Abstract
Forkhead O transcription factors (FOXO) are critical for the regulation of cell cycle arrest, cell death, and DNA damage repair. Inactivation of FOXO proteins may be associated with tumorigenesis, including breast cancer, prostate cancer, glioblastoma, rhabdomyosarcoma, and leukemia. Accumulated evidence shows that activation of oncogenic pathways such as phosphoinositide-3-kinase/AKT/IKK or RAS/mitogen-activated protein kinase suppresses FOXO transcriptional activity through the phosphorylation of FOXOs at different sites that ultimately leads to nuclear exclusion and degradation of FOXOs. In addition, posttranslational modifications of FOXOs such as acetylation, methylation and ubiquitination also contribute to modulating FOXO3a functions. Several anti-cancer drugs like paclitaxel, imatinib, and doxorubicin activate FOXO3a by counteracting those oncogenic pathways which restrain FOXOs functions. In this review, we will illustrate the regulation of FOXOs and reveal potential therapeutics that target FOXOs for cancer treatment.
Keywords: Forkhead transcriptional factor, breast cancer, cancer therapy
BACKGROUND
Forkhead Transcription Factors
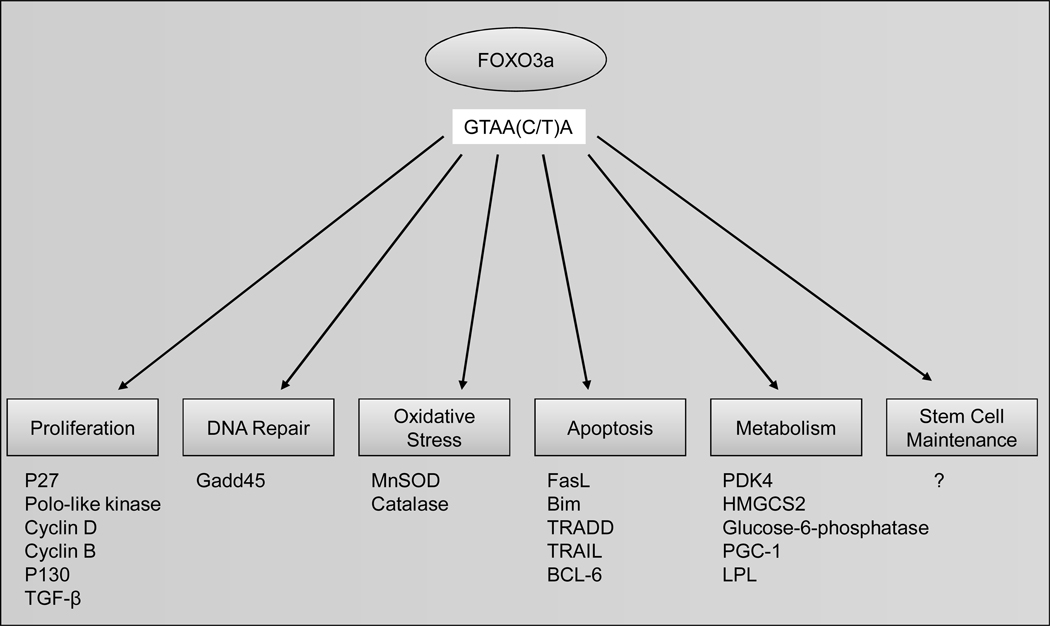
Forkhead proteins are not among the largest transcription factor families, but display a remarkable functional diversity and are involved in a wide variety of biological processes. The name is derived from the two spiked-head structures in the embryos of the Drosophila fork head mutant, which are defective in anterior and posterior gut formation [1]. With the 1990 discovery of a 110-amino-acid DNA binding domain that was almost perfectly conserved between FORK HEAD and the mammalian HNF-3 transcription factors, it became clear that this motif defined a novel transcription factor family [2]. A comprehensive review on Forkhead genes has been published by Kaufmann and Knochel [3]. Among the organisms for which the genome sequences are completed, or nearly so, there is indeed a correlation between anatomical complexity and forkhead gene number: 4 in Saccharomyces and Schizosaccharomyces, 15 in Caenorhabditis, 20 in Drosophila, and 39 in Homo. In 2000, the nomenclature of chordate forkhead transcription factors was revised [4, 5]. The new nomenclature, which uses Fox (for “Forkhead box”) as the root symbol, ensures that the same name is used for orthologous genes in different species and reflects phylogenetic relationships by including a letter that indicates subfamily. Within a subfamily, each gene is identified by a number (e.g., FoxO2), the typography follows the conventions used in each species (FOXO3a in Homo, Foxo3a in Mus, and FoxO3a in all others), and proteins are distinguished from genes by the use of roman type (e.g., FoxO3a). Forkhead proteins, the transcription factors of wingedhelix domain, are characterized by a conserved DNA-binding domain—the forkhead box among invertebrate and mammalian cells [1–3]. Based on the forkhead box domain, the forkhead genes are grouped into 19 subclasses of FOX genes [4, 5]. FOXO transcription factors, one of largest subgroups of forkhead family members, are characterized by a conserved DNA-binding domain--the forkhead box among invertebrate and mammalian cells [6, 7]. The FOXO subfamily contains four members (FOXO1, FOXO3, FOXO4, and FOXO6), which activate or repress multiple genes such as Bim and FasL involved in apoptosis [8, 9], p27kip [10] and cyclin D [11] in cell cycle regulation, GADD45a in DNA damage repair [7–9, 12], manganese superoxide dismutase (MnSOD) in stress response [13], and glycogenolytic gene glucose-6-phosphatase (G6pc) in metabolism [14]. Recent studies also reveal the importance of FOXOs in preserving the self-renewal capacity of hematopoietic stem cells [15, 16], however, the detailed mechanisms are currently a work in progress (Fig. 1).
Fig. (1). Transcriptional regulation by FOXO3a.
FOXO3a modulates proliferation, DNA repair, oxidative stress, apoptosis, metabolism and stem cell maintenance through upregulation of several its downstream targets. For instance, FOXO3a negatively control cell proliferation through transcriptional upregulation of p27KIP1 and promotes cell apoptosis by upregulating pro-apoptotic genes FAS ligand and Bim.
Forkhead Box O Family and Cancer
FOXO factors have been shown to be deregulated in several tumor types including breast cancer, prostate cancer, glioblastoma, rhabdomyosarcoma, and leukemia [6, 17]. Loss of FOXO1a through chromosomal deletion (13q14) promotes androgen-independent prostate cancer [18]. Moreover, down-regulation of FOXOs via AKT, IKK, and ERK-mediated phosphorylation is associated with breast cancer [19–21]. Increasing the activity of FOXOs may provide an effective therapeutic strategy since inactivation of FOXOs seems to be an important step in carcinogenic transformation. Although targeting oncoproteins with small molecule inhibitors is a prevailing strategy for current cancer therapy, developing small molecules that restore the function of tumor suppressors can be equally effective.
FOXO3a overexpression has been shown to inhibit breast tumor growth and tumor size [19, 20]. Furthermore, cytoplasmic location of FOXO3a correlates with poor survival of breast cancer [19]. In addition, genetic deletion of five FOXOs alleles (FOXO1, FOXO3 and FOXO4) shows a modest neoplastic phenotype. However, deletion of all of the FOXOs alleles generates progressive cancers including thymic lymphomas and hemangiomas. Together, these data elucidate FOXOs as bona fide tumor suppressor genes [19–22]. Recent studies also reveal the importance of FOXOs in preserving the self-renewal capacity of hematopoietic stem cells [15, 16]. The mechanisms are currently under investigation.
During tumor development, inhibition of FOXO3a’s transcriptional activity promotes cell transformation, tumor progression, and angiogenesis [6, 7, 19, 23]. FOXOs are known to be regulated by external stimuli, such as epidermal growth factor (EGF), insulin, insulin-like growth factor, nutrients, cytokines, neurotrophins, and oxidative stress. These stimuli regulate FOXO protein expression, subcellular localization, or DNA binding and transcriptional activity. In addition, FOXO proteins are regulated by stimuli through posttranslational modifications, including phosphorylation, acetylation, ubiquitination, etc. Many kinase pathways have been identified to activate FOXO activity, including the stress-activated c-Jun-NH2-kinase (JNK) [24, 25], the mammalian orthologue of the Ste20-like protein kinase (MST1) [26], and AMP-activated protein kinase (AMPK) [27, 28]. Some other kinases suppress FOXOs activity (Table 1) like phosphoinositide 3-kinase (PI3K)/AKT, serum and glucocorticoidinduced kinase (SGK) [29], casein kinase 1(CK1) [30], dual-specificity tyrosine-phosphorylated-regulated kinase 1A (DYRK1A) [31], IκB kinase β (IKKβ) [19], and extracellular signal-regulated kinases 1 and 2 [20] (ERK1/2; please see the following discussion).
Table 1.
Summary of Kinase and E3 Ligase Regulate FOXOs and its Effect
| Kinase | FOXO1 | FOXO3 | FOXO4 | FOXO6 | E3 Ligase | Effect |
|---|---|---|---|---|---|---|
| AKT/SGK | T24, S256, S319 | T32, S253, S315 | T28, S193, S258 | T26, S184 | Skp2 (FOXO1) | Inhibition |
| IKKb | S644 | ? | Inhibition | |||
| ERK1/2 | S294, S344, S425 | MDM2 (FOXO3a) | Inhibition | |||
| CK1 | S322, S325 | S318, S321 | S261, S264 | ? | Inhibition | |
| DYRK1A | S329 | S325 | S268 | ? | Inhibition | |
| CDK2 | S249 | ? | Inhibition | |||
| AMPK | T179, S399, S413, S555, S588, S626 | Activation | ||||
| JNK | T447, T451 | Activation | ||||
| MST1 | S207 | Activation |
THREE ONCOKINASES TARGETING FOXO3A
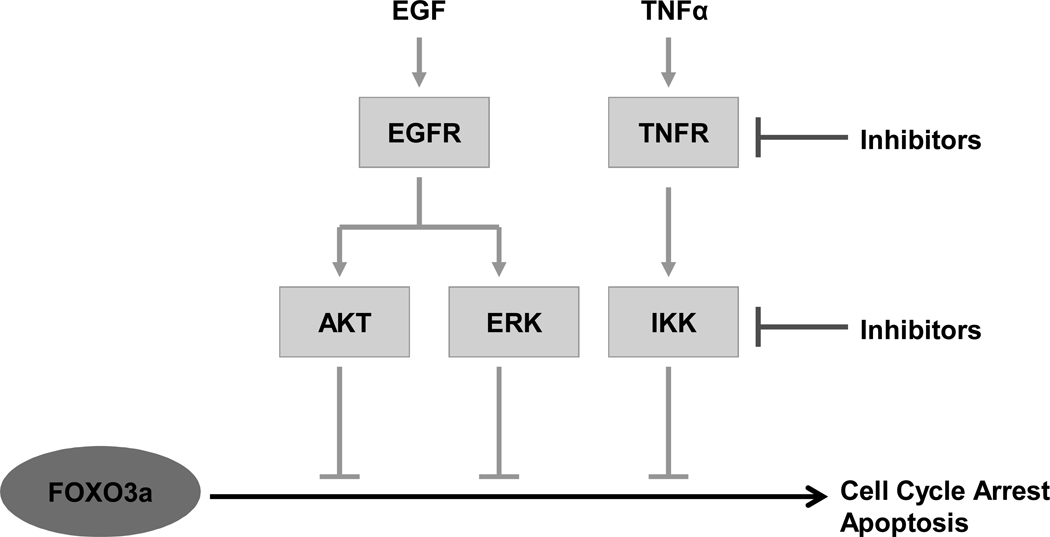
AKT, IKK and ERK are three commonly activated oncogenic kinases in human cancers. It is worth noting that all three kinases target the same tumor suppressor gene, FOXO3a. AKT, IKK and ERK phosphorylate FOXO3a at different phosphorylation sites in response to growth factor and insulin stimulation. Similarly, phosphorylation of FOXO3a by these three oncogenic kinases results in FOXO3a translocation from nucleus to cytoplasm and subsequent degradation. AKT phosphorylates FOXO3a at Thr32, Ser253, and Ser315 [7, 32], and IKKβ and ERK phosphorylate FOXO3a at Ser 644 and Ser 294, 344 and 425, respectively (Table 1 and Fig. 2). All three kinase-mediated phosphorylations stimulate FOXO3a ubiquitination, resulting in its proteasomal degradation. Phosphorylation of FOXO3a by ERK may cause FOXO3a conformational change and promote the physical interaction with the E3 ubiqitin ligase MDM2, leading to subsequent FOXO3a and FOXO4 degradation [20, 33]. MDM2 does not serve as the E3 ligase for degradation of AKT- and IKK-phosphorylated FOXO3a. However, another E3 ligase, Skp2 was reported to mediate AKT-dependant FOXO1 ubiquitination and degradation. FOXO1 loss in Skp2 overexpressing cells is associated with a known mouse lymphoma model [34]. At this moment, it is still not clear which E3 ligase is responsible for IKKβ-mediated degradation of FOXO3a.
Fig. (2). Inhibition of FOXO3a through three oncogenic kinases.
Upon EGF or TNF stimulation, PI3K/AKT, IKKβ and RAS/ERK pathways are activated. The primary oncogenic kinases AKT, IKKβ and ERK directly phosphorylate FOXO3a at different sites and lead to FOXO3a nuclear exclusion. FOXO3a in the cytoplasm loses its anti-tumor activity and is degraded via ubiquitin-proteosome pathway.
CK1 [30], DYRK1A [31] and CDK2 [35, 36] phosphorylate FOXOs at various sites to inhibit FOXOs activity. AKT and ERK were shown to up-regulate CK1 [37] and CDK2 [38] activity. Phosphorylation of FOXOs by AKT, IKK, ERK, CK1, CDK2 and DYRK1A all result in inhibition of FOXOs. However, whether CK1, DYRK1A and CDK2 are also involved in tumorigenesis is unclear. Since AKT, IKK and ERK govern most of the signaling pathways in regulating FOXOs, inhibition of AKT, IKK and ERK are likely to restore FOXOs function.
REGULATION OF FOXOS IN CLINICAL APPLICATION
FOXO families have been demonstrated to be therapeutic targets in various cancers by mediating the cytostatic and cytotoxic effects of chemotherapeutic drugs (Table 2). For example, the chemotherapeutic drugs paclitaxel [39, 40] and KP372-1 (a multiple kinase inhibitor) [41], currently used in treatment of breast carcinoma, activate FOXO3a by reducing AKT activity. Paclitaxel also activates JNK, which phosphorylates several sites within the DNA binding domain of FOXO3a [40]. These phosphorylation residues decrease the interaction between FOXO3a with the 14-3-3 protein, leading to impaired nuclear export of FOXO3a mediated by 14-3-3, and thereby increasing FOXO3a activity [32, 40]. Novel small molecules that regulate FOXOs subcellular localization and activity were identified by chemical screens [42–44]. For example, CRM1 inhibitors were shown to enhance FOXO1 activity by blocking FOXO1 nuclear export [45].
Table 2.
Clinical Anticancer Treatments Target FOXO3a through Three Oncogenic Kinases (AKT, IKK, and ERK) and Various Signaling Pathways
| Cancer type | Drug or therapy | Protein targeted |
AKT, IKK and ERK affected |
Signal pathway affected |
|---|---|---|---|---|
| Breast cancer | Paclitaxel | FOXO3a | AKT | Effected tubulin, Decrease in AKT and increase in JNK activity, leading to FOXO3a activation and induction of Bim [33, 34] |
| Acute myeloid leukaemia | KP372-1 | FOXO3a | AKT | Inhibition of AKT activity, activating FOXO3a and inducing apoptosis [35] |
| Chronic myeloid leukaemia | Imatinib (STI571 or Glivec) | FOXO3a | AKT and ERK | BCR-ABL inhibition, leading to FOXO3a dephosphorylation and Bim dependent apoptosis; downregulation of ID1 and erythroid differentiation [39, 40] |
| Osteosarcoma, breast cancer | Ionizing radiation (IR or UV) | FOXO3a | ? | Induction of FOXO3a in p53-null cells and enhanced ATM activity [9, 44] |
| Breast, prostate, kidney, ovarian, non-small-cell lung cancer | Trastuzumab, cetuximab, lapatinib, gefitinib | FOXO3a | AKT and ERK | EGFR blockade; induction of FOXO3a and expression of pro-apoptotic FOXO3a target BNIP3L1 [42] |
| Leukemia | Doxorubicin | FOXO3a | ? | Activation of FOXO3a and MDR1 [38] |
| Melanoma | Adenovirus-mediated transfer of constitutively active FOXO3A | FOXO3a | ? | Activation of FOXO3a and induction of apoptosis [46] |
| Breast cancer | OSU-03012/ tamoxifen | FOXO3a | AKT | Inhibition of PDK-1/ AKT activity, activating FOXO3a and sensitizing ER-negative breast cancer cells to Tamoxifen [41] |
| Breast, ovarian cancer | AZD6244 | FOXO3a | ERK | Inhibition of ERK activity, activate FOXO3a activity [64] |
The clinical importance of activating FOXO3a and its downstream genes in anti-cancer therapy has been revealed [17]. Doxorubicin activates FOXO3a to induce the expression of the multidrug resistance gene ABCB1 (MDR1) in K562 doxorubicin-sensitive leukemic cells [46]. Imatinib activates FOXO3a and induces Bim-dependent apoptosis through inhibition of BCR-ABL in chronic myeloid leukemia [47]. Imatinib also induces erythroid differentiation through repressing ID1 gene transcription by FOXO3a [48].
Due to the potent anti-tumor activity of FOXO3a, drugs that activate FOXO3a can be used in combination with other therapeutic agents for sensitizing tumor cells. A novel PDK-1/AKT inhibitor, OSU-03012, sensitizes ER negative breast cancer cells to Tamoxifen through activating FOXO3a and p27 [49]. It was also shown that inhibition of the epidermal growth factor receptor (EGFR) family by blocking antibodies (such as trastuzumab or cetuximab) inhibit the PI3K pathway and induce FOXO3a activity [50]. Single EGFR/HER2 blocking agents have been used alone and in combination with other agents in clinical trials against breast, prostate, kidney, ovarian and lung cancers [17]. Reintroduction of active FOXO3a may sensitize resistant cancer cells to EGFR/HER2 inhibitors like lapatinib and gefitinib [17].
AKT, IKK and ERK phosphorylate and downregulate the tumor suppressor FOXO3a (Fig. 2). Restoring FOXOs from the regulation of these kinases can be effective for cancer treatment. Activation of FOXO3a can also be effective in overcoming tumor resistance against radiotherapy [17]. FOXO3a activation induced Bim and apoptosis in osteosarcoma cell lines exposed to ionizing radiation [12]. FOXO3a was also shown to enhance ataxia telangiectesia mutated (ATM) activity through the binding of the carboxy-terminal domain of FOXO3a to the (FRAP, ATM, and TRRAP) FAT [51] domain of ATM, contributing to ATM activation [20]. These data suggest that combination of radiation with chemotherapy targeting FOXO3a may be able to sensitize resistant tumor cells to radiation therapy.
AZD6244 is known to promote cell cycle arrest and apoptosis through inhibiting ERK activation and is being tested in multiple clinical trials (From NIH website: WWW.Clinicaltrials.gov), it is therefore critical to understand the detailed molecular mechanisms and downstream target genes responsible for its tumor suppression activity. Based on recent study of FOXO3a regulation by the RASMEK-ERK pathway [20], it has been speculated that AZD6244 may also have potential in synergizing with cancer therapeutics through activating FOXO3a [21].
FOXOs are continually being adopted as therapeutic targets in cancers. However, targeting FOXO proteins may be complicated by feedback mechanisms. Evidence shows that activation of FOXO3a is important in mediating cell apoptosis in a broad spectrum of tumors [17]. Alternatively, FOXO1 was able to increase PKB phosphorylation by repressing TRB3, a pseudo-kinase inhibiting PKB phosphorylation [17, 52]. The FOXO homologue DAF16 in C. elegans has also been shown to induce the expression of the insulin receptor and increase PI3K activity [53].
It was reported that blocking HER2 or EGFR with a single agent might not be effective due to resistant mutations [54, 55]. In recent years, development of multi-targeted agents has been applied in treating cancers by inhibiting multiple receptor tyrosine kinase (RTK). For example, lapatinib has been shown to be effective in targeting two EGFR family members, EGFR and HER2 [56, 57]. Importantly, lapatinib is able to treat trastuzumab (Humanized monoclonal antibody for EGFR)-resistant breast cancer, where these cancer cells developed mutation or alteration in survival pathways.
Studies have also shown that both insulin-like growth factor-IR (IGF-1R) and c-MET pathways mediate the resistance of non-small cell lung cancer cells (NSCLC) to gefitinib treatment. Combining gefitinib with inhibitors targeting IGF-1R and c-MET can revert gefitinib resistance [58, 59]. Therefore, multi-targeted drugs aimed at oncogenic molecules will be a critical intervention for drug resistance. Herein, based on our model (Fig. 2), developing therapeutic agents to block multi-targets AKT, IKKβ and ERK will be expected to optimize the anti-tumor activity with the least resistance through maximal activation of FOXOs in cancer therapy.
FOXO3A ABERRATION AND DRUG RESISTANCE
Development of cancer cell resistance to cancer therapeutics is a major problem of clinical concern; therefore, it is important to understand the molecular mechanisms that contribute to drug resistance and to further identify molecular targets for novel therapeutics that can overcome resistance. Previous reports have suggested that cancer cells resistant to MEK inhibitors exhibit activation of PI3K/AKT signaling [60–63]. From Roth’s report, they found that the level of phosphorylated-AKT was much higher in AZD6244-resistant lung cancer cell lines than in AZD6244-sensitive cells. The same phenomenon was shown in gastric cancer cells from a different group [63]. These data are in concert with our results showing that FOXO3a is inactivated in AZD6244 resistant cells (manuscript to be accepted in Cancer Research), which likely results from AKT activation. In our study, we found that AZD6244 blocks ERK activity and thereby activates FOXO3a and its downstream gene Bim, which are essential for growth suppression in human cancer cells. Moreover, Taxol and LY290024 synergize with AZD6244 in killing cancer cells, however, such killing effect is reduced by knocking-down FOXO3a and Bim. We have concluded that the anti-tumor activity of AZD6244 falls significantly upon FOXO3a-mediated tumor suppression. Furthermore, we found that in several AZD6244-resistant cancer cells there is significant loss of FOXO3a activity, and thereby they have become resistant to AZD6244. We have also shown that further re-activation of FOXO3a by PI3K/AKT inhibitors sensitize AZD6244 resistant cancer cells to apoptosis. Our data demonstrates that the combination therapy of AZD6244 with pharmacological agents that enhance FOXO3a activity can effectively treat AZD6244-ressitant cells and by that modulating FOXO3a activation can convert an AZD6244-resistant tumor cell into one that is AZD6244-sensitive. Ultimately, our study implicates that FOXO3a activation may be an essential pharmacological target and therapeutic indicator to predict and mediate AZD6244 efficacy in clinical use.
SUMMARY
FOXO transcription factors are crucial for regulating a myriad of physiological processes, including cell cycle arrest, DNA repair, metabolism, and cell differentiation, FOXOs integrate multiple intracellular and extracellular signals and further orchestrate proliferation, differentiation, or apoptosis. FOXOs also play important roles in tumorigenesis since they have been shown to be deregulated in many types of human cancers. For example, downregulation of FOXO3a results from genomic deletion or post-translational regulation by oncogenic signaling pathways, such as AKT, IKK and ERK. Furthermore, restoring the expression/activity of FOXOs and their downstream genes has been shown to be effective in tumor suppression. Due to the potent anti-tumor activity of FOXO3a, clinical drugs that activate FOXO3a can also be used in combination with other therapeutic agents for sensitizing tumor cell to chemotherapy. In addition, activation of FOXO3a was able to overcome tumor resistance against radiotherapy [17], and FOXO3a activity predicts therapeutic efficacy of AZD6244 treatment [64]. Inhibitors blocking multi-targets AKT, IKK and ERK will be expected to optimize the anti-tumor activity with the least resistance through maximal activation of FOXO3a or possible other tumor suppressors (e.g. p21, p27) in cancer therapy (Fig. 2). Overall, FOXO3a may be well suited as an effective pharmacological target and therapeutic indicator for clinical cancer therapy.
ACKNOWLEDGMENTS
We thank Drs. Chun-Ju Chang and Stephanie A. Miller for reading this manuscript. We also thank Mr. Jung-Mao Hsu for the figure 2 diagram. This work was supported by NIH grant P01 CA 099031, Cancer Center Support Grant CA16672, MDACC SPOREs in Breast Cancer CA116199, Cancer Center Research of Excellence DOH-TD-C-111-005 (Taiwan), Sister Institution Fund of China Medical University and Hospital/MD Anderson Cancer Center and was partially supported by the National Breast Cancer Foundation, Inc. Patel Memorial Breast Cancer Research Foundation, Breast Cancer Research Foundation grant, Marcus Foundation and Kadoorie Charitable Foundation. This work was also partially supported by Andrew Sowell-Wade Huggins Scholarships Award (J.-Y.Y.).
REFERENCE
- 1.Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 2.Weigel D, Jackle H. The fork head domain: a novel DNA binding motif of eukaryotic transcription factors? Cell. 1990;63:455–456. doi: 10.1016/0092-8674(90)90439-l. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann E, Knochel W. Five years on the wings of fork head. Mech Dev. 1996;57:3–20. doi: 10.1016/0925-4773(96)00539-4. [DOI] [PubMed] [Google Scholar]
- 4.Kaestner KH. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol Metab. 2000;11:281–285. doi: 10.1016/s1043-2760(00)00271-x. [DOI] [PubMed] [Google Scholar]
- 5.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 6.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41:709–717. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 8.Finnberg N, El-Deiry WS. Activating FOXO3a, NF-kappaB and p53 by targeting IKKs: an effective multi-faceted targeting of the tumor-cell phenotype? Cancer Biol Ther. 2004;3:614–616. doi: 10.4161/cbt.3.7.1057. [DOI] [PubMed] [Google Scholar]
- 9.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 10.Dijkers PF, Medema RH, Pals C, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M, Fernandez de Mattos S, van der Horst A, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JY, Xia W, Hu MC. Ionizing radiation activates expression of FOXO3a, Fas ligand, and Bim, and induces cell apoptosis. Int J Oncol. 2006;29:643–648. [PMC free article] [PubMed] [Google Scholar]
- 13.Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 14.Onuma H, Vander Kooi BT, Boustead JN, Oeser JK, O'Brien RM. Correlation between FOXO1a (FKHR) and FOXO3a (FKHRL1) binding and the inhibition of basal glucose-6-phosphatase catalytic subunit gene transcription by insulin. Mol Endocrinol. 2006;20:2831–2847. doi: 10.1210/me.2006-0085. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 18.Dong XY, Chen C, Sun X, et al. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 19.Hu MC, Lee DF, Xia W, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang JY, Zong CS, Xia W, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nature Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–757. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potente M, Urbich C, Sasaki K, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Essers MA, de Vries-Smits LM, Barker N, et al. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 25.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Lehtinen MK, Yuan Z, Boag PR, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 28.Greer EL, Oskoui PR, Banko MR, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 29.Brunet A, Park J, Tran H, et al. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rena G, Woods YL, Prescott AR, et al. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J. 2002;21:2263–2271. doi: 10.1093/emboj/21.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woods YL, Rena G, Morrice N, et al. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J. 2001;355:597–607. doi: 10.1042/bj3550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 33.Brenkman AB, de Keizer PL, van den Broek NJ, Jochemsen AG, Burgering BM. Mdm2 induces mono-ubiquitination of FOXO4. PLoS One. 2008;3:e2819. doi: 10.1371/journal.pone.0002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Regan KM, Wang F, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 37.Giamas G, Hirner H, Shoshiashvili L, et al. Phosphorylation of CK1delta: identification of Ser370 as the major phosphorylation site targeted by PKA in vitro and in vivo. Biochem J. 2007;406:389–398. doi: 10.1042/BJ20070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lents NH, Keenan SM, Bellone C, Baldassare JJ. Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2. The J Biol Chem. 2002;277:47469–47475. doi: 10.1074/jbc.M207425200. [DOI] [PubMed] [Google Scholar]
- 39.Sunters A, Fernandez de Mattos S, Stahl M, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 40.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 41.Zeng Z, Samudio IJ, Zhang W, et al. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 2006;66:3737–3746. doi: 10.1158/0008-5472.CAN-05-1278. [DOI] [PubMed] [Google Scholar]
- 42.Link W, Oyarzabal J, Serelde BG, et al. Chemical interrogation of FOXO3a nuclear translocation identifies potent and selective inhibitors of phosphoinositide 3-kinases. J Biol Chem. 2009;284:28392–28400. doi: 10.1074/jbc.M109.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder FC, Kau TR, Silver PA, Clardy J. The psammaplysenes, specific inhibitors of FOXO1a nuclear export. J Nat Prod. 2005;68:574–576. doi: 10.1021/np049624z. [DOI] [PubMed] [Google Scholar]
- 44.Zanella F, Rosado A, Garcia B, Carnero A, Link W. Using multiplexed regulation of luciferase activity and GFP translocation to screen for FOXO modulators. BMC Cell Biol. 2009;10:14. doi: 10.1186/1471-2121-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kau TR, Schroeder F, Ramaswamy S, et al. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 46.Hui RC, Francis RE, Guest SK, et al. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008;7:670–678. doi: 10.1158/1535-7163.MCT-07-0397. [DOI] [PubMed] [Google Scholar]
- 47.Essafi A, Fernandez de Mattos S, Hassen YA, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 48.Birkenkamp KU, Essafi A, van der Vos KE, et al. FOXO3a induces differentiation of Bcr-Abl-transformed cells through transcriptional down-regulation of Id1. J Biol Chem. 2007;282:2211–2220. doi: 10.1074/jbc.M606669200. [DOI] [PubMed] [Google Scholar]
- 49.Weng SC, Kashida Y, Kulp SK, et al. Sensitizing estrogen receptor-negative breast cancer cells to tamoxifen with OSU-03012, a novel celecoxib-derived phosphoinositide-dependent protein kinase-1/Akt signaling inhibitor. Mol Cancer Ther. 2008;7:800–808. doi: 10.1158/1535-7163.MCT-07-0434. [DOI] [PubMed] [Google Scholar]
- 50.Real PJ, Benito A, Cuevas J, et al. Blockade of epidermal growth factor receptors chemosensitizes breast cancer cells through up-regulation of Bnip3L. Cancer Res. 2005;65:8151–8157. doi: 10.1158/0008-5472.CAN-05-1134. [DOI] [PubMed] [Google Scholar]
- 51.Bosotti R, Isacchi A, Sonnhammer EL. FAT: a novel domain in PIK-related kinases. Trends Biochem Sci. 2000;25:225–227. doi: 10.1016/s0968-0004(00)01563-2. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19:2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson MH, Dolder CR. Lapatinib: a novel dual tyrosine kinase inhibitor with activity in solid tumors. Ann Pharmacother. 2006;40:261–269. doi: 10.1345/aph.1G387. [DOI] [PubMed] [Google Scholar]
- 55.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 56.Wheatley-Price P, Shepherd FA. Epidermal growth factor receptor inhibitors in the treatment of lung cancer: reality and hopes. Curr Opin Oncol. 2008;20:162–175. doi: 10.1097/CCO.0b013e3282f335a3. [DOI] [PubMed] [Google Scholar]
- 57.Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–4065. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- 58.Morgillo F, Kim WY, Kim ES, et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–2803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- 59.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoeflich KP, O'Brien C, Boyd Z, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15:4649–4664. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 61.Meng J, Peng H, Dai B, et al. High level of AKT activity is associated with resistance to MEK inhibitor AZD6244 (ARRY-142886) Cancer Biol Ther. 2009;8 doi: 10.4161/cbt.8.21.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mirzoeva OK, Das D, Heiser LM, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–572. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon YK, Kim HP, Han SW, et al. Combination of EGFR and MEK1/2 inhibitor shows synergistic effects by suppressing EGFR/HER3-dependent AKT activation in human gastric cancer cells. Mol Cancer Ther. 2009;8:2526–2536. doi: 10.1158/1535-7163.MCT-09-0300. [DOI] [PubMed] [Google Scholar]
- 64.Yang JY, Chang CJ, Wang Y, et al. Activation of FOXO3a is sufficient to reverse MEK inhibitor chemoresistance in human cancer. Cancer Res. 2011;70:4709–4718. doi: 10.1158/0008-5472.CAN-09-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]