Abstract
Optimal root architecture is established by multiple intrinsic (e.g. hormones) and extrinsic (e.g. gravity and touch) signals and is established, in part, by directed root growth. We show that asymmetrical exposure of cytokinin (CK) at the root tip in Arabidopsis (Arabidopsis thaliana) promotes cell elongation that is potentiated by glucose in a hexokinase-influenced, G protein-independent manner. This mode of CK signaling requires the CK receptor, ARABIDOPSIS HISTIDINE KINASE4 and, at a minimum, its cognate type B ARABIDOPSIS RESPONSE REGULATORS ARR1, ARR10, and ARR11 for full responsiveness, while type A response regulators act redundantly to attenuate this CK response. Ethylene signaling through the ethylene receptor ETHYLENE RESISTANT1 and its downstream signaling element ETHYLENE INSENSITIVE2 are required for CK-induced root cell elongation. Negative and positive feedback loops are reinforced by CK regulation of the expression of the genes encoding these elements in both the CK and ethylene signaling pathways. Auxin transport facilitated by PIN-FORMED2 as well as auxin signaling through control of the steady-state level of transcriptional repressors INDOLE-3-ACETIC ACID7 (IAA7), IAA14, and IAA17 via TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX PROTEIN are involved in CK-induced root cell elongation. This action lies downstream of ethylene and CK induction. Intrinsic signaling in this response operates independently of the extrinsic signal touch, although actin filament organization, which is important in the touch response, may be important for this response, since latrunculin B can induce similar growth. This root growth response may have adaptive significance, since CK responsiveness is inversely related to root coiling and waving, two root behaviors known to be important for fitness.
Root architecture is developmentally plastic (Malamy, 2005) and affected by many intrinsic and extrinsic factors (Massa and Gilroy, 2003a, 2003b) in order to maximize nutrient and water acquisition. Among the intrinsic factors, auxin has an important role in shaping root growth pattern, since mutants with altered auxin response or transport show altered root growth, lateral root primordia formation, and tropic responses such as perturbed gravitropism, coiling, waving, and skewing responses (Okada and Shimura, 1990; Garbers et al., 1996; Luschnig et al., 1998; Rutherford et al., 1998; Marchant et al., 1999; Rashotte et al., 2001; Piconese et al., 2003; Buer and Muday, 2004). Ethylene has also been shown to inhibit root waving (Buer et al., 2003), root gravitropism (Buer et al., 2006), and lateral root formation (Negi et al., 2008). Ethylene in turn can regulate auxin biosynthesis as well as transport-dependent auxin distribution (Růžička et al., 2007).
Ethylene has been shown to be involved in controlling cytokinin (CK)-mediated root elongation but not meristem size (Růžička et al., 2009). CK signaling follows a multiple-step phosphorelay cascade (Heyl and Schmülling, 2003; Müller and Sheen, 2007; To and Kieber, 2008; Werner and Schmülling, 2009; Kieber and Schaller, 2010; Perilli et al., 2010; Müller, 2011) using, in this order of phosphorylation, (1) one of three hybrid His protein kinases (ARABIDOPSIS HISTIDINE KINASE2 [AHK2], AHK3, AHK4) that serve as CK receptors, (2) His phosphotransfer proteins (ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEINS [AHPs]), and (3) type A and type B response regulators (ARABIDOPSIS RESPONSE REGULATORS [ARRs]). After phosphorylation, AHPs move into the nucleus, where they phosphorylate type B and type A ARRs. Phosphorylated type B ARRs act as transcription factors and induce the transcription of type A ARRs and other CK early-responsive genes (Hwang and Sheen, 2001). CK is known to interact with other hormones so as to affect plant growth and development. Among these, ethylene and auxin are the two most important hormones with which CK exhibits extensive cross talk. CK stabilizes the ethylene biosynthetic enzyme ACS5, leading to increased ethylene production (Vogel et al., 1998a, 1998b; Chae et al., 2003; Hansen et al., 2009). CK and ethylene signaling also share in common the phosphorylation of ARR2 (Hass et al., 2004).
CKs negatively regulate PIN-FORMED (PIN)-dependent auxin distribution and consequently auxin transport direction and flux (Laplaze et al., 2007; Lee et al., 2009; Pernisová et al., 2009). CK and auxin have opposite effects on root development (Dello Ioio et al., 2008) mediated by SHORT HYPOCOTYL2 (SHY2). CK activates the transcription of the SHY2 gene, while auxin degrades the SHY2 protein (Dello Ioio et al., 2008). Auxin promotes cell division, while CK controls the switch from meristematic to differentiated cell-suppressing auxin signaling and transport to the transition zone (Dello Ioio et al., 2008). This CK-auxin antagonism is also crucial for specifying the embryonic root stem cell niche during Arabidopsis (Arabidopsis thaliana) embryogenesis. It has been shown that an auxin maxima at the hypophysis activates the transcription of type A ARR7 and ARR15 genes, which are negative regulators of CK signaling. This leads to abnormal embryonic stem cell niche formation (Müller and Sheen, 2008). The different aspects of the CK-auxin interaction have been recently reviewed (Aloni et al., 2006; Zhao, 2008; Chapman and Estelle, 2009; Kuderová and Hejátko, 2009; Moubayidin et al., 2009).
There are several reports that sugar can also influence plant root growth (Rolland et al., 2006). The increasing Glc concentration not only increases root length, number of lateral roots, and root hairs but also modulates the gravitropic response of the primary roots of young seedlings (Mishra et al., 2009). Increasing Glc concentrations can induce differential root length, lateral roots, gravitropism, and root hair elongation in auxin perception and signaling mutants, suggesting that auxin signaling is involved in controlling Glc-regulated root responses (Mishra et al., 2009). Glc has also been shown to act via G-protein signaling to attenuate auxin-mediated bimodality in controlling lateral root formation (Booker et al., 2010).
In summary, Glc, CK, auxin, and ethylene control a number of root-related phenotypes such as root elongation, root meristem size, specification of root stem cell niche, and lateral root production. CK along with auxin is also involved in controlling root gravitropic response (Aloni et al., 2004). Here, we show that apart from gravitropism, CK has an important role in controlling other root directional/tropic responses such as coiling and waving, aspects of optimal root architecture.
RESULTS
CK-Induced Root Growth Response Is CK Specific and Glc Enhanced
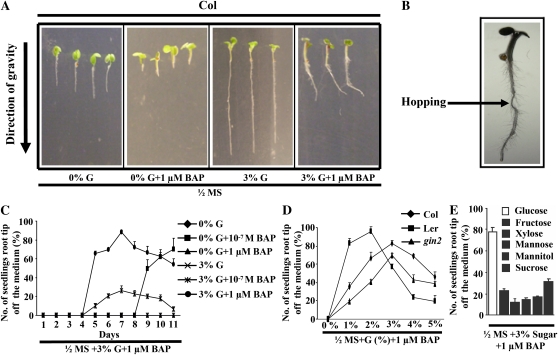
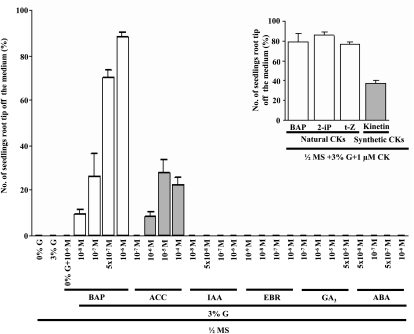
Wild-type seeds were grown vertically in 0% Glc (G)-, 0% G + 1 μm 6-benzylaminopurine (BAP)-, 3% G-, and 3% G + 1 μm BAP-containing half-strength Murashige and Skoog (MS) medium solidified with 0.8% agar in the light. Root tips grew away from 3% G + 1 μm BAP-containing medium after 5 d of growth, but then gravitropism returned them to the medium (Fig. 1A). This occurred in all roots multiple times during the test period; consequently, the history of this type of growth was preserved in the root morphology (“hopping”; Fig. 1B). In order to distinguish “instantaneous” changes in sensitivity, hereafter the data are presented as the percentage of total roots with tips off the medium at the indicated times (Fig. 1C). The presence of Glc enhanced this response, since CK-induced growth was delayed in medium lacking Glc. These roots were also shorter. The tip growth response became visible on day 5 but reached its maximum on day 7 and thereafter became less sensitive to CK (Fig. 1C). This tip growth response was attenuated in the Glc receptor gin2 mutant (Fig. 1D) but not other Glc signaling mutants such as rgs1 and gpa1 (Supplemental Fig. S1). Glc was found to be the most potent hexose among those tested (Fig. 1E). Glc decreased the steady-state level of transcripts for several signaling elements that operate either positively or negatively in the CK signaling pathway (Supplemental Fig. S2). The Arabidopsis seedlings, grown in auxin-, brassinolide-, abscisic acid (ABA)-, or GA3-containing medium, did not display the growth response, while seedlings grown in 1-aminocyclopropane-1-carboxylic-acid (ACC)-containing medium showed some CK-induced root growth but at high concentrations (Fig. 2) compared with the CK effect (Fig. 2, inset).
Figure 1.
Seeds were directly sown on treatment medium, and seedlings were grown vertically in the presence of light. The data presented are averages of three biological replicates, with each replicate having 30 seedlings and error bars representing sd. A, Col seeds were sown on medium containing half-strength MS medium with and without G, 1 μm BAP, 3% G, or 3% G + 1 μm BAP as indicated. The images represent 7-d-old vertically, light-grown Col seedlings on 3% G + 1 μm BAP-containing medium showing CK-induced root growth responses as opposed to seedlings grown either in G-free medium or medium containing only 3% G. B, Col seeds were sown on 3% G + 1 μm BAP-containing half-strength MS medium. The arrow indicates an example of a past growth event analogous to hopping across the surface. C, The kinetics of CK-induced root growth of Col in Glc-free and Glc-containing half-strength MS medium with and without BAP. The CK-induced root growth kinetics shows that the response becomes visible from day 5 and reaches its maximum on day 7 in 3% G + BAP-containing medium. The response was either delayed (0% G + 10−7 m BAP) or not visible (0% G + 1 μm BAP) in BAP-containing Glc-free half-strength MS medium. D, Comparison of CK-induced root growth of Col, Ler, and gin2 mutant seedling roots on day 7. Seeds were sown on half-strength MS + 1 μm BAP medium with different concentrations of Glc. The CK-induced root growth response was found to be highly reduced in gin2 at lower Glc concentrations while greater than Ler at higher Glc concentrations. E, Comparison of CK-induced root growth responses of 7-d-old Col seedling roots on various sugars. Seeds were sown on half-strength MS + 1 μm BAP medium with different sugars (3%). Glc was the most potent sugar for promoting CK-induced root growth among the various sugars tested. Student’s t test, P < 0.05, n = 3 biological replicates.
Figure 2.
The effects of different hormones and CKs on 7-d-old Col seedling roots to determine their roles in CK-induced root growth. Seeds were sown on 3% G and various hormone-containing half-strength MS medium and grown vertically in the light. The significant extent of the CK-induced root growth response was found only with BAP, while ACC could also bring about very little response but at a very high concentration. The CK-induced root growth response was not visible in 3% G and different concentrations of IAA-, epibrassinolide (EBR)-, GA3-, or ABA-containing half-strength MS medium. Among the various CKs tested, all naturally occurring CKs (trans-zeatin [t-Z] and 2-isopentenyladenine [2-iP]) could evoke CK-induced root growth to a similar extent as with BAP, in contrast to synthetic CK kinetin (inset). The data presented are averages of three biological replicates, with each replicate having 30 seedlings and error bars representing sd. Student’s t test, P < 0.05, n = 3 biological replicates.
Effect of Light and Different Medium Composition on CK-Induced Root Growth Response
The Glc enhancement suggests that the nutrient state is important for this root response. Dark-grown seedlings displayed the CK-induced root growth response, although the extent was less than in light-grown seedlings (Supplemental Fig. S3A).
To determine the effect of different medium composition, root growth was also tested in 1 mm KNO3 + 3% G + 1 μm BAP + 0.8% agar medium. The seedlings displayed CK-induced root growth in both media, suggesting that a basal medium along with Glc and BAP is sufficient (Supplemental Fig. S3B). The CK-induced root growth response did not require agar. For this experiment, 5-d-old wild-type seedlings grown on half-strength MS medium were transferred to square petri plates containing Whatman sheets saturated with half-strength MS (liquid) + 3% G + 1 μm BAP for the next 2 d (data not shown). Seedlings without a root tip did not exhibit CK-induced root growth (data not shown), suggesting that the root tip is the site of CK perception.
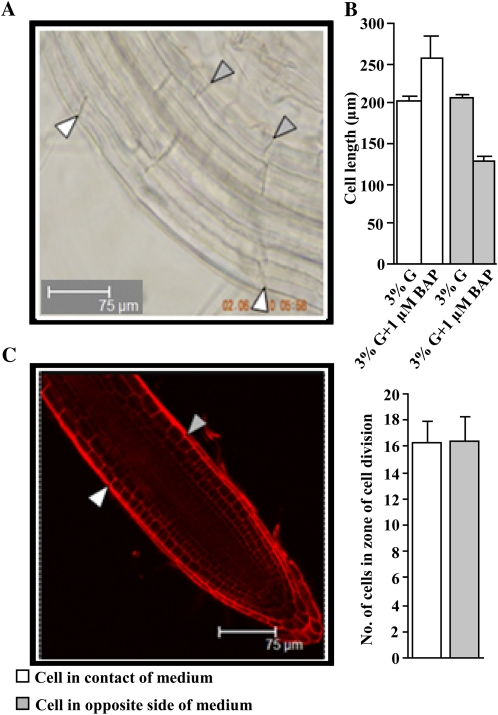
Differential Cell Elongation across Root Is Responsible for the CK-Induced Root Growth Response
The roots “move away” from CK-containing medium because of more cell elongation on the side of the root touching the medium and simultaneous cell length inhibition on the side facing away from the medium (Fig. 3, A and B). It is important to mention here that the average size of the cells generally decreases in the CK-containing medium except the cells where the root starts to move away from the medium. Cell division was statistically the same on the side touching the medium compared with the side facing away from the medium (Fig. 3C). Real-time gene expression analysis suggested that either Glc alone or Glc plus CK treatment together increased EXPANSIN (EXP) gene expression, providing a possible mechanism for the Glc enhancement (Supplemental Fig. S4A). However, increased EXP gene expression can be at best an indirect effect, as different mutant lines lacking individual EXP genes EXP1, EXP10, EXP13, EXP14, and EXPA9 had the wild-type root-growth phenotype (Supplemental Fig. S4B). Alternatively, EXPs operate here redundantly.
Figure 3.
Col seeds were sown on 3% G- and 3% G + 1 μm BAP-containing half-strength MS medium and grown vertically in the light for 7 d. The cell length and cell number data presented are averages of two biological replicates, with each replicate having 10 seedlings and error bars representing sd. A, Cells touching the medium were almost two times more elongated as compared with cells on the side facing away from the medium in seedlings grown on 3% G + 1 μm BAP-containing half-strength MS medium. B, The cell length of seedlings grown on 3% G medium was statistically the same on both sides. Cell length was quantified using ImageJ. C, Cell division was statistically the same on the sides touching the medium and away from the medium. Student’s t test, P < 0.05, n = 2 biological replicates. Bars = 75 μm.
Involvement of a Two-Component System in Controlling the CK-Induced Root Growth Response
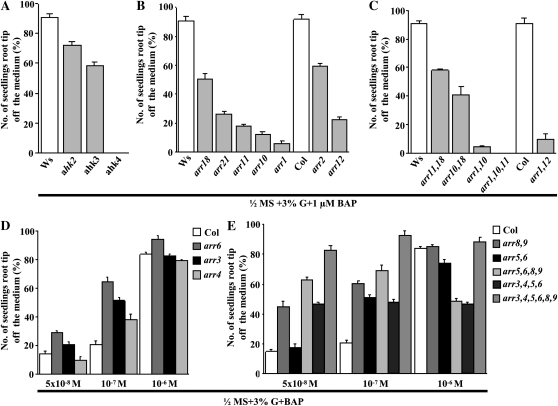
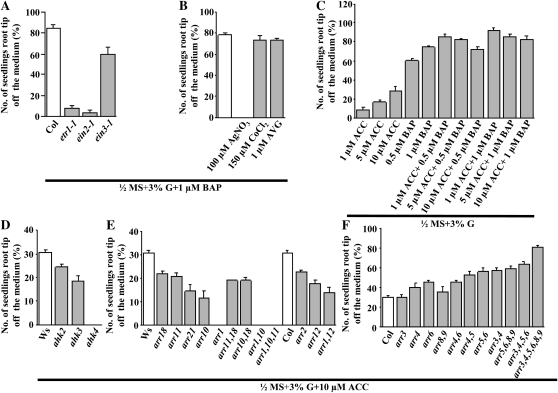
In order to elucidate the mechanism, several mutants in the CK signaling pathway were assayed. Loss of the CK receptor AHK4, but not AHK2 or AHK3, abrogated CK-induced root growth, suggesting that AHK4 mediates this CK response (Fig. 4A). AHK4 is localized predominantly in roots (Higuchi et al., 2004; Nishimura et al., 2004; Riefler et al., 2006).
Figure 4.
Comparison of the CK-induced root growth response of 7-d-old wild-type and CK signaling mutants. Wild-type and mutant seeds were sown on 3% G + BAP-containing half-strength MS medium and grown vertically in the light. The data presented are averages of three biological replicates, with each replicate having 30 seedlings and error bars representing sd. A, Comparison of the CK-induced root growth response of 7-d-old wild-type and CK receptor mutant seedling roots. The reduced CK-induced root growth response was found with ahk2 and ahk3, while no response was found in the ahk4 receptor mutant. B and C, Comparison of the CK-induced root growth response of 7-d-old wild-type and type B ARR signaling mutant seedling roots. The reduced CK-induced root growth response was found in many single and double mutants, while highly reduced CK-induced root growth was found in the arr1,10 double mutant and the arr1,10,11 triple mutant. D and E, Comparison of the CK-induced root growth response of 7-d-old Col and type A ARR signaling mutant seedling roots. Col and mutant seeds were directly sown on 3% G and different concentrations of BAP-containing half-strength MS medium and grown vertically in the light. Among the various lines tested, single mutants arr6 and arr3 and multiple mutants arr8,9, arr5,6,8,9, and arr3,4,5,6,8,9 display higher CK-induced root growth responses even at lower concentrations of BAP. Student’s t test, P < 0.05, n = 3 biological replicates.
Type B ARRs are phosphorylated by AHPs and act as CK-regulated transcription factors (To and Kieber, 2008). Type B ARRs do not act redundantly in this response because null mutations in individual ARR genes conferred different degrees of CK insensitivity. Combined loss of ARR1, ARR10, and ARR11 completely eliminated CK responsiveness, indicating that these three type B ARRs act in concert in this CK response (Fig. 4, B and C). The steady-state levels of type A ARRs are increased by CK; these ARRs negatively regulate CK signal transduction (To and Kieber, 2008). CK signaling is regulated by a mechanism involving proteasomal degradation of certain type A ARRs, which may act to derepress type B ARRs (Ren et al., 2009). Combined loss of type A ARR genes increased CK sensitivity. The arr3,4,5,6,8,9 sextuple and arr5,6,8,9 and arr3,4,5,6 quadruple mutants displayed significantly higher responsiveness, suggesting that these ARR proteins attenuate the CK response in a redundant manner, although not equally, because the single loss of ARR6 had a greater effect than other single arr mutations (Fig. 4, D and E). These results were confirmed in another manner, specifically by determining the angle between the plane of the medium and the growing root axis (Supplemental Fig. S5, A–E). In order to distinguish changes in sensitivity, the angles of only those seedlings in which root tips were off the medium were taken into account.
Elements of Ethylene Signaling Act Downstream of CK Induction of Root Tip Growth
Genetic ablation of the ethylene receptor ETHYLENE RESISTANT1 (ETR1) or the ethylene signal transducer and putative membrane transporter ETHYLENE INSENSITIVE2 (EIN2) lacked CK-induced root growth, suggesting the involvement of an ethylene signal transduction pathway in CK-induced root growth. In contrast, the loss of EIN3, a transcription factor acting downstream of ethylene signal pathways (Yoo et al., 2009), had little effect on CK-induced root growth (Fig. 5A). CK-induced root growth could also be abolished by the ethylene signaling inhibitor but not the ethylene biosynthetic inhibitor aminoethoxyvinylglycine (AVG) and CoCl2 (Fig. 5B). Both ethylene signaling and biosynthesis inhibitors affected overall root growth patterns (Supplemental Fig. S6), but biosynthesis inhibitors could not affect CK-induced root growth. The ethylene precursor ACC alone was able to cause only partial (30% instead of 90%) root tip growth at high concentration (Fig. 5C). Supplementing ACC and BAP together enhanced CK-induced root growth (Fig. 5C). The ethylene-overproducer mutant eto1 exhibited delayed but increased CK-induced root growth only after 10 d in BAP-supplemented medium (Supplemental Fig. S7A). As predicted, incrementally removing type A ARR negative regulators in the CK pathway also magnified the ACC effect on root tip growth to nearly the maximum level, in contrast to positive regulators like CK receptors and type B ARRs (Fig. 5, D–F).
Figure 5.
The role of ethylene signaling and biosynthesis in controlling the CK-induced root growth response. Wild-type and mutant seeds were sown on treatment medium and grown vertically in the light. The data presented are averages of three biological replicates, with each replicate having 30 seedlings and error bars representing sd. A, Comparison of the CK-induced root growth response of 7-d-old Col and ethylene receptor and signaling mutant seedling roots. Col and mutant seeds were sown on 3% G + 1 μm BAP-containing half-strength MS medium. Ethylene receptor mutant etr1-1 and signaling mutant ein2-1 showed reduced CK-induced root growth responses, while the response of ein3-1 was only a little less as compared with the wild type. B, Comparison of the CK-induced root growth response of 7-d-old Col in 3% G + 1 μm BAP-containing half-strength MS medium in the presence of an ethylene signaling inhibitor (AgNO3) and biosynthesis inhibitors (CoCl2 and AVG). The CK-induced root growth was completely abolished in the presence of AgNO3, while no significant reduction was found in the presence of CoCl2 or AVG. C, Comparison of the CK-induced root growth response of 8-d-old Col in 3% G + BAP- and/or ACC-containing half-strength MS medium. Supplementing ACC and BAP together enhanced CK-induced root growth. D to F, Comparison of the CK-induced root growth responses of 7-d-old wild-type and different CK signaling mutant seedling roots in ACC-containing medium. Wild-type and mutant seeds were sown on 3% G + 10 μm ACC-containing half-strength MS medium. The response was reduced in the CK receptor and type B ARR mutants and significantly enhanced in type A ARR multiple mutants. Student’s t test, P < 0.05, n = 3 biological replicates.
These results were confirmed using the root tip angle assay. The angle between the plane of the medium and the growing root axis of only those seedlings in which root tips were off the medium was taken into account (Supplemental Fig. S7B). The root angle was greater in arr3,4,5,6,8,9 mutants treated with ACC as compared with wild-type seedlings treated with ACC (Supplemental Fig. S7C).
CK Derepresses Auxin Signaling in CK-Induced Root Growth
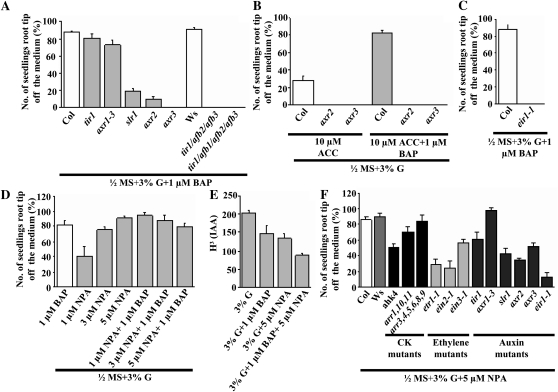
Auxin is classically known to cause differential growth either by itself or in combination with CK (Aloni et al., 2004) or ethylene (Žádníková et al., 2010). To determine any involvement of auxin in this CK-induced root growth response, the effect of CK was analyzed in mutants with altered auxin signaling or transport. AUXIN RESISTANT1 (AXR1) confers altered sensitivity to auxin, ethylene, methyl jasmonate, and CK (Timpte et al., 1995; Tiryaki and Staswick, 2002). Roots carrying the weak allele of axr1-3 had a nearly normal response, as did the null allele in TRANSPORT INHIBITOR RESPONSE1 (TIR1; encoding the F-box protein TIR1); however, combining loss-of-function alleles of tir with other members of this F-box gene family completely eliminated CK-induced root growth (Fig. 6A). Loss of AUXIN RESPONSE3 (AXR3)/INDOLE-3-ACETIC ACID17 (IAA17) abrogated CK-induced root growth, and the same was highly reduced in axr2/iaa7 and solitary root (slr1)/iaa14 mutants (Fig. 6A). These auxin-inducible genes encode for transcription regulators that repress auxin signal transduction (Gray et al., 2001). Auxin decreases the steady-state level of these IAA proteins by shunting them into the proteasomal pathway that includes the F-box proteins TIR1 and four related members, AUXIN SIGNALING F-BOX PROTEIN1 (AFB1) to AFB4, consistent with the loss of the CK response in the tir1/afb triple and quadruple mutants (Fig. 6A). The repressor mutants used here are gain-of-function mutants, and the mutation in them makes these repressors stable such that they are not degraded by auxin and are constitutively active.
Figure 6.
Comparison of the CK-induced root growth response of 7-d-old wild-type, auxin signaling, and transport mutant seedling roots. Wild-type and mutant seeds were sown on treatment medium and grown vertically in the light. The data presented are averages of three biological replicates, with each replicate having 30 seedlings and error bars representing sd. A, Wild-type and auxin signaling mutant seeds were sown on 3% G + 1 μm BAP-containing half-strength MS medium. The auxin signaling mutants, which lead to stability of the auxin signaling repressor protein, led to substantial reduction in the CK-induced root growth response, while no response was found in auxin receptor triple and quadruple mutants. B, Wild-type and mutant seeds were sown on 3% G + ACC and/or BAP-containing half-strength MS medium. ACC alone and ACC + BAP together did not induce root growth in auxin signaling mutants axr2 and axr3 even over an extended period (10 d). C, Wild-type and mutant seeds were sown on 3% G + 1 μm BAP-containing half-strength MS medium. The auxin transport- and root gravitropism-defective mutant eir1 did not display the CK-induced root growth response. D, Wild-type seeds were sown on 3% G + NPA and/or BAP-containing half-strength MS medium. Application of the auxin polar transport inhibitor NPA mimicked the CK induction of the root tip growth response. E, Basipetal auxin transport was measured by applying [3H]IAA to the root apex. The amount of radioactivity was determined in root segments (24-h treatment) at 2 h after application of the radiolabeled synthetic auxin (each replicate having 15 root segments). NPA and CK significantly reduced basipetal auxin transport in the roots. F, Wild-type and mutant seeds were sown on 3% G + 5 μm NPA-containing half-strength MS medium for comparison of the CK-induced root growth response of 7-d-old seedlings. Application of NPA induced root growth in all the mutant lines belonging to the CK, ethylene, and auxin signal transduction pathways. Student’s t test, P < 0.05, n = 3 biological replicates.
Unlike the type A ARR mutants, ACC had no effect on the auxin signaling mutants axr2 and axr3 (Fig. 6B), suggesting that either auxin signaling lies downstream to ethylene signaling or that the derepression of CK signaling as seen in type A ARR mutants is absolutely essential for executing CK-induced root growth. Combined ACC and CK treatment of axr2 and axr3 mutants also did not induce root growth (Fig. 6B), suggesting that an intact auxin signaling pathway is needed to execute CK-induced root growth and that auxin signaling lies downstream of both CK and ethylene signaling pathways. These root growth data were confirmed using the root angle assay (Supplemental Fig. S8A).
The auxin transport- and root gravitropism-defective mutant eir1 did not display the CK-induced root growth response, suggesting a role for auxin transport and gravitropism (Fig. 6C). Loss of PIN1, PIN3, PIN4, PIN7, PGP1, or MULTIPLE DRUG RESISTANCE1 protein did not decrease the CK-induced response (Supplemental Fig. S8B). Application of the auxin polar transport inhibitor 1-N-naphthylphthalamic acid (NPA) mimicked the CK induction of the root tip growth response (Fig. 6D), although, in addition, NPA made roots agravitropic, unlike CK. CK applied with NPA enhanced the CK-induced root growth response but restored gravitropism at lower concentrations of NPA (Supplemental Fig. S8C). NPA also caused more cell elongation at the side touching the medium, similar to BAP-treated roots (Supplemental Fig. S8D). NPA and CK significantly reduced basipetal auxin transport in the roots (Fig. 6E), indicating the involvement of differential auxin distribution in the CK-induced root growth response. Application of NPA induced root growth in all the mutant lines belonging to the CK, ethylene, and auxin signal transduction pathways, suggesting that all these pathways culminate to create an auxin gradient across the root (Fig. 6F).
Disruption of Actin Filament Polymerization Can Induce a CK-Induced Root Growth-Like Response
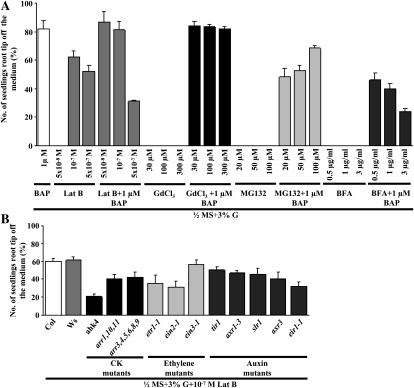
To determine which pathways may be responsible for exhibiting CK-induced root growth, seedlings were treated with inhibitors of various cytological processes in plants: latrunculin B (Lat B) to disrupt actin filament organization, GdCl3 to block calcium channels involved in mechanosensing, MG132 to inhibit proteolysis, and brefeldin A (BFA) to disrupt protein trafficking. The touch response as measured by TCH4::GUS lines was affected by GdCl3, indicating that it was nonetheless active despite any lack of an effect on CK-induced root growth (Supplemental Fig. S9A). Similarly, MG132 and BFA changed the overall root growth pattern (Supplemental Fig. S9B) but did not affect CK-induced root growth. In contrast, the application of Lat B, which disrupts actin polymerization, evokes a pronounced root growth response, suggesting that CK may work by disrupting actin filament assembly (Fig. 7A). It has been shown that differential auxin transport causes asymmetrical cell elongation across the root by disrupting actin filament organization (Rahman et al., 2007). Since we showed that Lat B induced a root growth response even in the eir1 auxin polar transport-defective mutant (Fig. 7B), actin disassembly probably lies downstream of auxin transport.
Figure 7.
Comparison of the CK-induced root growth response of 7-d-old seedling roots grown vertically in the presence of light. The data presented are averages of three biological replicates, with each replicate having 30 seedlings and error bars representing sd. A, Comparison of the CK-induced root growth response of 7-d-old Col seedling roots in the presence of different inhibitors involved in various cytological processes. Wild-type seeds were sown on medium with different inhibitors and 3% G-containing half-strength MS medium with and without 1 μm BAP. CK induced root growth with Lat B but not with GdCl3, MG132, or BFA even in longer periods. B, Wild-type and mutant seeds were sown on 3% G + 10−7 m Lat B-containing half-strength MS medium. Lat B can evoke the CK-induced root growth-like response in all the mutant lines belonging to the CK, ethylene, and auxin signal transduction pathways, even the eir1 auxin polar transport-defective mutant. Student’s t test, P < 0.05, n = 3 biological replicates.
Adaptive Significance for This Signal-Integrated Root Growth Response
The evidence using loss- and gain-of-function mutations in genes encoding elements of ethylene, CK, and auxin signaling indicates that the root tip growth described here integrates many signals in a hierarchical manner. However, it is not clear that this robust phenotype in the laboratory confers fitness to the plant in nature. To address this, we determined if the root tip growth phenotype correlated positively or negatively with a known adaptive response of the root, namely, coiling and waving. Root coiling on a hard surface is an estimate of the spiral-downward root pattern in the soil (Okada and Shimura, 1990; Garbers et al., 1996; Legué et al., 1997; Sack, 1997; Blancaflor et al., 1998; Luschnig et al., 1998; Rutherford et al., 1998; Marchant et al., 1999; Fasano et al., 2001; Rashotte et al., 2001; Massa and Gilroy, 2003a, 2003b; Piconese et al., 2003; Buer and Muday, 2004; Chen et al., 2009). Arabidopsis root waving is essentially a flattened-spiral growth pattern on a firm agar medium, positioned at an angle of less than 90° with respect to the gravity vector (Okada and Shimura, 1990; Simmons et al., 1995; Mullen et al., 1998; Thompson and Holbrook, 2004). The wave pattern results mainly from the interaction of gravitropic and thigmotropic responses (Okada and Shimura, 1990; Thompson and Holbrook, 2004).
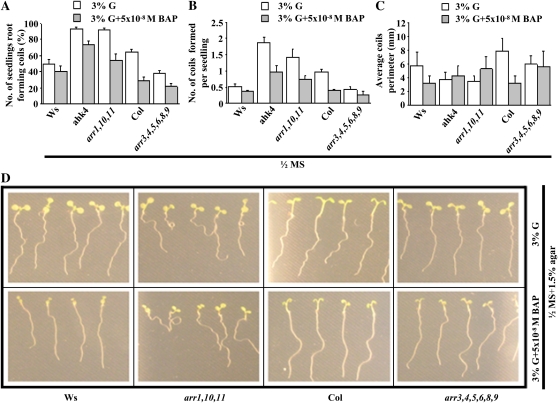
CK receptor (ahk4) and type B ARR (arr1,10,11) mutants, which lack the CK-induced root growth response, displayed (1) more seedlings exhibiting root coils (Fig. 8A), (2) more coils per seedling (Fig. 8B), and (3) tighter coils in horizontal plates (Fig. 8C). The reciprocal relationship was observed for the type A ARR (arr3,4,5,6,8,9) mutant (Fig. 8, A–C), which has an increased CK-induced root growth response. This suggests that CK sensitivity is directly related to the CK-induced root growth response while it is inversely related to root coiling (Fig. 8, A–C). The inverse relationship was also observed for root waving. The type B ARR (arr1,10,11) mutant exhibited coils on slanted plates without CK under normal conditions, while the type A ARR (arr3,4,5,6,8,9) mutant exhibited less waving under similar conditions (Fig. 8D).
Figure 8.
Evidence for a role in fitness: root tip growth negatively correlates with root coiling and waving. A to C, To assay root coiling, seeds were sown on square petri plates containing half-strength MS + 3% G + 0.8% agar with and without 5 × 10−8 m BAP. Plates were kept horizontal for 8 d. Numbers (%) of seedling root-forming coils (A), numbers of coils formed per seedling (B), and coil perimeters (C) represent averages of three biological replicates, with each replicate having at least 15 seedlings and error bars representing sd. Student’s t test, P < 0.05, n = 3 biological replicates. The numbers of seedling root-forming coils and numbers of coils formed per seedling were quantified as described in “Materials and Methods.” The numbers of seedling root-forming coils were expressed as percentages of all roots in the treatment/genotype group. Coil perimeter was quantified using ImageJ. D, To assay root waving, seeds were directly germinated on square petri plates containing half-strength MS + 3% G + 1.5% agar with and without 5 × 10−8 m BAP. Wild-type and mutant seeds were sown and grown vertically for 2 d, and then plates were slanted at 45° for the next 6 d for observation of waving. All images were captured on day 8. The waving response was quantified as described in “Materials and Methods.” The panel shows results of one replicate. The experiment was repeated twice, yielding similar results. An inverse relationship was found between CK-induced root growth and the coiling/waving response.
DISCUSSION
Asymmetrical root growth as described here implies either more cell elongation or more cell division at the side touching the medium as compared with the one facing away from the medium. While CK controls both cell division and elongation, asymmetrical root growth under these conditions is solely due to differential elongation of cells exposed to CK (Fig. 3). This is further supported by the requirement for actin bundling, which is known to be critical for elongation and less involved in cell division (Baluška et al., 2001).
Hexokinase-Mediated Glc Signaling Promotes CK-Induced Root Growth, Probably Indirectly
CK-induced root growth is potentiated by Glc mediated through hexokinase (HXK), since the CK effect is reduced in the gin2 mutant at lower concentrations of Glc. Sugar signaling in Arabidopsis also involves the heterotrimeric G protein complex (Ullah et al., 2002, 2003; Chen et al., 2003; Chen and Jones, 2004; Jones and Assmann, 2004; Huang et al., 2006; Temple and Jones, 2007; Trusov et al., 2007), but it is not yet determined how much cross talk between G proteins and HXK-based sugar perception occurs. In this aspect of CK-induced root growth, Glc imposes either an indirect effect, such as through altered metabolism, or acts as a signal through HXK1. Loss of HXK1 in gin2 confers increased sensitivity, while cytokinin resistant1 (gene not known) confers decreased sensitivity toward exogenous CK (Moore et al., 2003; Laxmi et al., 2006).
There is considerable overlap or cross talk between Glc signaling and other hormones. For example, Glc interacts with CK and auxin to control the cell cycle (Riou-Khamlichi et al., 2000; Hartig and Beck, 2006). Glc affects ethylene signaling (León and Sheen, 2003) and auxin signaling/transport (Mishra et al., 2009); thus, the mechanism of the Glc effect on CK signaling here may be direct or by modulating ethylene and auxin signaling/transport. Since ACC and IAA at similar concentrations did not cause a similar response as CK did in Glc-containing medium (Fig. 2), the promoting role of Glc on CK-root repulsion may be a result of a specific interaction between Glc and CK.
The Mechanism Revealed through Genetic Dissection
Among the CK receptors, AHK4 is the most important for this response. AHK4 is, to date, the most salient receptor for responses to exogenous CK application (Higuchi et al., 2004; Nishimura et al., 2004; Riefler et al., 2006). Type B ARR mutants displayed a reduced CK-induced root response, with ARRs ARR1, ARR10, ARR11, and ARR12 playing the most important roles. Type A ARRs are negative regulators of this response; mutants exhibited enhanced CK-induced root growth, with ARR3, -4, -5, -6, -8, and -9 having prominent but redundant functions. Some type A ARRs are degraded by CK through the proteasomal pathway, which is an integrator of a number of signaling pathways such as light and circadian rhythm (To and Kieber, 2008; Ren et al., 2009; Vierstra, 2009). Hence, it would be interesting to determine if CK-induced growth is also affected by other environmental or endogenous cues via the control of proteasomal degradation of type A ARRs.
CK-induced root growth requires the ethylene receptor ETR1 and a putative membrane transporter, EIN2, but minimally the transcription factor EIN3 in the ethylene signaling pathway. CK has been previously shown to be involved in ethylene biosynthesis by stabilizing the ethylene biosynthesis enzyme ACS5 (Vogel et al., 1998a, 1998b; Chae et al., 2003; Hansen et al., 2009). A number of CK-related responses have been shown to be mediated by ethylene (Cary et al., 1995; Golan et al., 1996; Tanaka et al., 2006). However, in the root growth response described here, increased ethylene production had only a small effect. This suggests that either the ethylene effect is above its signaling threshold under the experimental conditions used here (and that further reduction of ethylene is insufficient to reduce signaling) or that components of the ethylene pathway, including ethylene perception by ETR1, are recruited into the CK pathway. Another possibility is that an ethylene gradient is critical. The observation that increased ethylene has an effect when attenuation by the type A response regulators is relieved favors the former possibility. CK-induced change in root elongation has also been shown to be mediated by ethylene signaling, although the CK effect on root meristem size involves ethylene-independent modulation of asymmetric auxin distribution (Růžička et al., 2009). CK-induced inhibition of the elongation and formation of lateral roots and stimulated swelling of root tips are counteracted by the inhibition of ethylene biosynthesis as well as signaling in pea (Pisum sativum), suggesting that the CK effect on root growth occurs via the modulation of ethylene biosynthesis/signaling (Bertell and Eliasson, 1992).
Perturbation of the major flux of auxin in the root and disruption of auxin signal transduction, in particular, auxin-regulated proteasome-mediated degradation, had major effects on CK-induced growth (Fig. 6). Auxin and CK signaling interact in several ways (Aloni et al., 2006; Zhao, 2008; Chapman and Estelle, 2009; Kuderová and Hejátko, 2009; Moubayidin et al., 2009). For example, CK controls the steady-state level of type A ARRs through the proteasomal pathway, suggesting similar mechanisms for auxin and CK signaling (Ren et al., 2009). Degradation of AUX/IAA gene family member proteins is important for the CK-induced root growth response, since stabilization of these proteasome-targeted proteins inhibits CK-induced growth in the root (Fig. 6). Since ACC alone or in combination with CK does not exhibit CK-induced root growth in the AUX/IAA mutants, ethylene signaling seems to work upstream of auxin, and degradation of AUX/IAA repressor proteins seems to be essential for the CK-induced root growth response. Differential auxin polar transport may disrupt actin filament organization and cause asymmetric growth across the root, since Lat B can evoke the CK-induced root growth-like response in the eir1 (auxin polar transport-defective) mutant.
A Testable Model
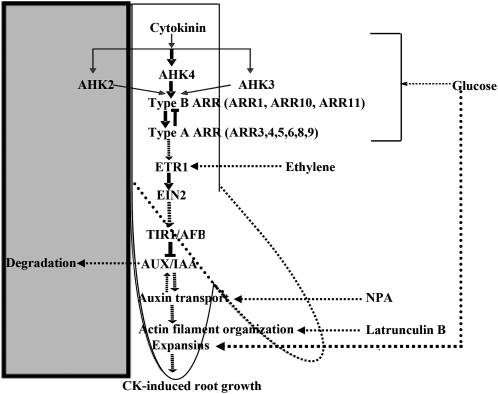
A testable model based on these findings and published literature is presented in Figure 9. CK leads to the CK-induced root tip growth response (Fig. 1, A–C), which is potentiated by Glc in a HXK-influenced (Fig. 1D), G protein-independent manner (Supplemental Fig. S1). Asymmetrical exposure of CK at the root tip promotes cell elongation without affecting cell division (Fig. 3). This CK-induced root growth response involves the two-component system employing AHKs and type A and type B ARRs (Fig. 4). Glc may affect the response either via affecting the expression of different CK signaling elements (Supplemental Fig. S2) or increasing the expression of expansin gene family members (Supplemental Fig. S4A). Ethylene signaling components ETR1 and EIN2 work downstream of CK to transduce the signal further (Fig. 5A). Ethylene signaling but not biosynthesis is important (Fig. 5B) for promoting this response. Auxin works further downstream, since auxin signaling gain-of-function mutants as well as loss-of-function alleles of tir with other members of this F box gene family either dramatically reduced or completely lacked CK-induced root growth (Fig. 6A). Auxin works downstream of both CK and ethylene, since auxin signaling gain-of-function mutants do not show this response when CK, ethylene, or both are applied together (Fig. 6B). Previously, various studies have also placed auxin signaling downstream of ethylene signaling (Roman et al., 1995; Stepanova et al., 2005; Chilley et al., 2006; Růžička et al., 2007; Swarup et al., 2007). The auxin transport- and root gravitropism-defective mutant eir1 did not display a CK-induced root growth response, suggesting a role for auxin transport and gravitropism (Fig. 6C). Differential distribution of auxin lies downstream of all the other factors mentioned above, since NPA can evoke the CK-induced root growth-like response in all CK, ethylene, and auxin signaling mutants (Fig. 6F). Differential auxin transport may cause differential cell elongation across the root by disrupting actin filament organization (Maisch and Nick, 2007; Rahman et al., 2007), since Lat B can induce the CK-induced root growth-like response even in the eir1 auxin polar transport-defective mutant (Fig. 7B). Alternatively, the whole signaling cascade may lead to differential expression of expansin gene family members, causing more cell elongation at the side touching the medium.
Figure 9.
Testable model explaining the interactions among various signals involved in the CK-induced root growth response. CK leads to the CK-induced root tip growth response (Fig. 1, A–C), which is potentiated by Glc. Asymmetrical exposure of CK at the root tip promotes cell elongation without affecting cell division (Fig. 3). This CK-induced root growth involves the two-component system employing AHKs and type A and type B ARRs (Fig. 4). Glc may affect the response either by affecting the expression of different CK signaling elements (Supplemental Fig. S2) or increasing the expression of expansin gene family members (Supplemental Fig. S4A). Ethylene signaling components ETR1 and EIN2 work downstream of CK to transduce the signal further (Fig. 5A). Ethylene signaling but not biosynthesis is important (Fig. 5B) for promoting this response. Auxin signaling is also involved, since auxin receptor and signaling mutants dramatically reduced CK-induced root growth (Fig. 6A). Auxin signaling works downstream of both CK and ethylene, since auxin signaling gain-of-function mutants do not show this response when CK, ethylene, or both are applied together (Fig. 6B). The auxin transport- and root gravitropism-defective mutant eir1 did not display the CK-induced root growth response, suggesting a role for auxin transport and gravitropism (Fig. 6C). Differential distribution of auxin lies downstream of all the other factors mentioned above, since NPA can promote this root growth in all CK, ethylene, and auxin signaling mutants (Fig. 6F). Differential auxin transport may cause differential cell elongation across the root by disrupting actin filament organization, since Lat B can induce root growth even in the eir1 auxin polar transport-defective mutant (Fig. 7B).
The Relevance of CK for Optimal Root Architecture
Optimal root architecture provides mechanical support to aerial tissue, for uptake of water and nutrients from the soil, and also maximizes the contact with growth-promoting microorganisms. Root architecture is determined by nutrient and water gradients, positive gravitropism, negative thigmotropism, and root circumnutation. Root plasticity is important for the optimal positioning of plants with respect to the changing microenvironment and facilitates growth of the root via the easiest route through the soil. Among the other factors, changes in auxin, ethylene, gravity signaling, or alteration in cell wall properties cause altered root directional growth (Okada and Shimura, 1990; Simmons et al., 1995; Mullen et al., 1998; Rutherford et al., 1998; Gibson, 2000; Buer et al., 2003; Hobe et al., 2003; Pandey et al., 2008; Chen et al., 2009). The cytoskeleton plays a crucial role in optimal root architecture, as is evident by the root phenotypes of seedlings harboring mutations in genes encoding various microtubule-interacting proteins such as spr1/sku6, spr2, wvd2-1, lefty1, and lefty2 (Thitamadee et al., 2002; Nakajima et al., 2004; Sedbrook et al., 2004; Perrin et al., 2007). Root waving and coiling are two important adaptive responses involved in regulating root architecture and thus contributing to seedling fitness in local environmental conditions. The wild-type seedlings treated with CK and different CK mutants did not show either proper root coiling or waving, suggesting that CK plays a prominent role in controlling Arabidopsis root tropic/differential growth responses. Moreover, an inverse correlation between CK-induced root growth and root coiling-waving implicates an indirect role of this response in seedling adaptability to local environments. Further work is required to dissect out the exact molecular mechanism of this response, which will eventually shed light on how roots respond to exogenous cues, which integrate with endogenous factors to modulate architecture and eventually determine seedling fitness under natural conditions.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The following Arabidopsis (Arabidopsis thaliana) seed stocks were obtained from the Arabidopsis Biological Resource Center at Ohio State University: ahk2 (AT5G35750, CS6561); ahk3 (AT1G27320, CS6562); ahk4 (AT2G01830, CS6563); arr1 (AT3G16857, CS6368); arr2 (AT4G16110, CS6975); arr10 (AT4G31920, CS6369); arr11 (AT1G67710, CS6370); arr12 (AT2G25180, CS6978); arr18 (AT5G58080, CS6371); arr21 (AT5G07210, CS6372); arr1,10 (AT3G16857/AT4G31920, CS6987); arr1,12 (AT3G16857/AT2G25180, CS6981); arr10,18 (AT4G31920/AT5G58080, CS6991); arr11,18 (AT1G67710/AT5G58080, CS6992); arr1,10,11 (AT3G16857/AT4G31920/AT1G67710, CS6993); arr3 (AT1G59940, CS25265); arr4 (AT1G10470, CS25266); arr6 (AT5G62920, CS25268); arr3,4 (AT1G59940/AT1G10470, CS25271); arr5,6 (AT3G48100/AT5G62920, CS25272); arr4,5 (AT1G10470/AT3G48100, CS25273); arr4,6 (AT1G10470/AT5G62920, CS25274); arr8,9 (AT2G41310/AT3G57040, CS25275); arr3,4,5,6 (AT1G59940/AT1G10470/AT3G48100/AT5G62920, CS25276); arr5,6,8,9 (AT3G48100/AT5G62920/AT2G41310/AT3G57040, CS25277); arr3,4,5,6,8,9 (AT1G59940/AT1G10470/AT3G48100/AT5G62920/AT2G41310/AT3G57040, CS25279); tir1-1 (AT3G62980, CS3798); axr1-3 (AT1G05180, CS3075); axr3-1 (AT1G04250, CS57504); eir1-1 (AT5G57090, CS8058); etr1-1 (AT1G66340, CS237); ein2-1 (AT5G03280, CS3071); ein3-1 (AT3G20770, CS8052); eto1 (AT3G51770, CS3072); gin2-1 (AT4G29130, CS6383); exp1 (AT1G69530, SALK_010506C); expA1 (AT1G69530, CS25050); exp10 (AT1G26770, SALK_053326C); exp13 (AT3G03220, SALK_093913C); exp14 (AT5G56320, SALK_089449C); and expA9 (AT5G02260, CS25024). The following seed stock was obtained from the European Arabidopsis Stock Centre: axr2-1 (AT3G23050, CS3077). The following lines were obtained from the original published sources: tir1/afb2-3/afb3-4 (AT3G62980/AT3G26810/AT1G12820) and tir1/afb1-3/afb2-3/afb3-4 (AT3G62980/AT4G03190/AT3G26810/AT1G12820; Dharmasiri et al., 2005); pin1-1 (AT1G73590; Gälweiler et al., 1998); pin3-4 (AT1G70940; Friml et al., 2002b); pin4-3 (AT2G01420; Friml et al., 2002a); pin7-2 (AT1G23080; Friml et al., 2003); mdr1-1 (AT3G28860; Noh et al., 2001); mdr1-101 (AT3G28860; Lin and Wang, 2005); pgp1-100 (AT2G36910; Lin and Wang, 2005); rgs1-1 (AT3G26090; Chen et al., 2003); and gpa1-1 and gpa1-2 (AT2G26300; Ullah et al., 2001). TCH4::GUS (AT5G57560; Xu et al., 1995) and slr-1 (AT4G14550; Fukaki et al., 2002) mutant lines were generously provided by Dr. Janet Braam at Rice University and Dr. Hidehiro Fukaki at the Nara Institute of Science and Technology, respectively. All mutant lines were in the ecotype Columbia (Col) background except the following: ahk2; ahk3; ahk4; arr1; arr10; arr11; arr18; arr21; arr1,10; arr10,18; arr11,18; arr1,10,11; tir1/afb2/afb3; tir1/afb1/afb2/afb3; mdr1-1; gpa1-1; and gpa1-2 were derived from the ecotype Wassilewskija (Ws) background. The gin2 mutant was in the Landsberg erecta (Ler) background. The pinl-1 mutant was in the Enkheim-2 background.
Seeds were surface sterilized and imbibed at 4°C for 48 h. Seed germination was carried out in a climate-controlled growth room under long-day conditions (16 h of light and 8 h of darkness, 80 μmol m−2 s−1 light intensity) at 22°C ± 2°C temperature. All chemicals were purchased from Sigma except agar and 2-isopentenyladenine, which were purchased from Himedia. 3-[5(n)-3H]Indolylacetic acid was purchased from GE Healthcare. The following were prepared as 10−2 m stock solutions in dimethyl sulfoxide: BAP, BFA, 2-isopentenyladenine, IAA, MG132, epibrassinolide, GA3, ABA, Lat B, and NPA. Kinetin and trans-zeatin were prepared as 10−2 m stock solutions in 1 n NaOH. AVG, ACC, CoCl2, AgNO3, and GdCl3 were prepared as sterile 10−2 m aqueous stock solutions. 5-Bromo-4-chloro-3-indolyl-β-d-glucuronic acid was prepared as a 100 mg L−1 stock solution in N,N-dimethylformamide. Propidium iodide (PI) was prepared as a sterile 10 mg mL−1 aqueous stock solution.
CK-Induced Root Growth Assay
Imbibed seeds were grown vertically on square (120 × 120 mm) petri plates containing 0.5× Murashige and Skoog medium buffered with 0.5× MES, supplemented with 3% (w/v) G (pH 5.7) and 0.8% (w/v) agar except where indicated otherwise. For some experiments, different concentrations of Glc (also without Glc) and various sugars instead of Glc were also used. Hormones, other chemicals, and solvents were added after autoclaving. For experiments testing the effect of medium supplements/hormones on the CK-induced root growth response, seeds were directly sown on square petri plates containing treatment medium (half-strength MS with and without Glc and/or BAP and/or other supplements) as mentioned separately and grown vertically in a climate-controlled growth room (16 h of light/8 h of dark, 80 μmol m−2 s−1 light intensity, and 22°C ± 2°C temperature). To determine the CK-induced root growth response in agar-free medium, 5-d-old wild-type seedlings grown vertically on half-strength MS medium in the light were transferred to square petri plates containing Whatman filter paper saturated with half-strength MS (liquid) + 3% G + 1 μm BAP for 2 d. To determine the role of the root tip in signal perception, 5-d-old wild-type seedlings grown vertically on half-strength MS medium in the light with and without intact root tips (a 0.5-mm-long section was dissected and discarded) were transferred to square petri plates containing half-strength MS + 3% G + 1 μm BAP medium solidified with 0.8% agar for 3 d. For the dark-grown seedlings, seeds on plates were first exposed to 12 h of light, then the plates were wrapped with aluminum foil and placed in the chamber as described for other treatments above. The instantaneous CK-induced root growth response was calculated by determining the number of primary roots deviating away (with root tips off) from the medium and expressed as the percentage of all roots in the treatment/genotype group. The percentage of seedlings with the root tip off the medium represents the average of three biological replicates, with each replicate sample having at least 30 seedlings and error bars representing sd. These results were confirmed using the root tip angle assay. In this assay, the angle between the plane of the medium and the growing root axis was measured by carefully placing the seedlings on transparent tape so that their deviation angle was preserved. Thereafter, digital images were captured using a Nikon Coolpix digital camera and angles were quantified using ImageJ (http://rsb.info.nih.gov/ij/). The angle data presented are averages of 10 seedlings with root tips off the medium, and error bars represent sd. For all experiments, Student’s t test with paired two-tailed distribution was used for statistical analysis. In all experiments, plates were sealed with gas-permeable tape to avoid ethylene accumulation. All the photographs were taken either using a Nikon Coolpix digital camera or a Nikon Coolpix digital camera connected with a Nikon SMZ1500 Stereo-Zoom microscope. All end point analyses were taken on day 7 unless otherwise specified, although plates were observed for longer periods up to 10 d.
Laser Confocal Scanning Microscopy
To study cell length and cell division, 7-d-old Col seedlings vertically grown on half-strength MS + 3% G and half-strength MS + 3% G + 1 μm BAP or 3 μm NPA medium were used. For cell length measurements, the root sections were observed using the differential image contrast optics of a TCS SP2 (AOBS) laser confocal scanning microscope (Leica Microsystems) and images were captured (bar = 75 μm). Cell length was quantified using ImageJ (http://rsb.info.nih.gov/ij/). The cell length data are represented as averages of two biological replicates, with each replicate having 10 seedlings and error bars representing sd. To determine cell length, roots were mounted on a calibrated glass slide and imaged with a Nikon Eclipse E100 compound microscope using a 40× objective, and digital images were captured using a Nikon Coolpix digital camera. Cell division was observed after staining with PI. For labeling of the cell wall with PI, seedlings were incubated in 10 μg mL−1 PI solution for 30 s before confocal imaging analysis. For imaging PI, the 514-nm line of the argon laser was used for excitation, and emission was detected at 600 nm. The number of cells in the division zone was the average of two biological replicates each having 10 seedlings, and error bars represent sd. The laser and pinhole settings of the confocal microscope were kept identical among different treatments. For all experiments, Student’s t test with paired two-tailed distribution was used for statistical analysis.
Auxin Transport Assay
Root basipetal auxin transport was measured as described previously (Shin et al., 2005) with the following modifications. Col seeds were sown on half-strength MS medium grown vertically in the light. Five-day-old seedlings were transferred to 3% G-, 3% G + 1 μm BAP-, 3% G + 5 μm NPA-, or 3% G + 1 μm BAP + 5 μm NPA-containing half-strength MS medium for 24 h. Agar blocks of 1-mm diameter containing 7.7 × 10−8 m [3H]IAA were applied immediately next to the root tips. Plates were incubated vertically in the dark for 2 h. Subsequently, a 0.5-mm section of the root close to the agar block was dissected and discarded. Two consecutive 2-mm root segments below the incision line were then collected separately and placed into two scintillation vials each containing 5 mL of scintillation fluid; these were subsequently incubated in the dark for 16 h. Radioactivities in these two pools of root segments (15 root segments per vial) were measured using a Perkin-Elmer Tri-Carb 2800TR liquid scintillation analyzer. The radioactivity in these segments was the average of three biological replicates, and error bars represent sd. Student’s t test with paired two-tailed distribution was used for statistical analysis.
Coiling and Waving Responses
To assay root coiling, seeds were sown on square petri plates containing 0.5× Murashige and Skoog medium buffered with 0.5× MES supplemented with 3% G (pH 5.7), with and without 5 × 10−8 M BAP, and solidified with 0.8% agar. Plates were kept horizontal for 8 d. The number of seedling roots forming coils and the number of coils formed per seedling were quantified by inspection with a dissecting microscope, while coil perimeter was quantified using ImageJ (http://rsb.info.nih.gov/ij/). All images were captured with a Nikon Coolpix digital camera. Roots were then pulled out from the medium, and the lengths of the roots were measured using a ruler (Supplemental Fig. S10). The number of roots forming coils was expressed as the percentage of all roots in the treatment/genotype group. The percentage of seedling roots forming coils represents the average of three biological replicates, with each replicate having at least 15 seedlings and error bars representing sd. The number of coils formed per seedling and coil perimeter represent averages of three biological replicates, with each replicate having at least 15 seedlings and error bars representing sd. The root length data presented are averages of at least 25 seedlings, and error bars represent sd. For all experiments, Student’s t test with paired two-tailed distribution was used for statistical analysis. For waving, the above medium was used except that 1.5% agar was used for solidification. The seedlings were vertically grown for 2 d, and then plates were slanted at 45° for the next 6 d for observation of waving. The waving response was observed by visual inspection. All images were captured with a Nikon Coolpix digital camera.
Gene Expression Analysis: Quantitative Real-Time PCR
For quantitative real-time PCR analysis, the imbibed Col seeds were germinated and grown vertically on square petri plates with 0% G-, 3% G-, or 3% G + 1 μm BAP-containing half-strength MS medium buffered with 0.5× MES and solidified with 0.8% agar in the light. Root tissue from 7-d-old seedlings was flash frozen in liquid nitrogen, and the mRNA was prepared from frozen tissue using the RNeasy Plant Mini Kit (Qiagen) following the manufacturer’s protocol. The RNA was quantified and tested for quality before it was used for subsequent analyses. First-strand cDNA was synthesized by reverse transcription using 4 μg of total RNA in a 40-μL reaction volume using the high-capacity cDNA Reverse Transcription kit (Applied Biosystems). Diluted cDNA samples (5 μm) were used for quantitative real-time PCR analysis, with 5 μm of each primer mixed with SYBR Green PCR Master Mix as per the manufacturer’s instructions. Primers for all the candidate genes were designed preferentially from the 3′ end of the gene using Primer Express version 3.0 (PE Applied Biosystems) with default parameters. The reaction was carried out on 96-well optical reaction plates (Applied Biosystems) using the ABI Prism 7900 HP fast real-time PCR system (Applied Biosystems). To normalize the variance among samples, 18S rRNA was used as the internal control. The mRNA levels for each candidate gene in different samples were determined using the ΔΔCT method (Livak and Schmittgen, 2001). The values represent averages of the two biological replicates (each with three technical replicates), and error bars represent sd. For all experiments, Student’s t test with paired two-tailed distribution was used for statistical analysis. The primer sequences for all the genes tested have been included in Supplemental Figure S11.
GUS Histochemical Staining
Five-day-old TCH4::GUS seedlings grown in half-strength MS medium were subsequently treated with 0% G- or 0% G + 300 μm GdCl3-containing liquid half-strength MS medium in a climate-controlled growth room for 4 h in the light. GUS activities were then determined by incubating the seedlings at 37°C in a GUS staining solution [sodium phosphate buffer, pH 7, 0.1 m; K3Fe(CN)6, 0.5 mm; K4Fe(CN)6, 0.5 mm; EDTA, 50 mm; 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, 1 mg mL−1] for 3 to 4 h. The seedlings were then kept in 70% ethanol for the removal of chlorophyll. The seedlings were observed with a Nikon SMZ1500 Stereo-Zoom microscope, and photographs were taken with a Nikon Coolpix digital camera connected to a Nikon SMZ1500 Stereo-Zoom microscope. The experiment was repeated twice, with each replicate having 10 seedlings and yielding similar results.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Comparison of CK-induced root growth responses of Col, Ws, the HXK-independent signaling mutant rgs1-1, and gpa1-1 and gpa1-2 mutant seedling roots on day 7.
Supplemental Figure S2. Effect of Glc on the expression of genes involved in CK signaling as revealed by real-time gene expression analysis.
Supplemental Figure S3. Comparison of CK-induced growth of 7-d-old wild-type roots under different conditions and in the presence of different media.
Supplemental Figure S4. The relative expression of EXP gene family members.
Supplemental Figure S5. Angle between the plane of the medium and the growing root axis
Supplemental Figure S6. Wild-type (Col) seeds were directly sown on 3% G + AgNO3/CoCl2/AVG-containing half-strength MS medium and grown vertically in the presence of light.
Supplemental Figure S7. The comparison of CK-induced root growth of Col and ethylene overproducer mutant eto1.
Supplemental Figure S8. The angle between the plane of the medium and the growing root axis of 7-d-old wild-type and auxin signaling mutants.
Supplemental Figure S9. Five-day-old TCH4::GUS seedlings first grown in half-strength MS medium were subsequently incubated in 0% G- and 0% G + 300 μm GdCl3-containing liquid half-strength MS medium for 4 h.
Supplemental Figure S10. To assay root coiling, wild-type and mutant seeds were sown on square petri plates containing half-strength MS + 3% G medium, with and without 5 × 10−8 M BAP, and solidified with 0.8% agar.
Supplemental Figure S11. List of primers used for PCR.
Acknowledgments
We are grateful to the staffs at the National Institute of Plant Genome Research confocal imaging facility and central instrument facility (real-time PCR division) for assistance and help.
References
- Aloni R, Aloni E, Langhans M, Ullrich CI. (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot (Lond) 97: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R, Langhans M, Aloni E, Ullrich CI. (2004) Role of cytokinin in the regulation of root gravitropism. Planta 220: 177–182 [DOI] [PubMed] [Google Scholar]
- Baluška F, Jasik J, Edelmann HG, Salajová T, Volkmann D. (2001) Latrunculin B-induced plant dwarfism: plant cell elongation is F-actin-dependent. Dev Biol 231: 113–124 [DOI] [PubMed] [Google Scholar]
- Bertell G, Eliasson L. (1992) Cytokinin effects on root growth and possible interactions with ethylene and indole-3-acetic acid. Physiol Plant 84: 255–261 [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116: 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker KS, Schwarz J, Garrett MB, Jones AM. (2010) Glucose attenuation of auxin-mediated bimodality in lateral root formation is partly coupled by the heterotrimeric G protein complex. PLoS ONE 5: e12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Muday GK. (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16: 1191–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Sukumar P, Muday GK. (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140: 1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Wasteneys GO, Masle J. (2003) Ethylene modulates root-wave responses in Arabidopsis. Plant Physiol 132: 1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary AJ, Liu W, Howell SH. (1995) Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol 107: 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ. (2003) The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15: 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M. (2009) Cytokinin and auxin intersection in root meristems. Genome Biol 10: 210.1–210.5 [Google Scholar]
- Chen J-G, Jones AM. (2004) AtRGS1 function in Arabidopsis thaliana. Methods Enzymol 389: 338–350 [DOI] [PubMed] [Google Scholar]
- Chen J-G, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovski DP. (2003) A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 301: 1728–1731 [DOI] [PubMed] [Google Scholar]
- Chen Z, Noir S, Kwaaitaal M, Hartmann HA, Wu MJ, Mudgil Y, Sukumar P, Muday G, Panstruga R, Jones AM. (2009) Two seven-transmembrane domain MILDEW RESISTANCE LOCUS O proteins cofunction in Arabidopsis root thigmomorphogenesis. Plant Cell 21: 1972–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilley PM, Casson SA, Tarkowski P, Hawkins N, Wang KLC, Hussey PJ, Beale M, Ecker JR, Sandberg GK, Lindsey K. (2006) The POLARIS peptide of Arabidopsis regulates auxin transport and root growth via effects on ethylene signaling. Plant Cell 18: 3058–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. (2008) A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M. (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9: 109–119 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao T-h, Gilroy S. (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13: 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, et al. (2002a) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108: 661–673 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. (2002b) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M. (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruére J, Bernasconi P, Söll D. (1996) A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J 15: 2115–2124 [PMC free article] [PubMed] [Google Scholar]
- Gibson SI. (2000) Plant sugar-response pathways: part of a complex regulatory web. Plant Physiol 124: 1532–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan A, Tepper M, Soudry E, Horwitz BA, Gepstein S. (1996) Cytokinin, acting through ethylene, restores gravitropism to Arabidopsis seedlings grown under red light. Plant Physiol 112: 901–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Hansen M, Chae HS, Kieber JJ. (2009) Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J 57: 606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig K, Beck E. (2006) Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol (Stuttg) 8: 389–396 [DOI] [PubMed] [Google Scholar]
- Hass C, Lohrmann J, Albrecht V, Sweere U, Hummel F, Yoo SD, Hwang I, Zhu T, Schäfer E, Kudla J, et al. (2004) The response regulator 2 mediates ethylene signalling and hormone signal integration in Arabidopsis. EMBO J 23: 3290–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyl A, Schmülling T. (2003) Cytokinin signal perception and transduction. Curr Opin Plant Biol 6: 480–488 [DOI] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, et al. (2004) In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA 101: 8821–8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobe M, Müller R, Grünewald M, Brand U, Simon R. (2003) Loss of CLE40, a protein functionally equivalent to the stem cell restricting signal CLV3, enhances root waving in Arabidopsis. Dev Genes Evol 213: 371–381 [DOI] [PubMed] [Google Scholar]
- Huang J, Taylor JP, Chen J-G, Uhrig JF, Schnell DJ, Nakagawa T, Korth KL, Jones AM. (2006) The plastid protein THYLAKOID FORMATION1 and the plasma membrane G-protein GPA1 interact in a novel sugar-signaling mechanism in Arabidopsis. Plant Cell 18: 1226–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sheen J. (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413: 383–389 [DOI] [PubMed] [Google Scholar]
- Jones AM, Assmann SM. (2004) Plants: the latest model system for G-protein research. EMBO Rep 5: 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Schaller GE. (2010) The perception of cytokinin: a story 50 years in the making. Plant Physiol 154: 487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderová A, Hejátko J. (2009) Spatiotemporal aspect of cytokinin-auxin interaction in hormonal regulation of the root meristem. Plant Signal Behav 4: 156–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, et al. (2007) Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19: 3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmi A, Paul LK, Raychaudhuri A, Peters JL, Khurana JP. (2006) Arabidopsis cytokinin-resistant mutant, cnr1, displays altered auxin responses and sugar sensitivity. Plant Mol Biol 62: 409–425 [DOI] [PubMed] [Google Scholar]
- Lee BH, Johnston R, Yang Y, Gallavotti A, Kojima M, Travençolo BA, Costa LdF, Sakakibara H, Jackson D. (2009) Studies of aberrant phyllotaxy1 mutants of maize indicate complex interactions between auxin and cytokinin signaling in the shoot apical meristem. Plant Physiol 150: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. (1997) Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol 114: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P, Sheen J. (2003) Sugar and hormone connections. Trends Plant Sci 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Lin R, Wang H. (2005) Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport. Plant Physiol 138: 949–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch J, Nick P. (2007) Actin is involved in auxin-dependent patterning. Plant Physiol 143: 1695–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE. (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28: 67–77 [DOI] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ. (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa GD, Gilroy S. (2003a) Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. Plant J 33: 435–445 [DOI] [PubMed] [Google Scholar]
- Massa GD, Gilroy S. (2003b) Touch and gravitropic set-point angle interact to modulate gravitropic growth in roots. Adv Space Res 31: 2195–2202 [DOI] [PubMed] [Google Scholar]
- Mishra BS, Singh M, Aggrawal P, Laxmi A. (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 4: e4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Moubayidin L, Di Mambro R, Sabatini S. (2009) Cytokinin-auxin crosstalk. Trends Plant Sci 14: 557–562 [DOI] [PubMed] [Google Scholar]
- Mullen JL, Turk E, Johnson K, Wolverton C, Ishikawa H, Simmons C, Söll D, Evans ML. (1998) Root-growth behavior of the Arabidopsis mutant rgr1: roles of gravitropism and circumnutation in the waving/coiling phenomenon. Plant Physiol 118: 1139–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B. (2011) Generic signal-specific responses: cytokinin and context-dependent cellular responses. J Exp Bot 62: 3273–3288 [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J. (2007) Advances in cytokinin signaling. Science 318: 68–69 [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J. (2008) Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Furutani I, Tachimoto H, Matsubara H, Hashimoto T. (2004) SPIRAL1 encodes a plant-specific microtubule-localized protein required for directional control of rapidly expanding Arabidopsis cells. Plant Cell 16: 1178–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. (2004) Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 16: 1365–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Shimura Y. (1990) Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 250: 274–276 [DOI] [PubMed] [Google Scholar]
- Pandey S, Monshausen GB, Ding L, Assmann SM. (2008) Regulation of root-wave response by extra large and conventional G proteins in Arabidopsis thaliana. Plant J 55: 311–322 [DOI] [PubMed] [Google Scholar]
- Perilli S, Moubayidin L, Sabatini S. (2010) The molecular basis of cytokinin function. Curr Opin Plant Biol 13: 21–26 [DOI] [PubMed] [Google Scholar]
- Pernisová M, Klíma P, Horák J, Válková M, Malbeck J, Souček P, Reichman P, Hoyerová K, Dubová J, Friml J, et al. (2009) Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc Natl Acad Sci USA 106: 3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RM, Wang Y, Yuen CYL, Will J, Masson PH. (2007) WVD2 is a novel microtubule-associated protein in Arabidopsis thaliana. Plant J 49: 961–971 [DOI] [PubMed] [Google Scholar]
- Piconese S, Tronelli G, Pippia P, Migliaccio F. (2003) Chiral and non-chiral mutations in Arabidopsis roots grown on the random positioning machine. J Exp Bot 54: 1909–1918 [DOI] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI. (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50: 514–528 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK. (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13: 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Liang Y, Deng Y, Chen Q, Zhang J, Yang X, Zuo J. (2009) Genome-wide comparative analysis of type-A Arabidopsis response regulator genes by overexpression studies reveals their diverse roles and regulatory mechanisms in cytokinin signaling. Cell Res 19: 1178–1190 [DOI] [PubMed] [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T. (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18: 40–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH. (2000) Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol 20: 4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R, Gallois P, Masson PH. (1998) Mutations in Arabidopsis thaliana genes involved in the tryptophan biosynthesis pathway affect root waving on tilted agar surfaces. Plant J 16: 145–154 [DOI] [PubMed] [Google Scholar]
- Růžička K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E. (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K, Simásková M, Duclercq J, Petrásek J, Zazímalová E, Simon S, Friml J, Van Montagu MC, Benková E. (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci USA 106: 4284–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD. (1997) Plastids and gravitropic sensing. Planta (Suppl 1) 203: S63–S68 [DOI] [PubMed] [Google Scholar]
- Sedbrook JC, Ehrhardt DW, Fisher SE, Scheible W-R, Somerville CR. (2004) The Arabidopsis sku6/spiral1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. Plant Cell 16: 1506–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Shin H-S, Guo Z, Blancaflor EB, Masson PH, Chen R. (2005) Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases. Plant J 42: 188–200 [DOI] [PubMed] [Google Scholar]
- Simmons C, Migliaccio F, Masson P, Caspar T, Söll D. (1995) A novel root gravitropism mutant of Arabidopsis thaliana exhibiting altered auxin physiology. Physiol Plant 93: 790–798 [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17: 2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S. (2006) Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J Exp Bot 57: 2259–2266 [DOI] [PubMed] [Google Scholar]
- Temple BRS, Jones AM. (2007) The plant heterotrimeric G-protein complex. Annu Rev Plant Biol 58: 249–266 [DOI] [PubMed] [Google Scholar]
- Thitamadee S, Tuchihara K, Hashimoto T. (2002) Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417: 193–196 [DOI] [PubMed] [Google Scholar]
- Thompson MV, Holbrook NM. (2004) Root-gel interactions and the root waving behavior of Arabidopsis. Plant Physiol 135: 1822–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpte C, Lincoln C, Pickett FB, Turner J, Estelle M. (1995) The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J 8: 561–569 [DOI] [PubMed] [Google Scholar]
- Tiryaki I, Staswick PE. (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130: 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To JPC, Kieber JJ. (2008) Cytokinin signaling: two-components and more. Trends Plant Sci 13: 85–92 [DOI] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Chen J-G, Jones AM, Botella JR. (2007) Heterotrimeric G protein γ subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell 19: 1235–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen J-G, Temple B, Boyes DC, Alonso JM, Davis KR, Ecker JR, Jones AM. (2003) The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15: 393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen J-G, Wang S, Jones AM. (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen J-G, Young JC, Im K-H, Sussman MR, Jones AM. (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292: 2066–2069 [DOI] [PubMed] [Google Scholar]
- Vierstra RD. (2009) The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10: 385–397 [DOI] [PubMed] [Google Scholar]
- Vogel JP, Schuerman P, Woeste K, Brandstatter I, Kieber JJ. (1998a) Isolation and characterization of Arabidopsis mutants defective in the induction of ethylene biosynthesis by cytokinin. Genetics 149: 417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ. (1998b) Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA 95: 4766–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Schmülling T. (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12: 527–538 [DOI] [PubMed] [Google Scholar]
- Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam J. (1995) Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell 7: 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y, Sheen J. (2009) Emerging connections in the ethylene signaling network. Trends Plant Sci 14: 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žádníková P, Petrásek J, Marhavý P, Raz V, Vandenbussche F, Ding Z, Schwarzerová K, Morita MT, Tasaka M, Hejátko J, et al. (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137: 607–617 [DOI] [PubMed] [Google Scholar]
- Zhao Y. (2008) The role of local biosynthesis of auxin and cytokinin in plant development. Curr Opin Plant Biol 11: 16–22 [DOI] [PubMed] [Google Scholar]