Abstract
This study investigates the role of extracellular nucleotides and apyrase enzymes in regulating stomatal aperture. Prior data indicate that the expression of two apyrases in Arabidopsis (Arabidopsis thaliana), APY1 and APY2, is strongly correlated with cell growth and secretory activity. Both are expressed strongly in guard cell protoplasts, as determined by reverse transcription-polymerase chain reaction and immunoblot analyses. Promoter activity assays for APY1 and APY2 show that expression of both apyrases correlates with conditions that favor stomatal opening. Correspondingly, immunoblot data indicate that APY expression in guard cell protoplasts rises quickly when these cells are moved from darkness into light. Both short-term inhibition of ectoapyrase activity by polyclonal antibodies and long-term suppression of APY1 and APY2 transcript levels significantly disrupt normal stomatal behavior in light. Stomatal aperture shows a biphasic response to applied adenosine 5′-[γ-thio]triphosphate (ATPγS) or adenosine 5′-[β-thio] diphosphate, with lower concentrations inducing stomatal opening and higher concentrations inducing closure. Equivalent concentrations of adenosine 5′-O-thiomonophosphate have no effect on aperture. Two mammalian purinoceptor inhibitors block ATPγS- and adenosine 5′-[β-thio] diphosphate-induced opening and closing and also partially block the ability of abscisic acid to induce stomatal closure and of light to induce stomatal opening. Treatment of epidermal peels with ATPγS induces increased levels of nitric oxide and reactive oxygen species, and genetically suppressing the synthesis of these agents blocks the effects of nucleotides on stomatal aperture. A luciferase assay indicates that treatments that induce either the closing or opening of stomates also induce the release of ATP from guard cells. These data favor the novel conclusion that ectoapyrases and extracellular nucleotides play key roles in regulating stomatal functions.
The swelling or shrinking of guard cells in the leaf epidermis controls stomatal aperture. Guard cells respond to a variety of stimuli, including abscisic acid (ABA) and blue light, to regulate stomatal apertures through changes in ion transport, water potential, and osmotic pressure. Swelling and shrinking events may be accompanied by changes in surface area of the plasma membrane, requiring membrane exocytosis and endocytosis to accommodate the fluctuating volume (Shope et al., 2003). In recent years, guard cell signaling pathways have been elucidated, revealing new roles for nitric oxide (NO) and reactive oxygen species (ROS) in stomatal closure (García-Mata and Lamattina, 2001; Bright et al., 2006; Desikan et al., 2006). These findings illustrate the complexity of guard cell responses, and an understanding of the signaling pathways remains incomplete (Neill et al., 2008).
Intracellular ATP has long been known as a cellular energy source, but now extracellular ATP (eATP) has become recognized as a signaling agent in both plants and animals (Roux and Steinebrunner, 2007; Clark and Roux, 2009; Tanaka et al., 2010a). In mammals, it binds to purinergic receptors of the P2 receptor family, which induces a rapid increase in cytosolic [Ca2+] that leads to diverse physiological responses (Burnstock, 2008). In plants, application of ATP also controls cytosolic [Ca2+] fluctuations (Demidchik et al., 2003, 2009; Jeter et al., 2004; Tanaka et al., 2010b), although the plant receptor that initiates these responses remains unknown. As it does in animals, eATP in plants also up-regulates transcripts for proteins involved in signal transduction (Jeter et al., 2004; Song et al., 2006). Downstream of the changes in cytosolic [Ca2+], but upstream of the gene expression changes, applied ATP can promote growth-altering accumulation of ROS and NO in diverse tissues of diverse plants (for review, see Tanaka et al., 2010a). These accumulations appear to be critical intermediates for eATP signaling, because genetic suppression of ROS or NO production can block cell and tissue responses to applied nucleotides (Song et al., 2006; Reichler et al., 2009; Clark et al., 2010b).
The involvement of NO and ROS in guard cell responses, and their production via eATP signaling in other plant cells, led us to hypothesize that eATP may also play an important role in stomatal signaling pathways. An additional rationale for testing the role of eATP in stomata function was the evidence of Wolf et al. (2007) that the apyrase (nucleoside triphosphate diphosphohydrolase) enzymes APY1 and APY2 were strongly expressed in guard cells of Arabidopsis (Arabidopsis thaliana). These observations led us to test whether the heightened presence of APY1 and APY2 in guard cells reflected a role for eATP in guard cell function and whether eATP signaling could be upstream of the NO and ROS signals known to regulate stomatal aperture.
Our results provide independent verification of the enhanced presence of APY1 and APY2 in guard cells and reveal that applied adenosine 5′-[γ-thio]triphosphate (ATPγS) and adenosine 5′-[β-thio] diphosphate (ADPβS), which activate eATP responses in plants and animals but are poorly hydrolyzable, can induce stomatal opening or closure in a dose-dependent manner. They also show that the effects of applied nucleotides on aperture are accompanied by increases in NO and ROS production and can be blocked either by genetically suppressing NO or ROS production or by using a purinoceptor inhibitor that blocks eATP responses in animals. These findings are linked to apyrase function by data demonstrating that apyrase expression is dynamically increased when stomates open and that the chemical inhibition or genetic suppression of apyrases can significantly alter rates of stomatal opening and closing. These results, plus a luciferase assay that shows that guard cells release ATP when stomates are induced to open or close, support the novel and unexpected postulate that eATP is an important factor in guard cell signaling pathways and that APY1 and APY2 impact stomatal opening and closure consistent with their hypothesized regulation of eATP.
RESULTS
Expression of APY1 and APY2 in Guard Cells
To determine if APY1 and APY2 are expressed in guard cells, we performed reverse transcription (RT)-PCR analyses of guard cell protoplasts and whole leaf extracts using gene-specific primers. The transcript levels of both APY1 and APY2 are enriched in protoplast preparations in which the ratio of guard cells to mesophyll cells is 1.0 or greater, compared with whole leaf extracts, in which the ratio of guard cells to mesophyll cells is 0.1 or less (Fig. 1A). Immunoblot analyses using polyclonal anti-APY1 antibodies were performed to confirm that APY protein expression in protoplast preparations is enriched in guard cells. APY1 and APY2 are 87% identical at the deduced amino acid level, and APY1 antibodies have previously been shown to cross-react with both APY1 and APY2 proteins (Wu et al., 2007). Immunoblot results reveal that the cross-reactive band near 50 kD, the approximate size of APY1 and APY2 proteins (Steinebrunner et al., 2000), is more abundant in the enriched guard cell preparation than in the whole-leaf extracts (Fig. 1B).
Figure 1.
Apyrase expression is enriched in preparations of guard cell protoplasts compared with extracts of whole leaves. A, As assayed by RT-PCR, APY1 and APY2 transcripts are present at a higher level in guard cell protoplast preparations compared with extracts of whole leaves. Control levels of an actin PCR product indicate equal amounts of cDNA as starting material prior to PCR. B, Immunoblot analysis using anti-APY1 antibodies shows that immunodetectable protein levels of APY1/2 are higher in guard cell protoplast preparations compared with extracts of whole leaves. Control levels of α-tubulin (α-Tub) show equal loading of protein. Leaves taken from 3-week-old plants grown under identical conditions were used for both the protoplast preparations and the whole leaf extracts.
APY1 and APY2 Promoter Activities and Protein Levels Correlate with Open Stomata
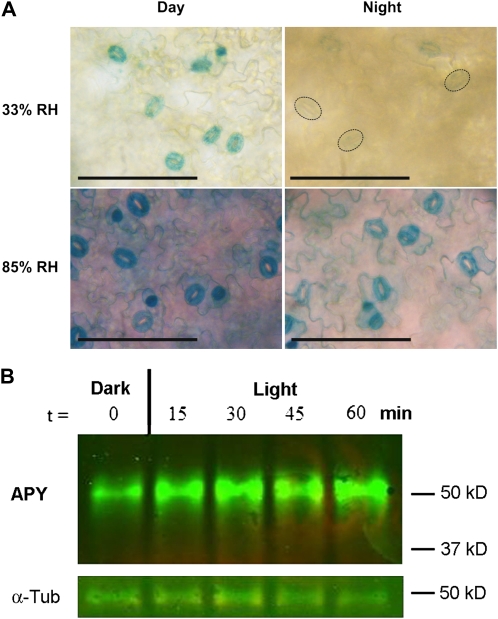
To help evaluate whether APY1 and APY2 are involved in the opening and closing of stomates, APY1 and APY2 promoter:GUS fusion lines were grown in conditions that either promoted opening or closing of stomata and analyzed for GUS activity. During the day, when stomates are generally open, APY1 and APY2 promoter activity was observed in guard cells (Fig. 2A, top left panel), as published previously (Wolf et al., 2007). Higher humidity levels of 85% relative air humidity (RH), which increase stomata opening, also increased the GUS staining of the guard cells (Fig. 2A, bottom left panel). On the other hand, closure of stomates in the dark correlated with the decrease of APY1 and APY2 promoter activity (Fig. 2A, top right panel). Under high-humidity conditions, stomates will remain open in the dark (Barbour and Buckley, 2007; Mott and Peak, 2010), and again, guard cells showed high GUS staining (Fig. 2A, bottom right panel). Taken together, APY1 and APY2 promoter activity was high under conditions that induced stomata opening, as analyzed by GUS staining. In order to determine if the promoter activities had the predicted effects at the protein level, we performed immunoblot analyses of APY1/APY2 protein levels in guard cell protoplasts after treatment with light at various time points. We found that after 15 min of light treatment, there was a corresponding increase in the level of immunodetectable APY1/APY2 protein and that this increase was maintained over a 1-h period (Fig. 2B).
Figure 2.
Open stomata have more active APY1/2 promoters, and light-treated guard cell protoplasts have higher APY1/2 protein levels. A, APY1:GUS and APY2:GUS plants were grown in low-humidity (33% RH) and high-humidity (85% RH) conditions. Leaves were harvested after 7 h of light (Day) and after 4 h in the dark (Night) and stained for GUS activity. Bright-field images of the abaxial epidermis of whole mount leaves from the APY2:GUS line 3-2-11 are shown representing the staining pattern of all four GUS lines analyzed. Dashed lines mark the outlines of some weakly stained guard cells in the top right panel. Bars = 100 μm. B, Western-blot analysis of APY1/APY2 protein levels in dark-adapted guard cell protoplasts after treatment with light at various time points. Treatment with light for 15 min results in an increase in immunodetectable APY1/APY2 protein levels. This result is representative of three biological repeats. α-Tub, α-Tubulin.
Chemical and Immunological Inhibition of Apyrase Activity Induces Stomatal Closure
In order to directly determine if apyrase activity plays a role in regulating guard cell aperture in Arabidopsis, we treated epidermal peels and whole leaves with apyrase antibodies and chemical apyrase inhibitors. Anti-APY1 antibodies have previously been shown to inhibit ectoapyrase activity in pollen tubes and cotton (Gossypium hirsutum) fiber cultures (Wu et al., 2007; Clark et al., 2010a), so we tested their effects on stomatal aperture. Treatment of epidermal peels and whole leaves with 10 μm ABA and with immune sera induced stomatal closure, while treatment with preimmune sera had no effect on stomatal aperture (Fig. 3A). The chemical apyrase inhibitors NGXT 191 and apyrase inhibitor 13, which were selected from a chemical library based on a screen for specific inhibition of potato (Solanum tuberosum) apyrase activity (Windsor et al., 2002), have previously been shown to inhibit the activity of APY1 and APY2 (Wu et al., 2007). Treatment of epidermal peels with 7.5 μg mL−1 apyrase inhibitor NGXT 191 induced stomatal closure, and this closure was blocked by coincubation with pyridoxalphosphate-6-azo-phenyl-2′,4′-disulfonic acid (PPADS), an antagonist of animal purinoceptors, at a concentration of PPADS (100 μm) that had no effect by itself (Fig. 3B). Treatment with 7.5 μg mL−1 NGXT 191 or apyrase inhibitor 13 also caused stomatal closure in whole leaf experiments (data not shown).
Figure 3.
Chemical and immunological inhibition of apyrase activity induces stomatal closure. A, Application of anti-apyrase immune sera induced stomatal closure in whole leaves, but application of control preimmune sera had no effect on the aperture (5.2 μm average width for control). B, Application of apyrase inhibitor NGXT 191 induced stomatal closure in epidermal peels, and 100 μm PPADS blocked this closure, but 100 μm PPADS had no effect alone (5.3 μm average width for control). Apertures were measured as width/length after 1 h of treatment for peels and after 2 h of treatment for leaves. Error bars represent se. Different letters above the bars indicate mean values that are significantly different from one another as determined by Student’s t test (P < 0.05; n ≥ 50). These data are representative of three or more biological repeats.
Treatment with ATPγS and ADPβS Induces Changes in Guard Cell Aperture
In order to test whether eATP and eADP might play a role in the regulation of stomatal aperture, we determined dose-response curves for stomatal opening and closure using poorly hydrolyzable ATP and ADP analogs, ATPγS and ADPβS. There was a biphasic response to treatment with these nucleotide analogs in epidermal and whole leaf experiments: low concentrations of ATPγS-induced stomatal opening in darkness and high concentrations of ATPγS-induced stomatal closure in the light. In epidermal peel experiments, the threshold for ATPγS-induced closure was between 150 and 200 μm; however, 250 μm adenosine 5′-O-thiomonophosphate had no effect on stomatal aperture (Fig. 4A). We found the same threshold for ATPγS-induced closure in leaf experiments and for ADPβS-induced closure in peel experiments (Supplemental Fig. S1). The mean stomatal aperture after the closure induced by 200 μm ATPγS was statistically the same as the aperture induced by treatment with 10 μm ABA. When maintained in darkness, epidermal peel experiments showed that 5 and 15 μm ATPγS induced opening and 15 μm AMPS had no effect on stomatal aperture (Fig. 4B). The mean stomatal aperture induced by low concentrations of ATPγS was smaller than the aperture induced by light treatment. In whole leaf experiments, the same biphasic response was observed, and the threshold concentration for closure for both ATPγS and ADPβS was the same as that found in the peel experiments; however, the threshold concentration for opening was shifted higher than 5 μm ATPγS (Supplemental Fig. S2).
Figure 4.
Dose-response curves for the effects of various concentrations of ATPγS on stomatal aperture in epidermal peel experiments. A, Treatment with 10 μm ABA induced stomatal closure in the light, as did 200 and 250 μm ATPγS. Treatment with 150 μm ATPγS or 250 μm AMPS had no statistically significant effect on stomatal aperture (6.3 μm average width for control). B, Treatment with 1 h of light induced stomatal opening, and application of 5 and 15 μm ATPγS induced stomatal opening in darkness. Treatment with 15 μm AMPS had no effect on stomatal aperture (2.1 μm average width for control). Apertures were measured as width/length after 1 h of treatment. Error bars represent se. Different letters above the bars indicate mean values that are significantly different from one another as determined by Student’s t test (P < 0.05; n ≥ 50). These data are representative of three or more biological repeats.
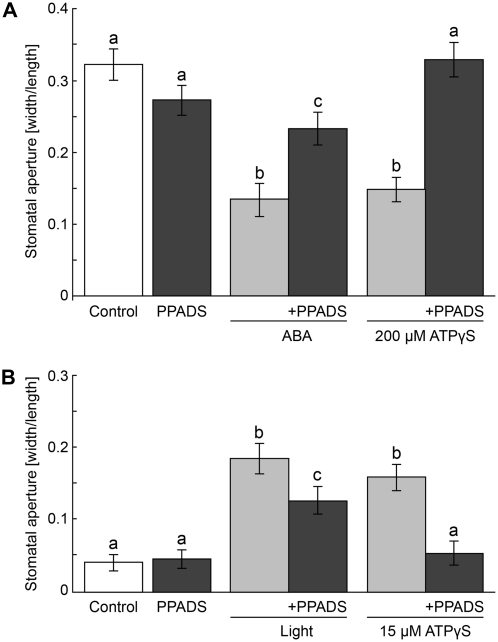
PPADS and Reactive Blue 2 Block the Ability of ATPγS to Regulate Guard Cell Aperture
PPADS and reactive blue 2 (RB2) are well-characterized purinoceptor antagonists that have previously been shown to block ATPγS-induced changes in plant growth responses (Clark et al., 2010a, 2010b). We tested the effects of coincubation of PPADS and RB2 with different agents on stomatal aperture. We found that 100 μm PPADS, which had no effect alone, blocked ATPγS-induced stomatal closure and partially blocked ABA-induced stomatal closure in leaves (Fig. 5A). In epidermal peel experiments, PPADS could also block stomatal closure by ATPγS and partially block ABA-induced closure (data not shown). Correspondingly, 100 μm PPADS, which had no effect alone, blocked ATPγS-induced stomatal opening and partially blocked light-induced opening in epidermal peels (Fig. 5B). We observed the same effects of PPADS in opening experiments using whole leaves (data not shown). In whole leaf experiments, RB2 was also able to block ATPγS-induced stomatal closure and to partially block ABA-induced stomatal closure in leaves at a concentration (30 μm) that had no effect alone (Supplemental Fig. S3A). RB2 was also able to block ATPγS-induced opening and to partially block light-induced opening at a concentration (30 μm) that had no effect by itself in leaves (Supplemental Fig. S3B).
Figure 5.
The animal purinergic receptor antagonist PPADS blocks ATPγS-induced changes in stomatal aperture and partially blocks the effects of ABA and light on stomatal aperture. A, Treatment with 200 μm ATPγS induced stomatal closure in leaves and cotreatment with 100 μm PPADS blocked this closure, but 100 μm PPADS alone had no effect on stomatal aperture. Treatment with 10 μm ABA induced stomatal closure and cotreatment with 100 μm PPADS partially blocked this ABA-induced stomatal closing (4.9 μm average width for control). B, Treatment with 15 μm ATPγS induced stomatal opening in epidermal peels and cotreatment with 100 μm PPADS blocked this opening, but 100 μm PPADS alone had no effect on stomatal aperture. Treatment with light induced stomatal opening, although cotreatment with 100 μm PPADS partially blocked this light-induced stomatal opening (1.7 μm average width for control). Apertures were measured as width/length after 1 h of treatment for peels and after 2 h of treatment for leaves. Error bars represent se. Different letters above the bars indicate mean values that are significantly different from one another as determined by Student’s t test (P < 0.05; n ≥ 50). These data are representative of three or more biological repeats.
Suppression of Apyrase Expression Affects the Regulation of Stomatal Aperture
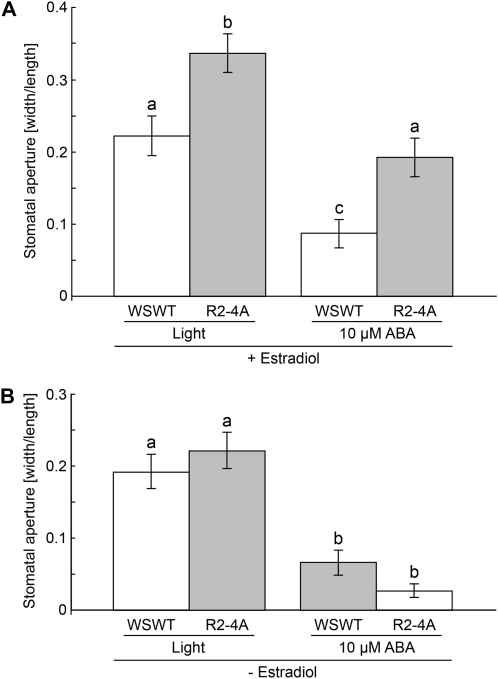
In order to test the effects of apyrase suppression on stomatal aperture, we used the RNA interference (RNAi) line R2-4A, which is an estradiol-inducible line for the RNAi suppression of APY1 in the background of the apy2 T-DNA knockout line (Wu et al., 2007). After 2 h of light treatment, stomata in estradiol-induced R2-4A peels were more open than stomata in Wassilewskija (Ws) plants (Fig. 6A). In all three biological repeats, the percentage of open stomata in R2-4A leaves was 82% to 93%, compared with 68% to 72% in Ws, and even when only open stomata are analyzed, R2-4A stomata were statistically significantly more open than Ws stomata after 2 h of light treatment (data not shown). Average stomatal aperture width in estradiol-induced R2-4A mutants ranged from 1.7 to 3.3 μm, while average stomatal aperture width in estradiol-treated Ws ranged from 1.0 to 1.5 μm (data not shown). These control average stomatal widths are low compared with the typical control average stomatal widths for ecotype Columbia (Col-0) in closing experiments but are in agreement with average stomatal widths (1.3–3.3 μm) previously reported for closing experiments with Ws plants (Klein et al., 2003). Treatment with 10 μm ABA caused closure in both estradiol-treated Ws wild-type and R2-4A plants, but after 2 h of exposure to ABA, stomata in R2-4A peels remained more open than stomata in the Ws wild type. In contrast, there were no observable differences in stomatal apertures after treatment with 2 h of light or 10 μm ABA when Ws wild-type or R2-4A (single apy2 knockout) plants were not treated with estradiol (Fig. 6B).
Figure 6.
RNAi suppression of APY1 in an apy2 single knockout results in increased stomatal apertures compared with the Ws wild type (WSWT). A, Treatments with light and 10 μm ABA induce more open stomata in leaves of RNAi plants treated with estradiol compared with leaves of Ws wild-type plants treated with estradiol (1.5 μm average width for control). B, Treatments with light and 10 μm ABA have no effect on stomatal apertures in non-estradiol-treated leaves of RNAi plants compared with leaves of non-estradiol-treated Ws wild-type plants (1.7 μm average width for control). Apertures were measured as width/length after 2 h of treatment. Error bars represent se. Different letters above the bars indicate mean values that are significantly different from one another as determined by Student’s t test (P < 0.05; n ≥ 50). These data are representative of three or more biological repeats.
NO and Hydrogen Peroxide Mediate Stomatal Closure Induced by 200 μm ATPγS
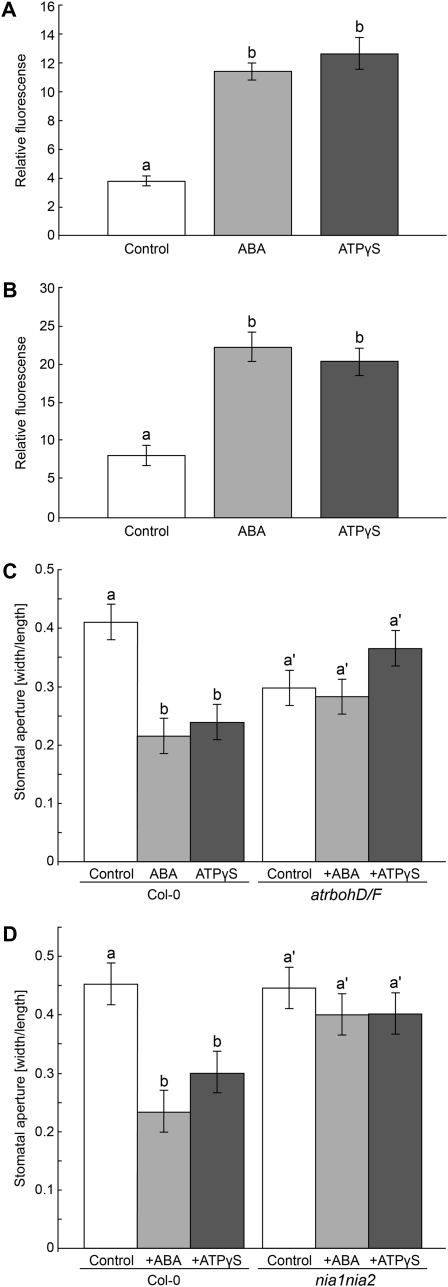
In order to detect mediators of ATPγS-induced stomatal closure, 2′,7′-dichlorodihydrofluorescein diacetate (DAF-2DA) and dichlorofluorescein diacetate (H2DCFDA) were used as fluorescent markers for the presence of NO and hydrogen peroxide (H2O2), respectively. Treatment of leaf epidermal tissue with 10 μm ABA or 200 μm ATPγS induced a 3-fold increase in H2DCFDA fluorescence after 30 min in guard cells but no change in fluorescence in the buffer-treated guard cells (Fig. 7A; Supplemental Fig. S4, A–C). Coincubation with N-acetyl-l-cysteine, a ROS scavenger (Joo et al., 2001), blocked both the ABA- and ATPγS-induced H2DCFDA fluorescence, indicating that this fluorescence is specific for ROS (data not shown). Treatment of leaf epidermal tissue with 10 μm ABA or 200 μm ATPγS caused a 2.5-fold increase in DAF-2DA fluorescence after 45 min in guard cells, while there was no change in fluorescence observed in the buffer-treated guard cells (Fig. 7B; Supplemental Fig. S4, D–F). Coincubation with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, a NO scavenger, blocked both the ABA- and ATPγS-induced DAF-2DA fluorescence, indicating that this fluorescence is specific for NO (data not shown).
Figure 7.
High concentrations of ATPγS induce stomatal closure via increased levels of NO and H2O2 in guard cells. A, Treatment of wild-type leaf tissue with 10 μm ABA or 200 μm ATPγS induces a differential accumulation of H2DCFDA fluorescence at 30 min in guard cells compared with control tissue. Different letters above the bars indicate mean values that are significantly different from one another (P < 0.05; n ≥ 25). These data are representative of three biological repeats. B, Treatment of wild-type leaf tissue with 10 μm ABA or 200 μm ATPγS induces a differential accumulation of DAF-2DA fluorescence at 45 min in guard cells compared with control tissue. C, Treatment with 200 μm ATPγS and 10 μm ABA induced stomatal closure in wild-type leaves but not in atrbohD/F leaves (4.5 μm average width for control). D, Treatment with 200 μm ATPγS and 10 μm ABA induced stomatal closure in wild-type leaves but not in nia1nia2 leaves (4.2 μm average width for control). Different letters above the bars indicate mean values that are significantly different from one another as determined by Student’s t test (P < 0.05; n ≥ 25 for fluorescence experiments and n ≥ 50 for stomatal aperture experiments). These data are representative of two biological repeats. Error bars represent se.
In order to test the connection between NO and H2O2 production and stomatal closure induced by treatment with 200 μm ATPγS, we tested the ability of stomata in leaves of nia1nia2 and atrbohD/F mutants to respond to 200 μm ATPγS. The double mutant of nitrate reductase, nia1nia2, has only 0.5% nitrate reductase activity compared with wild-type plants (Wilkinson and Crawford, 1993). The atrbohD/F mutant is disrupted in two subunits of NADPH oxidase that are expressed in guard cells and is deficient in H2O2 accumulation in guard cells (Kwak et al., 2003). In closing experiments with whole leaves, treatments of atrbohD/F and nia1nia2 mutants with 200 μm ATPγS or 10 μm ABA had no effect on stomatal aperture (Fig. 7, C and D).
ABA and Light Induce the Release of ATP from Guard Cells
In order to monitor the release of ATP from guard cells, transgenic lines expressing a secreted luciferase were generated. To determine if the ecto-luciferase lines could report the presence of eATP in leaves, we first tested the effects of 1 mm ATP on luminescence production. When they were treated with eATP for 5 min or longer, x-luc1 and x-luc9 lines showed high levels of luminescence, on average 4.75 counts per second (cps; Fig. 8). This luminescence was primarily centered around guard cells, although pavement cells of the epidermis also showed a significant increase in luminescence, on average 1.35 cps, after ATP treatment. In trials where no eATP was added and no stimulus was used to cause stomata to change their aperture, only background levels of luminescence were recorded, on average 0.73 cps.
Figure 8.
ABA treatment of light-adapted leaves induces the release of ATP in guard cells, as assayed by ecto-luciferase luminescence. A, Background levels of ecto-luciferase luminescence are observed in an epidermal peel from an untreated x-luc9 leaf (light control). B, An epidermal peel from an x-luc9 leaf treated with 10 μm ABA in light for 5 min shows ecto-luciferase luminescence in guard cells. C, An epidermal peel from an x-luc9 leaf treated with 1 mm ATP in light for 5 min shows ecto-luciferase luminescence in guard cells. Bars = 50 μm for A and B and 100 μm for C. Luminescence levels are represented in pseudocolor (blue, green, yellow, orange, and red, where red represents the highest and blue represents the lowest level of relative intensity). D, Quantification of luciferase activity from a representative data set of a closing experiment. Treatments with 10 μm ABA and 1 mm ATP were done in the light. Luminescence returned to untreated control levels 15 min after treatment with 10 μm ABA. Different letters above the bars indicate mean values that are significantly different from one another as determined by Student’s t test (P < 0.05; n ≥ 15 guard cell pairs). These data are representative of three biological repeats. Error bars represent se.
Once we had identified individual plants that were producing luciferase, we tested the effect of ABA on epidermal peels of x-luc9 plants that had been exposed to 24 h of continuous light and thus had fully open stomata. As early as 5 min after strips were floated on a solution of leaf buffer containing 10 μm ABA, luminescent signals were produced in the location of stomata, on average 3.57 cps (Fig. 8), a level that was statistically higher than control levels. This luminescence signal was maintained for up to 15 min, but after 15 min of continuous exposure to ABA, the luminescence signals returned back to control levels, about 0.80 cps (Fig. 8D). We also tested the effects 200 μm ADPβS on open stomata. After 5 min of this treatment, stomata exhibited luminescence levels that averaged 2.75 cps (data not shown), a value that was statistically higher than control levels of luminescence.
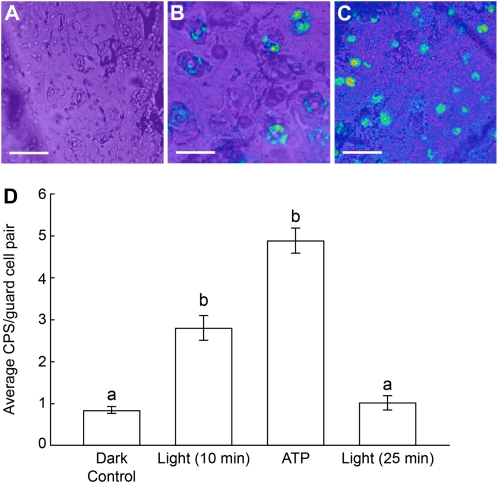
When closed stomata of x-luc9 leaves were exposed to a light stimulus that would cause them to open, the luminescence of stomata in epidermal peels from these leaves increased. After the leaves were exposed to 5 min of constant light and peels from these leaves were floated on leaf buffer for 5 additional min in the light, the addition of luciferin plus flash buffer increased stomatal luminescence levels to an average of 2.81 cps, a level statistically higher than the control levels observed after the initial 24 h of darkness (Fig. 9). Plants were continuously exposed to constant light, and stomatal luminescence levels of peels from their leaves were recorded every 5 min. Luminescence levels peaked after 10 min of irradiation, returned back to levels similar to control levels (average of 1.03 cps) after 25 min, and stayed at this low level for up to 35 min of constant light exposure (Fig. 9D).
Figure 9.
Light treatment of dark-adapted leaves induces the release of ATP in guard cells, as assayed by ecto-luciferase luminescence. A, Background levels of ecto-luciferase luminescence are observed in an epidermal peel from an untreated x-luc9 leaf (dark control). B, An epidermal peel from an x-luc9 leaf treated with 10 min of light shows ecto-luciferase luminescence in guard cells. C, An epidermal peel from an x-luc9 leaf treated with 1 mm ATP in the dark shows ecto-luciferase luminescence in guard cells. Bars = 50 μm for A and B and 100 μm for C. Luminescence levels are represented in pseudocolor (blue, green, yellow, orange, and red, where red represents the highest and blue represents the lowest level of relative intensity). D, Quantification of luciferase activity from a representative data set of an opening experiment. Treatment with 1 mm ATP was done in the dark. Luminescence returned to untreated control levels 25 min after treatment with light. Different letters above the bars indicate mean values that are significantly different from one another as determined by Student’s t test (P < 0.05; n ≥ 15 guard cell pairs). These data are representative of three biological repeats. Error bars represent se.
DISCUSSION
Promoter:GUS and RT-PCR analyses indicated that the level of expression of APY1 and APY2 in guard cells was dependent on conditions that control stomatal opening and closing. Higher levels were observed in guard cells of open stomates and lower levels were observed in guard cells of closed stomates, revealing that apyrase expression was linked to guard cell swelling and shrinking. Immunoblot results showed that the dynamic increase in APY transcript levels when guard cells open was accompanied by increases in apyrase protein, which occurred rapidly, similar to the dynamic changes in apyrase protein levels that occur during rapid growth changes in hypocotyl cells (Wu et al., 2007). Osmotic swelling of cells induces the release of ATP into the medium (Jeter et al., 2004), so the increased expression of APY1 and APY2 in swelling guard cells could be linked to the appearance of increased concentrations of eATP in the extracellular matrix (ECM). Increased eATP during cell expansion is correlated with increased APY1 and APY2 expression during the growth of cells and tissues (Kim et al., 2006; Wu et al., 2007). To the extent that the increase in APY in expanding guard cells is driven by changes in [eATP], it would be the consequence rather than the cause of guard cell swelling; nonetheless, these results suggest that there could be a role for these enzymes in regulating stomatal aperture.
Dark-adapted guard cell protoplasts expand when they are exposed to light (Zeiger and Hepler, 1977). When guard cells swell or shrink, the surface area of their plasma membrane changes to accommodate the fluctuating cell volume (Shope et al., 2003), and these volume changes require membrane trafficking (Shope and Mott, 2006; Meckel et al., 2007). Exocytosis and stretching of the plasma membrane were previously shown to promote ATP release from cells (Jeter et al., 2004; Kim et al., 2006; Weerasinghe et al., 2009), and similar events in expanding guard cells could be the changes that promote ATP release from these cells during stomatal opening.
In both plants and animals, ATP release also occurs during hypotonic shock and cell volume decrease (Light et al., 1999; Jeter et al., 2004; Blum et al., 2010). If this occurs when guard cells shrink in darkness or after ABA treatment, then this release of ATP would probably not be linked to an increase in APY1 and APY2, because the levels of these proteins are lower in dark-adapted guard cell protoplasts, as are the levels of the transcripts that encode them (Fig. 2). The combination of ATP release and a decrease in ectoapyrase levels would result in a higher [eATP] during stomatal closure than during its opening, since ectoapyrase levels increase during stomatal opening. Measuring changes in eATP levels during the swelling and shrinking of guard cell protoplasts would be somewhat problematic, for any breakage of the protoplasts during the incubation periods would increase the background [eATP] in the medium and reduce the signal-to-noise ratio. A method for dynamically assaying changes in eATP levels in the ECM around guard cells in intact leaves or epidermal strips would be one way to solve this problem.
Given these considerations, the question must be asked: Do intact guard cells release ATP when they expand and when they shrink? To address this question, we used transgenic lines expressing a luciferase modified to include a signal peptide that would direct it to be secreted. Results using these lines indicated that guard cells do release ATP both when stomata are induced by light to open and when they are induced by ABA to close.
The technique of engineering the secretion of cytoplasmic proteins by attaching a signal peptide has been successfully employed in many reports (Schnell et al., 2010). The signal peptide used to direct the secretion of luciferase into the ECM was that of S-locus Cys-rich protein (SCR) from Brassica oleracea, which definitely promotes the secretion of SCR in pollen (Schopfer et al., 1999; Watanabe et al., 2000). Evidence that it also promotes the secretion of luciferase is that the addition of ATP and luciferin to the incubation medium of x-luc lines 1 and 9 results in a strong luminescence signal that peaks in 3 s and then, as the applied ATP is hydrolyzed, goes down to baseline levels within 10 s, even though some of the luciferin has entered the cell during this time. In the same experiment, in endo-luciferase lines, even without an addition of ATP to the medium, the luminescence rises sharply as the luciferin enters the cell and continues to rise during a 1-min recording period, indicating that the luciferin-luciferase in the cytoplasm is reporting the internal ATP, which remains at nonlimiting levels during the recording period (data not shown). This indicates that there is very little luciferase in the cytoplasmic compartment of the x-luc lines to report the cytoplasmic [ATP]. In Figures 8 and 9, the applied 1 mm ATP would not be expected to readily cross the plasma membrane, and even if it did, it would not significantly increase the ATP concentration of the cytoplasm, which is typically near or above millimolar levels (Gout et al., 1992). Further evidence that luciferase is secreted is that in an earlier use of x-luc9 plants to assay the pattern of ATP release into the ECM of apical root regions, Roux et al. (2008) reported high [eATP] at the tip and in the elongation zone. This pattern is the same as observed by Kim et al. (2006) using the cellulose-binding domain-luciferase hybrid protein, which was demonstrated to report [eATP] in Arabidopsis roots.
Applied ATP increases the luminescence of both guard cells and the surrounding pavement cells of the epidermis in peels of x-luc leaves, but the signal in the guard cells is two to three times higher than that in the other epidermal cells. Since the cuticle layer covers all epidermal cells, there is no reason to believe that this differential luminescence is due to a more rapid penetration of luciferin into the guard cells. Rather, a more likely explanation is that there is relatively more secretory activity in guard cells (and thus higher ecto-luciferase levels) than in mature pavement cells of the epidermis. This conclusion would be consistent with the fact that there is significantly more membrane turnover in guard cells as they swell and shrink than would be expected in the mature, nongrowing pavement cells.
In principle, the lack of luciferase luminescence in stomata that are in a stable open state in light or a stable closed state in darkness (Figs. 8 and 9) could be due to a lack of expression of ecto-luciferase or to too low a level of eATP in these cells. However, the fact that applied ATP induces a strong luminescence in these cells demonstrates that the level of available luciferase is not limiting, thus favoring the interpretation that it is the [eATP] that is limiting. The increase in guard cell pair luminescence after ABA or light treatment, then, is most likely due to an increase in [eATP] induced by these stimuli.
The increase in luciferase luminescence after ABA treatment appears to be significantly greater than after light treatment. As discussed above, this would be expected if APY levels do not increase when stomates are induced to close but do increase when they are induced to open. This result would be consistent with the dose-response data predicting that higher levels of eATP would induce closing and lower levels would induce opening. However, data quantifying the exact [eATP] represented by luminescence would be needed before this relationship could be confidently established.
Because APY1 and APY2 are nucleoside triphosphate diphosphohydrolases, their increased expression when the [eATP] rises suggests that a role for these enzymes is to limit the [eATP]. In Arabidopsis, APY1 and APY2 are reported to function in part as ectoapyrases, because polyclonal antibodies that inhibit their activity transiently increase the [eATP] that accumulates in the medium of growing pollen tubes (Wu et al., 2007). However, as pointed out by Wu et al. (2007), APY1 and APY2 could influence [eATP] by their activity in the lumen of the Golgi as well as by their activity on the outer face of the plasma membrane.
We directly tested a role for ectoapyrase activity in the control of stomatal closure by treating leaves and peels with chemical inhibitors or anti-APY1/2 antibodies. Both of these treatments induced stomatal closure in the light, similar to the effects of an ABA treatment. Although the chemical inhibitors are small, hydrophobic molecules that could potentially cross the plasma membrane, it is unlikely that the larger antibody molecules could exert their effects inside the cell. These results, then, are consistent with the conclusion that ectoapyrase activity plays an important role in regulating guard cell apertures. The treatments with inhibitors or antibodies were short, 1 h in peel experiments and 2 h in leaf experiments, and they raised the question of whether genetic suppression of APY1 and APY2 expression over a longer period of time might also affect stomatal apertures.
Total suppression of both APY1 and APY2 expression is lethal (Steinebrunner et al., 2003; Wolf et al., 2007), so partial suppression of these genes by RNAi has been the preferred genetic approach to see how a reduction in apyrase expression affects plant growth and development (Wu et al., 2007; Clark et al., 2010b). These RNAi-suppressed plants are apy2 knockouts and are conditionally suppressed in APY1 expression via RNAi under the inducer estradiol (Wu et al., 2007). However, if plants in the RNAi-suppressed line R2-4A are continuously suppressed from germination through flowering, they are dwarf (Wu et al., 2007), and their leaves are too small to fairly compare their stomatal function with wild-type plants. Thus, for the experiments to examine stomata function in R2-4A plants, the plants were not treated with estradiol to suppress apyrase expression until after the development of the mature basal leaves. This late RNAi induction still suppressed APY1 expression by approximately 70% (data not shown), but it allowed the basal leaves to fully expand before being used in experiments.
Although chemical inhibition of apyrase activity and partial suppression of APY expression by RNAi would both reduce ectoapyrase activity, they would not be expected to do so equally. The direct chemical inhibition of the enzyme would likely reduce the ectoapyrase activity significantly more than a partial reduction of the transcript level would. If so, plants treated with the chemical inhibitors would be expected to have a significantly higher [eATP] than the transgenic RNAi-inhibited plants. According to the dose-response assays (Fig. 4), differences in the concentration of applied nucleotides can either promote or inhibit stomata opening.
The dose-response results provide one possible explanation for why RNAi suppression of apyrase expression leads to increased stomatal aperture in response to light in contrast to the results showing that chemical inhibition of apyrase promotes stomatal closure. That is, the “low” concentration (15 μm) of applied ATPγS that induces opening may be similar to the [eATP] in the apyrase-suppressed R2-4A mutant, but the concentration of applied ATPγS that induces closing (greater than 150 μm) may be similar to the [eATP] established after chemical inhibition of the apyrases. To test this hypothesis, a technology for measuring the exact [eATP] in the ECM of plants, which is currently not available, would have to be developed, as noted above.
An alternative explanation for the results observed in RNAi-suppressed plants is that APY1 and APY2 have ectoapyrase functions that affect stomata aperture while they are in the endoplasmic reticulum and/or Golgi en route to the plasma membrane. Antibody or other chemical suppression of only the ECM-localized ectoapyrase activities of APY1 and APY2 on the plasma membrane would not alter any endoplasmic reticulum or Golgi function of these apyrases, but genetic suppression of APY1/APY2 would.
The effects of ATPγS on stomatal aperture are likely mediated by a plant eATP receptor that is pharmacologically similar to animal purinoceptors, because PPADS and RB2 are able to block eATP-induced stomatal closure and opening. These inhibitors also partially block the ability of ABA to induce stomatal closure and of light to induce stomatal opening, suggesting that eATP may play a critical role in the complex signaling pathway mediated by ABA and light. Although a purinoceptor-like protein has been discovered in algae (Fountain et al., 2008), none has yet been found in higher plants. The discovery and characterization of a receptor for extracellular nucleotides in leafy plants will be a necessary prerequisite to clarify more completely the role of extracellular nucleotides in controlling guard cell responses to hormonal and environmental cues.
In Arabidopsis, the production of NO and ROS is both induced by extracellular nucleotides (Song et al., 2006; Reichler et al., 2009; Clark et al., 2010b; Tonón et al., 2010) and required in the signaling pathway that links applied nucleotides to growth changes (Reichler et al., 2009; Clark et al., 2010b). The induction of these signaling intermediates by eATP in other species is also well established (Kim et al., 2006; Foresi et al., 2007; Wu and Wu, 2008; Clark et al., 2010b; Terrile et al., 2010). Because H2O2 and NO play roles in stomatal closure in response to ABA and stressors (García-Mata and Lamattina, 2001; Desikan et al., 2002, 2006; Kwak et al., 2003; Neill et al., 2008), we tested the effects of applied nucleotides on their production in guard cells. Concentrations of ATPγS that induced closure also increased levels of H2O2 and NO in guard cells to near levels induced by a concentration of ABA that induces stomatal closure. Interestingly, ATPγS-induced H2O2 production appeared to occur faster than ATPγS-induced NO production in guard cells, consistent with an earlier observation that ABA-induced NO production in guard cells is dependent on H2O2 synthesis (Bright et al., 2006).
The production of NO is not absolutely required for ABA to induce stomatal closure in all circumstances. For example, NO is not required for ABA-induced closure when plants are experiencing dehydration (Ribeiro et al., 2009; Lozano-Juste and León, 2010). In our experiments, plants were well hydrated, and our results agree with previous reports that in these conditions, nia1nia2 and atrbohD/F mutant stomata are unable to close in response to ABA treatment (Desikan et al., 2002; Kwak et al., 2003; Bright et al., 2006; Hao et al., 2010). The fact that these mutants also do not respond to treatment with 200 μm ATPγS indicates that NO and H2O2 help mediate the effects of nucleotides on stomatal closure.
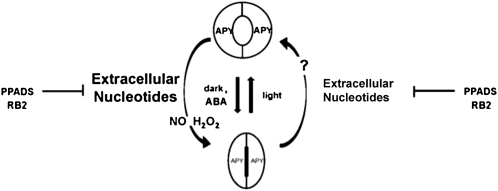
Figure 10 illustrates some of our main findings in a hypothetical model. The model shows that extracellular nucleotides can regulate both the opening and closing of stomata, with the closing response requiring a higher concentration of nucleotides. It shows that the mammalian purinoceptor antagonists PPADS and RB2 inhibit the ATPγS- or ADPγS-induced stomatal aperture changes, which suggests that the extracellular nucleotides are recognized by purinergic-like receptors. The model predicts that NO and H2O2 changes already documented to induce stomatal closure (García-Mata and Lamattina, 2001; Bright et al., 2006; Desikan et al., 2006) are downstream of increases in extracellular nucleotides. In principle, NO and H2O2 production could also be induced by the ATP released during guard cell swelling, but we present no data on this question. In this regard, we note that different levels of applied nucleotides induce different levels of NO and H2O2, which help mediate both the stimulation and the inhibition of root hair growth. The model indicates that the light and dark treatments that alter the volume of guard cells also alter their content of apyrase transcripts and protein.
Figure 10.
Model for the regulation of stomatal movements by extracellular nucleotides. Treatment with the nucleotides ATPγS and ADPβS at high concentrations (greater than 150–250 μm) induces stomatal closure and the release of NO and H2O2, whereas the addition of low concentrations of these nucleotides (15–35 μm) leads to the opening of stomata. These responses to either high (indicated by larger type) or low concentrations of nucleotides can be blocked by the mammalian purinoceptor inhibitors PPADS and RB2, which can also block the ability of ABA to induce stomatal closing and the ability of light to induce opening. The light treatment that induces stomatal opening also induces a higher expression of the transcripts and proteins of APY1 and APY2, and the text discusses the likelihood that these are ectoapyrases that would help regulate the concentrations of extracellular nucleotides during stomatal opening and closing.
In animal cells, hypotonic stress and cell swelling are accompanied by ATP release (Light et al., 1999; Blum et al., 2010), just as happens during hypertonic stress and cell shrinkage. Our model predicts that both the swelling and shrinking of guard cells would induce a release of ATP, but because the shrinking response is accompanied by a decrease in ectoapyrase content and the swelling is accompanied by an increase in ectoapyrase content, the equilibrium [eATP] would be higher during the closing response.
The data presented here strongly favor the conclusion that APY1 and APY2 and extracellular nucleotides play key roles in the control of stomatal aperture. Further studies will be needed to discover how APY and extracellular nucleotide changes intersect with the better-characterized hormonal and environmental cues that control guard cell function.
MATERIALS AND METHODS
RT-PCR Analysis of APY Transcripts in Guard Cell Protoplasts
The tissue source for isolating guard cell protoplasts was rosette leaves from Arabidopsis (Arabidopsis thaliana) Col-0 and Ws ecotypes grown in continuous light for 3 weeks. The isolation procedure used, which yields a protoplast preparation enriched in guard cells, was the overnight method previously described, excluding Histopaque purification steps (Pandey et al., 2002). Total RNA was isolated from the enriched guard cell protoplasts (more than 50% guard cells) and from whole leaves using the Sigma Spectrum Plant Total RNA Kit, following the manufacturer’s protocol. Two micrograms of RNA was treated with DNase (Invitrogen), and first-strand cDNA was synthesized with SuperScript III Reverse Transcriptase (Invitrogen) using the manufacturer’s protocol. APY1 and APY2 transcripts were amplified by adding 2 μL of first-strand cDNA as a template in 25-cycle PCRs. For APY1, the primers AraF172 (5′-GCAGCCGTAACTTGCAATC-3′) and AAR566 (5′-CACAGCGTAATTCTTCGGACC-3′) were used, and for APY2, the primers Arapy2F (5′-GCTTTCCCAAATTCACCGT-3′) and AAR566 (5′-CACAGCGTAATTCTTCGGACC-3′) were used (Wu et al., 2007). For ACT2, the primers 5′-AACTCTCCCGCTATGTATGTCGC-3′ and 5′-CCATCTCCTGCTCGTAGTCAACA-3′ were used. The PCR products were run on a 1% agarose gel and visualized under UV light.
Immunoblot Analysis of APY Proteins Extracted from Guard Cell Protoplasts
Guard cell protoplasts and whole leaf tissues were snap frozen with liquid nitrogen, and the whole leaves were ground while frozen. Samples were boiled in a protein extraction buffer containing 0.1 m Tris (pH 6.8), 20% (v/v) glycerol, 5% (w/v) SDS, 200 mm dithiothreitol, 200 μm phenylmethylsulfonyl fluoride, and SigmaFAST Protease Inhibitor Cocktail tablets for 3 to 4 min and were then centrifuged at 17,000g for 2 min. The pellet was discarded. The protein concentration of the supernatant was determined using the Bradford assay (Bio-Rad). Then, 16 μg of this protein was loaded in each lane, separated by SDS-PAGE, and transferred to nitrocellulose. To detect APY1 and APY2, the nitrocellulose was probed with polyclonal guinea pig anti-AtAPY1 antibody (GP1318; Wu et al., 2007) in a 1:1,000 dilution and polyclonal anti-guinea pig IgG antibody coupled to IRDye in a 1:5,000 dilution (Rockland) and then assayed with the Odyssey infrared imaging system (Li-Cor Biosciences). For Arabidopsis α-tubulin detection with the Odyssey system, monoclonal mouse anti-α-tubulin from sea urchin (1:2,500 dilution; Sigma) and polyclonal anti-mouse IgG antibodies coupled to IRDye (1:5,000 dilution; Rockland) were used. For immunoblot analyses of the effects of light treatments on APY1 and APY2 protein levels, guard cell protoplasts were dark adapted for 1 h on ice and equilibrated to room temperature for 10 min. Then, the guard cell protoplast preparation was separated into five 100-μL aliquots in 1.5-mL centrifuge tubes, and samples were either untreated (dark control) or treated for 15, 30, 45, or 60 min. Protoplast samples were boiled in extraction buffer and used for immunoblot analyses as described above.
Promoter:GUS Analyses
APY1:GUS and APY2:GUS fusion lines (described by Steinebrunner et al., 2003; Wu et al., 2007), both in the Ws background, were grown in short days (8 h of light; 150 μmol photons m−2 s−1) at 20°C (night) and 23°C (day). The light intensity was measured with the quantum sensor LI-190SA (Li-Cor Biosciences). High-humidity conditions (85% RH) were achieved by growing the plants covered. Low humidity (33% RH) represented the default condition in the plant growth chamber BrightBoy XL (CFL Plant Climatics). The RH was determined with a Lutron humidity meter (model HT-315). Two independent lines were used per GUS fusion construct. Plants were grown on well-watered soil for 25 to 42 d. Two rosette leaves per line from two different plants were harvested for each time point. The leaves were fixed in ice-cold 90% (v/v) acetone for 1 h at −20°C, washed three times with 50 mm sodium phosphate (pH 6.8), and stained overnight at 37°C in staining solution [50 mm sodium phosphate (pH 6.8), 20 mm K4Fe(CN)6, 20 mm K3Fe(CN)6, 0.2% (v/v) Triton X-100, and 1 mm 5-bromo-4-chloro-3-indolyl β-d GlcA]. After the staining procedure, the staining solution was removed and replaced by 70% (v/v) ethanol. Stomates on the abaxial side of the leaves were photographed. The experiment was performed three times with independent plant cultures.
Stomatal Aperture Experiments
Arabidopsis ecotypes Col-0 and Ws were used as wild types in this study. Col-0, nia1nia2, atrbohD/F, R2-4A, and Ws plants were all grown on autoclaved Metro-Mix 200 soil at 22°C under continuous light. The mutant seeds nia1nia2 (nia1-1, nia2-5; CS 2356; Col-0 background) were obtained from Dr. N.M. Crawford (University of California at San Diego), and the mutant seeds atrbohD/F (Col-0 background) were obtained from Dr. J.M. Kwak (University of Maryland). The RNAi mutant apyrase line (R2-4A) is in the Ws background, so ecotype Ws was used as the control wild type for these experiments. Ws plants and R2-4A were treated with 4 μm estradiol for 1 week after the development of mature basal leaves. Leaves from 2- to 3-week-old plants were used for peel and whole leaf experiments. Plants were placed in the dark and watered 24 h before an experiment. For opening experiments, plants were used immediately after 24 h of dark treatment, and all treatments were done in the dark except for the light-treated leaves or peels; thus the control stomata are dark controls. For closure experiments, plants were placed in the light 3 h before an experiment to induce stomatal opening, and all treatments were done in the light; thus the control stomata are light controls. For peel experiments, epidermal peels were made from the underside of the leaf, and treatments were applied to the peels for 1 h. For whole leaf experiments, leaves were removed, and treatments were applied for 2 h. For each treatment, about three or four peels collected from different leaves or three or four different leaves of at least two different plants were floated with the abaxial side up in petri dishes on 3 mL of Arabidopsis leaf buffer consisting of 10 mm KCl, 25 mm MES, pH 6.15 (Melotto et al., 2006), and the chemical being tested. For whole leaf experiments, peels were collected after the treatment.
For experiments testing the effects of ATPγS, ADPβS, AMPS, RB2, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, N-acetyl-l-cysteine, and PPADS (Sigma), 20 mm stocks were made by dissolving the compounds in deionized water. For experiments testing the effects of apyrase inhibitors, 7.5 mg mL−1 NGXT191 and inhibitor 13 (Windsor et al., 2002) was dissolved in dimethyl sulfoxide (DMSO) and then applied at a dilution of 1:1,000 for a final concentration of 0.1% DMSO. ABA (Sigma) was dissolved in ethanol in 10 mm stocks and then applied at a dilution of 1:1,000. The stocks were stored at −20°C while not in use. The production of the anti-AtAPY1 antibodies used is described by Steinebrunner et al. (2003). The crude immune and preimmune sera were purified using protein A-Sepharose following the protocol described by Martin (1982) with the slight modification that the buffers used were azide free. The protein A-purified sera were both used at a 1:1,000 dilution in leaf stomatal aperture experiments. The concentration of the immune and preimmune sera, determined by Bradford assay (Bio-Rad), was 10.2 and 7.7 μg mL−1, respectively.
After treatment, photographs of stomata were taken using a light microscope at 20×. Stomatal aperture width was measured using the image-processing software ImageJ. Typically, photographs were taken of 70 to 90 stomata per treatment, of which only 50 stomata were measured for each treatment. For each treatment, the ratio of closed stomata (width of 0) to open stomata was determined for all stomata imaged, and this ratio was maintained in the 50 stomata that were measured for each treatment. Data shown used apertures determined as width/length; however, data in all experiments were also obtained as width only, and generally, width/length and width only data are in agreement with each other. Statistical significance of the measurements for the treatments was determined using Student’s t test in Microsoft Excel.
Detection and Quantification of ROS and NO
Col-0 plants were grown on soil for 2 to 4 weeks under continuous light at 21°C. Plants were placed in the dark for 24 h to ensure closure and then transferred to the light for 1 h. After 1 h in the light, mature basal leaves were excised and blended in a Waring blender on the low setting for approximately 10 s to isolate epidermal tissues, and the tissues were placed in 3 mL of 30 mm KCl, 10 mm MES-KOH, pH 6.15, buffer in light for 2 h (Pei et al., 2000; Murata et al., 2001). DAF-2DA (Calbiochem) and H2DCFDA (Invitrogen) were dissolved in DMSO to produce 5 and 10 mm stock solutions, respectively, and these were stored at −20°C in 30-μL aliquots. Fifty micromolar H2DCFDA or 15 μm DAF-2DA was added to the medium in the dark, and after 30 min, 10 μm ABA, 200 μm ATPγS, or buffer (no-ATP control) was also added to the medium in the dark for 30 min. Peels were then rinsed by decanting the treatment solution and adding 3 mL of fresh leaf buffer to the peels. Peels were rinsed twice as described. Peels from each of the three treatments (ABA, ATPγS, and buffer) were placed on the same microscope slide and observed sequentially. A second experiment was staggered 30 to 45 min after the first by loading the H2DCFDA or DAF-2DA dye to new peels 30 to 45 min after the first round of dye was added. Confocal laser scanning microscopy was performed with a Leica SP2 AOBS confocal microscope (Leica Microsystems). Laser power was set at 15%, with an excitation of 488 nm and an emission of 525 nm. A series of 0.5-μm optical sections with average intensity projection along the z axis were collected and made into one two-dimensional image with greater focal depth. All images were obtained with the same software scanning settings, including detector gain and laser intensity settings.
Ecto-Luciferase Construct and Plant Transformation
The nucleotide sequence for the 24-amino acid, cleavable signal peptide from the Brassica oleracea pollen coat protein (SCR13) was used to target luciferase for secretion (Schopfer et al., 1999). The signal peptide was incorporated at the N terminus of the luciferase gene by PCR, and the SCR13 signal peptide-modified luciferase PCR product was then ligated into the binary vector pLBJ21. This construct was transformed into Agrobacterium tumefaciens strain GV3101 (pMP90) and then transformed into Arabidopsis plants (Col-0) using the floral dipping method (Clough and Bent, 1998). Transgenic plants were selected by planting on solidified Murashige and Skoog (MS) medium (4.3 g L−1 MS salts [Sigma], 0.5% [w/v] MES, and 1.0% [w/v] agar, raised to pH 5.7 with 5 m KOH) containing 50 μg mL−1 kanamycin. Segregation of the T3 generation on kanamycin plates was analyzed in order to obtain homozygous lines. Plants showing kanamycin resistance were checked for luciferase activity by growing transgenic plants in MS medium for 10 d and then transferring the whole seedlings into test tubes. Luminescence measurements were performed by placing the test tubes in the light-tight housing of the luminometer reader (Dynatech). Experimental solutions with luciferin substrate with and without 1 mm ATP were injected by an automatic injector, and after 3 s, counting started at 0.2-s time intervals. Two different transgenic lines, x-luc1 and x-luc9, were chosen for this study based on their positive signals without added ATP and increased signals with 1 mm ATP added, and the immediate luminescence of these lines was compared with endo-luciferase lines, which showed a delay in luminescence.
Imaging of Luminescence in Ecto-Luciferase Plants
Ecto-luciferase seeds were surface sterilized, stratified in the dark in 4°C for at least 3 d, and then planted directly on a cellophane membrane placed upon solidified MS with 1.0% (w/v) agar. Planted plates were placed upright in a culture chamber and grown at 23°C under a 24-h fluorescent light for 7 d. Plates were then reoriented so that the solidified MS medium was at the bottom of the plate and the seedling was able to grow up into the empty space of the petri dish. Seedlings were then allowed to mature for up to 4 weeks under identical temperature and light settings. For “dark” experiments, petri dishes of 3- to 4-week-old plants were placed in a dark chamber for 24 h prior to use in experiments.
Mature basal leaves were excised from x-luc1 and x-luc9 plants 3 to 4 weeks old. Peels were taken and then immediately floated with the abaxial side up in 40 μL of Arabidopsis leaf buffer on a microscope slide and the chemical being tested in either light or dark conditions depending on the experimental setup. The placing of the peels within their respective solution was considered time zero. Peels were treated for a minimum of 5 min before luciferin and flash buffer were added to the solution. At specified times, 40 μL of luciferin solution (20× stock of d-luciferin [catalog no. E160A]; Promega) was diluted to a final concentration of 5 mm in flash assay buffer (20 mm Tricine, 2.67 mm MgSO4, 0.1 mm EDTA, and 2 mm dithiothreitol) as described by Kim et al. (2006) and was added to the existing peel solution in low-light conditions, bringing the final concentration of luciferin to 2.5 mm. After placing a coverslip over the sample, imaging was performed immediately using a Leica DME microscope with a Leica HC PLAN APO 20×/0.7 numerical aperture or HI PLAN 40×/0.65 numerical aperture objective installed in a NightOwl II LB 983 instrument (Berthold Technologies). Luminescence was integrated over 120 s, High Gain, Read Out set to slow, using 4- × 4-pixel binning with Cosmic Suppression on, Background Correction off, and Flatfield Correction off with the filter set to Photo.
All luminescence analysis was conducted using the indiGO Analysis Software (version 2.0.0.26). All images consisted of a photographic image in grayscale with an overlaid luminescence image in pseudocolor. Areas of interest were defined manually using the Manual Areas command. This allowed us to manually define integration areas using free-form selection of guard cells only. This was accomplished by first lowering the luminescence overlay slider to 0%. This completely removed all luminescence signal from the photographic image. Then, we adjusted the intensity scale of the photographic image to improve the contrast of the image so that we could confidently identify guard cells from the surrounding pavement cells. Next, we used the Manual Areas command to select only guard cells as areas of interest for luminescence signal integration. Once we had manually selected all areas of interest, we brought the luminescence overlay slider back up to 65%. Finally, we adjusted the intensity scale for the luminescence signal until all background-level luminescence was displayed as a dark magenta color, while luminescence levels above background levels were displayed as blue, green, yellow, orange, and red, where red represented the highest level of relative intensity. It is important to note that while adjusting the intensity scale may change the visual representation of measured luminescence, it does not change the raw levels of luminescence present, which are used to calculate actual luminescence activity. After all areas of interest had been selected and adjusted, the Excel export function was used to create a complete measurement report, including all images and analysis data. All luminescence activity was expressed as average cps.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: ATRBOHD, At5g47910; ATRBOHF, At1g64060; APY1, At3g04080; APY2, At5g18280; NIA1, At1g77760; NIA2, At1g37130; SCR13, AF195626; and ACT2, At3g18780.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Dose-response assays for stomatal closure induced by ADPbS in epidermal peels and ATPgS in leaves.
Supplemental Figure S2. Dose-response assays for stomatal opening induced by ADPbS and ATPgS in leaves.
Supplemental Figure S3. RB2 blocks ATPgS-induced stomatal closure and opening in leaves.
Supplemental Figure S4. H2DCFDA and DAF-2DA staining of guard cells treated with 200 μm ATPgS.
Acknowledgments
We thank Delmy Gomez, Patricia Triece, and Desiree Waugh for help with stomatal aperture experiments. We also thank Marianna Grenadier for creating the final figures.
References
- Barbour MM, Buckley TN. (2007) The stomatal response to evaporative demand persists at night in Ricinus communis plants with high nocturnal conductance. Plant Cell Environ 30: 711–721 [DOI] [PubMed] [Google Scholar]
- Blum AE, Walsh BC, Dubyak GR. (2010) Extracellular osmolarity modulates G protein-coupled receptor-dependent ATP release from 1321N1 astrocytoma cells. Am J Physiol Cell Physiol 298: C386–C396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2008) Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7: 575–590 [DOI] [PubMed] [Google Scholar]
- Clark G, Roux SJ. (2009) Extracellular nucleotides: ancient signaling molecules. Plant Sci 177: 239–244 [Google Scholar]
- Clark G, Torres J, Finlayson S, Guan XY, Handley C, Lee J, Kays JE, Chen ZJ, Roux SJ. (2010a) Apyrase (nucleoside triphosphate-diphosphohydrolase) and extracellular nucleotides regulate cotton fiber elongation in cultured ovules. Plant Physiol 152: 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G, Wu M, Wat N, Onyirimba J, Pham T, Herz N, Ogoti J, Gomez D, Canales AA, Aranda G, et al. (2010b) Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygen species. Plant Mol Biol 74: 423–435 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM. (2003) Is ATP a signaling agent in plants? Plant Physiol 133: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, Mortimer JC, Chivasa S, Slabas AR, Glover BJ, et al. (2009) Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J 58: 903–913 [DOI] [PubMed] [Google Scholar]
- Desikan R, Griffiths R, Hancock J, Neill S. (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA 99: 16314–16318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill SJ. (2006) Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J 47: 907–916 [DOI] [PubMed] [Google Scholar]
- Foresi NP, Laxalt AM, Tonón CV, Casalongué CA, Lamattina L. (2007) Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol 145: 589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, Cao L, Young MT, North RA. (2008) Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J Biol Chem 283: 15122–15126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126: 1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Bligny R, Douce R. (1992) Regulation of intracellular pH values in higher plant cells: carbon-13 and phosphorus-31 nuclear magnetic resonance studies. J Biol Chem 267: 13903–13909 [PubMed] [Google Scholar]
- Hao FS, Zhao SL, Dong H, Zhang H, Sun LR, Miao C. (2010) Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J Integr Plant Biol 52: 298–307 [DOI] [PubMed] [Google Scholar]
- Jeter CR, Tang W, Henaff E, Butterfield T, Roux SJ. (2004) Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 16: 2652–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Bae YS, Lee JS. (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126: 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-Y, Sivaguru M, Stacey G. (2006) Extracellular ATP in plants: visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol 142: 984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Perfus-Barbeoch L, Frelet A, Gaedeke N, Reinhardt D, Mueller-Roeber B, Martinoia E, Forestier C. (2003) The plant multidrug resistance ABC transporter AtMRP5 is involved in guard cell hormonal signalling and water use. Plant J 33: 119–129 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light DB, Capes TL, Gronau RT, Adler MR. (1999) Extracellular ATP stimulates volume decrease in Necturus red blood cells. Am J Physiol 277: C480–C491 [DOI] [PubMed] [Google Scholar]
- Lozano-Juste J, León J. (2010) Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis. Plant Physiol 152: 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LN. (1982) Separation of guinea pig IgG subclasses by affinity chromatography on protein A-Sepharose. J Immunol Methods 52: 205–212 [DOI] [PubMed] [Google Scholar]
- Meckel T, Gall L, Semrau S, Homann U, Thiel G. (2007) Guard cells elongate: relationship of volume and surface area during stomatal movement. Biophys J 92: 1072–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Mott KA, Peak D. (2010) Stomatal responses to humidity and temperature in darkness. Plant Cell Environ 33: 1084–1090 [DOI] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J. (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. (2008) Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59: 165–176 [DOI] [PubMed] [Google Scholar]
- Pandey S, Wang X, Coursol S, Assmann S. (2002) Preparation and applications of Arabidopsis thaliana guard cell protoplasts. New Phytol 153: 517–526 [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Reichler SA, Torres J, Rivera AL, Cintolesi VA, Clark G, Roux SJ. (2009) Intersection of two signalling pathways: extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide. J Exp Bot 60: 2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro DM, Desikan R, Bright J, Confraria A, Harrison J, Hancock JT, Barros RS, Neill SJ, Wilson ID. (2009) Differential requirement for NO during ABA-induced stomatal closure in turgid and wilted leaves. Plant Cell Environ 32: 46–57 [DOI] [PubMed] [Google Scholar]
- Roux S, Wu J, Henaff E, Torres J, Clark G. (2008) Regions of growth are regions of highest release of ATP and highest expression of ectonucleotidases AtAPY1 and AtAPY2 in Arabidopsis. Purinergic Signal 4: S112 [Google Scholar]
- Roux SJ, Steinebrunner I. (2007) Extracellular ATP: an unexpected role as a signaler in plants. Trends Plant Sci 12: 522–527 [DOI] [PubMed] [Google Scholar]
- Schnell JA, Han SY, Miki BL, Johnson DA. (2010) Soybean peroxidase propeptides are functional signal peptides and increase the yield of a foreign protein. Plant Cell Rep 29: 987–996 [DOI] [PubMed] [Google Scholar]
- Schopfer CR, Nasrallah ME, Nasrallah JB. (1999) The male determinant of self-incompatibility in Brassica. Science 286: 1697–1700 [DOI] [PubMed] [Google Scholar]
- Shope JC, DeWald DB, Mott KA. (2003) Changes in surface area of intact guard cells are correlated with membrane internalization. Plant Physiol 133: 1314–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope JC, Mott KA. (2006) Membrane trafficking and osmotically induced volume changes in guard cells. J Exp Bot 57: 4123–4131 [DOI] [PubMed] [Google Scholar]
- Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. (2006) Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol 140: 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinebrunner I, Jeter C, Song C, Roux SJ. (2000) Molecular and biochemical comparison of two different apyrases from Arabidopsis thaliana. Plant Physiol Biochem 38: 913–922 [Google Scholar]
- Steinebrunner I, Wu J, Sun Y, Corbett A, Roux SJ. (2003) Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol 131: 1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Gilroy S, Jones AM, Stacey G. (2010a) Extracellular ATP signaling in plants. Trends Cell Biol 20: 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Swanson SJ, Gilroy S, Stacey G. (2010b) Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol 154: 705–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrile MC, Tonón CV, Iglesias MJ, Lamattina L, Casalongué CA. (2010) Extracellular ATP and nitric oxide signaling pathways regulate redox-dependent responses associated to root hair growth in etiolated Arabidopsis seedlings. Plant Signal Behav 5: 698–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonón C, Terrile MC, Iglesias MJ, Lamattina L, Casalongué C. (2010) Extracellular ATP, nitric oxide and superoxide act coordinately to regulate hypocotyl growth in etiolated Arabidopsis seedlings. J Plant Physiol 167: 540–546 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Suzuki G, Takayama S, Isogai A, Hinata K. (2000) Genomic organization of the SLG/SRK region of the S locus in Brassica species. Ann Bot (Lond) 85: 155–160 [Google Scholar]
- Weerasinghe RR, Swanson SJ, Okada SF, Garrett MB, Kim SY, Stacey G, Boucher RC, Gilroy S, Jones AM. (2009) Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett 583: 2521–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Crawford NM. (1993) Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol Gen Genet 239: 289–297 [DOI] [PubMed] [Google Scholar]
- Windsor JB, Thomas C, Hurley L, Roux SJ, Lloyd AM. (2002) Automated colorimetric screen for apyrase inhibitors. Biotechniques 33: 1024–, 1026, 1028–1030 [DOI] [PubMed] [Google Scholar]
- Wolf C, Hennig M, Romanovicz D, Steinebrunner I. (2007) Developmental defects and seedling lethality in apyrase AtAPY1 and AtAPY2 double knockout mutants. Plant Mol Biol 64: 657–672 [DOI] [PubMed] [Google Scholar]
- Wu J, Steinebrunner I, Sun Y, Butterfield T, Torres J, Arnold D, Gonzalez A, Jacob F, Reichler S, Roux SJ. (2007) Apyrases (nucleoside triphosphate-diphosphohydrolases) play a key role in growth control in Arabidopsis. Plant Physiol 144: 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Wu JY. (2008) Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. J Exp Bot 59: 4007–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E, Hepler PK. (1977) Light and stomatal function: blue light stimulates swelling of guard cell protoplasts. Science 196: 887–889 [DOI] [PubMed] [Google Scholar]