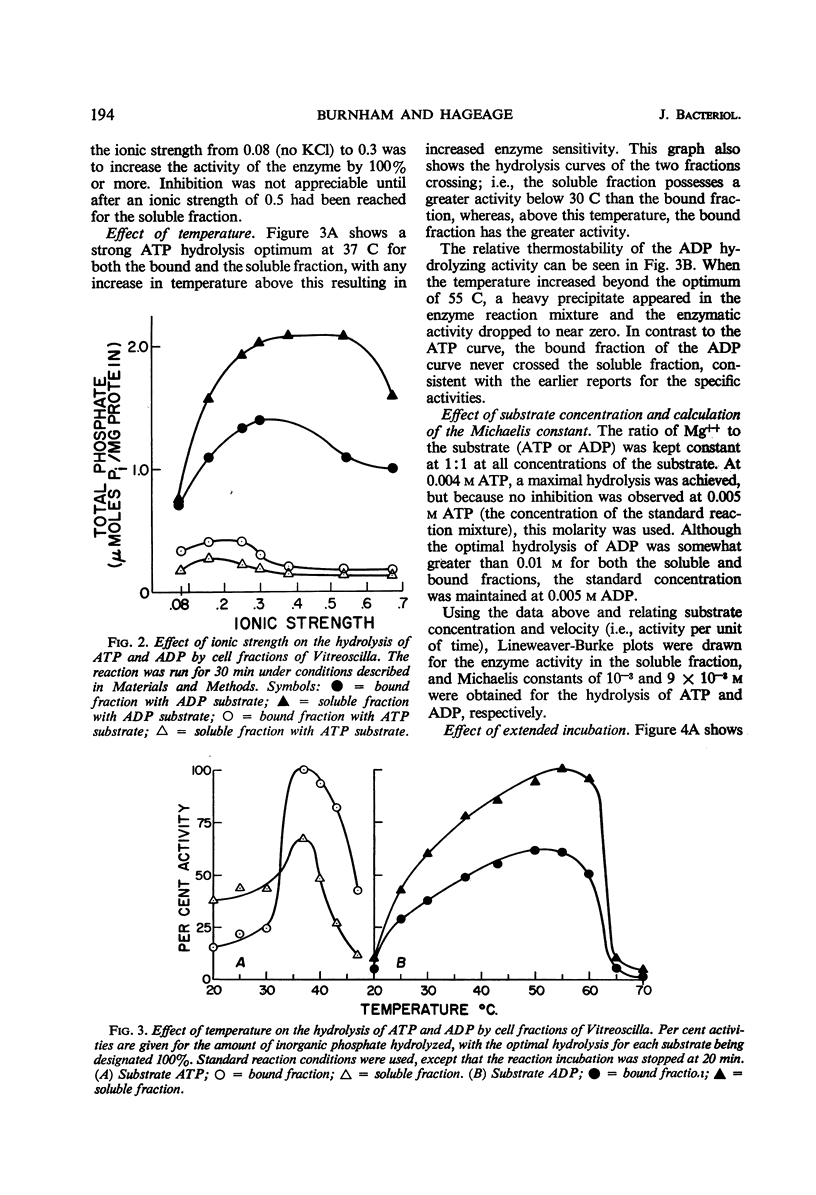
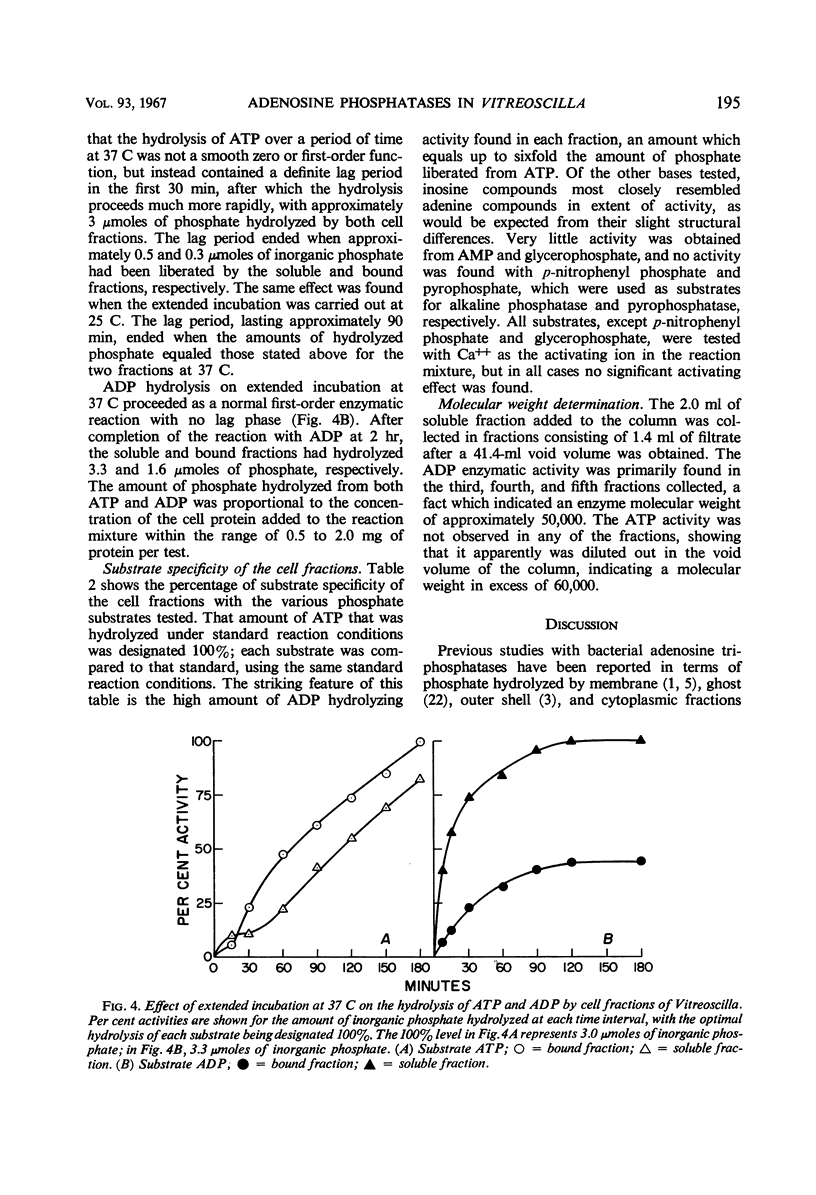
Bound, soluble, and whole-cell fractions of two strains of the gliding bacterium Vitreoscilla were found to contain two enzymes capable of hydrolyzing adenosine phosphates: a Mg++-activated adenosine triphosphatase with a temperature optimum of 37 C, and a Mg++-activated adenosine diphosphatase with a temperature optimum of 55 C. Both enzymes had an optimal pH response between 8.5 and 9.5. Maximal activation was achieved at an ionic strength of 0.2 for the adenosine triphosphatase and at 0.3 to 0.4 for the adenosine diphosphatase. Preliminary studies indicated a molecular weight of approximately 50,000 for the adenosine diphosphatase and a molecular weight greater than 60,000 for the adenosine triphosphatase. Comparisons are made with previously reported characteristics of these enzymes in other bacteria, and a hypothesis is offered as to the role these enzymes have in the gliding mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., McNAMARA P., JOHNSON F. B. Adenosine triphosphatase in isolated bacterial cell membranes. J Biol Chem. 1960 Dec;235:3649–3662. [PubMed] [Google Scholar]
- COSTERTON J. W., MURRAY R. G., ROBINOW C. F. Observations on the motility and the structure of Vitreoscilla. Can J Microbiol. 1961 Jun;7:329–339. doi: 10.1139/m61-040. [DOI] [PubMed] [Google Scholar]
- Chesbro W. R., Stuart D., Burke J. J., 2nd Multiple molecular weight forms of staphylococcal nuclease. Biochem Biophys Res Commun. 1966 Jun 21;23(6):783–792. doi: 10.1016/0006-291x(66)90555-9. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Hughes D. E. The enzymic activity of the outer shell of Lactobacillus arabinosus. J Gen Microbiol. 1965 Jul;40(1):81–95. doi: 10.1099/00221287-40-1-81. [DOI] [PubMed] [Google Scholar]
- DRAPEAU G. R., MACLEOD R. A. NUTRITION AND METABOLISM OF MARINE BACTERIA. XII. ION ACTIVATION OF ADENOSINE TRIPHOSPHATASE IN MEMBRANES OF MARINE BACTERIAL CELLS. J Bacteriol. 1963 Jun;85:1413–1419. doi: 10.1128/jb.85.6.1413-1419.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf W. Bewegungsorganellen bei Myxobakterien. Arch Hyg Bakteriol. 1965 Jul;149(5):518–526. [PubMed] [Google Scholar]
- LEVY H. M., KOSHLAND D. E., Jr Mechanism of hydrolysis of adenosinetriphosphate by muscle proteins and its relation to muscular contraction. J Biol Chem. 1959 May;234(5):1102–1107. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARSH C., MILITZER W. Thermal enzymes. VII. Further data on an adenosinetriphosphatase. Arch Biochem Biophys. 1956 Feb;60(2):433–438. doi: 10.1016/0003-9861(56)90448-9. [DOI] [PubMed] [Google Scholar]
- OGINSKY E. L., RUMBAUGH H. L. A cobalt-activated bacterial pyrophosphatase. J Bacteriol. 1955 Jul;70(1):92–98. doi: 10.1128/jb.70.1.92-98.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLAUT G. W. An inosinediphosphatase from mammalian liver. J Biol Chem. 1955 Nov;217(1):235–245. [PubMed] [Google Scholar]
- VOELZ H. SITES OF ADENOSINE TRIPHOSPHATASE ACTIVITY IN BACTERIA. J Bacteriol. 1964 Oct;88:1196–1198. doi: 10.1128/jb.88.4.1196-1198.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., GREENWALT J. W., LOW H. The hydrolysis of adenosine triphosphate by cell fractions of Bacillus megaterium. I. Localization and general characteristics of the enzymic activities. J Biol Chem. 1962 Mar;237:847–852. [PubMed] [Google Scholar]
- Yayashi M., Uchida R. A cation activated adenosinetriphosphatase in cell membranes of halophilic Vibrio parahaemolyticus. Biochim Biophys Acta. 1965 Oct 25;110(1):207–209. doi: 10.1016/s0926-6593(65)80113-8. [DOI] [PubMed] [Google Scholar]


