Abstract
Nonvolatile terpenoid phytoalexins occur throughout the plant kingdom, but until recently were not known constituents of chemical defense in maize (Zea mays). We describe a novel family of ubiquitous maize sesquiterpenoid phytoalexins, termed zealexins, which were discovered through characterization of Fusarium graminearum-induced responses. Zealexins accumulate to levels greater than 800 μg g−1 fresh weight in F. graminearum-infected tissue. Their production is also elicited by a wide variety of fungi, Ostrinia nubilalis herbivory, and the synergistic action of jasmonic acid and ethylene. Zealexins exhibit antifungal activity against numerous phytopathogenic fungi at physiologically relevant concentrations. Structural elucidation of four members of this complex family revealed that all are acidic sesquiterpenoids containing a hydrocarbon skeleton that resembles β-macrocarpene. Induced zealexin accumulation is preceded by increased expression of the genes encoding TERPENE SYNTHASE6 (TPS6) and TPS11, which catalyze β-macrocarpene production. Furthermore, zealexin accumulation displays direct positive relationships with the transcript levels of both genes. Microarray analysis of F. graminearum-infected tissue revealed that Tps6/Tps11 were among the most highly up-regulated genes, as was An2, an ent-copalyl diphosphate synthase associated with production of kauralexins. Transcript profiling suggests that zealexins cooccur with a number of antimicrobial proteins, including chitinases and pathogenesis-related proteins. In addition to zealexins, kauralexins and the benzoxazinoid 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one-glucose (HDMBOA-glucose) were produced in fungal-infected tissue. HDMBOA-glucose accumulation occurred in both wild-type and benzoxazine-deficient1 (bx1) mutant lines, indicating that Bx1 gene activity is not required for HDMBOA biosynthesis. Together these results indicate an important cooperative role of terpenoid phytoalexins in maize biochemical defense.
Maize (Zea mays) is one of the most significant and widely grown crop plants. According to the U.S. Department of Agriculture National Agricultural Statistics Service, 144,000 square miles of maize were planted in the United States alone in 2011 (Capehart and Allen, 2011). A number of phytopathogenic fungi successfully colonize maize plants. Predominant among these are the genera Fusarium and Aspergillus that produce a variety of toxic secondary metabolites, mycotoxins, which adversely affect both animals and humans. Mycotoxin-contaminated feed is detrimental to livestock health and reproduction, resulting in significant economic loss to farmers (Chassy, 2010). Contamination of food with mycotoxins is endemic in developing countries, and long-term chronic exposure to these toxins can cause numerous cancers and immune suppression. Acute high-level exposure can be lethal (Wagacha and Muthomi, 2008).
Despite the importance of maize and the prevalence of fungal-produced mycotoxin contamination, few of the direct chemical defenses employed by maize against fungal pathogens have been well characterized. An exception are the benzoxazinoid hydroxamic acids such as 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA). In the 1950s benzoxazinoid products were identified as factors for resistance against Fusarium rot in rye (Secale cereale) and leaf feeding by Ostrinia nubilalis in maize (Virtanen and Hietala, 1955; Smissman et al., 1957). The biosynthetic pathway through which indole, generated by an indole-3-glycerol phosphate lyase (BENZOXAZINE-DEFICIENT1 [BX1]), is decorated by a series of cytochrome P450 enzymes to yield benzoxazinoids has been elucidated since in detail (Frey et al., 1997, 2009; Melanson et al., 1997; Niemeyer, 2009). Benzoxazinoids largely have been considered phytoanticipins; defensive molecules accumulated constitutively as inactive glucosylated precursors that are liberated instantly in damaged tissue through the action of β-glucosidases (Morant et al., 2008; Niemeyer, 2009). More recently these defense chemicals have also been shown to be an induced response to biotic stress. For example, Spodoptera littoralis herbivory resulted in increased expression of the gene encoding BX1 and accumulation of free DIMBOA that was attributed to de novo synthesis (Erb et al., 2009). The benzoxazinoid 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one-Glc (HDMBOA-Glc) also accumulates in response to treatment with jasmonic acid (JA), pathogen infection, and herbivory (Oikawa et al., 2001, 2004). Recent research demonstrated that an endogenous peptide regulator of disease resistance in maize, ZmPep1, activates Bx1 gene expression and strongly induces HDMBOA-Glc accumulation through de novo synthesis (Huffaker et al., 2011).
Terpenoids are another well-characterized family of inducible defense chemicals, but in maize have been associated primarily with indirect defenses against herbivores. Insect attack elicits production of a suite of maize volatiles including terpenes such as β-ocimene, linalool, β-farnesene, β-caryophyllene, nerolidol, and β-bergamotene as predominant constituents (Turlings et al., 1995). These volatiles mediate tritrophic interactions; contributing to defense indirectly through attraction of parasitoids and predators that are natural enemies of maize herbivores (Turlings et al., 1990). Through a number of elegant studies, enzymes involved in production of these terpenes have been characterized and shown to be important to indirect defenses. Foliar production of α-bergamotene and β-farnesene by TERPENE SYNTHASE10 (TPS10) was sufficient to attract Cotesia marginiventris, a parasitoid of lepidopteran pests (Schnee et al., 2006). Similarly, volatile β-caryophyllene formed in roots through the activity of TPS23 attracted entomopathogenic nematodes that prey upon the root herbivore Diabrotica virgifera virgifera (Rasmann et al., 2005; Köllner et al., 2008a; Degenhardt et al., 2009).
Nonvolatile terpenoids comprise major classes of phytoalexins in many plants and play direct defensive roles through their antimicrobial activities (Brooks and Watson, 1991; Gershenzon and Dudareva, 2007). Sesquiterpenoid phytoalexins such as gossypol, capsidiol, and rishitin are important inducible defenses in cotton (Gossypium hirsutum) and solanaceous plants, respectively (Stoessla et al., 1976; Chappell and Nable, 1987; Desjardins et al., 1992; Delannoy et al., 2005). Similarly, rice (Oryza sativa) plants produce an array of diterpenoid phytoalexins that contribute to resistance against fungal diseases such as rice blast, caused by Magnaporthe grisea (Peters, 2006; Toyomasu, 2008). Despite the wide distribution of terpenoid phytoalexins in the plant kingdom, nonvolatile chemical defense in maize was believed to be mainly benzoxazinoid mediated. However, the recent discovery that maize produces diterpenoid phytoalexins, termed kauralexins, indicates that nonvolatile terpenoids are also important components of maize pathogen defense (Schmelz et al., 2011).
Inoculation of maize stalks with Rhizopus microsporus revealed the dominant inducible diterpenoids ent-kaur-19-al-17-oic acid and ent-kaur-15-en-19-al-17-oic acid (Schmelz et al., 2011). These acidic ent-kaurane-related phytoalexins, termed kauralexin A3 and B3, respectively, accumulated at the plant-pathogen interface in response to a number of maize fungal pathogens and displayed antimicrobial activity against both R. microsporus and the causative agent of anthracnose stalk rot, Colletotrichum graminicola. Kauralexin production was also induced in stems by O. nubilalis herbivory and in bioassays exhibited antifeedant activity. Accumulation of kauralexins was preceded by strongly elicited expression of the gene encoding the ent-copalyl diphosphate synthase ANTHER EAR2 (AN2). An2 expression is Fusarium graminearum inducible and the gene is an ortholog of rice genes encoding ent-copalyl diphosphate synthases that supply precursors to diterpenoid phytoalexins (Harris et al., 2005). A hypothesized function of fungal-induced ent-copalyl diphosphate production in maize is the increased supply of precursors for kauralexin biosynthesis (Schmelz et al., 2011).
Our current analysis of molecular responses induced in maize by inoculation with F. graminearum reveals a novel family of nonvolatile terpenoid phytoalexins. The structure and activity of a number of these acidic sesquiterpenoids, termed zealexins, is presented. The hydrocarbon skeleton of the zealexins closely resembles β-macrocarpene, and zealexin accumulation is preceded by strong induction of the genes encoding the terpene synthases TPS6 and TPS11. These terpene synthases are closely related and, in the presence of farnesyl pyrophosphate, both generate β-macrocarpene as the dominant product (Köllner et al., 2008b). Tps6 and Tps11 have been shown previously to be among the most highly elicited genes following Ustilago maydis infection (Basse, 2005; Dohlemann et al., 2008). Recent characterization of plants silenced in the expression of Tps6 and Tps11 revealed their increased susceptibility to U. maydis (van der Linde et al., 2011). Although genes encoding TPS6 and TPS11 are important contributors to maize resistance, the end product terpenoid metabolites responsible for this activity have not yet been identified. Consistent with conversion of β-macrocarpene to nonvolatile compounds, both genes are inducible in leaves, but volatile terpene products were not detected (Köllner et al., 2008b). We propose zealexins as candidates for TPS6 and TPS11-produced defenses and consider their protective roles in maize with respect to benzoxazinoids, kauralexins, and transcripts encoding antimicrobial proteins.
RESULTS
Zealexins Are a Family of Pathogen-Inducible Acidic Sesquiterpenoid Phytoalexins in Maize
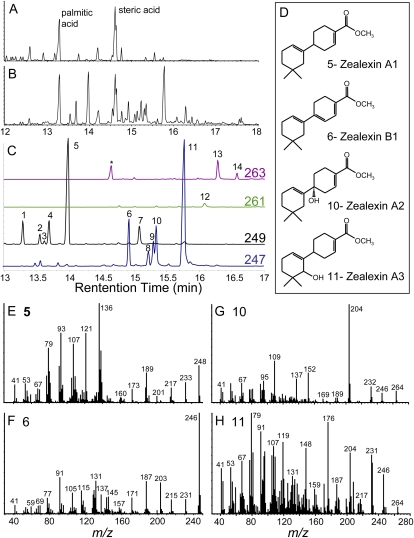
In maize stems inoculated with the stalk-rotting mycotoxigenic fungus F. graminearum, we previously observed a strong induction of kauralexins (Schmelz et al., 2011). Using metabolic profiling based on carboxylic acid methylation and vapor phase extraction coupled with gas chromatography/chemical ionization-mass spectrometry [GC/(+)CI-MS], these infected tissue samples were found to also contain a large number of additional unknown terpenoids (Schmelz et al., 2004). Following mock inoculation consisting of damage + water, abundant phytochemicals in this analysis include fatty acid methyl esters (Fig. 1A); however, following fungal infection many novel acidic sesquiterpenoids begin to dominate the chromatogram (Fig. 1B). Using extracted ions from the GC/CI-MS profile, 14 seemingly related induced compounds could be routinely detected (Fig. 1C). To identify these compounds, a large-scale purification of fungal-infected tissue was performed and resulted in isolation of the four most abundant sesquiterpenoids (analytes 5, 6, 10, and 11). Structural elucidation via NMR (Supplemental Tables S1–S4) revealed that all four were closely related to 4′,5,5-trimethyl-1,1′-bi(cyclohexane)-1,3′-diene, termed β-macrocarpene, from which we used the previously assigned NMR numbering scheme of carbon atoms (Cool, 2005). We term the C15 carboxylic acid of β-macrocarpene zealexin A1 (Fig. 1, C and D). In analytes 10 and 11, termed zealexin A2 and A3, respectively, additional modifications of zealexin A1 include hydroxylations at C1 and C8 (Fig. 1, C and D). Analyte 6, termed zealexin B1, likewise contains a C15 carboxylic acid and an additional C1-C6 double bond, resulting in a conjugated 1,1′,3′-triene system (Fig. 1, C and D). Diagnostic electron ionization (EI) spectra of the zealexin A1-A3, B1, and the remaining 10 unknown sesquiterpenoids all contain detectable [M]+ molecular ions (Fig. 1, E–H; Supplemental Fig. S1).
Figure 1.
Identity and GC/MS spectra of zealexin A1-A3 and B1 as methyl esters. A, Damage. B, Damage plus F. graminearum-inoculated (106 spores mL−1) maize stem samples after 48 h, analyzed as GC/(+)CI-MS total ion chromatograms (TIC). Predominant fatty acid methyl esters are labeled as palmitic and steric acid. C, Expanded GC/(+)CI-MS selected ion trace of predominant fungal-induced sesquiterpenoids as methyl esters. Analytes 1 to 5, denoted by [M+H]+ 249 ions, are unmodified sesquiterpene acids. For analyte 6, a partially unsaturated sesquiterpene acid, 247 is the parent [M+H]+ ion. As oxygenated acidic sesquiterpenoids, analytes 7 and 8 to 11 are denoted by 249 [M+H-H20]+ ions and 247 [M+H-H20]+ ions, respectively. Analytes 12 to 14 are denoted by 261 and 263 [M+H]+ ions, consistent with partially unsaturated, oxygenated acidic sesquiterpenoids. D, Zealexin structures deduced by NMR. EI spectra (mass-to-charge ratio) of: Zealexin A1 (E); analyte 5, Zealexin B1 (F); analyte 6, Zealexin A2 (G); analyte 10, and Zealexin A3 (H); analyte 11. In each section (A–C and E–H), the y axis denotes relative abundance of ions.
In reference to Figure 1C, low levels of an additional desaturated sesquiterpene acid methyl ester (245 [M+H]+ ion) cochromatographing with oleic acid (methyl ester, 297 [M+H]+ ion) was also detected at 14.55 min. In partially purified fractions, this compound displays the predominant EI mass-to-charge ratio (relative intensity) fragments [M]+ 244 (55), 229 (21), 201 (17), 188 (20), 129 (100), and 115 (14). With potential similarity, a 60-ng sample of compound 12-methyl ester displayed several one-dimensional 1H-NMR resonances in C6D6 consistent with the aromatic β-macrocarpene derivative previously described as compound 1 (Suzuki et al., 2007). The 1H-NMR spectrum was referenced to a residual proton signal in C6D6 at δ 7.16 ppm. Tentatively termed zealexin C2, this compound displayed AA’, BB’ pattern aromatic signals at δ 8.24 (2H, dm, J = 8.4 Hz) and δ 7.40 (2H, dm, J = 8.4 Hz), a signal attributed to olefinic proton at δ 5.77 (1H, t, J = 3.9 Hz), a signal for oxymethine proton at δ 3.78 (1H, d, J = 7.0 Hz), two methyl signals at δ 1.00 (3H, s) and δ 0.74 (3H, s), and methyl signal of methyl ester at δ 3.53 (3H, s). Following future confirmation, predicted aromatic zealexins with either no oxidation or an additional C8 hydroxylation could be reasonably assigned as zealexin C1 and C2, respectively.
Zealexins Are Strongly Induced by Most Fungal Inoculations, Except Anthracnose Stalk Rot
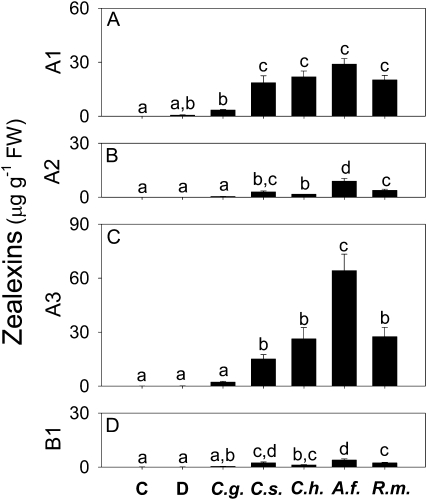
To examine whether zealexins are produced specifically in response to virulent pathogens such as C. graminicola and Cochliobolus heterostrophus or whether they are a more general response to fungi that are not maize stem pathogens such as R. microsporus, Colletotrichum sublineolum, and Aspergillus flavus, inoculated stems were analyzed after 24 h. Exposure to C. heterostrophus, R. microsporus, C. sublineolum, and A. flavus all resulted in accumulation of zealexins A1-3 and B1, whereas inoculation with C. graminicola elicited an attenuated response that was not statistically different from mock inoculation (Fig. 2, A–D). Treatment with A. flavus induced zealexin A1 accumulation to the same levels as other fungi, but elicited significantly higher levels of zealexins A2 and A3. Damaged stems that were mock inoculated with water showed a small mean increase in zealexin A1, but this change was not statistically significant. As with elicitation by F. graminearum, zealexin A1 and A3 accumulated to higher levels than did zealexin A2 and B1 (Figs. 1C and 2, A–D).
Figure 2.
With the exception of a maize specialist (C. graminicola), zealexins accumulate in response to an array of fungal pathogens. Average (n = 4, ±sem) of zealexin A1 (A), zealexin A2 (B), zealexin A3 (C), and zealexin B1 (D) levels in stems 24 h after plants were untreated (control, C), or damaged and treated with either water (D), or a water suspension of the following phytopathogenic fungi; C. graminicola (C.g.), C. sublineolum (C.s.), C. heterostrophus (C.h.), A. flavus (A.f.), and R. microsporus (R.m.) at a concentrated inoculum (1 × 107 spores mL−1). Within plots different letters (a–d) represent significant differences (all ANOVAs, P < 0.0001; Tukey test corrections for multiple comparisions, P < 0.05).
Accumulation of Zealexins Is Preceded by Expression of Tps6 and Tps11 Genes
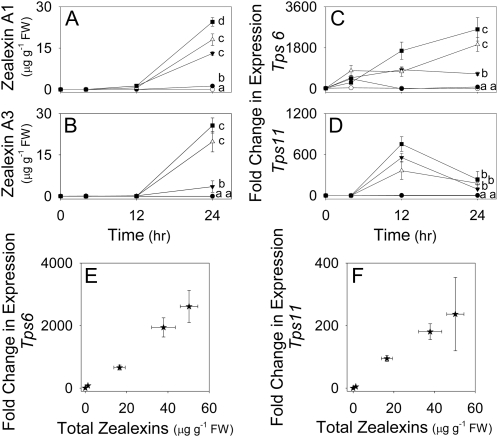
Given the similarity of zealexins and β-macrocarpene, we examined expression of these candidate pathway genes in response to fungal inoculation. The Tps6 and Tps11 genes are very similar, thus we developed quantitative real-time PCR (qRT-PCR) primers specific to each and validated this specificity through restriction digest of the subsequent amplicons using BmgBI, which cleaves the amplicon derived from Tps6 but not Tps11 (Supplemental Fig. S2; Supplemental Table S5). A time-course study of zealexin levels and abundance of transcripts encoding TPS6 and TPS11 revealed that all accumulated in response to fungal inoculation. Only trace levels of zealexin A1 and A3 were detectable within the first 12 h; however, significant increases resulted at 24 h (Fig. 3, A and B). Relative transcript abundance of Tps6 began to increase within 4 h of exposure to each fungus (Fig. 3C). F. graminearum and A. flavus induced significantly greater levels of both zealexin A3 and Tps6 transcripts as compared to R. microsporus (Fig. 3, B and C). Increased expression of Tps11 also occurred in response to fungal inoculation, but was more transient than for Tps6; not increasing until 12 h and then declining within 24 h (Fig. 3D). Zealexin A1 and A3 were consistently the most highly induced sesquiterpenoid phytoalexins. Thus, unless otherwise indicated, we simplified many estimations of pathway activation by defining total zealexins as a combination of these two metabolites. When the individual treatment averages of total zealexins were compared to gene expression levels at 24 h, a positive relationship between zealexin accumulation and expression of both Tps6 and Tps11 was observed (Fig. 3, E and F).
Figure 3.
Relationship of pathogen-induced zealexin concentrations with Tps6 and Tps11 transcript levels. Average (n = 4; ±sem). A, Zealexin A1. B, Zealexin A3 levels in maize stems experiencing no treatment (○), damage plus water (•), or damage plus water suspensions of R. microsporus (▾, 107 spores mL−1), A. flavus (△, 107 spores mL−1), or F. graminearum (106 spores mL−1) at time 0 or 4, 12- and 24-h postinoculation. C, Tps6. D, Tps11 corresponding average (n = 4; ±sem) qRT-PCR fold change of transcripts as compared to levels of Tps6 in control stems. E, Tps6. F, Tps11 relationship between fungal-induced fold change in gene expression and total zealexins at 24 h. Within plots, different letters (a–d) represent significant differences at 24 h (all ANOVAs, P < 0.0001; Tukey test corrections for multiple comparisons, P < 0.05).
Fungal-Induced Accumulation of Zealexins and Transcripts Encoding TPS6 and TPS11 Is Dose Dependent and Displays Coregulation with Kauralexins
To understand if quantitative dose-response relationships exist between applied fungal inoculum and defense activation, we examined the accumulation of total zealexins and transcripts for both Tps6 and Tps11 in response to F. graminearum and A. flavus at 48 h. Levels of both zealexin A1 and A3 increased with the application of increasing numbers of fungal spores (Fig. 4, A and B). In response to F. graminearum, both zealexin A1 and A3 reached maximum observed levels following application of 1 × 106 spores mL−1 (Fig. 4, A and B). In response to A. flavus, the average concentration of zealexin A1 and A3 continued to increase with application of up to 1 × 107 spores mL−1. Likewise, transcript accumulation for both Tps6 and Tps11 also increased with inoculations of increasing spore concentrations (Fig. 4, D and E). To examine the relationship of zealexins to other fungal-induced maize defenses, we also studied kauralexin levels (Schmelz et al., 2011). As for the zealexins, increased accumulation of total kauralexins also was observed with increasing quantities of applied F. graminearum and A. flavus spores (Fig. 4C). Maximal kauralexin induction in response to both F. graminearum and A. flavus occurred at the same inoculum levels as for maximal zealexin induction (Fig. 4, A–C). Accumulation of An2 transcript also increased with the increasing inoculum of F. graminearum or A. flavus spores (Fig. 4F).
Figure 4.
Dose-response relationships between fungal-induced terpenoid phytoalexins and fold change in candidate terpene synthase transcripts. Average (n = 4; ±sem). A, Zealexin A1. B, Zealexin A3. C, Total kauralexin levels in damaged stems treated with either water (D) or with water suspensions of increasing concentrations of F. graminearum (▪) or A. flavus (△) spores after 48 h. D, Tps11. E, Tps6. F, An2 corresponding average (n = 4; ±sem) qRT-PCR fold change in transcript abundance 48 h after fungal inoculation. G, β-Macrocarpene. H, Total zealexin relationship to fungal-induced fold change in Tps6 expression. I, Relationship between qRT-PCR fold change in transcript abundance of Tps6 and An2. J, Relationship between fungal-induced total zealexins and total kauralexins. Plots G to J represent all treatment groups from the corresponding 48-h F. graminearum and A. flavus dose-response experiment, and thus general trends. Within plots different letters (a–e) represent significant differences (all ANOVAs, P < 0.0001; Tukey test corrections for multiple comparisons, P < 0.05).
Comparisons of all inoculum concentrations and treatments revealed many positive relationships between fungal-induced production of zealexins, kauralexins, and candidate pathway Tps gene transcript levels. Fungal-induced Tps6 transcript levels and total zealexins were again found to display positive relationships (Figs. 4H and 3E). Tps6 transcript levels also displayed positive relationships with the concentrations of free β-macrocarpene in stem tissue (Fig. 4G). This relationship is expected if β-macrocarpene is to be considered as a logical candidate sesquiterpene precursor to the zealexins. Consistent with the coregulation of terpenoid defenses, Tps6 expression and An2 expression also displayed parallel increases (Fig. 4I). This trend was also well matched by the positive relationship between total zealexin and total kauralexin accumulation (Fig. 4J).
Genes Encoding TPS6/11 and Antifungal Protein Defenses Are Among the Most Highly Up-Regulated Transcripts in Response to F. graminearum
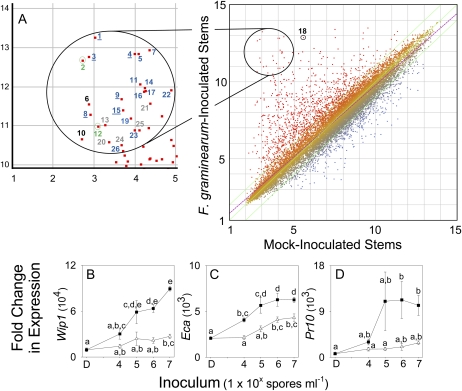
To gain insights as to how F. graminearum-induced zealexin accumulation corresponds with predicted protein-based defenses, we used microarray analyses to examine gene expression in inoculated and mock-inoculated stems. Using the Affymetrix GeneChip maize genome array, which contains probe sets representing 13,339 genes, we examined transcripts from samples 48 h postinoculation. This revealed 2-fold or greater changes in probe intensity for 226 genes at 99% confidence, 1,787 genes at 95%, and 3,265 genes at 90% confidence. Among these genes, 26 were up-regulated 100-fold or more in F. graminearum-inoculated stems. Tps6/11 and An2 corresponded to the second and 12th most highly induced probe sets, and increased 995- and 240-fold, respectively (Fig. 5A; Supplemental Table S6). The majority of the other 24 most highly induced probe sets corresponded to genes encoding directly antimicrobial defenses. These included genes encoding six chitinases, a β-1,3 glucanase, a zeamatin-like antifungal protein, and the pathogenesis-related proteins PR10 and PR5. Other highly induced defense genes encoded proteinase inhibitors, a Ser endopeptidase, a peroxidase, and a polygalacturonase inhibitor. Non-PR genes included those encoding an O-methyl transferase, GST 2, and the receptor complex component BRI-1 ASSOCIATED KINASE (BAK1).
Figure 5.
Transcripts encoding Tps6/11 and antimicrobial proteins are coregulated following F. graminearum infection. A, Scatter plot of average probe intensity (Log2 scale) for Affymetrix maize microarray chips hybridized with cDNA from mock-inoculated stems versus F. graminearum-inoculated stems. Probes corresponding to genes up-regulated in F. graminearum-inoculated stems are plotted in red whereas the down-regulated are plotted in blue. Linear regression (r2 = 0.901) is indicated in magenta; green lines indicate threshold of fold change >2. Student’s t test with Benjamini-Hochberg FDR analysis was applied. The 26 genes up-regulated >100-fold in response to F. graminearum (Ps < 0.015; t > 12.4) are numbered in descending order. Tps6/11 was the second-most up-regulated probe set, whereas An2 was 12th (green numbers). Among the other 24 genes, the majority were pathogenesis-protein related (PR, blue numbers); including chitinases (underlined;1, 3, 4, 8, 9, 15), Pr10 (5), Pr5 (11), proteinase inhibitors (6, 16, 23), Ser endopeptidase (14), peroxidase (17), β-1,3 glucanase (19), zeamatin-like protein (22), and polygalacturonase inhibitor (26). Non-PR genes (black numbers) included Bak1 (6), an O-methyl transferase (10) and GST 2 (18). Genes of unknown function are marked with gray numbers (13, 20, 21, 24, 25). B to D, Average (n = 4; ±sem) qRT-PCR fold change in transcript levels of genes encoding antimicrobial proteins at 48 h in damaged stems treated with either water (D) or water containing increasing inoculum concentrations of F. graminearum (▪) or A. flavus (△) spores after 48 h. B, Bowman-Birk proteinase inhibitor Wip1. C, Endochitinase A (Eca). D, Pathogenesis-response protein 10 (Pr10). Within each plot different letters (a–e) represent significant differences (all ANOVAs, P < 0.001; Tukey test corrections for multiple comparisons, P < 0.05).
To ensure validity of the microarray data, we independently evaluated expression of three genes encoding antimicrobial proteins through qRT-PCR, and analyzed their response to inoculation with F. graminearum and A. flavus. These antimicrobial proteins included WIP1, a Bowman-Birk wound-inducible proteinase inhibitor; ECA, an inducible endochitinase; and PR10, a pathogenesis response protein that contributes to resistance against mycotoxigenic fungi. In each case, accumulation of transcripts encoding these defense proteins was induced by infection with F. graminearum (Fig. 5, B–D). Transcripts corresponding to Pr10 and Eca reached maximal levels of induction in response to applications of 1 × 106 spores mL−1; the same concentration at which maximal accumulation of zealexins, kauralexins, and transcripts encoding TPS6 and AN2 occurred. Average expression of Wip1 continued to increase in response to inoculation levels as high as 1 × 107 spores mL−1 F. graminearum. Expression of Eca and Wip1 were moderately responsive to A. flavus, with maximal induced transcript levels occurring in response to 1 × 107 spores mL−1; however, Pr10 transcript was only marginally increased.
Both Zealexins and Benzoxazinoid Defenses Are Triggered by Fungal Infection
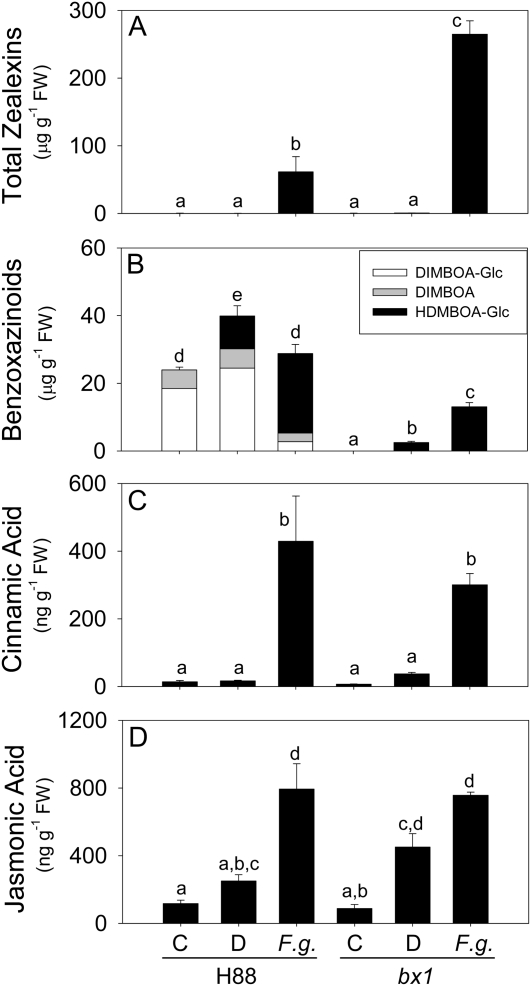
To understand the production of zealexins in relation to benzoxazinoids, we quantified both in response to F. graminearum at 48 h. The bx1 inbred mutant has a lesion in the indole-glycerol lyase (BX1) that catalyzes production of indole as the first committed step in benzoxazinoid biosynthesis (Frey et al., 1997; Melanson et al., 1997). In an attempt to examine these relationships in the presence and absence of benzoxazinoids, fungal-induced metabolites in bx1 were compared to the corresponding H88 inbred parent line. Not surprisingly, zealexins accumulated in both lines when they were inoculated with F. graminearum; however, bx1 displayed a significant 4.3-fold greater total zealexin induction than H88. As predicted from the literature, untreated H88 plants contained a pool of DIMBOA and glucosylated DIMBOA (DIMBOA-Glc) whereas these benzoxazinoid metabolites were absent in untreated bx1 (Fig. 6B; Hamilton, 1964; Frey et al., 1997; Melanson et al., 1997). After damage and mock inoculation with water, total benzoxazinoids increased in H88 and HDMBOA-Glc became a prominent constituent. In F. graminearum-inoculated H88 plants, there was no increase in total benzoxazinoid content above the undamaged controls, but most of the detected benzoxazinoid pool was in the form of HDMBOA-Glc rather than DIMBOA or DIMBOA-Glc (Fig. 6B). Unexpectedly, benzoxazinoids were also inducible in bx1 stems but only in the form of HDMBOA-Glc and not DIMBOA or DIMBOA-Glc. In bx1 stems, HDMBOA-Glc was significantly induced by F. graminearum to 3.7-fold greater levels than mock inoculation (Fig. 6B). Despite the F. graminearum-induced levels of HDMBOA-Glc in bx1, total benzoxazinoid levels in this mutant remained significantly lower than those of similarly treated H88. Thus while not devoid of benzoxazinoids during pathogen attack, bx1 remains accurately classifiable as hydroxamic acid deficient (Hamilton, 1964). In contrast to these altered patterns in total zealexins and benzoxazinoids, the induced levels of other defense-related metabolites and phytohormones were identical. As a precursor to phenylpropanoid defenses, cinnamic acid levels increased to the same levels in both bx1 and H88 lines following inoculation (Fig. 6C). Similarly, F. graminearum-induced levels of JA also did not differ between these lines (Fig. 6D). Together, these results are suggestive of compensatory induced zealexin production in bx1 following F. graminearum inoculation.
Figure 6.
Zealexin and benzoxazinoid defenses are concurrently induced by fungal infection. Average levels (n = 4; ±sem) of defense metabolites in H88 and the H88-derived bx1 mutant line. Stems were either untreated (C), or damaged and treated with water (D) or water containing 1 × 106 spores mL−1 F. graminearum (F.g.). A, Total zealexins. B, Total measured benzoxazinoid hydroxamic acids. C, Cinnamic acid. D, JA. Within each plot different letters (a–d) represent significant differences (all ANOVAs, P < 0.0001; Tukey test corrections for multiple comparisions, P < 0.05).
Concurrent Production of JA and Ethylene Promotes Zealexin Accumulation
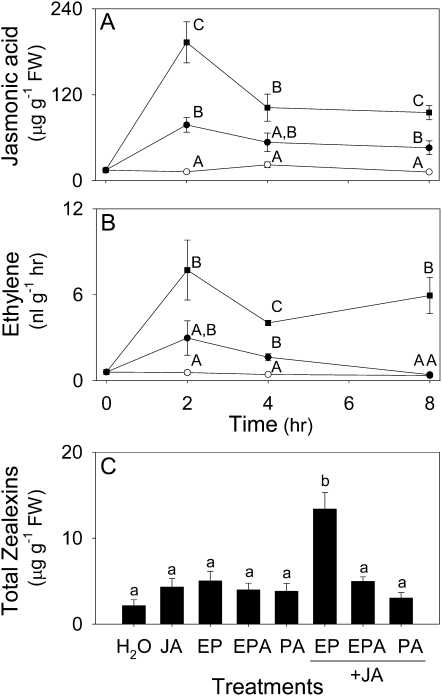
In the context of herbivore-induced sesquiterpene volatile emission in maize, a combination of both JA and ethylene has been previously demonstrated to synergize foliar production (Schmelz et al., 2003a, 2003b). Based on the observed JA increases in F. graminearum-infected stems, we considered the role of these two commonly interacting phytohormones in regulating zealexin production. A time-course study of JA and ethylene production following F. graminearum inoculation revealed sustained increases in both over an 8 h period (Fig. 7, A and B). Importantly, this fungal-induced phytohormone production precedes significant increases in zealexins (Fig. 3, A and B). To ascertain a role for JA and ethylene in the regulation of zealexin production, stems were treated with either JA or ethephon (2-chloroethylphosphonic acid), which locally decomposes to release ethylene (Fig. 7C). Neither single treatment resulted in zealexin levels higher than control plants treated with water alone. However, when applied together, JA and ethephon exerted a significant synergistic action on the elicitation of total zealexin accumulation (Fig. 7C). In contrast, coapplication of JA with the nonethylene generating controls, phosphonic acid and ethylphosphonic acid, did not alter total zealexin production.
Figure 7.
Fungal-induced JA and ethylene production promotes zealexin accumulation as demonstrated by the synergistic activity of pharmacological applications. Average (n = 4; ±sem). A, JA. B, Ethylene levels in stems that were either untreated (○), or damaged and treated with water (•) or water containing F. graminearum (1 × 106 spores mL−1, ▪). Within each plot and time point, different letters (A–C) represent significant differences (all ANOVAs, P < 0.003; Tukey test corrections for multiple comparisons, P < 0.05). C, Average (n = 4, ±sem) total zealexins in maize stems 24 h following damage plus either water, the ethylene releasing chemical ethephon (EP), ethylphosphonic acid (EPA), phosphonic acid (PA), JA, or a combination of JA + EP, JA + EPE, or JA + P. JA was applied at 100 nmol plant−1 as a Na+ salt while EP, EPA, and PA were applied at 33 nmol plant−1 in 10 μL water. Within this plot different letters (a and b) represent significant differences (all ANOVAs, P < 0.0001; Tukey test corrections for multiple comparisons, P < 0.05).
Zealexins Are Ubiquitous in Maize and Broadly Inducible by Biotic Stimuli Including Insect Herbivory and Nontoxigenic Fungi
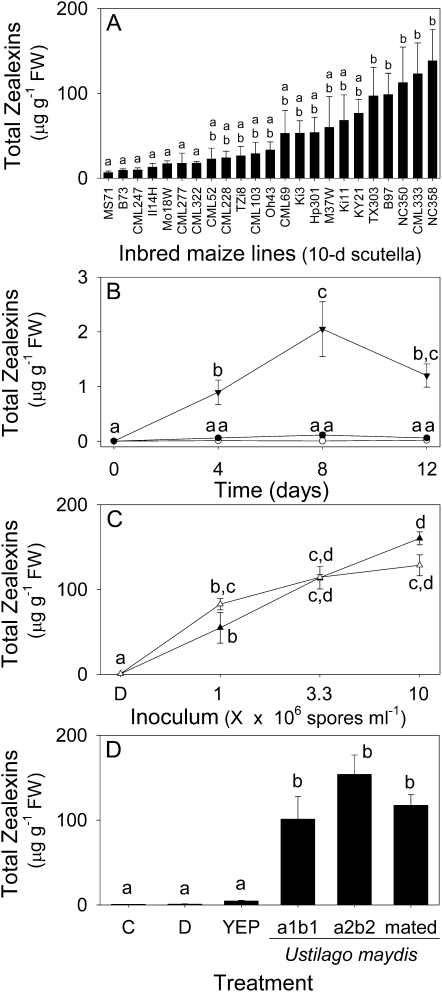
Thus far we have observed zealexin production in three maize lines. To examine if zealexin biosynthesis occurs widely in maize we screened 23 diverse parental inbred maize lines previously developed for use in the maize nested association mapping population (Yu et al., 2008). Given that kauralexins were previously demonstrated to accumulate in the scutella of germinated seedlings, we performed a similar analysis of zealexins 10 d after germination (Kiesselbach, 1999; Schmelz et al., 2011). Zealexins were present in all maize lines examined and demonstrated a significant 21-fold difference between the lowest and highest accumulating lines, namely MS71 and NC358, respectively (Fig. 8A). While these seedlings received no experimental treatments, the variation of total zealexin levels in the highest lines (TX303, B97, NC350, CML333, NC358) is consistent with the potential for unintentional seed- or soil-derived fungal elicitation.
Figure 8.
Zealexins are ubiquitous in maize and inducible in stems by insect herbivory, mycotoxigenic fungi, and nontoxigenic fungi. A, Average (n = 4, ±sem) total zealexins, arranged from low to high, in the scutella of 10-d-old maize seedlings from 23 diverse inbred maize lines. B, Time course of average total zealexin (n = 3, ±sem) accumulation in maize stems following no treatment (○), damage (•) or damage + O. nubilalis herbivory (▾). C, Average (n = 4, ±sem) total zealexins in maize stems 48 h after the plants were damaged and treated with either water (D) or water containing mycotoxigenic A. flavus (△) and nonmycotoxigenic A. sojae (▲) at a range of inoculum levels. D, Average (n = 4, ±sem) total zealexins in maize stems 48 h after either no treatment (C) or damage plus either water (D), YEP, or U. maydis: a1b1 a2b2, or mated, each at final concentration of 1 × 106 cells mL−1 in YEP. Within plots, different letters (a–d) represent significant differences (ANOVA P value < 0.001; Tukey test corrections for multiple comparisons: P < 0.05).
To examine if induced zealexin accumulation occurs in response to insect attack, we conducted a long-term experiment with O. nubilalis herbivory in stems. Within 4 d of stem herbivory, total zealexin levels were significantly higher than those caused by mechanical damage alone (Fig. 8B). Total zealexins continued to increase at 8 d and declined slightly by 12 d. Overall, total O. nubilalis-induced zealexin levels were modest (2 μg g−1 fresh weight [FW]) as compared to most of the aforementioned experiments; however, by necessity of the insect’s behavior, these trials utilized nearly mature maize whereas the fungal elicitation trials were performed on smaller plants. Despite these bioassay differences, fungal attack is likely a stronger stimulus for zealexin elicitation than herbivory.
In these studies, the fungi that most strongly induce zealexins, namely A. flavus and F. graminearum, also are coincidently known producers of aflatoxin and trichothecene class mycotoxins (Bhatnagar et al., 2002). To examine if mycotoxigenic capacity influences defense elicitation, we compared inoculum dose responses of toxigenic A. flavus, nontoxigenic Aspergillus sojae, and resulting zealexin production. At each increasing inoculum level, the production of zealexins did not significantly differ between A. flavus and A. sojae (Fig. 8C). Thus while mycotoxins could conceivably interact with maize zealexin production, they are not necessary for strong fungal elicitation. This point is further supported by the strong elicitation of zealexins following inoculation of damaged stems with both haploid (a1b1 and a2b2) and mated diploid U. maydis cells (Fig. 8D). U. maydis is the causal agent of edible corn smut and does not produce mycotoxins harmful to human health.
Zealexins Inhibit the Growth of Fungal Pathogens at Physiologically Relevant Concentrations
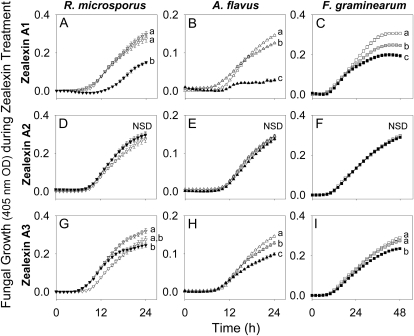
Following damage and inoculation with F. graminearum spores, zealexins are induced to high levels at the immediate infected area. After 48 h, targeted surface analyses revealed zealexin A1 and A3 concentrations of 230 and 290 μg g−1 FW (Supplemental Fig. S3). To examine the antimicrobial activity of these phytoalexins, we assayed the growth of R. microsporus, A. flavus, and F. graminearum in nutrient broth spiked separately with zealexin A1, A2, and A3 at final concentrations of 0, 25, and 100 μg mL−1. Unfortunately we were unable to obtain sufficient amounts of pure zealexin B1 to include in these trials. At 100 μg mL−1, zealexin A1 significantly inhibited the growth of R. microsporus, A. flavus, and F. graminearum by 45%, 80%, and 37%, respectively (Fig. 9, A–C). Even at 25 μg mL−1, zealexin A1 retained measurable inhibitory activity against A. flavus and F. graminearum, but not R. microsporus. In contrast, zealexin A2 displayed no significant growth inhibitory activity against the three fungi at any dose (Fig. 9, D–F). When tested at 100 μg mL−1, zealexin A3 significantly inhibited the growth of A. flavus and F. graminearum by 32% and 20%, respectively (Fig. 9, H and I). However, zealexin A3 did not inhibit the growth of R. microsporus at any concentration (Fig. 9G). Zealexin A1 exhibits significant broad-based inhibition of fungal growth at physiologically relevant concentrations; however, hydroxylations resulting in the production of zealexin A2 and A3 appear to reduce or even abolish antifungal activity in these assays.
Figure 9.
Zealexins inhibit fungal growth at physiologically relevant concentrations. Average (n = 8, ±sem). A, Zealexin A1. D, Zealexin A2. G, Zealexin A3 influence on the time course of R. microsporus growth in nutrient broth at 0 (▿), 25 (gray triangle), and 100 (▾) μg mL−1. B, Zealexin A1. E, Zealexin A2. H, Zealexin A3 influence on the time course of A. flavus growth in nutrient broth at 0 (△), 25 (gray triangle), and 100 (▲) μg mL−1. C, Zealexin A1. F, Zealexin A2. I, Zealexin A3 influence on the time course of F. graminearum growth in nutrient broth at 0 (□), 25 (gray square), and 100 (▪) μg mL−1. Within plots, different letters (a–c) represent significant differences (all ANOVAs P < 0.006; Tukey test corrections for multiple comparisons: P < 0.05). Not statistically different (NSD) indicates ANOVA P > 0.05.
DISCUSSION
While characterizing the molecular and biochemical responses of maize following attack by mycotoxigenic fungi, we uncovered a novel class of acidic sesquiterpenoid phytoalexins, termed zealexins. Zealexins accumulate to very high levels at local sites of infection and are produced in response to both fungi and stem herbivory by O. nubilalis. In the absence of intentional infection, zealexins were detected in the seedling scutella of the 23 diverse parental inbred nested association mapping population maize lines that were investigated. Given these observations, it is curious that zealexins, which copurify with free fatty acids, have not been widely observed by others. This may be due to a previous focus on benzoxazinoid metabolites as the primary biochemical agents of defense or possibly due to the reactive and labile nature of terpenoids. In this study, our analytical methods and focus on tissues specific to the plant-pathogen interface likely aided in the discovery of these defense chemicals.
Based upon the 1H- and 13C-NMR spectroscopic analyses of the four major observed acidic sesquiterpenoids, the following nomenclature is proposed. Compounds comprised of a β-macrocarpene hydrocarbon skeleton with an additional C15 carboxylic acid are defined as the zealexin A class. This includes oxidations, such as those present in zealexin A2 and A3, which result in a minimally modified hydrocarbon skeleton. The zealexin B class are C15 carboxylic acids that contain an additional C1-C6 desaturation of the β-macrocarpene skeleton to yield a conjugated 1,1′3′ triene system, as in zealexin B1. We also obtained one-dimensional 1H-NMR data on analyte 12 that is consistent with an additional C2-C3 double bond and C8 alcohol imposed upon zealexin B1, resulting in a skeleton with aromatic and nonaromatic carbon ring systems. While the proposed identity of analyte 12 is tentative, the existence of this structure is certain, as it has been previously isolated and identified from a 30-kg extraction of maize style tissue (Suzuki et al., 2007). Clearly there is additional chemical diversity yet to be discovered within the current analyses and additional maize varieties, tissues, and infection time courses. For example, when farnesyl pyrophosphate is used as a substrate, the second most abundant enzymatic product of both TPS6 and TPS11 is β-bisabolene (Köllner et al., 2008b). Thus acidic sequiterpenoid phytoalexins could also contain this hydrocarbon skeleton and, while speculative, analytes 1 to 4 exist as potential candidates (Fig. 1C). This idea is also inspired by the existence of sesquiterpenoids such as juvabione and related defenses, derived from the carboxylic acid of α-bisabolene, which are produced by fir trees of the genus Abies (Bowers et al., 1966; Bohlmann et al., 1998).
While at least 14 fungal-induced acidic sesquiterpenoids are detectable, only zealexins A1, A2, and A3 were isolated in sufficient quantity and purity for bioassays. The instability and lower abundance of the additional analytes has thus far delayed their examination. Importantly, zealexin A1 exhibits significant antimicrobial activity at concentrations lower than those naturally occurring within infected maize tissues. For example, at 100 μg mL−1 in liquid culture, zealexin A1 inhibited the growth of A. flavus by 80% (Fig. 9D). While zealexin A3 is the dominant fungal-induced metabolite at 48 h, it exhibits lower antimicrobial activity than zealexin A1. There are numerous potential explanations for this result worth considering. First, it is possible that zealexin A2 or A3 exist as stable precursors to more reactive/bioactive molecules. Second, these zealexins may act in synergy to enhance activity of other defensive molecules, much as chitin-binding proteins have no direct antimicrobial action but potentiate chitinase activity (Selitrennikoff, 2001). Third, zealexin A2 and A3 may be reacted catabolites of bioactive intermediates such as hydroperoxides. Fourth, zealexin A2 and A3 could be plant-derived inactivation products to reduce autotoxicity issues associated with high levels of zealexin A1 or B1. Fifth, extraction conditions may have caused alteration of endogenous product ratios or a failure to detect the most bioactive compounds. We offer additional thoughts regarding the first explanation: While zealexin B1 could be purified as a methyl ester, as a free acid it appeared to degrade in storage much more rapidly than zealexin A1. If plant-derived enzymatic action upon zealexin A1 is responsible for the C8 hydroxylation and C1-C6 desaturation, present in zealexin A3 and B1, respectively; then, a combination of these activities would result in a highly unstable and reactive molecule envisioned as zealexin B2. A candidate for this phytoalexin is analyte 13 with a GC/CI-MS [M+H]+ ion of 263, which to date has eluded proper analysis of NMR spectroscopic properties or antimicrobial activity (Fig. 1C).
Phytoalexins are inducible antimicrobial compounds that accumulate locally to very high levels upon attack by invading organisms (Smith, 1996). We demonstrate that zealexins and related acidic sesquiterpenoids accumulate to levels greater than 800 μg g−1 FW following inoculation with F. graminearum (Supplemental Fig. S3). With the exception of the specialized maize pathogen C. graminicola, zealexins are produced in response to all fungi examined. Furthermore, low levels of zealexins also accumulate in stem tissue surrounding feeding tunnels produced by O. nubilalis larvae. The promiscuous nature of this elicitation is characteristic of phytoalexins, which are highly regulated yet produced in response to a wide variety of biotic and abiotic stresses (Kodama et al., 1988; Smith, 1996). Although the mycotoxin-producing fungal species we studied were very strong inducers of zealexin accumulation, elicitation by numerous species was not dependent upon the ability to produce toxins (Fig. 8, C and D). A similar pattern is found for soybean (Glycine max) phytoalexins, where comparable accumulation occurs following challenge with mycotoxigenic and nonmycotoxigenic fungi (Boué et al., 2000).
The presence and bioactivity of zealexins and kauralexins indicates that terpenoid phytoalexins are both important and chemically diverse components of maize innate immunity. Kauralexins are antimicrobial ent-kaurane-related acidic diterpenoids hypothesized to be downstream end products derived in part from the activity of fungal-induced ent-copalyl diphosphate synthase AN2 (Schmelz et al., 2011). The gene encoding AN2 was originally discovered through a differential display analysis of transcripts induced in maize silks by F. graminearum (Harris et al., 2005). Through our microarray and qRT-PCR analyses, we also found F. graminearum to strongly induce An2 transcript accumulation in maize stalks. Furthermore, Tps6/Tps11 proved to be the second-most up-regulated probe set in our microarray analysis with probe intensity increasing 995-fold for F. graminearum-inoculated tissue as compared to mock inoculations. Highly induced expression of Tps6/Tps11 and An2 corresponds well to the large increases in levels of both zealexins and kauralexins at the site of infection.
Evidence that the Tps6 and Tps11 genes are involved in the production of plant defenses against pathogens has been accumulating for some time. Early ESTs deposited for these genes originated from libraries of F. graminearum-infected silks (GenBank AW267347 and AW282370) and from a subtraction library of leaves inoculated with an incompatible Puccinia sorghi isolate (GenBank CA452709). The association of these genes with plant-pathogen interactions became increasingly evident in a study identifying umi2, a gene strongly induced by U. maydis, that was later identified to be Tps6 (Basse, 2005; Köllner et al., 2008b). Detailed microarray analysis also revealed that Tps6/Tps11 is the single-most highly up-regulated probe set in U. maydis-infected maize ears (Dohlemann et al., 2008). Moreover, the genetic role of Tps6/Tps11 in resistance to U. maydis has been confirmed. In a recent elegant study, maize lines silenced in Tps6/11 using both viral-induced gene silencing and stable transgenic RNAi approaches, displayed increased susceptibility to U. maydis infection (van der Linde et al., 2011). To date, the potential function of these genes in defense against other pathogens has not been broadly considered. Our observations of induced Tps6 and Tps11 gene expression in response to a variety of fungal pathogens suggests that they play a wide role in maize innate immunity. One challenge in understanding the roles of Tps6 and Tps11 is their close homology with one another. Regulation of important biochemical pathways commonly occurs through complex parallel processes, and we could discern no discrete role for one Tps gene over the other in this study. However, our digest method to enable discrimination between the two genes should facilitate future studies of individual expression patterns for other maize tissue and pathogen combinations.
The Tps6/Tps11 genes were induced in leaf tissue following S. littoralis herbivory (Köllner et al., 2008b). This result is consistent with the induction of zealexins in maize stem tissue following O. nubilalis tunneling (Fig. 8B). In the case of S. littoralis herbivory, no volatile β-macrocarpene was detected in foliage that led authors to hypothesize that β-macrocarpene is converted with high efficiency to nonvolatile compounds (Köllner et al., 2008b). While our current study lacks unequivocal genetic or biochemical proof, based upon the following four observations we currently hypothesize that the dominant enzymatic product of TPS6 and/or TPS11, namely β-macrocarpene, is a precursor to the zealexins. First, zealexins A1, A2, and A3 are consistent with C15 carboxylic acids directly imposed upon the hydrocarbon skeleton of β-macrocarpene. Second, levels of transcripts encoding both TPS6 and TPS11 increased prior to zealexin accumulation, as would be predicted for enzymes that catalyze the first committed step in a biosynthetic pathway. Third, direct positive relationships between expression of both genes and zealexin levels were demonstrated in diverse data sets derived from maize tissue challenged with different fungi and inoculum concentrations. Fourth, a modest pool of β-macrocarpene was detected in infected stem tissue and, similar to zealexins, displayed a positive relationship to Tps6 transcript levels. Future proof of this hypothesis will come from isotopic labeling studies in planta and use of developed transgenic resources.
While investigating maize defenses against F. graminearum, we confirmed that terpenoid phytoalexins act in conjunction with a variety of other defenses, including a collection of highly up-regulated transcripts encoding antimicrobial proteins (Fig. 5A). Outside of Tps transcripts, most other genes highly up-regulated by F. graminearum infection encode antifungal proteins, including chitinases, PR proteins, zeamatins, proteinase inhibitors, and β-1,3, endoglucanase. Expression of Eca, Wip1 Bowman-Birk proteinase inhibitor, and Pr10 were all confirmed by qRT-PCR to be induced by F. graminearum inoculation concurrent with zealexin and kauralexin production. Of the genes encoding antimicrobial proteins, Pr10 is strongly associated with resistance against fungal infection and mycotoxin accumulation. For example, recent studies have demonstrated that maize plants harboring RNAi Pr10 silencing constructs display both increased susceptibility to A. flavus colonization and higher levels of aflatoxin contamination (Chen et al., 2010). How antifungal proteins and small molecule chemical defenses function together in maize has not been widely considered.
Maize defense against pathogens is clearly based on a complex mixture of defense proteins and a veritable cocktail of bioactive chemicals. To better understand the participants and interactions of these defenses, we sought to examine the role of zealexins in the absence of benzoxazinoids through experiments with bx1 plants. However, while there were no benzoxazinoids detected in untreated bx1 plants, HDMBOA-Glc was significantly induced by wounding and to an even greater extent by fungal inoculation. The HDMBOA aglycone displays a half-life of less than 10 min (pH = 7), a portion of which decomposes into the more stable 6-methoxy-2H-benzoxalin-2(3H)-one, and thus greatly exceeds the reactivity of commonly studied DIMBOA (Zhang et al., 2000). HDMBOA-Glc is known to accumulate in response to biotic attack; however, until very recently this accumulation was thought to occur primarily through methoxylation of the existing DIMBOA-Glc pool rather than through de novo benzoxazinoid synthesis (Oikawa et al., 2001; Huffaker et al., 2011). We confirm that HDMBOA-Glc synthesis can occur de novo while unexpectedly demonstrating that this does not require either a functional Bx1 gene or the detectable presence of DIMBOA or DIMBOA-Glc. Given that both Igl and Bx1 encode enzymes with indole-3-glycerol phosphate lyase activity, Igl exists as a potential candidate pathway gene enabling the inducible synthesis of HDMBOA-Glc in bx1 plants (Frey et al., 2000, 2009). Correspondingly, expression of the Igl gene is induced by a number of biotic stimuli, including herbivore elicitors, herbivory, and treatment with JA (Frey et al., 2000; Erb et al., 2009). Despite the accumulation of HDMBOA-Glc in fungal-inoculated bx1 plants, total benzoxazinoid content was less than half of that observed in the comparable H88 parent line. Conversely, total fungal-induced zealexins in bx1 plants was over 4-fold greater than H88. These results suggest that the zealexins may be induced to higher levels as a compensatory response to reduced benzoxazinoid defenses in bx1 plants. Cinnamic acid was also among the chemical cocktail induced by F. graminearum in both H88 and bx1 plants (Fig. 6C) and is a precursor to modified phenylpropanoids that likewise have defensive roles against pathogens in maize (Bily et al., 2003; Kim et al., 2008).
In this work a novel class of phytoalexins was discovered in one of the most widely studied and grown crop plants worldwide. This was accomplished through a survey of metabolites induced in response to common pathogens of monocot species. The identification of zealexins opens many lines of inquiry fruitful to the understanding of maize defense mechanisms against fungal pathogens and biotic stresses in general. Future studies to determine mechanisms regulating production of these phytoalexins and enzymes involved in their biosynthesis will be valuable in the development of strategies to manipulate and improve maize disease resistance. Further elucidation of other detectable acidic sesquiterpenoids, and the relative bioactivity of these chemicals is likely to identify precisely which molecular components, or combinations thereof, are most potent in impeding fungal infection. Together with similar studies into kauralexins, this research will begin to illuminate the role of nonvolatile terpenoid phytoalexins in maize disease resistance.
MATERIALS AND METHODS
Plant, Insect, and Fungal Materials
Unless otherwise stated, all experiments used hybrid maize (Zea mays var. Golden Queen; Southern States Cooperative, Inc.). Additional experiments used inbreds (bx1, H88, Ms71, B73, CML247, Il14H, Mo18W, CML277, CML322, CML52, CML288, TZi8, CML103, Oh43, CML69, Ki3, Hp301, M37W, Ki11, Ky21, Tx303, B97, NC350, CML333, and NC358 obtained from the National Genetic Resources Program, Germplasm Resources Information Network). All plants were germinated in MetroMix 200 (Sun Gro Horticulture Distribution, Inc.) supplemented with 14-14-14 Osmocote (Scotts Miracle-Gro) and grown as previously described (Schmelz et al., 2009). Ostrinia nubilalis (Benzon Research Inc.) were reared on artificial diet at 29°C and received as late first instars. Fungal stock cultures of Rhizopus microsporus (Northern Regional Research Laboratory [NRRL] stock no. 54029), Cochliobolus heterostrophus (field isolate), Aspergillus sojae (NRRL 1988), and Aspergillus flavus (NRRL 3357) were grown on one-half times potato dextrose agar for 1 week prior to use in assays. Colletotrichum graminicola (strain M1.001), Colletotrichum sublineolum (strain S12.001), and Fusarium graminearum (NRRL 31084) were grown on V8 agar for 1.5 to 2 weeks prior to use. Spores for inoculum were collected with a moist sterile cotton swab, suspended in sterile water, and quantified for use. Spores from Colletotrichum species were washed prior to inoculum preparation as described by Venard and Vaillancourt (2007) to eliminate sporulation inhibitors. Both mating strains of Ustilago maydis (a1b1; Fungal Genetics Stock Center nos. 9021 and a2b2; Fungal Genetics Stock Center no. 9926; McCluskey et al., 2010) were grown on yeast extract peptone medium (YEP) agar for 2 d and used to inoculate YEP broth cultures. YEP broth cultures were grown on a rotary shaker and after 2 d combined to produce a mated culture 1 h prior to inoculum quantification and assay.
Terpenoid Isolation
Maize stem tissue (500 g) infected by F. graminearum was ground to a fine powder in liquid N2, extracted with 2 L 2:1 MeCl2:propanol for 2 h, separated into organic and aqueous layers, and concentrated under vacuum. The organic layer was applied to a 30-g bulk C18 flash chromatography column, washed with water, 1:1 MeOH:water, and eluted with 100% MeOH and concentrated under vacuum. The residue was then separated by preparative flash chromatography (CombiFlashRf, Teledyne ISCO, Inc.) on a 130-g C18 (RediSepRF Gold) column using a water:MeOH gradient and flow rate of 60 mL min−1. At approximately 65%, 85%, and 88% MeOH, eluted fractions highly enriched in zealexin A3, B1, and A1, respectively, were collected and concentrated under vacuum. These fractions were then solubilized in EtOAc, loaded onto 1-g LC-Si SPE (Supelclean) columns, eluted with 1:1 EtOAc:Hexane, and again concentrated under vacuum. At this point the zealexin A1, A3, and B1 samples were approximately 85% pure by GC based on aliquots analyzed as methyl esters. To isolate zealexin A2, early steps of above extraction were repeated; however, in this case the extracted material was applied to a 30-g bulk C18 flash chromatography column, washed with water, 1:1 MeOH:water, eluted with 87% MeOH, and concentrated under vacuum. This fraction was passed through a 10-g bulk silica flash chromatography column using 1:1 pentane:EtOAc and concentrated under vacuum. This material was then separated by preparative flash chromatography (CombiFlashRf, Teledyne ISCO, Inc.) on a 4-g Silica (RediSepRF Gold) column using a pentane:EtOAc gradient and flow rate of 18 mL min−1. At approximately 37% EtOAc, an eluted fraction highly enriched in zealexin A2 was collected. The sample was then separated by HPLC using a Zorbax RX-silica (250 × 4.6 mm, 5 μm; Agilent) column with flow rate of 1 mL min−1 and linear gradient of 100% pentane to diethyl ether (99%; stabilized with ethanol) over 30 min resulting in purification of zealexin A2 for NMR analysis and bioassays. At each step, the content and purity of all zealexin fractions were analyzed as methyl esters by either GC-flame ionization detector or GC/MS. To achieve maximal sample purity for NMR, samples of zealexin A1, B1, and A3 were derivatized with 10 μL of 2 m (trimethylsilyl)diazomethane, concentrated under a N2 stream, and subjected to micropreparative GC. This separation utilized an Agilent 6890 chromatograph (He carrier gas; 5.7 mL min−1; cool on-column injector set to track oven) equipped with a DB-35MS (Aligent Technologies) column (30 m × 530 μm i.d., 0.50 μm film thickness), and with the temperature programmed from 35°C (2 min hold) at 10°C min−1 to 280°C (hold for 5.5 min). Recovery of GC fractionated biochemicals follows from Heath and Dueben (1998) with the slight modification of a glass press-fit splitter at the end of the column, coupling a 0.5-m (150 μm i.d. fused silica) capillary to the flame ionization detector and a second 0.5-m (350-μm i.d. fused silica) capillary directed to the heated transfer line. A chilled glass capillary was used for sample collection.
NMR Analysis of Isolated Acidic Sesquiterpenoids
GC-purified zealexin methyl esters (30–300 μg) were eluted directly into dry CDCl3 or C6D6 (Cambridge Isotope Laboratories Inc.) and transferred to a 2.5 mm × 100 mm NMR tube (Norell). One- and two-dimensional NMR spectroscopy, including double-quantum filtered correlation spectroscopy, or correlation spectroscopy, heteronuclear single-quantum coherence, heteronuclear multiple-bond correlation (HMBC), and nuclear overhauser enhancement spectroscopy (NOESY) was used to characterize the zealexins (Supplemental Tables S1–S4). NMR spectra for zealexin A1 methyl ester, A3 methyl ester, B1 methyl ester, and a possible C2 were acquired at 22°C using a 5-mm TXI CryoProbe and a Bruker Avance II 600 (Bruker Corporation) console (600 MHz for 1H, 151 MHz for 13C). NMR spectra for zealexin A2 were acquired at 27°C using a 2.5-mm TXI CryoProbe and Bruker Avance 500 console (500 MHz for 1H, 126 MHz for 13C). Solvent residual peaks were used to reference chemical shifts to δ(CHCl3) = 7.26 ppm or δ(C6D5H) = 7.16 ppm for 1H and δ(CH2 of ethyl acetate) = 60.49 ppm, δ(CHCl3) = 77.36 ppm, δ(C6D5H) = 128.2 ppm for 13C (Gottlieb et al., 1997). Directly detected 13C-NMR data for zealexin A1 methyl ester was collected on Bruker Avance 500 console (500 MHz for 1H, 126 MHz for 13C) with 5 mm Broadband probe with a sample temperature of 22°C. NMR spectra were processed using Topspin 2.0 (Bruker Corporation) and MestReNova (MestreLab Research) software packages. The numbering of the zealexin carbon atoms refers to that previously described for β-macrocarpene (Cool, 2005). Supplemental Tables S1 to S4 detail the 1H, 13C, HMBC, and NOESY correlations for zealexin A1, B1, A2, and A3, respectively.
Zealexin A1 methyl ester was analyzed in CDCl3 and its 1H and 13C chemical shifts were consistent with β-macrocarpene (Cool, 2005) except at C15 (carboxylic acid) and nearby carbons at C4, C3, and C5 (Supplemental Table S1). The correlation spectroscopy, HMBC, and NOESY data are also in agreement with the β-macrocarpene core structure and the presence of a carboxylic acid at C15. Zealexin B1 methyl ester was analyzed in C6D6 and its 1H and 13C chemical shifts (for C8, C9, C10, C11, C12, C13, and C14) are consistent with β-macrocarpene except for a C15 carboxylic acid and the presence of a C1-C6 double bond (Supplemental Table S2; Köllner et al., 2008b). Zealexin A2 in CDCl3 contained an OH substitution at the C1 position (Supplemental Table S3). Consistent with this assignment, zealexin A2 displayed different 1H and 13C chemical shifts at C1, C2, C3, C6, and C8 from both the zealexin A1 methyl ester (Supplemental Table S1) and β-macrocarpene (Cool, 2005). Zealexin A3 methyl ester contains an OH substitution at the C8 position (Supplemental Table S4). One-dimensional and two-dimensional NMR data for zealexin A3 is in agreement with the β-macrocarpene core.
Quantification of Maize Metabolites
Sample preparation via solvent extraction, methylation, and vapor-phase extraction followed by analysis using GC/CI-MS was performed using previously developed methods (Schmelz et al., 2004, 2011). To include kauralexins and zealexins in these analyses, we utilized a DB-35MS (30m × 0.25mm × 0.25 μm; Agilent) GC column held at 70°C for 1 min after injection, followed by a programmed temperature gradient of 15°C min−1 to 300°C (7 min), with helium as the carrier gas (0.7 mL/min). Quantity estimates of kauralexins and zealexins were based on 13C18-linolenic acid. In reference to Figure 1C, β-macrocarpene displays a retention time of 10.31 min and a 205 CI [M+H]+ ion. In representative samples analyzed by GC/EI-MS, this substance was provisionally identified based on its known presence in maize and a 99% EI match within the Robert P. Adams essential oil MS library (Allured Books). β-Macrocarpene was quantified based upon an external standard curve of β-caryophyllene (Schmelz et al., 2003c). Ethylene emitted by maize stems was measured by GC as described by Schmelz et al. (2009). Benzoxazinoids were extracted and analyzed by HPLC as previously described (Huffaker et al., 2011). After 48 h, leaf tissue surrounding the treatment sites was harvested in liquid nitrogen, freeze dried, and extracted in 49:1 methanol:acetic acid prior to HPLC analysis. Quantities were estimated using 2-benzoxazolinone (Sigma-Aldrich) as an internal standard.
Maize Stem Phytoalexin Elicitation Assays
Fungal and chemical elicitation assays were performed on 21 to 28 d maize var. ‘Golden Queen’ grown in 1-L plastic pots as described previously (Schmelz et al., 2011). Stems of mock-inoculated plants were slit with a surgical scalpel to create a 5-cm-long parallel longitudinal incision through the center that spanned the upper nodes, internodes, and unexpanded leaves. Essential comparisons included damaged plants treated with either 100 μL of water (D) or 100 μL of fungal spores suspended in water. For the phytohormone interaction study, JA was applied at 100 nmol plant−1 in 10 μL water as a Na+ salt, while ethephon, ethylphosphonic acid, and phosphonic acid were applied at 33 nmol plant−1 in 10 μL water. The O. nubilalis stem herbivory trial was initiated with 35 d plants grown in 3-g pots, each with 12 visible leaves. Damaged plants were treated by inserting a number 1 cork borer 75% through the stem above the first basal node. For additional insect damage, 3rd instar larvae, previously maintained for 24 h on maize leaves, were placed such that they initiated feeding tunnels at this selected location. Each sample collected (n = 3) was a separate pool of three identically treated plants. Control, damage, and O. nubilalis samples were taken at 0, 4, 8, and 12 d.
RNA Isolation and qRT-PCR
Total RNA was isolated with TRIzol (Invitrogen) as per the manufacturer’s protocol and cDNA synthesized using the RETROscript reverse transcriptase kit (Applied Biosystems) and random decamer primers. cDNA samples were diluted 2-fold and analyzed by qRT-PCR using power SYBR green master mix (Applied Biosystems) and each primer at a concentration of 300 nm. Amplification was performed using the ABI 7300 sequence detection system (Applied Biosystems) following standard thermal profile conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were analyzed with SDS 1.3.1 software (Applied Biosystems). Relative expression levels were determined for four independent biological replicates; all reactions were run in triplicate. Threshold cycle (Ct) values for Tps6, Tps11, An2, and Eca were normalized to the housekeeping gene encoding elongation factor-1α (Ef-1α), and the values for Pr10 and Wip1 were normalized to ribosomal protein 17 (Rpl17). Both Ef-1α and Rpl17 have previously been used as endogenous controls in maize (Kirchberger et al., 2007; Tomasi et al., 2009). The abundance of each gene transcript was calculated relative to its corresponding untreated control, except for Tps11 that was not detectable in untreated controls, and was therefore compared to transcript levels of Tps6 in untreated controls. Fold-change calculations were performed with the Livak and Schmittgen (2001) method using the equation 2−ΔΔCt. Specificity of real-time PCR products was verified on 1% agarose gels. To confirm the specificity of Tps6 and Tps11 primers, the PCR products of each primer pair were digested with BmgBI (New England Biolabs), which specifically digests the Tps6 amplicon but not Tps11. All primer sequences are listed in Supplemental Table S5.
Microarray Analysis Using Affymetrix GeneChip Maize Genome Arrays
Total RNA was prepared as described above for three biological replicates each of stem tissue 48 h postinoculation with F. graminearum or mock inoculation with water. To eliminate DNA contamination, RNA was DNAse treated with the TURBO DNA-free kit from Ambion (Applied Biosystems). Microarray sample preparation and analysis was performed by the University of Florida Interdisciplinary Center for Biotechnology Research (ICBR) gene expression core. For each sample, 1 μg of total RNA was provided to the ICBR Gene Expression Core for cDNA synthesis, reverse transcription, and biotin labeling using the Affymetrix GeneChip 3′ IVT express kit and protocol (Affymetrix). This was followed by hybridization to the Affymetrix GeneChip maize genome array, washing, and scanning as per Affymetrix protocols. Data extraction was executed by the ICBR gene expression core and provided as CEL files. Data analysis was performed using ArrayStar 4 software (DNASTAR, Inc.). To minimize probe-specific affinity differences, all data were preprocessed using Robust MultiArray Analysis and quantile normalization. Annotation was assigned using NetAffx Maize Annotation Files in MAGE-ML XML format, release 31 and CSV format, release 31 (Affymetrix).
Zealexin Activity Assays
Antifungal assays were modified from the Clinical and Laboratory Standards Institute M38-A2 guidelines as described previously (Schmelz et al., 2011). Using 96-well microtiter plates, fungal inoculum (2 × 104 spores mL−1) was incubated with 0.5 μL of either phytoalexin in ethanol or an ethanol control; final ethanol concentration for all wells was 0.5%. Fungal growth at either 37°C or 28°C in broth media was monitored through periodic measurements of changes in 405 nm optical density using a Synergy4 (BioTek Instruments, Inc.) reader.
Statistical Analysis
ANOVAs were performed on the quantified levels of terpenoid phytoalexins, β-macrocarpene, phytohormones, benzoxazinoids, cinnamic acid, qRT-PCR transcripts, and fungal growth. Treatment effects were investigated when the main effects of the ANOVAs were significant (P < 0.05). Tukey tests were used to correct for multiple comparisons between control and treatment groups. Prior to statistical analyses, data were subjected to square root transformation to compensate for elevated variation associated with larger mean values. The analysis was accomplished with JMP 4.0 statistical discovery software (SAS Institute). To determine genes for which a significant fold change in microarray probe intensity occurred, a Student’s t test with Benjamini-Hochberg false discovery rate (FDR) analysis was applied. Changes in expression levels were considered statistically significant if P < 0.015 after FDR correction.
Sequence Data
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: NM_001112204, NM_001112480, NM_001111420, NM_001112465, NM_001137023, NM_001148760, NM_001165432, NM_001152914, NM_001153886, NM_001112514, NM_001112071, NM_001111994, NM_001147289, NM_001112232, NM_001111787, NM_001147894, NM_001112513, NM_001158529, NM_001154892, NM_001139095, NM_001111896, NM_001146860, LOC100275716, NM_001149511, NM_001111886, NM_001111979, NM_001150422, NM_001176804, and NM_001153759.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. GC/EI-MS spectra of additional fungal-induced acidic sequiterpenoids in maize derivatized as methyl esters.
Supplemental Figure S2. Discernment of Tps6 amplicon from Tps11 through restriction digests with BmgBI.
Supplemental Figure S3. Quantitative profile of acidic terpenoids in maize stems 48 h after wounding and inoculation.
Supplemental Table S1. Zealexin A1 NMR.
Supplemental Table S2. Zealexin A2 NMR.
Supplemental Table S3. Zealexin A3 NMR.
Supplemental Table S4. Zealexin B1 NMR.
Supplemental Table S5. Primers used for qRT-PCR analysis of gene expression.
Supplemental Table S6. Microarray analysis of gene expression 48 h posttreatment in F. graminearum-inoculated stems as compared to mock-inoculated stems.
Acknowledgments
We thank the following people: G. Pearce (Institute of Biological Chemistry, Washington State University) and S. Walse (U.S. Department of Agriculture, Agricultural Research Service, San Joaquin Valley Agricultural Sciences Center) for critical reading of the manuscript; B. Forguson, H. Tang, T. Cox, K. Friman, M. Legaspi, and E. Mok (U.S. Department of Agriculture, Agricultural Research Service Center for Medical, Agricultural, and Veterinary Entomology) for technical support.
References
- Basse CW. (2005) Dissecting defense-related and developmental transcriptional responses of maize during Ustilago maydis infection and subsequent tumor formation. Plant Physiol 138: 1774–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar D, Yu J, Ehrlich KC. (2002) Toxins of filamentous fungi. Chem Immunol 81: 167–206 [DOI] [PubMed] [Google Scholar]
- Bily AC, Reid LM, Taylor JH, Johnston D, Malouin C, Burt AJ, Bakan B, Regnault-Roger C, Pauls KP, Arnason JT, et al. (2003) Dehydrodimers of ferulic acid in maize grain pericarp and aleurone: resistance factors to Fusarium graminearum. Phytopathology 93: 712–719 [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Crock J, Jetter R, Croteau R. (1998) Terpenoid-based defenses in conifers: cDNA cloning, characterization, and functional expression of wound-inducible (E)-alpha-bisabolene synthase from grand fir (Abies grandis). Proc Natl Acad Sci USA 95: 6756–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boué SM, Carter CH, Ehrlich KC, Cleveland TE. (2000) Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J Agric Food Chem 48: 2167–2172 [DOI] [PubMed] [Google Scholar]
- Bowers WS, Fales HM, Thompson MJ, Uebel EC. (1966) Juvenile hormone: identification of an active compound from balsam fir. Science 154: 1020–1021 [DOI] [PubMed] [Google Scholar]
- Brooks CJW, Watson DG. (1991) Terpenoid phytoalexins. Nat Prod Rep 8: 367–389 [DOI] [PubMed] [Google Scholar]
- Capehart T, Allen E. (2011) Feed outlook. United States Department of Agriculture Economic Research Service Report: Report Number ERS-FDS-11d. http://usda.mannlib.cornell.edu/usda/current/FDS/FDS-04-12-2011.pdf
- Chappell J, Nable R. (1987) Induction of sesquiterpenoid biosynthesis in tobacco cell suspension cultures by fungal elicitor. Plant Physiol 85: 469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy BM. (2010) Food safety risks and consumer health. New Biotechnol 27: 534–544 [DOI] [PubMed] [Google Scholar]
- Chen ZY, Brown RL, Damann KE, Cleveland TE. (2010) PR10 expression in maize and its effect on host resistance against Aspergillus flavus infection and aflatoxin production. Mol Plant Pathol 11: 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool LG. (2005) Sesquiterpenes from Cupressus macrocarpa foliage. Phytochemistry 66: 249–260 [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Hiltpold I, Köllner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TC. (2009) Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci USA 106: 13213–13218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannoy E, Lyon BR, Marmey P, Jalloul A, Daniel JF, Montillet JL, Essenberg M, Nicole M. (2005) Resistance of cotton towards Xanthomonas campestris pv. malvacearum. Annu Rev Phytopathol 43: 63–82 [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Gardner HW, Weltring KM. (1992) Detoxification of sesquiterpene phytoalexins by Gibberella pulicaris (Fusarium sambucinum) and its importance for virulence on potato tubers. J Ind Microbiol Biotechnol 9: 201–221 [Google Scholar]
- Dohlemann G, Wahl R, Horst RJ, Voll LM, Usadel B, Poree F, Stitt M, Pons-Kühnemann J, Sonnewald U, Kahmann R, et al. (2008) Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J 56: 181–195 [DOI] [PubMed] [Google Scholar]
- Erb M, Flors V, Karlen D, de Lange E, Planchamp C, D'Alessandro M, Turlings TC, Ton J. (2009) Signal signature of aboveground-induced resistance upon belowground herbivory in maize. Plant J 59: 292–302 [DOI] [PubMed] [Google Scholar]
- Frey M, Chomet P, Glawischnig E, Stettner C, Grün S, Winklmair A, Eisenreich W, Bacher A, Meeley RB, Briggs SP, et al. (1997) Analysis of a chemical plant defense mechanism in grasses. Science 277: 696–699 [DOI] [PubMed] [Google Scholar]
- Frey M, Schullehner K, Dick R, Fiesselmann A, Gierl A. (2009) Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants. Phytochemistry 70: 1645–1651 [DOI] [PubMed] [Google Scholar]
- Frey M, Stettner C, Pare PW, Schmelz EA, Tumlinson JH, Gierl A. (2000) An herbivore elicitor activates the gene for indole emission in maize. Proc Natl Acad Sci USA 97: 14801–14806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, Dudareva N. (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3: 408–414 [DOI] [PubMed] [Google Scholar]
- Gottlieb HE, Kotlyar V, Nudelman A. (1997) NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem 62: 7512–7515 [DOI] [PubMed] [Google Scholar]
- Hamilton RH. (1964) A corn mutant deficient in 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one with an altered tolerance of atrazine. Weeds 12: 27–30 [Google Scholar]
- Harris LJ, Saparno A, Johnston A, Prisic S, Xu M, Allard S, Kathiresan A, Ouellet T, Peters RJ. (2005) The maize AN2 gene is induced by Fusarium attack and encodes an ent-copalyl diphosphate synthase. Plant Mol Biol 59: 881–894 [DOI] [PubMed] [Google Scholar]
- Heath RR, Dueben BD. (1998) Analytical and preparative gas chromatography. Millar JG, Haynes KF, , Methods in Chemical Ecology: Chemical Methods, Vol 1. Kluwer, New York, pp 85–126 [Google Scholar]
- Huffaker A, Dafoe NJ, Schmelz EA. (2011) ZmPep1, an ortholog of Arabidopsis Elicitor Peptide 1, regulates maize innate immunity and enhances disease resistance. Plant Physiol 155: 1325–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesselbach TA. (1999) The Structure and Reproduction of Corn. 50th Anniversary Edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Kim JH, Yu J, Mahoney N, Chan KL, Molyneux RJ, Varga J, Bhatnagar D, Cleveland TE, Nierman WC, Campbell BC. (2008) Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int J Food Microbiol 122: 49–60 [DOI] [PubMed] [Google Scholar]
- Kirchberger S, Leroch M, Huynen MA, Wahl M, Neuhaus HE, Tjaden J. (2007) Molecular and biochemical analysis of the plastidic adp-glucose transporter (zmbt1) from Zea mays. J Biol Chem 282: 22481–22491 [DOI] [PubMed] [Google Scholar]
- Kodama O, Suzuki T, Miyakawa J, Akatsuka T. (1988) Ultraviolet-induced accumulation of phytoalexins in rice leaves. Agric Biol Chem 52: 2469–2473 [Google Scholar]
- Köllner TG, Held M, Lenk C, Hiltpold I, Turlings TC, Gershenzon J, Degenhardt J. (2008a) A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 20: 482–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köllner TG, Schnee C, Li S, Svatos A, Schneider B, Gershenzon J, Degenhardt J. (2008b) Protonation of a neutral (S)-β-bisabolene intermediate is involved in (S)- β-macrocarpene formation by the maize sesquiterpene synthases TPS6 and TPS11. J Biol Chem 283: 20779–20788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 254: 402–408 [DOI] [PubMed] [Google Scholar]
- McCluskey K, Wiest A, Plamann M. (2010) The Fungal Genetics Stock Center: a repository for 50 years of fungal genetics research. J Biosci 35: 119–126 [DOI] [PubMed] [Google Scholar]
- Melanson D, Chilton MD, Masters-Moore D, Chilton WS. (1997) A deletion in an indole synthase gene is responsible for the DIMBOA-deficient phenotype of bxbx maize. Proc Natl Acad Sci USA 94: 13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, Bak S. (2008) β-Glucosidases as detonators of plant chemical defense. Phytochemistry 69: 1795–1813 [DOI] [PubMed] [Google Scholar]
- Niemeyer HM. (2009) Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: key defense chemicals of cereals. J Agric Food Chem 57: 1677–1696 [DOI] [PubMed] [Google Scholar]
- Oikawa A, Ishihara A, Hasegawa M, Kodama O, Iwamura H. (2001) Induced accumulation of 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside (HDMBOA-Glc) in maize leaves. Phytochemistry 56: 669–675 [DOI] [PubMed] [Google Scholar]
- Oikawa A, Ishihara A, Tanaka C, Mori N, Tsuda M, Iwamura H. (2004) Accumulation of HDMBOA-Glc is induced by biotic stresses prior to the release of MBOA in maize leaves. Phytochemistry 65: 2995–3001 [DOI] [PubMed] [Google Scholar]
- Peters RJ. (2006) Uncovering the complex metabolic network underlying diterpenoid phytoalexin biosynthesis in rice and other cereal crop plants. Phytochemistry 67: 2307–2317 [DOI] [PubMed] [Google Scholar]
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TC. (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434: 732–737 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Engelberth J, Tumlinson JH. (2003a) Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol 133: 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH. (2003b) Synergistic interactions between volicitin, jasmonic acid and ethylene mediate insect-induced volatile emission in Zea mays. Physiol Plant 117: 403–412 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Alborn HT, O’Donnell P, Sammons M, Toshima H, Tumlinson JH., III (2003c) Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc Natl Acad Sci USA 100: 10552–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, Teal PEA. (2009) Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc Natl Acad Sci USA 106: 653–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT. (2004) The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J 39: 790–808 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, Ni X, Rocca JR, Alborn HT, Teal PE. (2011) Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc Natl Acad Sci USA 108: 5455–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee C, Köllner TG, Held M, Turlings TC, Gershenzon J, Degenhardt J. (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103: 1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selitrennikoff CP. (2001) Antifungal proteins. Appl Environ Microbiol 67: 2883–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smissman EE, LaPidus JB, Beck SD. (1957) Corn plant resistance factor. J Org Chem 22: 220 [Google Scholar]
- Smith CJ. (1996) Accumulation of phytoalexins: defence mechanism and stimulus response system. New Phytol 132: 1–45 [DOI] [PubMed] [Google Scholar]
- Stoessla A, Stothersb JB, Ward EWB. (1976) Sesquiterpenoid stress compounds of the solanaceae. Phytochemistry 15: 855–872 [Google Scholar]
- Suzuki R, Iijima M, Okada Y, Okuyama T. (2007) Chemical constituents of the style of Zea mays L. with glycation inhibitory activity. Chem Pharm Bull (Tokyo) 55: 153–155 [DOI] [PubMed] [Google Scholar]
- Tomasi N, Monte R, Rizzardo C, Venuti S, Zamboni A, Cesco S, Pinton R, Varanini Z. (2009) Effects of water-extractable humic substances on molecular physiology of nitrate uptake in two maize inbread lines with different nitrogen use efficiency. The Proceedings of the International Plant Nutrition Colloquium XVI. Department of Plant Sciences, University of California, Davis, CA [Google Scholar]
- Toyomasu T. (2008) Recent advances regarding diterpene cyclase genes in higher plants and fungi. Biosci Biotechnol Biochem 72: 1168–1175 [DOI] [PubMed] [Google Scholar]
- Turlings TC, Loughrin JH, McCall PJ, Röse US, Lewis WJ, Tumlinson JH. (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92: 4169–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TC, Tumlinson JH, Lewis WJ. (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251–1253 [DOI] [PubMed] [Google Scholar]
- van der Linde K, Kastner C, Kumlehn J, Kahmann R, Dohlemann G. (2011) Systemic virus-induced gene silencing allows functional characterization of maize genes during biotrophic interaction with Ustilago maydis. New Phytol 189: 471–483 [DOI] [PubMed] [Google Scholar]
- Venard C, Vaillancourt L. (2007) Colonization of fiber cells by Colletotrichum graminicola in wounded maize stalks. Phytopathology 97: 438–447 [DOI] [PubMed] [Google Scholar]
- Virtanen AI, Hietala PK. (1955) 2(3)-Benzoxazolinone, an anti-Fusarium factor in rye seedlings. Acta Chem Scand 9: 1543–1544 [Google Scholar]
- Wagacha JM, Muthomi JW. (2008) Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int J Food Microbiol 124: 1–12 [DOI] [PubMed] [Google Scholar]
- Yu J, Holland JB, McMullen MD, Buckler ES. (2008) Genetic design and statistical power of nested association mapping in maize. Genetics 178: 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Boone L, Kocz R, Zhang C, Binns AN, Lynn DG. (2000) At the maize/Agrobacterium interface: natural factors limiting host transformation. Chem Biol 7: 611–621 [DOI] [PubMed] [Google Scholar]