Abstract
Delphinidin, a major anthocyanidin present in many pigmented fruits and vegetables, possesses antioxidant, anti-inflammatory, and antiangiogenic properties. In this study, we provide evidence that it could be developed as a novel agent against human prostate cancer (PCa). We observed that delphinidin treatment to human PCa LNCaP, C4-2, 22Rν1, and PC3 cells resulted in a dose-dependent inhibition of cell growth without having any substantial effect on normal human prostate epithelial cells. We selected PC3 cells as a test model system because of their highly aggressive proliferative nature. Delphinidin treatment of cells resulted in a dose-dependent induction of apoptosis and arrest of cells in G2-M phase. This induction of apoptosis seems to be mediated via activation of caspases because N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluromethylketone significantly reduced apoptosis induced by delphinidin. We also observed that delphinidin treatment of cells resulted in a dose-dependent decrease in (a) phosphorylation of IκB kinase γ (NEMO), (b) phosphorylation of nuclear factor-κB (NF-κB) inhibitory protein IκBα, (c) phosphorylation of NF-κB/p65 at Ser536 and NF-κB/p50 at Ser529, (d) NF-κB/p65 nuclear translocation, and (e) NF-κB DNA binding activity. Delphinidin administration (2 mg, i.p. thrice weekly) to athymic nude mice implanted with PC3 cells resulted in a significant inhibition of tumor growth. Analysis of tumors from delphinidin-treated mice showed significant decrease in the expression of NF-κB/p65, Bcl2, Ki67, and PCNA. Taken together, our data suggest that delphinidin could be developed as an agent against human PCa.
Introduction
The public health effect of prostate cancer (PCa) poses a serious concern in the world, especially in Western countries. In the year 2008, in the United States alone 186,320 new cases of PCa will be diagnosed and a total of 28,660 deaths are predicted (1). PCa is generally detected in men >50 years of age, usually at an advanced stage of the disease. Because of unsatisfactory outcomes associated with treating advanced cases of PCa, there is a need to develop novel preventive approaches to control this disease. One such preventive approach is through chemoprevention by using naturally occurring dietary substances (2). In recent years, there is considerable activity in establishing the usefulness of naturally occurring dietary agents for chemoprevention as well as chemotherapy of PCa. Polyphenols are one group of such agents, many of which have been shown to be multitarget agents interfering with several processes involved in cancer development and progression (3). Among polyphenols, the anthocyanidins and their glycosylated forms (anthocyanins) are gaining considerable popularity on the fast-expanding market of food supplements as they seem to possess potentially beneficial effects against various diseases (4, 5) including cancer. These anthocyanidins are widely found in pigmented fruits and vegetables such as pomegranate, berry, grape, sweet potato, red cabbage, red radish, and eggplant (6, 7). Delphinidin (Fig. 1A, inset), one of the major anthocyanidin present in these fruits and vegetables, is a diphenylpropane-based polyphenolic ring structure that carries a positive charge in its central ring (8). It has been reported that delphinidin possesses antioxidant (9), anti-inflammatory (10), and antimutagenic properties (11), and we recently showed that it inhibits invasion of breast cancer cells (12). Other studies have revealed the antiangiogenic effects of delphinidin in in vitro and in vivo model systems (13–15).
Figure 1.
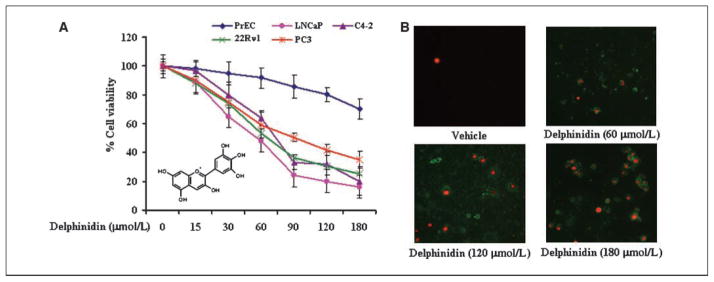
Effect of delphinidin on cell viability and apoptosis of LNCaP, C4-2, 22Rν1, and PC3 cells. A, human PCa cells were treated with vehicle alone (0.1% DMSO) and specified concentrations of delphinidin in 0.1% DMSO for 48 h, and cell viability was determined by MTT assay as detailed in Materials and Methods. The values are represented as the percent viable cells, with vehicle-treated cells regarded as 100% viable. Points, mean of three independent experiments; bars, SD. Inset, delphinidin structure. B, representative micrographs of PC3 cells undergoing apoptosis induced by treatment with specified concentrations of delphinidin for 48 h as assessed by fluorescence microscopy. Green fluorescence of Annexin V staining represents the cells undergoing apoptosis and red fluorescence of propidium iodide shows the cells undergoing either necrosis or late apoptosis as detected with a Zeiss Axiovert 100 microscope.
We reported chemopreventive and chemotherapeutic effects of pomegranate fruit juice against PCa (16) and speculated that these effects are mediated by the anthocyanidins present therein. Pomegranate fruit extract, which we have used in our earlier studies, was found to contain six anthocyanidins, among which delphinidin was the major component (17). In this study, we show for the first time that delphinidin induces apoptosis and cell growth inhibition of highly aggressive human PCa PC3 cells in in vitro system and significantly inhibits tumor growth in in vivo xenograft mouse model and that these effects are mediated through the inhibition of nuclear factor κB (NF-κB) signaling.
Materials and Methods
Cell lines and reagents
PC3, LNCaP, and 22Rν1 cells were obtained from American Type Culture Collection and were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and 1% antibiotics. Prostate epithelial cells and their growth media were procured from Cambrex BioScience. C4-2 cells were a kind gift from Dr. G.N. Thalmann (University of Bern, Bern, Switzerland) and grown in T-media (Invitrogen). The cells were maintained under standard cell culture conditions at 37°C and 5% CO2 in a humid environment. Delphinidin (>99% pure) was obtained from Extrasynthese. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was procured from Sigma. Bcl2, cyclin-dependent kinase (cdk)-1, cdk2, proliferating cell nuclear antigen (PCNA), Ki67, KIP1/p27, and lamin antibodies were obtained from Santa Cruz Biotechnology. Cyclin D1, cyclin A, active caspase-3, caspase-9, pNF-κB/p65S(ser536), pNF-κB/p50(ser529), IκBα, pIκBα, pIKKγ, IKKα, and WAF1/p21 antibodies were procured from Cell Signaling Technology. Full-length poly(ADP-ribose) polymerase (PARP), NF-κB/p65, and Bax antibodies were purchased from Upstate Biotechnology. Cleaved PARP antibody was procured from Promega. The general caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluromethylketone (Z-VAD-FMK) was obtained from R&D Systems, Inc. Rhodamine Red-X–conjugated antibody was procured from Jackson ImmunoResearch Laboratories, Inc. Antimouse or antirabbit secondary antibody horseradish peroxidase conjugate was obtained from Amersham Pharmacia Life Sciences. Novex precast 12% Tris-glycine gels were obtained from Invitrogen. Matrigel was procured from BD Bioscience.
Treatment of cells
Cells were grown to 50% to 70% confluence and then treated with freshly prepared delphinidin solution in DMSO. The final concentration of DMSO used was 0.1% (v/v) for each treatment. Control cells treated with 0.1% DMSO served as the vehicle group. After 48 h of treatment with delphinidin (30–180 μmol/L), the cells were harvested, and cell lysates were prepared and stored at −80°C for subsequent use. For caspase inhibition experiments, the PC3 cells were pretreated with 10 μmol/L Z-VAD-FMK, a general caspase inhibitor, 4 h before the addition of delphinidin (120 μmol/L) in the culture media.
Cell viability assay
The effect of delphinidin on cell viability of PC3, LNCaP, C4-2, 22Rν1, and prostate epithelial cells was determined by the MTT assay. Cells were plated at a density of 1 × 104 per well in 200 μL of complete culture medium and treated with delphinidin (30–180 μmol/L) in 96-well microtiter plates for 48 h. After incubation for a specified time at 37°C in a humidified incubator, MTT (5 mg/mL in PBS; diluted in 10 mL of serum-free medium) was added to each well and incubated for 2 h, after which the plate was centrifuged at 1,000 rpm for 5 min at 4°C. After careful removal of the medium, 0.1 mL buffered DMSO was added to each well. The absorbance was recorded on a microplate reader at the wavelength of 540 nm. The effect of delphinidin on cell growth inhibition was assessed as percent cell viability, where vehicle-treated cells were taken as 100% viable.
Detection of apoptosis by fluorescence microscopy
The Annexin V-FLUOS staining kit (Roche Diagnostic Corp.) was used for the detection of apoptotic bodies following the vendor’s protocol. This kit uses a dual-staining protocol in which the cells show green fluorescence of Annexin V (apoptotic cells) and red fluorescence of propidium iodide (necrotic cells or late apoptotic cells). PC3 cells were grown to ~60% confluence and then treated with delphinidin as described above. The fluorescence was detected with a Zeiss Axiophot DM HT microscope. Images were captured with an attached camera.
Quantification of apoptosis by flow cytometry
For quantification of apoptosis, PC3 cells were grown at a density of 50% to 60% confluence in 100-mm culture dishes and were treated with 10 μmol/L Z-VAD-FMK (a general caspase inhibitor) for 4 h, washed with PBS, and then treated with delphinidin for 48 h. The cells were trypsinized, washed with PBS, and were processed for labeling with fluorescein-tagged dUTP and propidium iodide by the use of an APO-DIRECT apoptosis kit (Phoenix Flow Systems) as per manufacturer’s protocol. The labeled cells were analyzed by flow cytometry.
DNA cell cycle analysis
PC3 cells (50–60% confluent) were synchronized by overnight serum starvation and treated with delphinidin (30–180 μmol/L) for 48 h in complete medium. The cells were trypsinized, washed twice with chilled PBS, and centrifuged. The cell pellet was resuspended in 50 μL cold PBS to which cold methanol (450 μL) was added, and the cells were incubated for 1 h at 4°C. The cells were centrifuged at 1,000 rpm for 5 min; the pellet was washed twice with chilled PBS, suspended in 500 μL PBS, and incubated with 5 μL RNase (20 μg/mL final concentration) at 37°C for 30 min. The cells were chilled on ice for 10 min and incubated with propidium iodide (50 μg/mL final concentration) for 1 h and analyzed by flow cytometry. Flow cytometry was done with a FACScan (Becton Dickinson). A minimum of 10,000 cells per sample were counted and the DNA histograms were further analyzed by using ModiFitLT software (Verity Software House) for cell cycle analysis.
Immunoblot analysis
The total cell lysates were prepared, and immunoblot analysis was done as described earlier (18). The protein concentration was determined by using a bicinchoninic acid protein assay kit.
ELISA
Following treatment of cells with delphinidin (30–180 μmol/L) for 48 h, cells were harvested and nuclear lysates were prepared as described earlier (18). The commercially available kit for NF-κB/p65 (purchased from Active Motif) contains the specific oligos with the specific consensus sequence for NF-κB/p65 binding. Five micrograms of nuclear lysate protein from each group were taken for quantification of NF-κB activity. The experiment was done according to the manufacturer’s instructions. Absorbance was taken at 450 nm by using ELISA reader (Multiscan MCC/340, Fisher Scientific).
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) for NF-κB was done using a LightShift chemiluminescent EMSA kit (Pierce) following the manufacturer’s protocol. NF-κB oligo was biotin labeled using the Biotin 3′ End DNA Labeling Kit (Pierce Biotechnology). The sequence of NF-κB oligonucleotide was 5′-AGTT-GAGGGGACTTTCCCAGGC-3′; 3′-TCAACTCCCCTGAAAGGGTCCG-5′. Briefly, 5 μg of nuclear extract were incubated with 20 μg of NF-κB/p65 antibody at room temperature for 30 min, followed by further incubation with 1 pmol of biotin-labeled NF-κB oligonucleotide for 30 min at 37°C. The DNA protein complex formed was resolved on 6% DNA retardation gel and transferred onto a nylon membrane. After transfer was completed, DNA was cross-linked to the membrane at 120 mJ/cm2 using a UV cross-linker. The biotin end-labeled DNA was detected using the streptavidin-horseradish peroxide conjugate and LightShift chemiluminescent substrate according to the manufacturer’s instructions. The membrane was exposed to X-ray film (XAR-5 Amersham Life Science, Inc.) and developed using a Kodak film processor.
Immunocytochemical staining of NF-κB/p65
Cells were seeded in two chambered tissue culture glass slides and treated with delphinidin as described previously. Cells were washed with PBS and fixed in 2% paraformaldehyde in PBS for 10 min at room temperature and then permeabilized in cold methanol (−20°C). After that, cells were washed thrice with PBS and blocked with 2% donkey serum in 1× PBS for 1 h and incubated with NF-κB/p65 antibody [1:50 in 5% donkey serum (1× PBS)] overnight at 4°C. After three washes with PBS, cells were incubated with 1:50 of donkey anti-rabbit Rhodamine Red-X–conjugated antibody for 1 h at room temperature. Slides were mounted using Prolong antifade kit (Invitrogen) and photographed with a Zeiss Axiophot DMHT microscope.
Luciferase assay
PC3 cells (2 × 106) were electroporated with human NF-κB luciferase reporter plasmid (pNF-κB-TA-Luc; 1 μg; Clontech Laboratories, Inc.) along with 50 ng of Renilla luciferase reporter plasmid pRL-TK (Promega), which was used as internal control to normalize transfection efficiency using AMAXA nucleofection kit. Parallel to that, cells were also transfected with empty pTA-Luc reporter vector (1 μg). After electroporation, 30,000 cells were distributed per well of a 24-well plate and allowed to grow for 16 h followed by delphinidin treatment for another 24 h with the above described concentrations. Dual luciferase assay reagent kit was procured from Promega and luciferase activity was measured according to the manufacturer’s protocol.
In vivo tumor xenograft model
Athymic (nu/nu) male nude mice, obtained from NxGen Biosciences, were housed under pathogen-free conditions with a 12 h light/12 h dark schedule and fed with an autoclaved diet and water ad libitum. To establish tumor xenografts in mice, PC3 cells (1 × 106) were suspended in 1:1 medium mixed with Matrigel and were s.c. inoculated on the left and right flanks of each mouse. Twelve animals were then randomly divided into two groups with six animals each. The first group of animals received i.p. injection of 100 μL of 1:10 ratio of DMSO and normal saline, whereas animals of the second group received i.p. injection of delphinidin (2 mg/animal in 100 μL of 1:10 ratio of DMSO and normal saline) thrice a week. Tumor sizes were measured every week, and tumor volume was calculated by the formula 0.5238 × L1 × L2 × H, where L1 is the long diameter, L2 is the short diameter, and H is the height of the tumor. All animals in non–delphinidin-treated group were sacrificed and removed from the protocol when the tumor crossed the targeted volume of 1,200 mm3. Because this occurred at 8 wk on test, at this time three animals from delphinidin-treated group were also sacrificed, and tumors were excised and pooled for immunoblotting and imunohistochemistry. The remaining animals of group 2 were allowed to remain in the protocol till tumors reached the targeted volume of 1,200 mm3, at which time the animals were sacrificed. All procedures were conducted in accordance with the guidelines for the Use and Care of Laboratory Animals and approved by the Institutional animal care and use committee.
Immunohistochemical analysis
Sections (5 μm thick) were cut from paraffin-embedded tumor tissues from vehicle- and delphinidin-treated animals. Immunostaining was done for Ki67, PCNA, NF-κB/p65, and Bcl2 using specific antibodies with appropriate dilutions and replaced with either normal host serum or block for negative controls followed by staining with appropriate horseradish peroxidase–conjugated secondary antibodies. The slides were developed in diaminobenzidine and counterstained with a weak solution of hematoxylin. The stained slides were dehydrated and mounted in Permount and visualized on a Zeiss Axiophot DM HT microscope.
Statistical analysis
Results were analyzed using a two-tailed Student’s t-test to assess statistical significance. P < 0.05 was considered statistically significant.
Results
Delphinidin inhibits the growth of LNCaP, C4-2, 22Rν1, and PCa PC3 cells
To evaluate the effect of delphinidin on cell viability of human PCa and normal prostate epithelial cells, MTT assay was done. Delphinidin treatment to PCa cells resulted in a significant dose-dependent inhibition of cell growth (Fig. 1A). The IC50 value at 48 hours posttreatment with delphinidin for LNCaP, C4-2, 22Rν1, and PC3 cells was 50, 70, 65, and 90 μmol/L, respectively, suggesting differential dose response of PCa cells to delphinidin (Fig. 1A). Similar doses of delphinidin showed insignificant effect on cell viability of prostate epithelial cells (Fig. 1A). Taking into account the more aggressive and highly metastatic nature of PCa, PC3 cells were selected then as a model system to conduct mechanistic studies in in vitro and in vivo xenografts.
Delphinidin induces apoptosis in PC3 cells
To test whether delphinidin-mediated decrease in cell growth is due to induction of apoptosis, we conducted Annexin V and propidium iodide stainings in delphinidin-treated cells. Data showed a significant induction of apoptosis by delphinidin at doses of 60 to 180 μmol/L in cells, which was evident from the significant enhancement in Annexin V staining (Fig. 1B).
Delphinidin induces PARP cleavage and increases Bax/Bcl2 ratio in PC3 cells
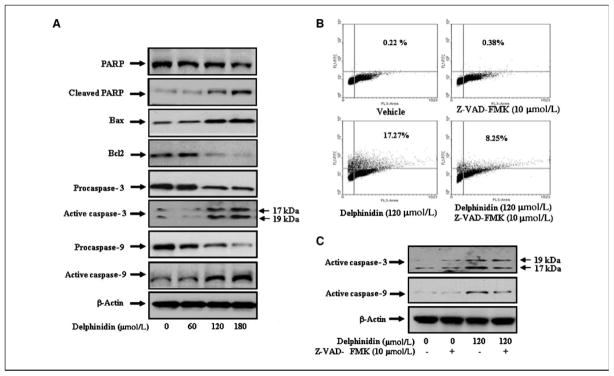
Poly (ADP-ribose) polymerase (PARP) protein is activated following DNA damage and is cleaved into a 85-kDa COOH-terminal fragment, with a reduced catalytic activity, and a 24-kDa NH2-terminal peptide, which retains the DNA binding domains. During induction of apoptosis, DNA fragmentation leads to activation of PARP; therefore, cleavage of PARP protein is considered as an important biomarker of apoptosis. Immunoblot analysis showed a significant dose-dependent increase in cleaved PARP in cells treated with delphinidin (Fig. 2A). It has been shown that Bcl2 forms a heterodimeric complex with the apoptotic Bax protein, thereby neutralizing its apoptotic effects. Therefore, the ratio of Bax/Bcl2 is often considered as a decisive factor in determining whether cells will undergo death or survive. We observed that delphinidin treatment of cells resulted in a decrease in Bcl2 expression with a concomitant increase in the protein level of Bax (Fig. 2A). This resulted in a substantial increase in Bax/Bcl2 ratio, which favors apoptosis. Taken together, these findings suggest that cleavage in PARP protein, up-regulation of Bax, and down-regulation of Bcl2 may collectively form a molecular basis for the apoptotic action of delphinidin.
Figure 2.
Effect of delphinidin on apoptotic biomarkers in PC3 cells. PC3 cells were treated with vehicle alone (0.1% DMSO) or specified concentrations of delphinidin in 0.1% DMSO for 48 h as detailed in Materials and Methods. A, protein levels of PARP, cleaved PARP, Bcl2, Bax, procaspase-3, procaspase-9, active caspase-3, and caspase-9 in PC3 cells as determined by immunoblot analysis. Equal loading of protein was confirmed by stripping and reprobing the blots with β-actin antibody. Representative of three independent experiments with similar results. B, PC3 cells were treated with 10 μmol/L concentration of the general caspase inhibitor Z-VAD-FMK for 4 h, followed by the treatment with the indicated doses of delphinidin for 48 h. Cells showing dUTP fluorescence above that of control population, as indicated by the line in each histogram, are considered as apoptotic cells. C, protein levels of active caspase-3 and caspase-9 in PC3 whole cell lysates were determined by immunoblot analysis. Equal loading of protein was confirmed by stripping and reprobing the blots with β-actin.
Delphinidin induces apoptosis via activation of caspases in PC3 cells
In most cancer cells, caspases are present in the pro-forms (inactive) and require site-specific cleavage of the protein to become active and participate in the process of apoptosis. To test whether caspases are involved in apoptosis induction by delphinidin, we first evaluated the protein levels of procaspases and active caspases in delphinidin-treated cells. Data presented in Fig. 2A showed a significant and progressive increase in the levels of active caspase-3 and caspase-9 proteins in delphinidin-treated cells. To test whether delphinidin induces apoptosis via activation of caspases, we used a general caspases inhibitor, Z-VAD-FMK. Delphinidin (120 μmol/L)–treated cells exhibited 17.3% terminal deoxyribonucleotidyl transferase–mediated dUTP nick end labeling–positive cells, which was significantly reduced to 8.25% with the treatment of cells with Z-VAD-FMK (Fig. 2B). Immunoblot analysis showed that delphinidin-induced activation of caspase-3 and caspase-9 was significantly reduced after caspase inhibitor treatment (Fig. 2C). These results suggest that induction of caspases may be a possible mechanism by which delphinidin induces apoptosis in PC3 cells.
Delphinidin causes G2-M phase cell cycle arrest in PC3 cells
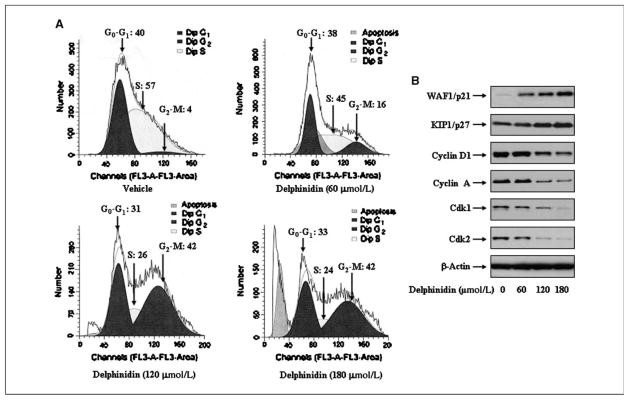
Several studies have shown that the induction of apoptosis might be due to cell cycle arrest (19, 20). Therefore, inhibition of the cell cycle has been appreciated as a target for the management of cancer (21, 22). To assess the effect of delphinidin treatment on the distribution of cells in the cell cycle, we performed DNA cell cycle analysis. As shown in Fig. 3A, compared with vehicle treatment, delphinidin treatment resulted in a dose-dependent accumulation of cells in the G2-M phase of the cell cycle by 16% and 42% at 60 and 120 μmol/L concentrations of delphinidin, respectively. These data suggest that delphinidin induces cell cycle arrest in G2-M phase.
Figure 3.
Effect of delphinidin on cell cycle and cell cycle modulatory proteins in PC3 cells. Cell cycle analysis was done by flow cytometry as detailed in Materials and Methods. A, cell cycle analysis in PC3 cells treated with delphinidin. B, protein levels of p21, p27, cyclin D1, cyclin A, cdk1, and cdk2 in PC3 cells as determined by immunoblot analysis. Equal loading of protein was determined by stripping and reprobing the blots with β-actin antibody. Representative of three independent experiments with similar results.
Delphinidin modulates cell cycle regulatory proteins in PC3 cells
Molecular analysis of human cancers has revealed that cell cycle regulators are frequently mutated in most common malignancies (23, 24). We next examined the effect of delphinidin on cell cycle inhibitory proteins p27/KIP1 and p21/WAF1, which are involved in cell cycle progression. Immunoblot analysis showed a significant induction of these proteins in a dose-dependent manner (Fig. 3B). We next evaluated the effect of delphinidin on the protein levels of cyclins and cdks, which are known to be regulated by KIP1/p27 and WAF1/p21. Delphinidin treatment of cells resulted in a significant dose-dependent decrease in the protein levels of cyclin D1 and cyclin A as well as cdk1 and cdk2 (Fig. 3B). These results suggest that delphinidin restores proper checkpoint control via modulation of the cyclins, cdks, and the expression of their inhibitors.
Delphinidin inhibits expression, translocation, and DNA binding activity of NF-κB in PC3 cells
It has been shown that activation of NF-κB blocks apoptosis and promotes cell proliferation (25). We tested whether delphinidin treatment inhibits constitutive NF-κB activation. Using immunoblot analysis, we observed that delphinidin treatment of cells resulted in decreased phospho-NF-κB/p50 at Ser529 and phospho-NF-κB/p65 at Ser536 in the nuclear fraction (Fig. 4A). We further confirmed the inhibition of NF-κB/p65 DNA binding activity by performing EMSA and immunocytochemistry. Delphinidin treatment of cells resulted in a dose-dependent decrease in NF-κB DNA binding activity (Fig. 4B). Delphinidin-treated cells exhibited a marked decrease in NF-κB nuclear localization, further suggesting inhibition of NF-κB nuclear translocation (Fig. 4C). Delphinidin treatment of cells showed no cytoplasmic immunostaining with anti-p65 antibody at doses of 120 to 180 μmol/L, whereas control cells showed an intense nuclear fluorescence (Fig. 4C). However, delphinidin treatment at doses of 60 μmol/L showed weak expression of NF-κB/p65 in the nucleus (Fig. 4C). Using a specific NF-κB/p65 ELISA, we also found that treatment of cells with delphinidin resulted in a significant inhibition of nuclear translocation of NF-κB/p65 in a dose-dependent manner (Fig. 4D). These results correspond with the NF-κB DNA binding activity data and further support the inhibition of nuclear translocation of NF-κB/p65 subunit into the nucleus by delphinidin. To further examine the effects of delphinidin on NF-κB, we performed NF-κB promoter activity assay using NF-κB binding sites containing luciferase reporter plasmid. Delphinidin treatment of cells resulted in a significant dose-dependent decrease in NF-κB luciferase activity (Fig. 5A), suggesting its effect at the transcriptional level.
Figure 4.
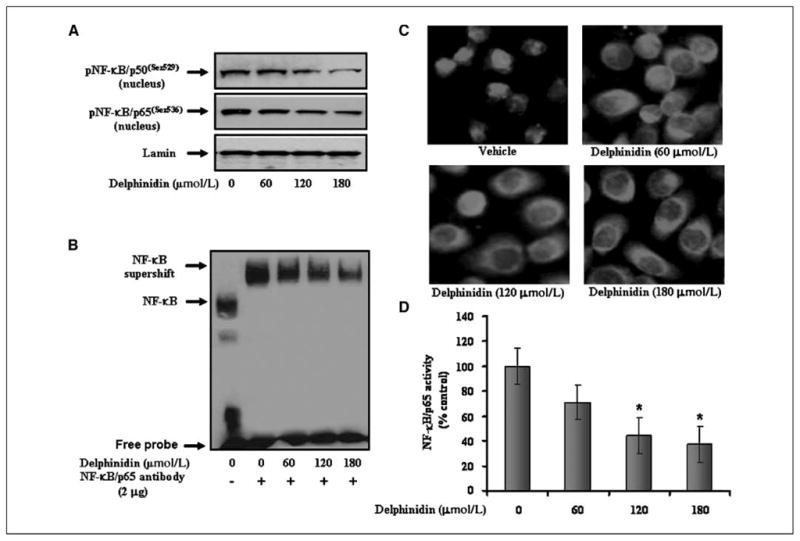
Effect of delphinidin on phospho-NF-κB/p50 and p65 and NF-κB DNA binding activity in PC3 cells. Nuclear extracts were prepared from cells treated with vehicle alone (0.1% DMSO) and specified concentrations of delphinidin in 0.1% DMSO for 48 h. A, protein levels of phospho-NF-κB/p50(ser529) and phospho-NF-κB/p65(ser536) in nuclear lysates of PC3 cells as determined by immunoblot analysis. Equal loading of protein was assessed by stripping and reprobing the blots with lamin antibody. B, NF-κB DNA binding activity in PC3 cells as detailed in Materials and Methods. Lane 1, NF-κB-DNA complex. Lane 2, the shift in NF-κB-DNA complex due to binding with NF-κB/p65 specific antibody. Lanes 3 to 5, change in the NF-κB/p65 oligo-immune complex due to treatment with delphinidin. C, immunocytochemistry of NF-κB/p65 in PC3 cells. D, specific ELISA for NF-κB/p65 was done in nuclear lysates of PC3 cells treated with vehicle alone (0.1% DMSO) and specified concentrations of delphinidin.
Figure 5.
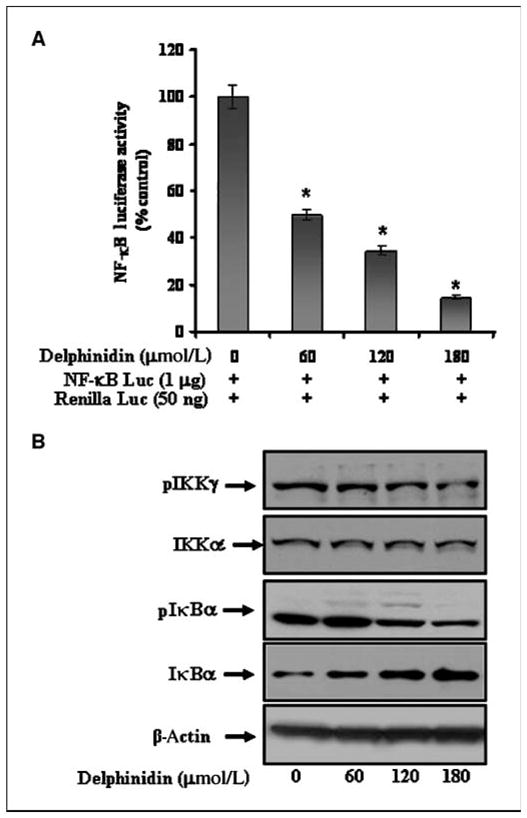
Effect of delphinidin on NF-κB/p65 transcriptional activation and the protein levels of pIKKγ, pIκBα, and total IκBα in PC3 cells. A, effect of delphinidin treatment on NF-κB promoter activity. Cells were transiently cotransfected with NF-κB luciferase plasmid and Renilla luciferase plasmid for 12 h and cells were treated with vehicle only or specified concentrations of delphinidin for 24 h and harvested as detailed in Materials and Methods. B, protein levels of pIKKγ, pIκBα, and total IκBα in cells treated with vehicle alone (0.1% DMSO) and specified concentrations of delphinidin in 0.1% DMSO as determined by immunoblot analysis. Equal loading of protein was confirmed by stripping and reprobing the blots with β-actin antibody.
Delphinidin inhibits protein levels of phospho-IκBα and its upstream kinase phospho-IKKγ (NEMO) in PC3 cells
In the absence of stimulatory signals, NF-κB resides in the cytoplasm in the form of hetrodimeric complex with its inhibitory proteins IκBα. Stimulation to the cells with various stimuli activates the IκB kinase (IKK) complex, which is composed of two catalytic subunits (IKKα and IKKβ) and a regulatory subunit (IKKγ/NEMO). Activated IKK phosphorylates NF-κB–bound IκB proteins and targets them for polyubiquitination and rapid degradation by creating a binding site for SCFβ-TRCP-ubiquitin ligase complex (26). Delphinidin treatment resulted in a dose-dependent decrease in the phosphorylation of the NF-κB inhibitory protein IκBα with a concomitant increase in total IκBα protein (Fig. 5B). IκB is regulated by upstream kinase IKKγ (NEMO), which phosphorylates IκB. We observed that delphinidin treatment of cells resulted in a significant inhibition of pIKKγ protein (Fig. 5B), but no such effect was observed on the protein level of the IKKα catalytic subunit. These results suggest that delphinidin inhibits NF-κB signaling via inhibition of upstream kinases.
Delphinidin inhibits tumorigenicity of PC3 cells in xenograft mouse model
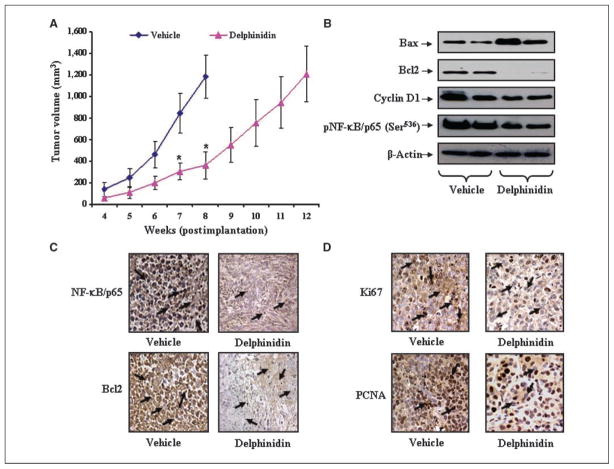
Because delphinidin was observed to be effective in inhibiting the growth of PCa cells in vitro, we next determined whether these results could be translated into an in vivo xenograft mouse model. Delphinidin treatment did not cause any loss in the body weight and food intake (data not shown), indicating that the dosages used were not toxic to the animals. The average volume of tumors in non–delphinidin-treated mice increased as a function of time and reached the targeted volume of 1,200 mm3 at 8 weeks postinoculation. However, at this time, in mice treated with delphinidin, the average tumor volume was only 361 mm3 (Fig. 6A). Next, we evaluated the extent of tumor growth inhibition in delphinidin-treated animals and found that the targeted tumor volume of 1,200 mm3 was reached at 12 weeks (Fig. 6A). The observed differences for tumor development in delphinidin-treated mice compared with control mice were statistically significant (P<0.01; Fig. 6A). From these data, we conclude that delphinidin is an effective anticancer agent that has the potential to inhibit or slow the tumorigenicity of PCa PC3 cells in in vivo system.
Figure 6.
Effect of delphinidin administration on tumorigenecity of PC3 cells and the expression levels of Bax, Bcl2, NF-κB/p65, and known proliferation markers, Ki67 and PCNA, under in vivo conditions. A, average tumor volume of vehicle- or delphinidin-treated animals was plotted over weeks as detailed in Materials and Methods. Points, mean of 12 tumors; bars, SD. P < 0.01, versus vehicle-treated animals. B, protein levels of Bax, Bcl2, cyclin D1, and phospho-NF-κB/p659(Ser536) as determined by immunoblot analysis in pooled tumors excised from mice treated with vehicle or delphinidin. Equal loading of protein was confirmed by stripping and reprobing the blots with β-actin antibody. C and D, representative photomicrographs (magnification, ×200) showing immunohistochemical staining for NF-κB/p65, Bcl2, Ki67, and PCNA in tumor sections of vehicle- or delphinidin-treated mice harvested at 8 wk. Arrows, regions exhibiting immunoreactivity.
Delphinidin modulates the protein levels of Bcl2 and Bax and inhibits the protein levels of NF-κB and cyclin D1 in PC3 originated tumors in athymic nude mice
Because delphinidin treatment was observed to modulate the expression levels of Bax and Bcl2 under in vitro conditions, we determined the effect of delphinidin administration on the expression levels of Bax and Bcl2 in tumors excised from both groups of animals. As shown in Fig. 6B, delphinidin administration was observed to decrease the expression level of Bcl2 protein. Tumor sections from delphinidin-administered mice also exhibited significantly reduced Bcl2-positive cells compared with vehicle-treated animals (Fig. 6C). Inversely, a significant increase in the expression level of Bax was observed in tumor tissues of animals treated with delphinidin. Further, immunoblot analysis of NF-κB and cyclin D1 in tumor tissues of delphinidin-treated mice also showed a significant decrease in these proteins (Fig. 6B). In addition, immunohisto-chemical analysis of NF-κB showed a weak nuclear staining of phospho-NF-κB in delphinidin-treated tumor tissues as compared with vehicle-treated tissues (Fig. 6C). These data further show the involvement of NF-κB signaling in the inhibition of tumor growth by delphinidin.
Delphinidin inhibits Ki67 and PCNA expression in PC3 originated tumors in athymic nude mice
As delphinidin treatment was observed to inhibit or decrease the tumorigenic potential of PC3 cells in vivo, we next determined the effect of delphinidin on the protein levels of Ki67 and PCNA, which are known markers of proliferation. It was evident from the immunohistochemical analysis of tumors that animals receiving delphinidin exhibited significant decrease in Ki67- and PCNA-positive cells compared with vehicle-treated animals (which exhibited intense staining for these proteins), suggesting the antiproliferative efficacy of delphinidin under in in vivo conditions (Fig. 6D).
Discussion
Prostate cancer is the second leading cause of cancer-related deaths in American men, being next only to lung cancer. Therefore, there is an urgent need to intensify our efforts to identify novel agents that could delay or prevent the development of PCa. Recently, chemoprevention and other intervention strategies using naturally occurring agents have emerged as a promising alternative option that could prevent or slow the tumor growth and thus improve the quality of life of PCa patients. In this regard, several fruit- and vegetable-derived chemopreventive agents have been reported that induce apoptosis in cancer cells in both in vitro and in vivo systems (27, 28). We have previously reported that pomegranate fruit extract induces apoptosis in PCa cells and inhibits tumor growth in athymic nude mice (16). Because pomegranate fruit extract contains several anthocyanidins, among which delphinidin is one of the most abundant constituent (17), we hypothesized that pomegranate fruit extract–induced effects might be largely due to the presence of delphinidin. In the present study, we observed the antiproliferative and apoptotic effects of delphinidin on PCa PC3 cells both in vitro and in vivo. We observed that the primary mode of delphinidin-mediated inhibition of tumor growth is through the induction of apoptosis. Because caspases have been shown to be involved in apoptosis via activation of downstream effector molecules such as PARP (29, 30), we found that the induction of apoptosis by delphinidin is governed primarily by the activation of caspases because pretreatment with Z-VAD-FMK, a caspase inhibitor, significantly prevented delphinidin-mediated apoptosis.
Bcl2 is an upstream effector molecule in the apoptotic pathway and has been identified as a potent suppressor of apoptosis (31), and most cancers including PCa generally overexpress Bcl2 (32, 33) thereby escaping apoptosis and undermining therapy. Bcl2 forms a heterodimer with the apoptotic protein Bax and thereby neutralizes its apoptotic effects. Therefore, alteration in the ratio of Bax/Bcl2 is a decisive factor that plays an important role to determine whether cells will undergo apoptosis. We and others have previously shown the involvement of caspases in the induction of apoptosis by naturally occurring, potentially useful cancer chemo-preventive agents (34, 35). We observed that delphinidin significantly down-regulated Bcl2 protein and up-regulated levels of Bax protein in PCa cells as well as in tumors from PC3 xenografts, suggesting the involvement of an intrinsic apoptotic pathway by which delphinidin induces apoptosis in PCa cells and inhibits tumor growth in athymic nude mice.
Inhibition of tumorigenesis often involves modulation of signal transduction pathways, leading to cell cycle arrest and, consequently, apoptosis. It has been shown that activation of NF-κB induces resistance to apoptosis induced by various chemotherapeutic agents (36). Other studies suggest that activation of NF-κB signaling induces inflammation and leads to carcinogenesis (37, 38). In clinical studies of PCa specimens, overexpression of NF-κB/p65 protein was shown to be an independent predictor of poor prognosis in PCa patients (39). In prostatectomy specimens of PCa with relapsed tumor, NF-κB was found to be concentrated in the nuclear fraction (40). Therefore, effective inhibition of NF-κB could be critical in providing a targeted pathway for PCa prevention. We observed that treatment of PC3 with delphinidin led to a dose-dependent decrease in the DNA binding potential of NF-κB, thereby making it transcriptionally incompetent to drive the expression of target genes. In addition, NF-κB nuclear exclusion induced by delphinidin seems to further synergize with its inability of binding to DNA to repress its function. Our observations suggest that delphinidin inhibits NF-κB signaling through a sequence of events that include inhibition of phosphorylation of IKKγ (NEMO) followed by inhibition of phosphorylation of IκBα and restoration of its subsequent degradation.
It has been reported that G2 abrogation prevents cancer cells from repairing DNA damage, forcing them into M phase. Thus, the G2 checkpoint has emerged as an attractive therapeutic target for cancer therapy (41). Our study indicates that delphinidin exerts strong growth inhibitory effects on human PCa cells by arresting cells in G2-M phase. It is known that cell cycle is primarily regulated by complexes containing cdks and cyclins, which are critical for the progression of cell cycle and whose inactivation leads to cell cycle arrest (42, 43). The observed inhibitory effects of delphinidin particularly on cyclin D1, cyclin A, cdk1, and cdk2 in PCa cells suggest its interference in cell cycle. Cdk activity is additionally regulated by cdk inhibitors such as the p21/WAF1 and p27/KIP1 families of proteins. Our data show that delphinidin arrests PCa PC3 cells in G2-M phase via modulation of cell cycle regulatory molecules, suggesting yet another important molecular mechanism through which delphinidin inhibits the growth of PCa cells.
To establish the relevance of these in vitro findings to in vivo situation, athymic nude mice were implanted with PC3 cells, which are derived from bone metastasis and known to be highly proliferative. Importantly, we observed that delphinidin significantly reduced tumor growth of PC3 xenograft (Fig. 6A). These in vivo growth inhibitory effects of delphinidin could be correlated well with the induction of apoptosis and inhibition of known cell proliferative biomarkers. A significant increase in PARP cleavage, an increase in Bax/Bcl2 protein ratio, and the inhibition of NF-κB/p65, Ki67, and PCNA protein levels in delphinidin-treated tumors suggest the involvement of similar molecular events as those observed in the in vitro system. Our findings are significant because the xenograft mouse model is extremely useful for preclinical studies of anticancer agents (16, 27, 28).
The present study is the first report showing the effect of delphinidin in inhibiting human PCa cell growth in in vitro model as well as in in vivo preclinical setting via interference with the NF-κB signaling pathway. In summary, based on the present findings, it is tempting to suggest that delphinidin could be developed as a potential anticancer agent against human PCa.
Acknowledgments
Grant support: USPHS grants RO1 CA78809 and RO1 CA120451.
We thank Dr. G.N. Thalmann for providing C4-2 cells.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Syed DN, Khan N, Afaq F, Mukhtar H. Chemo-prevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 3.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 4.Bell DR, Gochenaur K. Direct vasoactive and vaso-protective properties of anthocyanin-rich extracts. J Appl Physiol. 2006;100:1164–70. doi: 10.1152/japplphysiol.00626.2005. [DOI] [PubMed] [Google Scholar]
- 5.Vuorela S, Kreander K, Karonen M, et al. Preclinical evaluation of grape seeds, raspberry, and pine bark phenolics for health related effects. J Agric Food Chem. 2005;53:5922–31. doi: 10.1021/jf050554r. [DOI] [PubMed] [Google Scholar]
- 6.Afaq F, Malik A, Syed D, Maes D, Matsui MS, Mukhtar H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor κB in normal human epidermal keratinocytes. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- 7.Mazza G. Anthocyanins in grapes and grape products. Crit Rev Food Sci Nutr. 1995;35:341–71. doi: 10.1080/10408399509527704. [DOI] [PubMed] [Google Scholar]
- 8.Hou DX, Fujii M, Terahara N, Yoshimoto M. Molecular mechanisms behind the chemopreventive effects of anthocyanidins. J Biomed Biotechnol. 2004;2004:321–5. doi: 10.1155/S1110724304403040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric Food Chem. 2002;50:166–71. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 10.Hou DX, Yanagita T, Uto T, Masuzaki S, Fujii M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: structure-activity relationship and molecular mechanisms involved. Biochem Pharmacol. 2005;70:417–25. doi: 10.1016/j.bcp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Azevedo L, Alves de Lima PL, Gomes JC, Stringheta PC, Ribeiro DA, Salvadori DM. Differential response related to genotoxicity between eggplant (Solanum melanogena) skin aqueous extract and its main purified anthocyanin (delphinidin) in vivo. Food Chem Toxicol. 2007;45:852–8. doi: 10.1016/j.fct.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Syed DN, Afaq F, Sarfaraz S, et al. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol Appl Pharmacol. 2008;231:52–60. doi: 10.1016/j.taap.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamy S, Blanchette M, Michaud-Levesque J, et al. Delphinidin, a dietary anthocyanidin, inhibits vascular endothelial growth factor receptor-2 phosphorylation. Carcinogenesis. 2006;27:989–96. doi: 10.1093/carcin/bgi279. [DOI] [PubMed] [Google Scholar]
- 14.Lamy S, Beaulieu E, Labbé D, et al. Delphinidin, a dietary anthocyanidin, inhibits platelet derived growth factor ligand/receptor (PDGF/PDGFR) signaling. Carcinogenesis. 2008;29:1033–41. doi: 10.1093/carcin/bgn070. [DOI] [PubMed] [Google Scholar]
- 15.Afaq F, Zaman N, Khan N, et al. Inhibition of epidermal growth factor receptor signaling pathway by delphinidin, an anthocyanidin present in pigmented fruits and vegetables. Int J Cancer. 2008;123:1508–15. doi: 10.1002/ijc.23675. [DOI] [PubMed] [Google Scholar]
- 16.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–8. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–33. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 18.Saleem M, Afaq F, Adhami VM, Mukhtar H. Lupeol modulates NF-κB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–14. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- 19.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–8. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen K, Berneman ZN, Van Bockstaele DR. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:165–75. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald ER, El-Deiry WS. Cell cycle control as a basis for cancer drug development. Int J Oncol. 2000;16:871–86. [PubMed] [Google Scholar]
- 22.Owa T, Yoshino H, Yoshimatsu K, Nagasu T. Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr Med Chem. 2000;8:1487–503. doi: 10.2174/0929867013371996. [DOI] [PubMed] [Google Scholar]
- 23.Kastan MB, Canman CE, Leonard CJ. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995;14:3–15. doi: 10.1007/BF00690207. [DOI] [PubMed] [Google Scholar]
- 24.Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–74. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou M, Gu L, Zhu N, Woods WG, Findley HW. Transfection of a dominant-negative mutant NF-κB inhibitor (IκBm) represses p53-dependent apoptosis in acute lymphoblastic leukemia cells: interaction of IκBm and p53. Oncogene. 2003;22:8137–44. doi: 10.1038/sj.onc.1206911. [DOI] [PubMed] [Google Scholar]
- 26.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NFκB activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 27.Saleem M, Kweon MH, Yun JM, et al. A novel dietary triterpene Lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005;65:11203–13. doi: 10.1158/0008-5472.CAN-05-1965. [DOI] [PubMed] [Google Scholar]
- 28.Siddiqui IA, Zaman N, Aziz MH, et al. Inhibition of CWR22Rν1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006;27:833–9. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–42. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 31.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 32.Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–6. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Revelos K, Petraki C, Gregorakis A, Scorilas A, Papanastasiou P, Koutsilieris M. Immunohistochemical expression of Bcl2 is an independent predictor of time-to-biochemical failure in patients with clinically localized prostate cancer following radical prostatectomy. Anticancer Res. 2005;25:3123–33. [PubMed] [Google Scholar]
- 34.Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor κB and induction of apoptosis. Oncogene. 2004;23:2507–22. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- 35.Hafeez BB, Ahmed S, Wang N, Gupta S, Zhang A, Haqqi TM. Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with down-regulation of nuclear factor-κB. Toxicol Appl Pharmacol. 2006;216:11–9. doi: 10.1016/j.taap.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–9. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 37.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 38.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 39.Ross JS, Jennings TA, Nazeer T, et al. Prognostic factors in prostate cancer. Am J Clin Pathol. 2003;120:85–100. doi: 10.1309/PW69K48RRFJLXKBD. [DOI] [PubMed] [Google Scholar]
- 40.Fradet V, Lessard L, Begin LR, Karakiewicz P, Masson AM, Saad F. Nuclear factor-κB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res. 2004;10:8460–4. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- 41.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer. 2008;98:523–8. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devault A, Cavadore JC, Fesquet D, et al. Concerted roles of cyclin A, cdc25+ mitotic inducer, and type 2A phosphatase inactivating the cyclin B/cdc2 protein kinase at the G2/M phase transition. Cold Spring Harb Symp Quant Biol. 1991;56:503–13. doi: 10.1101/sqb.1991.056.01.057. [DOI] [PubMed] [Google Scholar]
- 43.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–4. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]