Abstract
Rice (Oryza sativa) and wheat (Triticum aestivum) are the most important starch crops in world agriculture. While both germinate with an anatomically similar coleoptile, this tissue defines the early anoxia tolerance of rice and the anoxia intolerance of wheat seedlings. We combined protein and metabolite profiling analysis to compare the differences in response to anoxia between the rice and wheat coleoptiles. Rice coleoptiles responded to anoxia dramatically, not only at the level of protein synthesis but also at the level of altered metabolite pools, while the wheat response to anoxia was slight in comparison. We found significant increases in the abundance of proteins in rice coleoptiles related to protein translation and antioxidant defense and an accumulation of a set of enzymes involved in serine, glycine, and alanine biosynthesis from glyceraldehyde-3-phosphate or pyruvate, which correlates with an observed accumulation of these amino acids in anoxic rice. We show a positive effect on wheat root anoxia tolerance by exogenous addition of these amino acids, indicating that their synthesis could be linked to rice anoxia tolerance. The potential role of amino acid biosynthesis contributing to anoxia tolerance in cells is discussed.
Rice (Oryza sativa) and wheat (Triticum aestivum) are economically important crops that are adversely affected by multiple environmental stresses. Both these monocotyledon grasses operate similar central metabolic processes yet notably differ in aspects of their development and anatomy as well as in their optimal growth conditions: rice is typically cultivated in tropical regions on flooded/anaerobic soils, whereas wheat is almost exclusively a dry-land winter crop (Nagai and Makino, 2009). Despite these differences, their critical role as the main source of nutrition for humanity makes the comparative study of these crops under yield-reducing stresses important. Furthermore, the study of two species, as opposed to two cultivars of the same species, may be useful in highlighting mechanisms of anoxia adaptation in plants differing in the contexts of their domestication. Rice is an ideal model species for elucidating the mechanisms of anoxia tolerance in plants; its full genome sequence is available (Yu et al., 2002), it can survive under prolonged anoxia, and it can even display elongation of its coleoptiles under anoxic conditions (Menegus et al., 1991; Perata et al., 1997). A critical aspect of rice anoxia tolerance is the induction of the starch-degrading enzyme α-amylase under anoxia, providing a continuing supply of substrates for metabolism (Perata et al., 1992). Rice growth under anoxia is largely restricted to the coleoptile, with root and leaf development halted in the absence of oxygen (Öpik, 1973). From an evolutionary perspective, successful coleoptile growth under anoxia provides rice seedlings with the opportunity to reach more air-saturated conditions above anaerobic mud or standing water (Kordan, 1974), thus increasing the chance of survival. In some regions of the world, wheat also encounters waterlogging, causing oxygen deficiency, but unlike rice, this normally leads to major or even total yield losses (Setter and Waters, 2003). Compared with rice seedlings, wheat seedlings are widely considered to be anoxia intolerant, despite possessing an anatomically similar coleoptile tissue under normal growth conditions (Menegus et al., 1991). Although wheat seeds are starchy like rice, they cannot germinate and grow a coleoptile under anoxia due to an absence of the starch-degrading enzyme α-amylase in anaerobic seeds (Perata et al., 1992).
Without oxygen, the glycolytic pathway that is linked with ethanolic fermentation is the predominant mechanism of energy production in plants (Gibbs et al., 2000; Bailey-Serres and Voesenek, 2008). However, there is still much less energy production during anoxia than in aeration per unit of carbohydrate metabolized. As a consequence, the synthesis rate of macromolecules such as proteins decreases well below that seen in aerated tissues (Alpi and Beevers, 1983). Even so, rice coleoptiles still exhibit a complex pattern of newly synthesized proteins under anoxia (Mocquot et al., 1981; Ricard and Pradet, 1989; Huang et al., 2005). Along with the classical anaerobic proteins first reported in maize (Zea mays; Sachs et al., 1996), anoxic rice coleoptiles also synthesize a range of proteins with unknown functions (Huang et al., 2005). To date, the identified anoxically synthesized protein data set in rice does not form complete biochemical pathways (Ricard et al., 1991; Huang et al., 2005). Evidently missing from this set are a range of enzymes in glycolysis and the enzymes that could explain the observed amino acid accumulation in rice coleoptiles under anoxia (Fan et al., 1997; Kato-Noguchi and Ohashi, 2006). It remains unclear whether these dramatically induced amino acid pools are derived from specific protein degradation under anoxia or de novo amino acid synthesis. With the improved techniques in protein identification using peptide mass spectrometry, it is feasible to analyze proteins on a large scale using combinations of gel-based or non-gel-based methods to address these issues and provide an in-depth understanding of the mechanism(s) of anoxia tolerance. Direct comparisons of proteome responses that occur during anoxia in tolerant rice coleoptiles and intolerant wheat coleoptiles also provide an opportunity to differentiate proteome changes under anoxia associated with cellular stress and damage from those associated with continued growth and adaptation.
At the metabolite level, the accumulation of fermentation end products such as ethanol, lactate, and Ala has been extensively studied in plants responding to oxygen deprivation (Raymond et al., 1985; Menegus et al., 1989, 1991; Gibbs and Greenway, 2003). A recent metabolomic analysis of Lotus japonicus suggests that the accumulation of succinate and Ala under low oxygen might function to generate ATP that is additional to what the glycolytic pathway offers (Rocha et al., 2010). In rice coleoptiles, the anaerobic assimilation of inorganic nitrogen into amino acids, particularly Ala and γ-aminobutyrate/Glu, may serve to supplement ethanolic fermentation in sustaining glycolytic energy production (Fan et al., 1997). There are also several studies that report changes of carbohydrates (Suc, Glc, Fru) and sugar phosphates in coleoptiles and shoots (composed of both leaves and coleoptiles) of rice seedlings in response to anoxia (Menegus et al., 1991; Guglielminetti et al., 1995; Huang et al., 2003). An analysis of the early germination stages of rice embryos at the metabolite level highlighted sets of 10 and 13 metabolites, respectively, as aerobic and anaerobic responders (Narsai et al., 2009). However, a broad picture of the changes of metabolites in the anoxic rice coleoptile itself remains unclear, and measuring only a few compounds, as has been reported in most of the earlier studies (Menegus et al., 1989, 1991), makes it hard to understand the flow of both carbon and nitrogen between metabolic pools under anoxia. Furthermore, there are no reported studies on how wheat coleoptiles respond to anoxia across a broad set of metabolite pools.
In this study, we combined protein and metabolite-profiling analyses to compare the differences in response to anoxia between anoxia-tolerant rice coleoptiles and anoxia-intolerant wheat coleoptiles. Rice coleoptiles, but not wheat coleoptiles, responded to anoxia dramatically, not only at the level of new protein synthesis but also at the level of altered metabolite pools. We also found significant increases in anoxic rice coleoptile proteins related to protein translation, such as 40S ribosomal proteins and elongation factors. The possibility of selected mRNA translation and protein turnover in anoxic rice coleoptiles, but not in anoxic wheat coleoptiles, which are remarkably unchanged, is discussed in light of the observed low correlation between protein abundance and reported gene expression data. A set of enzymes that increased in abundance in anoxic rice, a change that was not apparent in wheat, are involved in Ser, Gly, and Ala biosynthesis from glycolytic metabolites. This correlates with the observed accumulation of these amino acids in anoxic rice coleoptiles. The potential role of amino acid biosynthesis contributing to anoxia tolerance is discussed, and we show a positive effect on tolerance upon exogenous supplementation of these amino acids in wheat but not in rice.
RESULTS
Physiological Analyses Highlight Differences between Responses of Rice and Wheat Seedlings to Anoxia
Germination of the rice and wheat seeds used in this study under anoxic conditions replicated widely reported differences that rice can germinate and grow its coleoptile under anoxia while wheat seeds fail to germinate under similar conditions (Alpi and Beevers, 1983; Supplemental Fig. S1A). We compared rice coleoptiles from seedlings germinated and grown under anoxia for 6 d with those under aeration for 4 d to characterize rice metabolism under prolonged anoxia. Rice coleoptiles from seedlings grown under aeration for 4 d followed by a 1-d switch to anoxia were also studied. This allowed a comparison to be made between prolonged protein changes from germination and more rapid changes associated with the loss of oxygen from aerobic tissue. The treatment involving a switch to anoxia also generated a data set comparable to publicly available microarray data characterizing coleoptiles from 4-d-old anoxically germinated rice seedlings (Lasanthi-Kudahettige et al., 2007). As they are unable to germinate under anoxia, we were constrained to studying wheat seedlings that were germinated and grown under aeration for 4 d and then switched to 1 d of anoxia. By using the Evans blue viability stain, a distinction in the anoxia tolerance of rice and wheat was confirmed in that the viability of rice roots was much greater than that of wheat after a switch from aeration to anoxia (Supplemental Fig. S1B).
Detailed study of the 24-h anoxic response in rice and wheat coleoptiles was performed tracking growth, sugar content, and metabolic activities (Table I). This showed that aerobically grown rice coleoptiles, but not wheat coleoptiles, displayed significant elongation after 1 d of anoxia (Table I). Sugars are the primary carbon source for energy production via glycolysis and ethanolic fermentation. The sugar content of rice coleoptiles under anoxia for 6 d was considerably lower than that measured in 4-d-old aerobic coleoptiles (Table I). The sugar content of coleoptiles, leaves, and roots of both rice and wheat seedlings was also significantly lower even after a 1-d switch from aeration to anoxia (Table I). Induction of alcohol dehydrogenase (ADH) is a key step in the switch to anaerobic energy production, for it is ethanolic fermentation that regenerates NAD+, an oxidant necessary for the continuation of glycolysis. In both anoxic rice and wheat coleoptiles, ADH activity was induced compared with the aerated control (Table I). However the apparent inducibility of ADH activity during anoxia was greater in coleoptiles of rice (3.4-fold) than those of wheat (2.4-fold; Table I). The highest ADH activity recorded was observed in rice coleoptiles after 6 d under anoxia (Table I). In the leaves and roots of both rice and wheat seedlings, ADH activities were also induced by anoxia, but the final specific activity of ADH was 5- to 10-fold higher in rice than in wheat (Table I).
Table I. Growth, sugar concentration, oxygen consumption rate, and ADH activity of coleoptiles from rice and wheat seedlings exposed to aeration or anoxia.
Rice seeds were germinated and grown under aeration for 4 d (control), anoxia for 6 d (anoxically germinated; 6 d N2), or subsequently switched to 1 d of anoxia (anoxically switched; 4 d air + 1 d N2) or 1 d of air (aerobically switched; 6 d N2 + 1 d air). Wheat seeds were treated with control conditions or were anoxically switched.
| Parameters | Treatments | Rice |
Wheat |
||||
| Coleoptiles | Leaves | Roots | Coleoptiles | Leaves | Roots | ||
| Growth (mm) | 4 d air | 11.9 ± 0.9 | – | – | 28.1 ± 2.7 | – | – |
| 4 d air + 1 d N2 | 14.2 ± 1.0a | – | – | 26.2 ± 1.5 | – | – | |
| 6 d N2 | 7.1 ± 0.7a | – | – | – | – | – | |
| 6 d N2 + 1 d air | 9.7 ± 0.7b | – | – | – | – | – | |
| Sugar concentration (mg hexose g−1 fresh wt) | 4 d air | 44.8 ± 1.4 | 55.8 ± 2.1 | 28.0 ± 2.8 | 37.0 ± 0.7 | 64.9 ± 2.5 | 12.4 ± 1.2 |
| 4 d air + 1 d N2 | 21.6 ± 0.9a | 31.3 ± 0.7a | 13.5 ± 0.7a | 26.4 ± 1.2a | 40.4 ± 1.0a | 3.4 ± 0.3a | |
| 6 d N2 | 3.5 ± 0.2a | – | – | – | – | – | |
| 6 d N2 + 1 d air | 7.7 ± 1.1b | – | – | – | – | – | |
| Oxygen consumption rate (nmol min−1 g−1 fresh wt) | 4 d air | 126.3 ± 5.1 | 302.2 ± 10.8 | 398.8 ± 20.2 | 140.6 ± 6.0 | 478.8 ± 36.2 | 436.8 ± 14.4 |
| 4 d air + 1 d N2 | 104.7 ± 2.8a | 132.7 ± 20.0a | 82.1 ± 1.2a | 146.1 ± 8.9 | 336.7 ± 10.1 | 177.3 ± 1.0a | |
| 6 d N2 | 67.4 ± 6.0a | – | – | – | – | – | |
| 6 d N2 + 1 d air | 134.0 ± 11.8b | – | – | – | – | – | |
| ADH activity (units mg−1 protein) | 4 d air | 0.75 ± 0.06 | 0.94 ± 0.07 | 0.94 ± 0.05 | 0.25 ± 0.01 | 0.09 ± 0.01 | 0.32 ± 0.02 |
| 4 d air + 1 d N2 | 2.68 ± 0.13a | 3.42 ± 0.04a | 5.22 ± 0.70a | 0.59 ± 0.01a | 0.28 ± 0.01a | 1.08 ± 0.01a | |
| 6 d N2 | 6.61 ± 0.48a | – | – | – | – | – | |
| 6 d N2 + 1 d air | 4.38 ± 0.24b | – | – | – | – | – | |
The value of continuously anoxic/anoxically switched samples is P < 0.05 when compared with continuously aerated samples.
The value of aerobically switched samples is P < 0.05 when compared with continuously anoxic samples.
The 6-d-old anoxic rice coleoptiles were shorter than those from seedlings germinated and grown under aeration for 4 d (Table I). This contrasts with previous reports that rice coleoptiles grown under anoxia were much longer than aerated ones (Atwell et al., 1982; Alpi and Beevers, 1983). The explanation for this difference could be the different cultivars used or the use of N2 bubbling as a means to achieve anoxia, rather than the stagnant conditions used in other studies (Magneschi et al., 2009). Bubbling removes other gases such as CO2 and even ethylene produced by coleoptiles if trace amounts of oxygen are available, reducing the complexity of comparing anoxic and aerated conditions.
We also monitored the capacity for mitochondrial respiratory function by measuring tissue oxygen consumption rate. The rate of oxygen consumption in coleoptiles from seedlings continuously grown under anoxia, but returned to aeration for the measurements, was significantly less than that of continuously aerated coleoptiles (Table I). This is consistent with the need for oxygen for the biosynthesis of heme groups for the cytochromes of the plant respiratory chain (Millar et al., 2004). The oxygen consumption rate capacity in roots and leaves from rice and wheat seedlings was significantly lowered by 1 d of anoxia (Table I), indicating tissue adaptation to anoxia and/or damage or loss of mitochondrial function. Interestingly, this effect was more dramatic in rice than in wheat tissues. In comparison, the respiratory capacity of wheat coleoptiles was not significantly affected by 1 d of anoxia and in rice the reduction was significant yet slight (Table I), suggesting minimal mitochondrial damage occurred during this period of anoxia and the immediate ability of mitochondrial function to return upon the aeration required for the measurements to be carried out. In conclusion, rice coleoptiles grown continuously under anoxia or aeration had significant differences in all the parameters investigated. When switched from aerated to anoxic conditions, coleoptiles of rice responded to anoxia to a greater degree than those of wheat. In comparison, leaves and roots of rice and wheat seedlings responded to anoxia similarly in all parameters investigated, distinct from the coleoptile response in both species, and despite the longer term differences noted from Evans blue viability staining of roots (Supplemental Fig. S1B).
Quantitative Proteomic Analysis of Coleoptiles Shows a Significant Rice Response But a Minimal Wheat Response to Anoxia
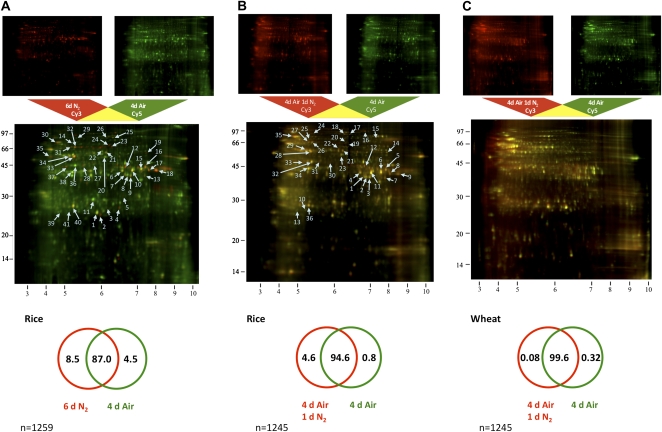
A number of molecular responses that underlie the differences noted in Table I have been investigated in published reports (Menegus et al., 1991; Perata et al., 1992). There are also a number of studies on global gene expression in response to anoxia or oxygen deficiency in plants including rice (Lasanthi-Kudahettige et al., 2007; Narsai et al., 2009) and Arabidopsis (Arabidopsis thaliana; Klok et al., 2002; Branco-Price et al., 2005; Liu et al., 2005; Loreti et al., 2005; Mustroph et al., 2010). But so far, information on the changes of protein abundance in response to anoxia is limited (Mocquot et al., 1981; Ricard and Pradet, 1989; Huang et al., 2005). Because there were such dramatic differences at the physiological level between 4-d aerated and 6-d anoxic coleoptiles (Table I), we started analyzing differences in protein profiles using differential in-gel electrophoresis (DIGE), which is based on staining protein samples with different fluorescent dyes (Fig. 1A). Out of 1,259 protein spots detected on isoelectric focusing (IEF)/SDS-PAGE gels (pI range, 3–10), 164 (13%) protein spots met the criteria of significant 2-fold differences in protein abundance on three replicate gels (P < 0.05). A total of 107 of these protein spots were more abundant under anoxia and 57 were more abundant under aeration (Fig. 1A). This suggested a significant difference in the protein profiles of samples from 6-d anoxic and 4-d aerated rice coleoptiles. We also compared rice coleoptiles from 4-d aerated seedlings with those grown in the same control conditions but switched to an additional 1 d of anoxia (Fig. 1B). There were 1,245 protein spots detected, and 67 (5%) of these met the above-mentioned significance criteria (Fig. 1B). Eighty-five percent of these changing protein spots were more abundant in coleoptiles subjected to the anoxic switch (Fig. 1B), suggesting a rapid 24-h response to anoxia at the protein level. This is in agreement with the results of van Dongen and colleagues (2009), who reported a tendency for gene expression to increase in response to 0.5 to 48 h of hypoxia as opposed to cessation of transcription in roots of Arabidopsis seedlings. We then compared coleoptile proteomes of wheat using the same anoxic-switch experimental setup used in rice (Fig. 1C). According to the same significance criteria, only five (0.4%) of the 1,245 protein spots detected differed in abundance between the two treatments (Fig. 1C), suggesting a very limited response to anoxia at the protein level in wheat coleoptiles.
Figure 1.
DIGE on two-dimensional IEF/SDS gels. A and B, Comparisons were made between coleoptile proteomes of rice seedlings treated with 4 d of aeration versus 6 d of anoxia (A) as well as 4 d of aeration with an additional 1 d under anoxia (B). C, Wheat responses to anoxia were also analyzed by comparing coleoptiles from seedlings treated in the same way as in B. The top panels are gel images of each fluorescence signal, and the bottom panel is a combined image electronically overlaid using ImageQuant TL software (GE Healthcare). Yellowish spots represent proteins of equal abundance between the two samples. The numbered arrows indicate proteins identified by MS (listed in Table II) with abundances that were significantly different between treatments (identified in all nine gel images; P < 0.05; abundance difference > 1.5). Below the DIGE image is a Venn diagram representing the percentage of protein spots significantly changing in abundance between the two treatments. The percentage of protein spots significantly more abundant under anoxia or aeration is shown on the left or right side in each Venn diagram, respectively. The percentage of proteins that did not significantly differ in abundance is shown in the middle.
We then conducted two additional independent analyses to further quantify the difference between 4-d aerated and 6-d anoxic coleoptiles in order to overcome limitations of the pH 3 to 10 nonlinear (3-10 NL) DIGE analysis. We used a broader pH range to show more basic protein spots in a DIGE analysis (using pI 3–11 gels) and a non-gel-based iTRAQ (for isobaric tag for relative and absolute quantitation) experiment to remove the bias against protein size and solubility that is inherent to IEF-based analysis. Out of the 1,007 spots detected in the pI 3 to 11 DIGE, 140 (13.9%) spots met the criteria of significance. Forty-six of these prospective proteins were more abundant under anoxia and 94 were more abundant under aeration (Supplemental Figs. S2 and S4). Using iTRAQ analysis, we identified 142 proteins, 126 of which could be quantified in a three-biological-replicate experiment comparing coleoptile proteomes extracted from 6-d anoxic and 4-d aerated rice seedlings (Supplemental Table S1). Among them, 34 were significantly more abundant under anoxia and 29 were significantly more abundant under aeration (Supplemental Fig. S2). The fold differences in protein abundance as revealed by iTRAQ were proportional to those revealed by the DIGE analysis, with the r2 being 0.61 (Supplemental Fig. S3A). However, the DIGE analysis resolved much larger fold differences between the two treatments than those calculated in the iTRAQ analysis (Supplemental Fig. S3A). For example, according to the DIGE analysis, peroxiredoxin (Os07g44430.1) was reported to be 3.38- to 22.69-fold more abundant in 6-d anoxic rice coleoptiles than in the 4-d aerated control, whereas this difference was only 2.48-fold according to the iTRAQ analysis (Table II). Similar discrepancies between the linearity of responses by the two methods have been previously reported (Wu et al., 2006).
Table II. Proteomic analysis of rice coleoptiles in response to anoxia or switch from air to anoxia.
Proteins selected from DIGE corresponding to Figure 1, A and B, and iTRAQ (Supplemental Table S2) were identified by MS/MS (Protein ID) with corresponding rice gene numbers (Os Gene No.). Details of the matched protein size, number of peptides identified, and percentage coverage are shown in Supplemental Table S1. Transcript abundance differences between anoxic (4 d) and aerated (4 d) rice coleoptiles derived from independent microarray data (Lasanthi-Kudahettige et al., 2007) have been incorporated (significant increase [positive, boldface], significant decrease [negative, boldface italic]). Protein spots chosen for MS/MS analysis met the following criteria in at least one analysis: a protein abundance difference of 1.5 or greater where proteins were higher in anoxic samples (positive, boldface) and those higher in aeration (negative, boldface italic), P < 0.05, and an abundance high enough on preparative gels for subsequent MS identification. FAD, Fold abundance difference; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; iPGAM, 2,3-bisphosphoglycerate-independent phosphoglycerate mutase; MetSyn, cobalamin-independent Met synthase; N/S, not significant; 3-PGDH, d-3-phosphoglycerate dehydrogenase; PSAT, phospho-Ser aminotransferase; S, significant; SHMT, Ser hydroxymethyltransferase; Sig., significantly different from 1 or not; TGM, transformed geometric mean; X, no data.
| Functional Category | Os Gene No. | Protein ID | DIGE: 3-10NL |
DIGE: 3-11NL |
DIGE: 3-10NL |
iTRAQ |
Array | |||||||
| 6 d N2/4 d Air |
6 d N2/4 d Air |
4 d Air 1 d N2/4 d Air |
6 d N2/4 d Air |
4 d N2/4 d Air | ||||||||||
| Spot | FAD | P | Spot | FAD | P | Spot | FAD | P | TGM | Sig. | FAD | |||
| Sugar metabolism, | Os03g28330.1 | Suc synthase | – | X | X | – | X | X | – | X | X | 1.6 | S | 2 |
| glycolysis, | Os06g09450.1 | Suc synthase | – | X | X | – | X | X | – | X | X | 1.7 | S | 2 |
| fermentation, | Os01g60190.1 | iPGAM | 31 | 3.3 | 4.E-04 | 20 | 5.7 | 5.E-04 | 27 | 1.3 | 1.E-01 | −1.0 | N/S | 2 |
| and TCA cycle | Os01g60190.1 | iPGAM | 32 | 4.3 | 4.E-06 | 21 | 6.1 | 3.E-05 | 26 | 1.5 | 9.E-03 | −1.0 | N/S | 2 |
| Os10g08550.1 | Enolase | 33 | 7.5 | 5.E-09 | 18 | 5.7 | 9.E-04 | 28 | 2.4 | 7.E-04 | 1.2 | S | 2 | |
| Os10g08550.1 | Enolase | 34 | 2.2 | 3.E-05 | 19 | 2.8 | 2.E-03 | 29 | 1.5 | 8.E-04 | 1.2 | S | 2 | |
| Os08g02120.1 | Fructokinase-2 | – | X | X | – | X | X | – | X | X | −2.2 | S | −2 | |
| Os05g33380.1 | Aldolase | 16 | 4.7 | 5.E-06 | – | X | X | 5 | 2.1 | 3.E-04 | 2.0 | S | N/S | |
| Os01g67860.1 | Aldolase | – | X | X | – | X | X | – | X | X | 2.2 | S | N/S | |
| Os10g08022.1 | Aldolase | – | X | X | – | X | X | – | X | X | 2.3 | S | X | |
| Os04g40950.1 | GAPDH | 6 | 2.2 | 7.E-04 | 6 | 2.4 | 2.E-03 | 4 | 1.1 | 8.E-02 | 1.4 | S | N/S | |
| Os04g40950.1 | GAPDH | 10 | 3.6 | 7.E-05 | – | X | X | 11 | 1.0 | 7.E-01 | 1.4 | S | N/S | |
| Os04g40950.1 | GAPDH | – | X | X | 8 | 2.6 | 1.E-03 | – | X | X | 1.4 | S | N/S | |
| Os02g38920.1 | GAPDH | 18 | 13.2 | 5.E-06 | – | X | X | 9 | 1.7 | 2.E-03 | 1.8 | S | 3 | |
| Os02g38920.1 | GAPDH | 17 | 6.7 | 4.E-08 | – | X | X | 8 | 1.7 | 1.E-02 | 1.8 | S | 3 | |
| Os08g03290.1 | GAPDH | 15 | 2.5 | 6.E-04 | – | X | X | 6 | 1.2 | 4.E-02 | X | X | N/S | |
| Os08g03290.2 | GAPDH | – | X | X | – | X | X | – | X | X | 1.3 | S | N/S | |
| Os03g50480.1 | Phosphoglucomutase | 14 | 6.3 | 2.E-05 | – | X | X | 25 | 2.7 | 2.E-03 | −1.1 | S | N/S | |
| Os02g07260.1 | Phosphoglycerate kinase | 28 | 5.3 | 1.E-04 | – | X | X | 31 | 2.4 | 1.E-02 | 1.2 | S | 2 | |
| Os01g05490.1 | Triosephosphate isomerase | 40 | 2.9 | 5.E-05 | – | X | X | 36 | 1.2 | 9.E-03 | 1.4 | S | 4 and N/S | |
| Os06g13810.1 | PPi-phosphofructokinase | 23 | 2.8 | 2.E-02 | – | X | X | 19 | 2.1 | 9.E-04 | 1.4 | S | 2 | |
| Os05g39310.1 | Pyruvate decarboxylase 1 | – | X | X | – | X | X | – | X | X | 2.0 | S | 594 | |
| Os11g10480.1 | ADH1 | 12 | 6.0 | 2.E-05 | – | X | X | 12 | 3.6 | 4.E-05 | 2.2 | S | 4 | |
| Os01g46070.1 | Malate dehydrogenase | – | X | X | – | X | X | – | X | X | −1.1 | S | N/S | |
| Amino acid synthesis | Os02g50240.1 | Gln synthetase | 38 | −2.3 | 7.E-05 | – | X | X | 34 | −1.3 | 3.E-04 | −2.3 | N/S | −2 |
| Os12g42884.1 | MetSyn | – | X | X | – | X | X | – | X | X | −1.7 | S | −2 and N/S | |
| Os12g42876.1 | MetSyn | 25 | 2.3 | 2.E-03 | – | X | X | 17 | 3.3 | 2.E-03 | −1.7 | S | X | |
| Os12g42876.1 | MetSyn | 26 | 1.1 | 6.E-01 | – | X | X | 18 | 2.7 | 3.E-03 | −1.7 | S | X | |
| Os03g06200.1 | PSAT | 5 | 4.7 | 9.E-07 | 5 | 6.5 | 1.E-03 | – | X | X | 1.7 | N/S | 5 | |
| Os03g06200.1 | PSAT | – | X | X | 9 | 5.6 | 1.E-03 | – | X | X | 1.7 | N/S | 5 | |
| Os12g22030.1 | SHMT | 19 | 1.6 | 9.E-02 | – | X | X | 14 | 2.0 | 4.E-04 | X | X | N/S | |
| Os04g55720.1 | 3-PGDH | 24 | 5.8 | 2.E-05 | – | X | X | 20 | 2.0 | 1.E-03 | 1.9 | S | 4 | |
| Os10g25130.1 | Ala aminotransferase | – | X | X | 15 | 2.5 | 4.E-03 | – | X | X | X | X | N/S | |
| Stress responsive | Os07g44430.1 | Peroxiredoxin | 4 | 5.5 | 6.E-05 | 4 | 11.0 | 5.E-05 | – | X | X | 2.5 | S | 32 |
| Os07g44430.1 | Peroxiredoxin | 3 | 3.4 | 2.E-03 | 3 | 22.7 | 1.E-06 | – | X | X | 2.5 | S | 32 | |
| Os05g25850.1 | Manganese-superoxide dismutase | 1 | 1.6 | 1.E-02 | 1 | 1.9 | 1.E-02 | – | X | X | 1.4 | N/S | N/S | |
| Os03g17690.1 | Ascorbate peroxidase | 39 | −4.6 | 3.E-06 | – | X | X | 13 | −2.3 | 1.E-04 | −1.8 | S | N/S | |
| Os07g49400.1 | Ascorbate peroxidase | – | X | X | – | X | X | – | X | X | −1.9 | S | N/S | |
| Os03g07180.1 | Embryotic protein DC-8 | – | X | X | 10 | 3.5 | 4.E-05 | – | X | X | X | X | X | |
| Os03g07180.1 | Embryotic protein DC-8 | – | X | X | 11 | 3.9 | 3.E-05 | – | X | X | X | X | X | |
| Os03g07180.1 | Embryotic protein DC-8 | – | X | X | 12 | 3.6 | 4.E-05 | – | X | X | X | X | X | |
| Os03g07180.1 | Embryotic protein DC-8 | – | X | X | 13 | 4.7 | 1.E-04 | – | X | X | X | X | X | |
| Os05g46480.1 | LEA group 3 | 2 | 4.1 | 7.E-07 | 2 | 7.1 | 2.E-04 | – | X | X | X | X | −2 | |
| Os02g15250.1 | LEA domain containing | – | X | X | 14 | 5.9 | 8.E-05 | – | X | X | – | X | X | |
| Translation | Os03g14530.1 | S10/S20 ribosomal protein | – | X | X | – | X | X | – | X | X | 1.3 | S | 2 |
| Os11g29190.1 | 40S ribosomal protein S5 | – | X | X | – | X | X | – | X | X | −1.3 | S | 2 | |
| Os03g08010.1 | Elongation factor 1-α | – | X | X | – | X | X | – | X | X | 1.3 | S | N/S | |
| Os01g52470.1 | Elongation factor | – | X | X | – | X | X | – | X | X | 1.4 | S | X | |
| Os02g32030.1 | Elongation factor | – | X | X | – | X | X | – | X | X | −1.1 | S | N/S | |
| Miscellaneous | Os08g04210.1 | Protein kinase | – | X | X | – | X | X | – | X | X | 3.2 | S | 1,007 |
| Os08g04250.1 | Protein kinase | – | X | X | – | X | X | – | X | X | 2.3 | S | 248 | |
| Os08g04240.1 | Protein kinase | – | X | X | – | X | X | – | X | X | 2.8 | S | 525 | |
| Os04g56430.1 | CRK5 | – | X | X | – | X | X | – | X | X | −1.9 | S | 3 | |
| Os01g03340.1 | BBTI4 | 11 | −6.0 | 2.E-06 | – | X | X | – | X | X | −1.9 | S | −3 and −2 | |
| Os01g03360.1 | BBTI5 | – | X | X | – | X | X | – | X | X | 1.1 | S | −2 | |
| Os03g62060.1 | IAA-amino acid hydrolase | 36 | −5.3 | 3.E-04 | 17 | −4.6 | 6.E-03 | 32 | −1.0 | 4.E-01 | −3.0 | S | −271 | |
| Os05g04510.1 | S-Adenosyl-Met synthetase | – | X | X | – | X | X | – | X | X | −1.6 | S | N/S | |
Identified Proteins in Rice Coleoptiles with Changes in Abundance under Anoxia
The identified proteins with significant changes in abundance between aerated and anoxic treatments are listed in Table II. The protein identification evidence from all protein analysis is shown in Supplemental Table S1, and further details for iTRAQ data analysis are provided in Supplemental Table S2. We have incorporated microarray data (4-d-old anoxic and 4-d-old aerobic rice coleoptiles from Lasanthi-Kudahettige et al. [2007]) into Table II and Supplemental Table S1 for further comparison. The enzymes detected, which are involved in glycolysis, fermentation, and amino acid biosynthesis, were also incorporated into a metabolite pathway map in Figure 2. We could not identify the five protein spots that were significantly different between treatments in wheat coleoptiles due to their very low abundance on gels.
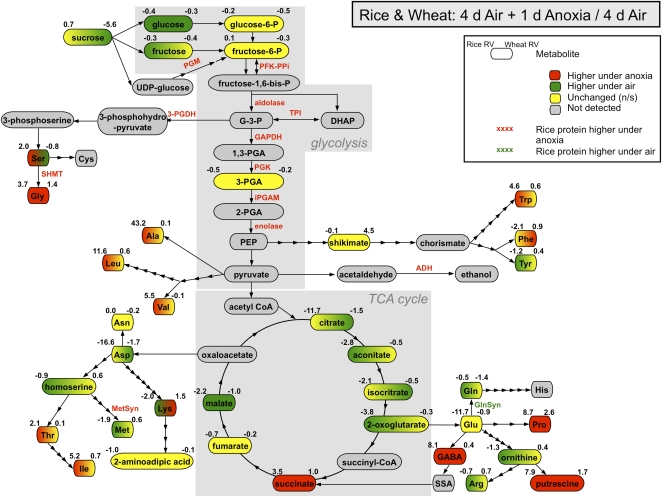
Figure 2.
Effect of prolonged anoxia on carbohydrate metabolism, glycolysis, fermentation, amino acid metabolism, and the TCA cycle in rice coleoptiles. Rice seeds were germinated and grown under anoxia for 6 d or aeration for 4 d. The green and red boxes represent metabolites significantly more abundant under aeration and anoxia, respectively (P < 0.05). The yellow boxes represent metabolites whose abundances are unchanged. Enzyme names on arrows are also colored in this fashion. The numbers on the top left side of each box represent the response value (RV) of the corresponding metabolite (anoxic/aerated) in rice coleoptiles. All data were extracted from Tables II and III. Metabolite abbreviations are as follows: DHAP, dihydroxyacetonephosphate; GABA, γ-aminobutyrate; G-3-P, glyceraldehyde-3-phosphate; PEP, phosphoenolpyruvate; 1,3-PGA, 1,3-bisphosphoglycerate; 2-PGA, 2-phosphoglycerate; 3-PGA, 3-phosphoglycerate; SSA, succinic semialdehyde. Protein abbreviations are as follows: ADH, alcohol dehydrogenase; AlaAT, Ala aminotransferase; aldolase, Fru-bisP aldolase; FK, fructokinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GlnSyn, Gln synthetase; iPGAM, 2,3-bisphosphoglycerate-independent phosphoglycerate mutase; MDH, malate dehydrogenase; MetSyn, 5-methyltetrahydropteroyltri-Glu-homo-Cys methyltransferase (cobalamin-independent Met synthase); PDC, pyruvate decarboxylase; PFK-PPi, PPi-Fru-6-P 1-phosphotransferase; 3-PGDH, d-3-phosphoglycerate dehydrogenase; PGK, phosphoglycerate kinase; PGM, phosphoglucomutase; PSAT, phospho-Ser aminotransferase; SHMT, Ser hydroxymethyltransferase; SS, Suc synthase; TPI, triosephosphate isomerase.
Enzymes Involved in Glycolysis and Ethanolic Fermentation
We identified the accumulation of enzymes involved in ethanolic fermentation such as ADH1 (Os11g10480) and pyruvate decarboxylase 1 (Os05g39310) in anoxic rice coleoptiles (Table II; Fig. 2), consistent with published reports (Mocquot et al., 1981; Ricard and Pradet, 1989; Bailey-Serres and Chang, 2005; Huang et al., 2005; van Dongen et al., 2009). Proteins significantly increasing in abundance and involved in multiple steps of glycolysis were also identified and listed in Table II and Figure 2. Those enzymes were pyrophosphate (PPi)-Fru-6-P 1-phosphotransferase β-subunit (Os06g13810), Fru-bisP aldolase (Os05g33380, Os01g67860, Os10g08022), triosephosphate isomerase (Os01g05490), glyceraldehyde-3-phosphate dehydrogenase (Os04g40950, Os02g38920, Os08g03290), phosphoglycerate kinase (Os02g07260), phosphoglucomutase (Os03g50480), 2,3-bisphosphoglycerate-independent phosphoglycerate mutase (Os01g60190), and enolase (Os10g08550; Table II; Fig. 2). Exceptions to these anoxia increases were fructokinase 2 (Os08g02120), which was observed to decrease in abundance under anoxia, as well as discrepancies in the direction of change for phosphoglucomutase (Os03g50480) between the DIGE and iTRAQ analyses.
We also identified two Suc synthase enzymes (Os03g28330, Os06g09450) that were significantly more abundant in rice coleoptiles of anoxically germinated seedlings (Table II). Our data agree with reports that under anoxia, there is a switch from invertase to Suc synthase as a means of degrading Suc into sugars that can then enter the glycolytic pathway (Guglielminetti et al., 1995).
These observations reinforced the evidence for general enhancement of glycolysis and ethanolic fermentation for rice coleoptiles during adaptation to anoxia and highlight the gene-specific identification of changing proteins that would promote a Pasteur effect. Only about two-thirds of the transcripts for these glycolytic proteins are transcriptionally more abundant under anoxia in rice (Lasanthi-Kudahettige et al., 2007). But notably, the decreased abundance of fructokinase 2 was also seen at the transcript level under anoxia (Table II).
Enzymes Involved in Amino Acid Metabolism
We identified several enzymes involved in the synthesis of Ser and Gly that increased in abundance under anoxic stress, notably, d-3-phosphoglycerate dehydrogenase (Os04g55720), phospho-Ser aminotransferase (Os03g06200), and Ser hydroxymethyltransferase (Os12g22030; Table II; Fig. 2). Ala aminotransferase 2 (Os01g25130) was also more abundant in anoxic rice coleoptiles. Gln synthetase root isozyme 3 (Os02g50240) was less abundant under anoxia when compared with aeration, and different isoforms of 5-methyltetrahydropteroyltri-Glu-homo-Cys methyltransferase (Met synthase; Os12g42876 up in DIGE, Os12g42876/Os12g42884 down in iTRAQ) showed discrepancies in their direction of change. Met generation is particularly interesting, as this amino acid is involved in the synthesis of ethylene, a plant hormone involved in submergence-induced gene expression (Fukao et al., 2006; Xu et al., 2006). With the exception of 6-d anoxia samples that showed anoxic accumulation, Met tended toward a higher abundance under aerobic conditions in rice (Table III). The enzyme involved in the first step of ethylene production, S-adenosyl-Met synthase (Os05g04510), was also detected in our iTRAQ analysis (Table II). However, it was significantly less abundant in coleoptiles of anoxically germinated rice, an observation in line with the oxygen dependence of the ethylene biosynthetic pathway.
Table III. Metabolomic analysis of rice and wheat coleoptiles in response to anoxia.
Rice plants were grown under (1) 4 d of aeration, (2) 6 d of anoxia, (3) 4 d of aeration and then 4 h of anoxia, or (4) 4 d of aeration and then 1 d of anoxia. Wheat plants were grown under all treatments except number 2. Coleoptile tissues were separated from leaves for analysis. Complex polar metabolite extracts were taken from all tissues and analyzed by GC-MS. Raw GC-MS data processing and statistical analyses were then carried out using MetabolomeExpress software (version 1.0; http://www.metabolome-express.org). Metabolite signal intensity ratios were calculated by dividing the mean tissue mass- and internal standard-normalized signal intensity for each metabolite in treated samples by its corresponding value in control samples (RV columns). The statistical significance of each ratio was tested by Welch’s t test (P < 0.05; n = 5). Values highlighted in boldface are significantly more abundant in anoxic coleoptiles, whereas negative boldface italic indicates a metabolite that is more abundant under aeration, with values that meet a threshold for significance of P < 0.05. N/D, No data; RV, response value.
| Sample | Metabolite | 6 d N2/4 d air |
4 d Air + 1 d N2/4 d Air |
4 d Air + 4 h N2/4 d Air |
|||||||
| Rice |
Rice |
Wheat |
Rice |
Wheat |
|||||||
| RV | P | RV | P | RV | P | RV | P | RV | P | ||
| Amino acids and polyamines | l-Pro | 98.6 | 5.8E-06 | 8.7 | 2.5E-02 | 2.6 | 1.7E-03 | 0.5 | 4.9E-01 | 0.5 | 1.4E-01 |
| l-Leu | 43.8 | 1.1E-05 | 11.6 | 1.7E-03 | 0.6 | 6.6E-02 | 0.6 | 4.6E-01 | 0.1 | 6.0E-01 | |
| β-Ala | 43.4 | 1.4E-08 | 0.7 | 2.6E-01 | 1.0 | 3.3E-03 | 0.9 | 4.4E-01 | 0.9 | 4.9E-04 | |
| l-Ala | 42.6 | 2.3E-06 | 43.2 | 8.1E-04 | 0.1 | 6.3E-01 | 6.9 | 1.5E-03 | 0.5 | 3.5E-01 | |
| l-Glu | 36.5 | 1.7E-06 | −11.7 | 6.9E-02 | −0.9 | 5.8E-02 | −39.2 | 7.7E-02 | −0.4 | 2.8E-01 | |
| l-Tyr | 22.5 | 4.4E-06 | −1.2 | 4.3E-03 | 0.4 | 2.7E-01 | −2.3 | 9.6E-02 | 0.2 | 3.0E-01 | |
| 2-Aminoadipic acid | 17.3 | 6.6E-07 | −1.0 | 8.1E-02 | −0.1 | 7.4E-01 | 0.1 | 7.4E-01 | −0.4 | 1.6E-01 | |
| l-Arg | 15.6 | 1.2E-05 | −0.7 | 2.7E-03 | 0.7 | 1.0E-01 | 0.0 | 9.6E-01 | 0.1 | 5.7E-01 | |
| l-Homo-Ser | 15.5 | 1.9E-02 | −0.9 | 1.0E-02 | 0.6 | 3.2E-01 | −0.9 | 9.6E-02 | −0.2 | 3.2E-01 | |
| l-Ile | 12.0 | 3.2E-05 | 5.2 | 6.6E-04 | 0.7 | 1.1E-01 | 0.3 | 4.7E-01 | 0.3 | 3.3E-01 | |
| l-Lys | 10.9 | 3.6E-05 | −2.0 | 3.1E-03 | 1.5 | 3.5E-02 | −1.8 | 1.1E-01 | 0.5 | 1.0E-01 | |
| l-Val | 10.2 | 5.8E-07 | 5.5 | 3.4E-07 | −0.1 | 6.6E-01 | −0.1 | 8.8E-01 | 0.3 | 4.2E-01 | |
| l-Ser | 9.0 | 5.9E-12 | 2.0 | 2.3E-03 | −0.8 | 4.4E-03 | 0.1 | 8.3E-01 | −0.4 | 4.2E-01 | |
| γ-Aminobutyric acid | 8.9 | 1.5E-08 | 8.1 | 1.2E-03 | 0.4 | 3.5E-03 | 2.3 | 1.4E-01 | 0.6 | 8.6E-04 | |
| l-Met | 6.0 | 2.8E-02 | −1.9 | 3.9E-02 | 0.6 | 1.4E-01 | −123.6 | 5.4E-02 | −0.2 | 4.4E-01 | |
| l-Trp | 5.2 | 1.8E-05 | 4.6 | 1.7E-02 | 0.6 | 1.9E-01 | −0.4 | 3.5E-01 | 0.5 | 8.8E-02 | |
| l-Thr | 2.4 | 1.1E-06 | 2.1 | 6.4E-06 | 0.1 | 4.3E-01 | 0.6 | 1.4E-01 | 0.5 | 1.2E-01 | |
| Gly | 1.9 | 2.5E-03 | 3.7 | 1.2E-03 | 1.4 | 4.4E-03 | 0.6 | 1.9E-01 | 0.4 | 2.0E-01 | |
| l-Gln | 1.8 | 4.6E-04 | −0.5 | 4.4E-03 | −1.4 | 9.0E-02 | −0.2 | 2.3E-01 | −1.5 | 2.0E-03 | |
| 4-Hyp | 1.4 | 1.1E-06 | −0.1 | 4.9E-01 | −0.1 | 4.9E-01 | 0.1 | 5.0E-01 | −0.9 | 2.2E-02 | |
| Orn | 0.6 | 3.5E-03 | −1.3 | 2.8E-04 | 0.4 | 1.8E-01 | −10.0 | 4.3E-02 | −0.9 | 1.2E-01 | |
| l-Asn | −0.6 | 4.5E-01 | 0.0 | 9.7E-01 | −0.2 | 5.9E-01 | −40.0 | 4.7E-02 | 0.1 | 6.1E-01 | |
| l-Asp | −1.5 | 1.5E-01 | −16.6 | 2.2E-01 | −1.7 | 3.7E-02 | −448.4 | 4.7E-02 | −3.2 | 1.2E-03 | |
| l-Phe | N/D | N/D | −2.1 | 1.5E-01 | 0.9 | 5.3E-03 | −0.2 | 2.1E-01 | 0.4 | 6.6E-02 | |
| l-α-Aminobutyric acid | N/D | N/D | 2.5 | 1.5E-04 | −2.7 | 2.7E-01 | 0.2 | 4.6E-01 | −1.2 | 2.4E-03 | |
| Putrescine | 52.4 | 6.9E-08 | 7.9 | 1.1E-04 | 1.7 | 4.2E-03 | 0.2 | 5.9E-01 | −0.2 | 1.5E-01 | |
| Sugars and glycolytic substrates | 6-Phosphogluconate | 9.7 | 1.0E-03 | −0.8 | 3.6E-01 | −1.6 | 1.1E-01 | −1.4 | 3.9E-02 | −1.0 | 1.0E-02 |
| d-Rib | −0.8 | 9.2E-05 | −1.2 | 9.2E-04 | −1.3 | 3.1E-03 | −0.5 | 2.9E-04 | −0.6 | 1.3E-02 | |
| Trehalose | −1.0 | 2.3E-02 | −2.1 | 3.1E-02 | −1.6 | 3.3E-04 | −0.3 | 1.8E-01 | −0.9 | 1.9E-03 | |
| d-Xyl | −2.7 | 5.8E-06 | −0.5 | 1.4E-01 | 0.1 | 6.4E-01 | 0.1 | 2.2E-01 | 0.1 | 4.0E-01 | |
| 3-Phosphoglyceric acid | 18.0 | 1.3E-04 | −0.5 | 5.5E-01 | −0.2 | 7.6E-01 | −0.3 | 5.6E-01 | −0.2 | 1.2E-01 | |
| Suc | 4.8 | 3.4E-04 | 0.7 | 9.3E-02 | −5.6 | 3.6E-05 | −0.3 | 1.3E-01 | −11.5 | 1.5E-03 | |
| Fru-6-P | 2.9 | 2.9E-04 | 0.1 | 8.7E-01 | −0.3 | 2.8E-01 | −0.2 | 3.4E-01 | −1.2 | 8.8E-03 | |
| Glc-6-P | 2.8 | 4.4E-05 | −0.2 | 6.9E-01 | −0.5 | 1.0E-01 | −0.1 | 1.7E-01 | −1.4 | 2.2E-03 | |
| d-Fru | −2.4 | 9.9E-07 | −0.3 | 1.7E-05 | −0.4 | 3.9E-01 | 0.0 | 8.1E-01 | −0.4 | 3.8E-02 | |
| d-Glc | −4.2 | 2.1E-05 | −0.4 | 8.8E-05 | −0.3 | 6.3E-03 | −0.1 | 2.0E-01 | 0.1 | 4.9E-01 | |
| TCA cycle substrates and other acids | Succinate | 116.5 | 1.8E-08 | 3.5 | 4.2E-03 | 1.0 | 1.1E-03 | 6.5 | 1.5E-03 | −1.1 | 2.7E-03 |
| Fumarate | 7.0 | 2.9E-07 | −0.7 | 2.7E-01 | −0.2 | 1.6E-01 | 1.1 | 5.1E-02 | −0.4 | 1.7E-01 | |
| Aconitate | 4.3 | 5.3E-04 | −2.8 | 3.2E-02 | −0.5 | 1.5E-01 | −1.6 | 1.1E-02 | −0.2 | 2.4E-01 | |
| Citrate | 2.5 | 4.0E-05 | −11.7 | 5.5E-02 | −1.5 | 8.3E-03 | −3.2 | 1.3E-02 | −0.7 | 1.4E-04 | |
| Malate | 0.0 | 1.5E-01 | −2.2 | 8.8E-05 | −1.0 | 3.1E-05 | −0.1 | 9.2E-02 | 0.0 | 7.5E-01 | |
| Isocitrate | −0.1 | 1.9E-01 | −2.1 | 8.7E-02 | −0.5 | 3.7E-02 | −5.3 | 6.0E-03 | −0.5 | 1.1E-03 | |
| 2-Oxoglutarate | −2.2 | 1.3E-03 | −3.8 | 2.6E-02 | −0.3 | 1.8E-01 | −24.5 | 6.4E-04 | −0.8 | 2.8E-03 | |
| Glycerate | −1.8 | 1.3E-04 | −1.5 | 2.6E-06 | −0.9 | 7.3E-03 | −0.5 | 1.6E-02 | −0.2 | 8.9E-03 | |
| Threonate | −118.6 | 1.0E-05 | −1.1 | 7.0E-04 | −0.2 | 4.3E-03 | 0.3 | 8.7E-03 | −0.2 | 2.0E-01 | |
| Ascorbate | N/D | N/D | −1.8 | 3.8E-04 | 1.2 | 2.2E-01 | −0.1 | 2.0E-01 | −0.1 | 2.7E-01 | |
| Glucarate | 0.9 | 1.1E-04 | −0.3 | 3.0E-02 | −0.4 | 3.9E-03 | −0.2 | 1.9E-01 | −0.2 | 2.1E-01 | |
| Citramalate | −0.5 | 5.7E-02 | −0.8 | 1.9E-02 | −0.2 | 6.8E-02 | 0.2 | 6.1E-02 | −1.4 | 4.9E-03 | |
| 4-Hydroxycinnamate | N/D | N/D | −0.1 | 3.0E-01 | 0.5 | 1.2E-03 | 0.2 | 2.2E-02 | 0.8 | 8.0E-04 | |
| Urate | 561 | 2.1E-06 | 0.2 | 5.8E-01 | 15.2 | 2.9E-03 | −1.4 | 3.3E-01 | 15.4 | 3.4E-04 | |
| Other | Shikimic acid | −0.7 | 1.0E-03 | −0.1 | 4.1E-01 | 4.5 | 6.2E-02 | 0.0 | 9.3E-01 | 3.4 | 1.5E-01 |
| Phosphate | 1.6 | 5.5E-08 | 0.3 | 2.9E-02 | −0.1 | 6.0E-01 | −0.1 | 6.2E-01 | −0.2 | 2.4E-01 | |
| Cytosine | N/D | N/D | −1.6 | 2.0E-04 | −0.6 | 1.5E-05 | −0.2 | 4.0E-01 | −0.3 | 3.0E-01 | |
Enzymes or Proteins Involved in Reactive Oxygen Species Detoxification
Reactive oxygen species (ROS) play an important role in signaling under oxygen deficiency (Baxter-Burrell et al., 2002; Bailey-Serres and Chang, 2005). We identified several proteins involved in ROS degradation that changed in abundance. As examples, peroxiredoxin (Os07g44430) was significantly more abundant in rice coleoptiles derived from 6-d-old anoxic seedlings than in 4-d-old aerated seedlings (Table II). In concordance, the transcript for this gene was 32-fold higher in abundance in anoxic rice coleoptiles (Lasanthi-Kudahettige et al., 2007). Peroxiredoxin is an antioxidant enzyme that can reduce both hydrogen peroxide (H2O2) and alkyl hydroperoxides. In contrast, we identified another H2O2-decomposing enzyme, ascorbate peroxidase (Os03g17690, Os07g49400), that was less abundant in anoxic coleoptiles (Table II). The Bowman-Birk-type trypsin inhibitor (BBTI; Os01g03340) found to decrease in abundance under anoxia may have other functions beyond its role in proteolysis. BBTIs have been reported to act as monodehydroascorbate reductases and dehydroascorbate reductases in etiolated mung bean (Vigna radiata) seedlings (Hou et al., 2000) and roots of sweet potato (Ipomoea batatas; Hou and Lin, 1997) and thus can be involved in the regeneration of ascorbate. These results suggest that rice coleoptiles may use different detoxification systems under anoxia/hypoxia and reaeration from those used during continual aeration. It has been reported that anoxia can cause an increase in H2O2 in the rice root apoplast and plasma membrane (Blokhina et al., 2001), suggesting a protective function of these antioxidant defense enzymes in anoxic rice coleoptiles. However, we have previously measured markers for lipid oxidation and demonstrated that damage was lower in anoxic coleoptiles relative to aerobic or reoxygenated coleoptiles (Millar et al., 2004), suggesting that either (1) oxidative stress under anoxia is minimal and that peroxiredoxin has a protective role upon the return of oxygen or (2) peroxiredoxin has a function under anoxia that we have not anticipated. The former seems more likely, given that oxygen is necessary for the formation of ROS. Interestingly, manganese-superoxide dismutase was detected in two DIGE analyses, and although statistically significant in its accumulation under 6 d of anoxia (P < 0.05), manganese-superoxide dismutase did not meet all of the criteria set for significance; specifically, the abundance change did not exceed 2 (Table II), indicating the quantitatively differential role of peroxiredoxin in responding to the availability of oxygen. What is clear from the literature is that oxygen deprivation perturbs the redox status of cells, whether it be ROS levels (Blokhina et al., 2001), oxidative damage (Blokhina et al., 1999; Millar et al., 2004), or regulation of genes and small-molecule antioxidants involved in redox regulation (Yan et al., 1996; Biemelt et al., 1998; Blokhina et al., 2000, 2003; van Dongen et al., 2009).
Proteins Involved in the Process of Translation
Selective translation of cytoplasmic mRNAs in plants under oxygen deficiency has been discussed (Bailey-Serres, 1999). We identified several proteins in the iTRAQ analysis involved in translation processes, which were more abundant in 6-d-old anoxic coleoptiles relative to the control. Those proteins were elongation factor 1-α (Os03g08010), elongation factor 2 (Os01g52470), and S10/S20 domain-containing ribosomal protein (Os03g14530; Table II). Others involved in translation that were less abundant under anoxia were 40S ribosomal protein S5 (Os11g29190) and elongation factor (Os02g32030). Whether these abundance changes in the translational machinery are responsible for the perturbations in the levels of glycolytic, amino acid biosynthetic, and ROS-defense proteins is currently unknown.
Other Proteins of Interest
The lower abundance of indole-3-acetic acid (IAA)-amino acid hydrolase precursor (ILR1; Os03g62060) we report under anoxia can be related to a long history of research on auxin-regulated coleoptile elongation dating back to the famous experiment of Went (1942). The IAA-amino acid hydrolase is involved in the cleavage of conjugates between IAA and amino acids (Bartel and Fink, 1995). The dramatic decrease of ILR1 (Table II) could indicate that IAA is maintained in its conjugate form under anoxia. Microarray data also suggest that the transcript of this protein was dramatically down-regulated (271-fold) in anoxic rice coleoptiles (Lasanthi-Kudahettige et al., 2007). This supports the observation that auxin-binding activities were decreased in anoxic coleoptiles (Mapelli and Locatelli, 1995) and that there was no synergistic effect of IAA and anaerobiosis on rice coleoptile elongation (Pegoraro et al., 1988). The repressive effect of anoxia on auxin-related genes has also been observed in Arabidopsis through a global gene expression analysis (Loreti et al., 2005).
Also of interest was the finding that several proteins with unknown functions accumulated under anoxia. These proteins are annotated as protein kinases that contain the domain of unknown function 26. Their transcript fold increases under anoxia range from 248-to 1,007-fold (Os08g04250, Os08g04210, Os08g04240; Lasanthi-Kudahettige et al., 2007). In addition, the Arabidopsis ortholog (At5g48540; Supplemental Table S3) is up-regulated in response to 2 and 9 h of hypoxia both within the total and polysomal mRNA pools of Arabidopsis seedlings (Branco-Price et al., 2008). Notably, we found anoxic accumulation of two group 3 late embryogenesis abundant proteins (LEA; Os05g46480, Os02g15250) as well as embryonic protein DC-8 (Os03g07180). LEAs are hydrophilic unstructured proteins rich in Gly, Ala, and Ser (Baker et al., 1988; Campos et al., 2006). It has been reported that a LEA protein (Os04g52110) accumulates in anoxic rice embryos (Howell et al., 2007), and other groups have demonstrated the inducibility of GUS reporters when fused to a carrot (Daucus carota) group 3 LEA promoter under hypoxia, salinity, and dehydration (Siddiqui et al., 1998). Recently, it was shown that SUB1A increased the accumulation of transcripts encoding for proteins involved in dehydration tolerance. Most interestingly, the LEA3 transcript level increased to a greater degree during desubmergence in M202(Sub1) rice relative to wild-type M202 (Fukao et al., 2011). This is especially interesting given that dehydration is a stress inherent to desubmergence. This LEA3 transcript actually showed a decrease during submergence, a change in opposition to what we have found. This may be attributable to the use of different cultivars, the measurement of transcript levels and not protein abundance levels, and that submergence and anoxia are not perfectly comparable. Despite these differences, in our experimental system, it is tempting to speculate that LEA up-regulation is a response that provides a protective and anticipatory function for when plants return to air. Clearly, LEAs are stress responsive; however, the role of these proteins in anoxic environments awaits further insights into their molecular function.
Metabolomic Analysis Reveals a Greater Response to Anoxia in Rice Than in Wheat Coleoptiles
To investigate the impact of the changes in primary metabolism on metabolite pools, we considered the overall changes in the gas chromatography-mass spectrometry (GC-MS) profiles of primary metabolites in wheat and rice coleoptiles exposed to anoxia. Consistent with the physiological and proteomic data, there were dramatic differences in metabolite profiles between rice coleoptiles derived from 4-d-old aerated and 6-d-old anoxic seedlings, with very high accumulation of amino acids under anoxia (Table III; Fig. 2). Many of these responses were also observed in rice coleoptiles that were switched to anoxia for 1 d, although these tended to be considerably more subtle (Table III; Fig. 3).
Figure 3.
Effect of a 1-d anoxic switch on carbohydrate metabolism, glycolysis, fermentation, amino acid metabolism, and the TCA cycle in rice and wheat coleoptiles. Rice and wheat seeds were germinated and grown under aeration for 4 d or for 4 d with a switch to 1 d of anoxia. Green or red boxes represent metabolites significantly more abundant during aeration or the anoxic switch, respectively (P < 0.05). The yellow boxes represent metabolites whose abundances are unchanged. Enzyme names that accompany arrows are also colored in this fashion for the rice response only (anoxia-responsive proteins were not identified in wheat). The numbers on the top left and right side of each square represent the response value (RV) of the corresponding metabolite (anoxia/aeration) in rice and wheat coleoptiles, respectively. All data were extracted from Tables II and III. (For abbreviations, see Fig. 2 legend).
A number of major differences observed in 6-d-old anoxic seedling coleoptiles relative to 4-d-old aerobic seedling coleoptiles were not observed at all as responses in switched seedling coleoptiles (e.g. 17- to 45-fold increases in 3-phosphoglycerate, β-Ala, and 2-aminoadipate and a remarkable 560-fold increase in urate; Supplemental Table S7). Moreover, some metabolites responded in opposite directions to the two treatments (e.g. Arg, homo-Ser, Lys, and Tyr; Supplemental Table S7). These discrepancies are consistent with oxygen-dependent biogenesis of cellular components involved in the regulation of these metabolites in rice.
The wheat coleoptile metabolite profile also responded to anoxia (Table III; Fig. 3; Supplemental Table S4). Certain features were found to be common to responses of wheat and rice coleoptiles to 1-d anoxic transfer. These included accumulations of γ-aminobutyrate, Gly, Ile, Pro, Thr, succinate, and putrescine and decreases in Asp, Fru, Rib, trehalose, citrate, isocitrate, citramalate, glucarate, malate, glycerate, threonate, and cytosine (Table III; Supplemental Table S5). However, in wheat, these responses tended to be much less pronounced than those observed in anoxically switched rice coleoptiles (Table III; Fig. 3).
While some metabolite responses to the 1-d anoxic shift were common to both species (Fig. 3; Table III), we did identify a number of species-specific responses that may be linked to the differential anoxia tolerance of these species (Supplemental Tables S5 and S6). Rice-specific responses included moderate to strong increases in Ser, Ala, Leu, and Trp and decreases in Arg, Met, Tyr, Orn, 6-phosphogluconate, and aconitate (with the aconitate response being the most consistently strong between experiments). Wheat-specific responses included moderate increases in β-Ala, 4-hydroxycinnamate, and shikimate, strong increases in urate, and moderate decreases in 4-Hyp and Suc. Interestingly, a small number of metabolites responded moderately strongly in opposite directions between the two species. For example, α-aminobutyrate and phosphate increased in rice while decreasing in wheat; conversely, Lys, Phe, Xyl, and ascorbate decreased in rice while increasing in wheat. The distinctive and significant accumulation of Ala and Ser in rice was consistent with our evidence of increased abundance of enzymes in these pathways in rice (Fig. 3).
Surprisingly, l-Ala did not significantly differ in abundance between control and anoxically switched wheat coleoptiles (Table III), contradictory to a previous report where Ala levels accumulated in wheat shoots to the same degree as that of rice shoots after 8 h of anoxia (Menegus et al., 1989) as well as a range of reports from other species (van Dongen et al., 2009; Narsai et al., 2011). Such a difference might be explained by differences in the experimental system, the specific dissection of the coleoptile tissue used in this report, or the timing of the amino acid accumulation. For example, accumulation of Ala in roots of Arabidopsis was found by treatment with 48 h of 4% and 8% oxygen but not when the concentration of oxygen was reduced to 1% (van Dongen et al., 2009). To consider the last of these, we repeated metabolite profiling at 4 h after the switch to anoxia in both rice and wheat, but again we saw an increase in l-Ala in rice but not in wheat (Table III).
In addition to changes in amino acids, variations in intermediates in the tricarboxylic acid (TCA) cycle were also observed. In the TCA cycle, the step converting succinate into fumarate by succinate dehydrogenase requires the operation of an electron transport chain and reduction of oxygen to water. Without oxygen, the TCA cycle will stop at succinate dehydrogenase and succinate will accumulate, as we observed in both rice and wheat coleoptiles (Table III; Fig. 3) and as other studies have reported (Menegus et al., 1991; Fan et al., 1997; Rocha et al., 2010). This claim was also supported by the decrease in other TCA cycle intermediates such as malate and citrate in both anoxically switched cereals, yet it was contradicted when observing 6-d-old anoxic rice coleoptiles (Table III; Figs. 2 and 3) and suggests that the other intermediates in the TCA cycle were utilized under anoxia. The advantage of the accumulation of succinate under anoxia has been widely discussed in the context of the extra ATP production that can result (Gibbs and Greenway, 2003; Bailey-Serres and Voesenek, 2008; Rocha et al., 2010).
Under prolonged anoxia, higher abundances of Suc, Glc-6-P, and Fru-6-P in rice coleoptiles were observed (Table III; Fig. 3). Rice coleoptiles treated with shorter periods of anoxia (24 or 4 h) revealed no significant differences in these sugars between stress and control treatments. In wheat, however, a 4-h anoxic treatment resulted in significant decreases in all three sugars, and the 24-h treatment resulted in a decrease of Suc (Table III; Fig. 3). Decreases in the levels of the transportable sugar Suc as well as decreases in glycolytic intermediates might be indicative of a delayed transition to anaerobic metabolism in wheat when compared with rice.
Database-Driven Metabolic Phenotype Analysis Reveals Conserved and Divergent Responses to Low Oxygen in Rice and Wheat
Having established that wheat and rice coleoptiles display differential responses to oxygen deprivation, we thought it would be informative to compare these responses with those previously observed in other species. To this end, we used the PhenoMeter tool of MetabolomeExpress (https://www.metabolome-express.org; for details, see “Materials and Methods”) to search the MetabolomeExpress database of metabolic phenotypes, MetaPhenDB (see “Materials and Methods”), for previously reported metabolic phenotypes of statistically significant qualitative overlap (codirectional responses) or inverse overlap (opposite direction responses) with the rice and wheat responses to anoxia that we report.
As expected, the metabolic responses of rice and wheat coleoptiles to anoxic transfer retrieved significant hits (P < 0.05; Fisher’s exact test) to a number of previously reported responses to oxygen deprivation (Gibon et al., 2002; Narsai et al., 2009, 2011; Rocha et al., 2010; summarized in Supplemental Table S9) while retrieving very few matches to any of the many other functionally less closely related metabolic responses in the MetaPhenDB database (for detailed results, including Fisher’s exact test P values, see Supplemental Table S9). In addition, a number of species-specific positive and negative hits were also observed to diverse treatments (Supplemental Table S8). For example, rice gave highly significant positive hits to the responses of Arabidopsis cell suspensions to inhibition of the mitochondrial electron transport chain complex I (Garmier et al., 2008), while wheat did not give any significant hits to this phenotype. Conversely, only wheat gave significant positive hits to low-oxygen responses of potato (Solanum tuberosum) tubers (Geigenberger et al., 2000), castor bean (Ricinus communis) phloem (van Dongen et al., 2003), or the sulfur depletion-mediated hypoxia response of the Chlamydomonas reinhardtii stm6 mutant (Timmins et al., 2009; Supplemental Table S8). In two cases, waterlogging of Populus × canescens roots (Kreuzwieser et al., 2009) and low-oxygen treatment of Arabidopsis roots (van Dongen et al., 2009), rice gave positive hits while wheat gave inverse hits (Supplemental Table S8), indicating significant divergence between rice and wheat in metabolites that define these responses. Given the large difference that these two species display in their responses to anoxia at the metabolite level, we wanted to consider whether the ability to generate a particular metabolite pool contributes to anoxia tolerance and we set out to test this hypothesis.
Amino Acid-Induced Improvement of Cell Integrity in Wheat under Anoxia
A range of reports in mammalian cells have highlighted that exogenous addition of Gly, Ser, and/or Ala can enhance the survival of cells to oxygen deprivation (Brecht and Groot, 1994; Tijsen et al., 1997; Wang et al., 2010). To test whether the differential accumulation of these amino acids could be part of plant anoxia tolerance and to define the functional importance of the divergence of rice and wheat metabolic responses to anoxia, we supplemented the medium used for rice and wheat growth under anoxia. We supplemented with a combination of amino acids and assessed plant performance with the Evans blue root cell viability assay after 3 d in anoxia (Fig. 4A). This showed that amino acid supplementation significantly increased cell viability in wheat but not in rice roots, consistent with the differential accumulation of these amino acids in rice. To confirm this finding from whole wheat seedlings, we used the measurement of electrical conductivity as a direct indicator of electrolyte leakage, and thus cell integrity, from anoxically treated seedlings in the presence or absence of these three amino acids in several different combinations (Fig. 4B). This showed that the combination of Ser/Ala/Gly significantly lowered electrolyte leakage, as did Ser/Ala, but the presence of only one of the amino acids did not protect wheat seedlings from electrolyte leakage (P < 0.01). We also confirmed the absence of this positive effect in anoxia-tolerant rice seedlings. This suggests that the disparity between rice and wheat metabolite pool responses (Fig. 3) may contribute to the degree of anoxia tolerance and that partial generation of these pools (via exogenous supplementation) in sensitive plants can improve cellular integrity.
Figure 4.
The effect of exogenous amino acid feeding on cell integrity after prolonged anoxia in wheat and rice seedlings. A, Rice and wheat seeds were germinated and grown under 4 d of aeration. Fresh culture medium in the presence or absence of 10 mm Ala, Ser, and/or Gly was then added to seedlings. Seedlings were returned to 3 d of aeration (green) or transferred to 3 d of anoxia (nonsupplemented in red; supplemented in dark red). Roots were then analyzed using the Evans blue viability stain (n = 3). An increase in cell death is proportional to increased A600. B, Cell membrane permeability in whole rice and wheat seedlings was also analyzed (n = 10–23). This was done by measuring electrical conductivity after seedlings were incubated in distilled, deionized water for 1 h (C1). A second measurement was taken after sample boiling (C2) to obtain the proportion of cell leakage in different samples. Larger C1/C2 values indicate higher electrolyte leakage and thus lower cell integrity. *** P < 0.001, ** P < 0.01, * P < 0.05 when compared with anoxic seedlings that were not supplemented (red bars).
DISCUSSION
We have analyzed the differential responses of coleoptiles from rice and wheat seedlings to anoxia at the physiological, metabolomic, and proteomic levels. Our data suggest that despite wheat having an anatomically similar coleoptile, it responds to anoxia to a lesser degree at the molecular level than the coleoptiles of rice. Our findings are consistent with previous reports that rice seedlings were much more tolerant to anoxia than wheat seedlings and that this involves an adaptive response (Menegus et al., 1989, 1991). Our results also suggest that the changes in the capacity of metabolic pathways, via alterations in protein synthesis or degradation rates, are important at least in the rice coleoptile for anoxia tolerance.
Transcriptional Versus Translational Control of Rice Anoxia Response
There is an increasing body of literature on the transcriptional response of rice to anoxia that shows great complexity in the response (Howell et al., 2007; Lasanthi-Kudahettige et al., 2007; Narsai et al., 2009), and the role of differential translation of only an active pool of RNAs during germination and anoxia further complicates its interpretation (Branco-Price et al., 2008). We have extracted published gene expression data from 4-d-old anoxic and aerobic rice seedlings (Lasanthi-Kudahettige et al., 2007) to make comparisons with our protein profiling data here. Overall, the correlation between differences in protein abundance and differences in mRNA expression in anoxically germinated rice versus aerated rice was poor (r2 = 0.39 when comparing log10 ratios; Supplemental Fig. S3B). However, there were some positive correlations observed where the direction of change in response to anoxia or aeration was the same for both the protein and its transcript (Table II). For example, peroxiredoxin (Os07g44430) and protein kinases (Os08g04250, Os08g04210, Os08g04240) were highly accumulated under anoxia alongside clear up-regulation of the corresponding transcripts (Table II). The BBTI (Os01g03340) and ILR1 (Os03g62060) were less abundant under anoxic conditions, which was in concordance with the extracted transcript data indicating their strong down-regulation (Table II). Such results suggest that these particular proteins might be regulated at the transcriptional level. However, another BBTI (Os01g03360) was reported as more abundant in 6-d anoxic coleoptiles according to iTRAQ quantitation, despite its transcript showing the opposite direction of change under anoxia (Table II).
Selective mRNA translation under oxygen deficiency has been previously observed in plants (Bailey-Serres, 1999). In Arabidopsis, it was recently reported that selective mRNA translation coordinates “energetic and metabolic adjustments” to oxygen deficiency and recovery (Branco-Price et al., 2008). This claim was also supported by our proteomic data for the accumulation of proteins from the glycolytic pathway in anoxic rice coleoptiles (Table II; Fig. 3). For example, Fru-bisP aldolase (Os05g33380) and glyceraldehyde-3-phosphate dehydrogenase cytosolic 3 (Os04g40950, Os08g03290) were significantly more abundant at the protein level, while the extracted microarray data indicated that both genes were not responsive to anoxia (Table II). Other isoforms of glyceraldehyde-3-phosphate dehydrogenase cytosolic 3 (Os02g38920) showed abundance differences that were in agreement at the protein and mRNA levels. This suggests that the selected translation of different mRNAs might require modification of the cytosolic ribosome. However, the actual mechanism of selective translation in plants remains unknown. Matching of the rice genes studied here to their Arabidopsis orthologs showed no apparent correlation between the rice proteins, whose abundance was not reflected in rice transcript data, and the ribosomal loading of orthologous mRNA under anoxia in Arabidopsis (Supplemental Table S3). Exceptions include some proteins with unknown functions as well as classical anaerobic proteins.
Alternatively, difference in protein abundance between treatments could be accounted for by alterations of the rate of synthesis and/or degradation of each protein. The abundance of cytosolic ascorbate peroxidase (Os03g17690) was significantly decreased without any apparent change in gene expression (Table II), suggesting that the translation of this gene was inhibited by some downstream consequence of anoxia or that this protein underwent selective degradation. The mechanism of selective protein degradation under anoxia also deserves further investigation. Because the wheat coleoptile proteome was largely unchanged even after 24 h of anoxia (Fig. 1C), selective mRNA translation or protein degradation might not be occurring as frequently in this species as is apparent in rice coleoptiles. The consequence of a smaller upstream response for the regulation of translation and protein turnover in wheat coleoptiles under anoxia was also reflected in metabolic and physiological responses.
Amino Acid Metabolism Is Perturbed during Anoxia
The accumulation of amino acids in anoxic rice and wheat coleoptiles is consistent with the well-documented observation of this phenomenon when plants are exposed to differing degrees of oxygen deprivation (Fan et al., 1997; Kato-Noguchi and Ohashi, 2006; Narsai et al., 2009; van Dongen et al., 2009; Rocha et al., 2010). We also detected the accumulation of enzymes involved in Ala, Ser, and Gly biosynthesis concomitant with the accumulation of those amino acids (Fig. 2). The proposed benefit of the accumulation of Ala under oxygen deprivation in different plant species has been discussed in detail (Gibbs and Greenway, 2003; Bailey-Serres and Voesenek, 2008). Also, Ala synthesis through Ala aminotransferase 2 does not contribute to the oxidation of NADH, as does lactate or ethanol production, but rather serves as a retainable carbon source upon return to air (Good and Crosby, 1989; Miyashita et al., 2007). However, the role of Gly and Ser accumulation is less clear. The transcripts for a number of these biosynthetic proteins are more abundant under anoxia (Lasanthi-Kudahettige et al., 2007; Table II), indicating that amino acid synthesis rather than protein degradation is likely to be responsible. But, to our knowledge, direct evidence for the benefits of feeding exogenous amino acids to seedlings growing under anoxia in an anoxia-intolerant but not an anoxia-tolerant species (Fig. 4) has not previously been reported.
We initiated these exogenous feeding experiments on the basis of an intriguing report on the positive effects of Gly, Ser, and Ala on mammalian cells under hypoxic stress. Of the 23 standard amino acids tested, only Gly, l-Ala, and l-Ser provided significant protection from hypoxic injury of cultured hepatocytes (Brecht and Groot, 1994). For some years, hypoxic or energy deficiency injury to hepatocytes and kidney tubules has been treated with Gly as a method of cell preservation (Weinberg et al., 1991; Carini et al., 1997; Tijsen et al., 1997). Although the literature agrees that protection by Gly is not simply an enhancement of the energetic state of the hypoxic cells, the mechanism of protection is still unclear. Research favors two different mechanisms associated with the modification of the rise in intracellular Na+ during hypoxia due to energy-induced loss of Na+-K+-ATPase activity: indirectly via the activation of Gly receptor neurotransmitters (Carini et al., 1997) or directly by blocking nonselective sodium transport (Frank et al., 2000).
Subsequent literature examination also shows that while the addition of a range of amino acids into external medium can result in cytoplasmic acidification of plant cells (Felle, 1981), the addition of Ala and Ser does not acidify the cytoplasm but instead results in a pH increase of some 0.2 to 0.3 units (Felle, 1996). This suggests a selective benefit of these amino acids in avoiding cytoplasmic acidification under anoxia. Additionally, Ser is the entry point for sphingolipid biosynthesis in plants. The transcript of the gene controlling the first step of sphingolipid biosynthesis, the condensation of palmitate and Ser to form 3-keto-dihydrosphingosine (Ser palmitoyltransferase [Os01g70370]), was up-regulated 20-fold in anoxic coleoptiles (Lasanthi-Kudahettige et al., 2007) and was classified as a core anaerobic responder in germinating rice embryos (Narsai et al., 2009). Recent research in Caenorhabditis elegans (Crowder, 2009; Menuz et al., 2009) suggests that ceramides play a critical role in anoxia tolerance. The possible role of Ser in ceramide biosynthesis through Ser palmitoyltransferase in plant adaptation to anoxia deserves further investigation to identify novel mechanisms conferring anoxia tolerance.
Hence, there are a range of possible explanations for the beneficial effects of combinations of Ala/Ser/Gly on plant cell anoxia tolerance through the retention of carbon skeletons, modification of biosynthetic processes, and cellular ion balance.
CONCLUSION
In summary, our study reinforced the importance of glycolysis and ethanolic fermentation in the adaptation to anaerobiosis and suggests that glycolysis might also be important in providing substrates for amino acid synthesis. Rice, but not wheat, coleoptiles responded to anoxia dramatically at the physiological, proteomic, and metabolomic levels, in concordance with the respective tolerance and intolerance of these species to anoxia. Further investigation into the role of machinery differences in selected mRNA translation and/or protein turnover between rice and wheat coleoptiles is needed based on the targets identified here. We provide novel protein and metabolite evidence of the enhancement of Ser/Gly biosynthesis as well as support observations that Ala accumulates in anoxic rice. We also show a benefit for wheat by exogenous application of these amino acids and highlight a range of mechanisms that could be responsible for conferring anoxia tolerance.
MATERIALS AND METHODS
Plant Material
Dehulled rice (Oryza sativa ‘Amaroo’) and wheat (Triticum aestivum ‘Calingiri’) seeds were surface sterilized for 10 min using 50% (v/v) NaOCl and then thoroughly rinsed with distilled, deionized water. Fifty to 75 seeds were placed in conical flasks containing 250 mL of culture medium (0.5 mm MES, 0.4 mm CaSO4, pH 6.5) and bubbled with air or N2 (6–7 L min−1). Plastic tubing delivered the gas to the seeds, and the system was sealed using Parafilm and aluminum foil. Seedlings were grown in the dark at 30°C for (1) 4 d under aeration, (2) 4 d under aeration with an additional 1-d switch to anoxia, or (3) 6 d under anoxia. To ensure that stress conditions were anoxic, oxygen concentrations were monitored, and after 10 min of N2 bubbling, the oxygen concentration was below the level of detection. This was measured using the LabQuest Vernier oxygen meter with a sensitivity of 0.01% oxygen. Rice seed was kindly provided by the New South Wales Department of Primary Industries and wheat seed by the Western Australian Department of Agriculture and Food.
Evans Blue Viability Stain
This protocol was adapted from the method described by Baker and Mock (1994). Fresh tissues were excised from seedlings, weighed (0.05–0.1 g), and placed in a 10-mL Falcon tube containing 100 μL of distilled, deionized water. Two milliliters of 0.25% (w/v) Evans blue was added to each sample, and horizontally lying tubes were shaken at room temperature for 20 min at 300 rpm. The stain was rinsed from tissues in a sieve until the water ran clear, snap frozen, and then ground in a 2-mL Eppendorf microfuge tube containing a carborundum ball for 3 min at 17 shakes s−1. A total of 0.5 mL of 1% (w/v) SDS was added, and samples were ground for 3 min again. One milliliter of distilled, deionized water was added to samples, which were then centrifuged at 8,800g for 3 min. The absorbance of diluted supernatants (1:3) was measured at 600 nm. The average masses of tissue samples (0.075 g) were used to normalize absorbance measurements so that different samples could be compared (n = 3).
Measurement of Electrical Conductivity
We measured electrical conductivity as an estimation of membrane integrity (Yan et al., 1996). This was done in whole rice and wheat seedlings germinated and grown for 4 d in aeration and subsequently transferred to anoxia or air for 3 d in fresh culture medium (0.5 mm MES, 0.4 mm CaSO4, pH 6.5). Some samples were supplemented with the amino acids l-Ala, l-Ser, and/or Gly at 10 mm concentrations. Seedlings were then washed with distilled, deionized water, patted dry with tissue paper, and placed in 15-mL Falcon tubes containing 10 mL of distilled, deionized water for 1 h at 19.5°C. The electrical conductivity of these solutions was measured (C1; TPS Aqua-C conductivity TDS temperature meter). Samples were then microwaved for 2 min. Care was taken to ensure boiling of each sample. After 1 h, the electrical conductivity was measured again (C2) at 19.5°C and used as the denominator in the calculation of percentage electrolyte leakage.
ADH Activity
Measurement of ADH activity was performed as described by Waters et al. (1991). Briefly, protein was extracted by grinding approximately 70 mg of snap-frozen plant tissue with acid-washed sand and 1 mL of extraction buffer (125 mm MES, 110 mm NaCl, 1 mm EDTA, 0.5 mm thiamine PPi, 2.5 mm MgSO2, and freshly added dithiothreitol at 2 mm, pH 6.8). After centrifugation at 10,000g for 4 min at 4°C, the supernatant was removed for analysis. The ADH activity was measured at 340 nm in a 1-mL cuvette in reaction medium (10 mm acetaldehyde, 50 mm TES, 0.17 mm NADH, pH 7.5).
Carbohydrate Measurement
Carbohydrate levels were measured using a modified method (Trevelyan and Harrison, 1952). Tissue extracts were prepared by heating 20-mg samples submerged in 2 mL of 80% ethanol at 70°C for 20 min in a tightly sealed tube. Extracts were then removed from tissue, and 100 μL of extract was added into 1 mL of freshly prepared anthrone reagent (mixture of 0.2 g of anthrone to 100 mL of 70% [v/v] sulfuric acid). After vortexing, samples were boiled at 100°C for 10 min exactly, promptly placed in an icy-water bath for 5 min, and then removed and stored at room temperature for 5 min before measuring A627.
Oxygen Uptake Measurements
Oxygen uptake measurements followed a procedure described previously (Lee et al., 2008) using a computer-controlled Clark-type oxygen electrode unit. Slight modifications include the use of approximately 90 mg of fresh tissue and 2 mL of oxygen-saturated buffer composed of 5 mm KH2PO4, 10 mm TES, 10 mm NaCl, and 2 mm MgSO4, pH 7.2.
Protein Purification
Snap-frozen coleoptiles were ground with acid-washed sand and a solubilizing solution (7% [w/v] SDS, 125 mm Tris-HCl, and 10% [w/v] β-mercaptoethanol at a 5:8 [w/v] ratio, pH 7). Protein purification was carried out using the chloroform-methanol method (Wessel and Flügge, 1984). Protein pellets were then incubated with 80% acetone for 1 h at −20°C. The solution was centrifuged at 14,000 rpm for 10 min at 4°C, and the pellets were air dried. Protein was resolubilized with rehydration buffer (6 m urea, 2 m thiourea, 2% [w/v] CHAPS, 2% [v/v] immobilized pH gradient buffer, and 18 mm dithiothreitol) for preparative gels or lysis buffer (6 m urea, 2 m thiourea, 2% [w/v] CHAPS, and 40 mm Tris) for DIGE gels by shaking in an orbital rocker at 1,400 rpm at 25°C for 45 min. Centrifugation at 20,000g for 15 min was then carried out. Protein was quantified using the 2D Quant Kit (GE Healthcare).
IEF/SDS-PAGE Gel Separations
For preparative gels, 800 μg of protein resolubilized in rehydration buffer containing equal amounts of both samples was loaded onto IEF strips (3-10NL, 3-11NL, 24 cm; GE Healthcare) and separated for 24 h (up to 50 μA per strip, 5 W, 21°C). Six-step program parameters were as follows: 30 V for 12 h and hold (step and hold [stp]), 500 V for 1-h stp, 1,000 V for 1-h gradient, 3,000 V for 2-h gradient, 8,000 V for 2-h gradient, and 8,000 V for 6-h stp). IEF strips were then dipped in 1× gel buffer, placed on top of a 12% acrylamide gel, and run at 45 mA per gel for 6 to 7 h. For DIGE gels (Eubel et al., 2007), 50 μg of treated, control, and a 1:1 internal standard mixture of the above-mentioned coleoptile proteins was labeled separately with 400 μm fluorescent CyDye. The labeling procedure was carried out according to the recommendations of GE Healthcare. Samples were pooled and separated in the same manner that preparative gels were. DIGE gels were scanned using a Typhoon laser scanner (GE Healthcare), and quantitative analysis was carried out using the DeCyder software package (version 6.5; GE Healthcare). Three independent dye-swapping replicates were carried out. Statistically significant spots were selected for MS identification according to their appearance in nine of nine fluorescent images, a ratio of abundance difference of 2 or greater, and a value of P ≤ 0.05. Both DIGE and preparative gels were Coomassie Brilliant Blue stained and destained.
Protein Identification Using MS
Selected protein spots were excised from gels and digested in gel as described previously (Taylor et al., 2005). Vacuum-dried samples were redissolved in 5% (v/v) acetonitrile and 0.1% (v/v) formic acid for analysis on an XCT Ultra Ion Trap mass spectrometer (Agilent Technologies), and MS/MS spectra were exported for data analysis. MS spectra were examined against an in-house rice database of The Institute for Genomic Research Rice Pseudomolecules and Genome Annotation and mitochondrial and plastid protein sets (Rice version 6) using version 2.2.03 (Matrix Science) of the Mascot search engine. The following settings were selected for searching: MS error tolerance of ±1.2 D, MS/MS error tolerance of ±0.6 D, maximum missed cleavages tolerated as one, two variable modifications including carbamidomethyl (C) and oxidation (M), peptide charge as 2+ and 3+, and finally, the instrument selected as ESI-TRAP. After results were retrieved from the Mascot search engine, “Require bold red” and “Standard scoring” checkboxes were selected with the ion score cutoff set at 37, the “Significance threshold” set at P < 0.05, and the “Max. number of hits” set at AUTO. MS spectra files are available for analysis via the Proteome Commons Tranche Project under hash Wiq7A0erU/p/zv9IJSKf+5pyjbFZXnERbYRQYwrglOB3N/kmT/Sp9qJ4ksGmr9J76AmipUl3xMLTO1I07LFWa19V0K8AAAAAAAACEg = =.
Metabolite Extraction, GC-MS Sample, and Data Analysis
Metabolite extraction from coleoptiles followed a modified procedure described previously (Howell et al., 2009). Tubes containing ground tissue samples (100 mg of stressed or nonstressed coleoptiles) and grinding balls (cooled) were placed in a liquid N2-cooled solid rack. Cold metabolite extraction medium (0.5 mL; 85% [w/v] HPLC-grade methanol, 15% [w/v] untreated MilliQ water, and 100 ng μL−1 ribitol) was added to each tube, immediately vortexed, and then shaken at 1,400 rpm for 20 min at 65°C. To pellet cell debris, samples were centrifuged at 20,000g for 10 min. Aliquots (60 μL) of extract were dried in a vacuum centrifuge for approximately 2 h. Twenty microliters of 20 mg mL−1 methoxylamine-HCl (98% purity; Sigma) was added to each of the dried samples. Samples were then shaken at 1,400 rpm for 90 min at 30°C. To each sample, 30 μL of N-methyl-N-(trimethylsilyl)-trifluoroacetamide (derivatization grade; Sigma) was added, followed by shaking again at 1,400 rpm for 30 min at 37°C. After this, 10 μL of n-alkane standard mix (0.029% [v/v] n-dodecane, 0.029% [v/v] n-pentadecane, 0.029% [w/v] n-nonadecane, 0.029% [w/v] n-docosane, 0.029% [w/v] n-octacosane, 0.029% [w/v] n-dotriacontane, and 0.029% [w/v] n-hexatriacontane dissolved in anhydrous pyridine) was added and vortexed. Samples were transferred to GC-MS amber vials with screw-top seals and low-volume inserts (Agilent Technologies). These were then incubated for 4 h at room temperature for equilibration. Analysis of samples on the GC-MS device followed the procedure described previously (Howell et al., 2009). GC-MS data were collected using Chemstation GC/MSD Data Analysis Software (Agilent Technologies). Raw GC-MS data preprocessing and statistical analysis were performed using MetabolomeExpress software (version 1.0; http://www.metabolome-express.org). Detailed methods have been reported (Carroll et al., 2010).
Metabolic Phenocopy Analysis Using the MetabolomeExpress PhenoMeter
To systematically characterize relationships between the metabolic phenotypes observed in this study and metabolic phenotypes reported in previous studies, we used the PhenoMeter tool of MetabolomeExpress (https://www.metabolome-express.org) to search the MetabolomeExpress metabolic phenotype database, MetaPhenDB, for metabolic phenotypes having statistically significant qualitative overlap with the responses observed in this study (submitted as a batch of “bait” responses). The PhenoMeter uses the following procedure for each bait response. The bait response is compared with each and every reference response in MetaPhenDB, one at a time. Each comparison is done by first counting (1) the number of metabolites increased (i.e. having a greater than 1-fold change) in both bait and reference; (2) the number of metabolites decreased in both bait and reference; (3) the number of metabolites increased in bait but decreased in reference; and (4) the number of metabolites decreased in bait but increased in reference. These counts were then used as input in a two-tailed Fisher’s exact test to calculate the P value of obtaining the observed positive (codirectional) or negative (inverse) response overlap by chance alone. P > 0.05 was used to indicate statistically significant overlaps or inverse overlaps. To minimize biases caused by the presence of different sets of “unknown” metabolites in metabolic phenotypes acquired from different studies, only metabolites of known structure (and hence having the same name in each study) were considered in comparisons. Only metabolites present in both bait and reference were considered. So that the anoxia responses observed in this study could be compared with previously published plant responses to anoxia and hypoxia, we added the complete set of 36 metabolic phenotypes associated with seven peer-reviewed publications (Geigenberger et al., 2000; Gibon et al., 2002; van Dongen et al., 2003, 2009; Branco-Price et al., 2008; Timmins et al., 2009; Rocha et al., 2010) from other groups presented in a recent review (Narsai et al., 2011) of the topic to MetaPhenDB prior to PhenoMeter analysis. At the time of analysis, MetaPhenDB contained 12,379 publicly available metabolite response statistics representing 116 metabolic phenotypes, including oxygen deprivation-related metabolic phenotypes for a total of six plant species (Arabidopsis [Arabidopsis thaliana], potato [Solanum tuberosum], Lotus japonicus, Populus × canescens, castor bean [Ricinus communis], and rice) in addition to metabolic phenotypes associated with a wide variety of other environmental, developmental, and genetic perturbations.
iTRAQ Analysis
Proteins were purified and quantified as described above. For each sample, a total of 100 μg of protein was precipitated by the addition of 4 volumes of cold acetone and stored in −20°C overnight. The precipitated protein was then resuspended in dissolution buffer and denatured, and Cys residues were blocked according to the manufacturer’s instructions (AB Sciex). Each sample was then digested with 20 μL of 0.25 μg μL−1 trypsin (Invitrogen) at 37°C overnight and labeled with the iTRAQ tags in triplicate. iTRAQ reagents were resuspended in 50 μL of 2-propanol and added to each sample, pH adjusted, and allowed to incubate at room temperature for 2 h. The labeled samples were pooled prior to further analysis. To remove excess labeling reactants and to reduce the interference of salts during liquid chromatography-MS/MS analysis, the pooled samples were diluted 4-fold with strong cation-exchange buffer A (10 mm KH2PO4 in 25% acetonitrile, pH 3.0) and subjected to strong cation-exchange chromatography using an OPTI-LYNX cartridge (Optimize Technologies). The eluent was dried in a vacuum concentrator and stored at −20°C for liquid chromatography-MS/MS analysis.
Samples were analyzed on an Agilent 6510 quadrupole-time-of-flight (Q-TOF) mass spectrometer with an HPLC Chip Cube source. The chip consisted of a 160-nL enrichment column (Zorbax 300SB-C18 5μm) and a 150-mm separation column (Zorbax 300SB-C18 5μm) driven by an Agilent Technologies 1100 series nano/capillary liquid chromatography system. Peptides were loaded onto the trapping column at 4 μL min−1 in 5% (v/v) acetonitrile and 0.1% (v/v) formic acid with the chip switched to enrichment and using the capillary pump. The chip was then switched to separation, and peptides were eluted during a 1-h gradient (5%–60% [v/v] acetonitrile) using the nano pump at 300 nL min−1 directly into the mass spectrometer. The Q-TOF mass spectrometer was run in positive ion mode, and MS scans were run over a mass-to-charge ratio range of 275 to 1,500 and at 4 spectra s−1. Precursor ions were selected for auto-MS/MS at an absolute threshold of 500 and a relative threshold of 0.01, with a maximum of three precursors per cycle and active exclusion set at two spectra, and released after 1 min. Precursor charge-state selection and preference was set to [M+H]2+ and then [M+H]3+, and precursors were selected by charge and then abundance. Resulting MS/MS spectra were searched against The Institute for Genomic Research Rice Pseudomolecules and Genome Annotation and mitochondrial and plastid protein sets (Rice_osa6) using version 2.2.03 (Matrix Science) of the Mascot search engine. The following settings were selected for database searching: MS error tolerance of ±100 ppm; MS/MS error tolerance of ±0.5 D; maximum missed cleavages tolerated as one; fixed modifications methylthio (C), iTRAQ8plex (N-term), iTRAQ8plex (K); variable modifications carbamidomethyl (C) and oxidation (M) iTRAQ8plex (Q), peptide charge of 2+ or greater; and finally, the instrument selected as ESI-Q-TOF. The resulting searches were then exported, and all peptides identified (P < 0.05) were extracted to create an exclusion list for the subsequent run. All five runs were performed and combined using mzdata Combinator version 1.0.4 (West Australian Centre of Excellence in Computational Systems Biology) for database searching as outlined below. MS spectra files are available for analysis via the Proteome Commons Tranche Project under hash 5AJQpzyi1I5adgPNIGdJ+oQf8nlIoXnjLVhePv9x39srDtRpuZe9gQu9ij62NLKetNEdx6t1MqirlvSglVAAUHcQZAYAAAAAAAABcA = =.
Quantitation was carried out using default settings in Mascot version 2.2.03 (Matrix Science) for protein identifications as outlined above and quantitation on isobaric mass tags (iTRAQ) at the peptide level. In more detail, ratios for individual peptide matches were obtained from peptides meeting the minimum criteria outlined above and were then combined to determine ratios for protein hits using a weighted average. Outlier removal was carried out by Dixon’s method for up to 25 data points per protein or by Rosner’s method, where more than 25 data points were present and normalization was carried out by median ratio. Values are reported as geometric means with sd, and those significantly different from 1 at a 95% confidence interval are marked with asterisks (Supplemental Table S1). For proteins reported to have a nonnormal distribution, the geometric sd was determined manually. Here, a geometric mean for the individual peptide ratios and a 95% confidence interval window was calculated as a t test in Analyze-it version 2.21.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Germination, growth, and cell integrity of rice and wheat under anoxia.
Supplemental Figure S2. Number of protein spots detected in each proteomic analysis that significantly differed in abundance between treatments.
Supplemental Figure S3. Correlations between protein abundance detected from iTRAQ and DIGE and between transcript and protein abundance differences between continuously anoxic and aerated rice coleoptiles.
Supplemental Figure S4. DIGE on two-dimensional 3-11NL IEF/SDS gels.
Supplemental Table S1. Entire list of rice coleoptile proteins significantly changing between treatments that were identified by DIGE and iTRAQ proteomic analyses.
Supplemental Table S2. Quantitative analysis of protein abundance from 4-d aerated and 6-d anoxic rice coleoptiles using iTRAQ.
Supplemental Table S3. Comparison of steady-state and polysomal Arabidopsis mRNAs under aeration and hypoxia, whose genes are orthologous to rice genes encoding proteins significantly changing in abundance in at least one of our rice proteome analyses.
Supplemental Table S4. Whole set of relative metabolite levels in wheat and rice coleoptiles under aerated and anoxic conditions.
Supplemental Table S5. Comparison of metabolite responses between wheat and rice coleoptiles from seedlings switched to 1 d of anoxia in two independent experiments.
Supplemental Table S6. Grouping of metabolite responses in coleoptiles of 4-d-old rice and wheat seedlings transferred to anoxia for 1 d (anoxic switch conditions).
Supplemental Table S7. Comparison of metabolite responses in coleoptiles of anoxically switched and anoxically germinated rice seedlings.
Supplemental Table S8. Metabolic phenocopy analysis: comparison of the low-oxygen responses of rice and wheat coleoptiles with low-oxygen and respiratory-perturbation responses in other species and tissues.
Supplemental Table S9. Expanded results of MetabolomeExpress PhenoMeter analysis.
References
- Alpi A, Beevers H. (1983) Effects of O2 concentration on rice seedlings. Plant Physiol 71: 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwell B, Waters I, Greenway H. (1982) The effect of oxygen and turbulence on elongation of coleoptiles of submergence-tolerant and -intolerant rice cultivars. J Exp Bot 33: 1030–1044 [Google Scholar]
- Bailey-Serres J. (1999) Selective translation of cytoplasmic mRNAs in plants. Trends Plant Sci 4: 142–148 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Chang R. (2005) Sensing and signaling in response to oxygen deprivation in plants and other organisms. Ann Bot (Lond) 96: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339 [DOI] [PubMed] [Google Scholar]
- Baker CJ, Mock NM. (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tissue Organ Cult 39: 7–12 [Google Scholar]
- Baker J, Steele C, Dure L. (1988) Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol Biol 11: 277–291 [DOI] [PubMed] [Google Scholar]
- Bartel B, Fink GR. (1995) ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science 268: 1745–1748 [DOI] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J. (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028 [DOI] [PubMed] [Google Scholar]
- Biemelt S, Keetman U, Albrecht G. (1998) Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings. Plant Physiol 116: 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot (Lond) 91: 179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina OB, Chirkova TV, Fagerstedt KV. (2001) Anoxic stress leads to hydrogen peroxide formation in plant cells. J Exp Bot 52: 1179–1190 [PubMed] [Google Scholar]
- Blokhina OB, Fagerstedt KV, Chirkova TV. (1999) Relationships between lipid peroxidation and anoxia tolerance in a range of species during post-anoxic reaeration. Physiol Plant 105: 625–632 [Google Scholar]
- Blokhina OB, Virolainen E, Fagerstedt KV, Hoikkala A, Wahala K, Chirkova TV. (2000) Antioxidant status of anoxia-tolerant and -intolerant plant species under anoxia and reaeration. Physiol Plant 109: 396–403 [Google Scholar]
- Branco-Price C, Kaiser KA, Jang CJH, Larive CK, Bailey-Serres J. (2008) Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J 56: 743–755 [DOI] [PubMed] [Google Scholar]
- Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J. (2005) Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann Bot (Lond) 96: 647–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Groot H. (1994) Protection from hypoxic injury in cultured hepatocytes by glycine, alanine, and serine. Amino Acids 6: 25–35 [DOI] [PubMed] [Google Scholar]
- Campos F, Zamudio F, Covarrubias AA. (2006) Two different late embryogenesis abundant proteins from Arabidopsis thaliana contain specific domains that inhibit Escherichia coli growth. Biochem Biophys Res Commun 342: 406–413 [DOI] [PubMed] [Google Scholar]
- Carini R, Bellomo G, Grazia De Cesaris M, Albano E. (1997) Glycine protects against hepatocyte killing by KCN or hypoxia by preventing intracellular Na+ overload in the rat. Hepatology 26: 107–112 [DOI] [PubMed] [Google Scholar]
- Carroll AJ, Badger MR, Harvey Millar A. (2010) The MetabolomeExpress Project: enabling Web-based processing, analysis and transparent dissemination of GC/MS metabolomics datasets. BMC Bioinformatics 11: 376–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder CM. (2009) Ceramides: friend or foe in hypoxia? Science 324: 343–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubel H, Lee CP, Kuo J, Meyer EH, Taylor NL, Millar AH. (2007) Free-flow electrophoresis for purification of plant mitochondria by surface charge. Plant J 52: 583–594 [DOI] [PubMed] [Google Scholar]
- Fan TW-M, Higashi RM, Frenkiel TA, Lane AN. (1997) Anaerobic nitrate and ammonium metabolism in flood-tolerant rice coleoptiles. J Exp Bot 48: 1655–1666 [Google Scholar]
- Felle H. (1981) Stereospecificity and electrogenicity of amino acid transport in Riccia fluitans. Planta 152: 505–512 [DOI] [PubMed] [Google Scholar]
- Felle HH. (1996) Control of cytoplasmic pH under anoxic conditions and its implication for plasma membrane proton transport in Medicago sativa root hairs. J Exp Bot 47: 967–973 [Google Scholar]
- Frank A, Rauen U, de Groot H. (2000) Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J Hepatol 32: 58–66 [DOI] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. (2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18: 2021–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. (2011) The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 23: 412–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmier M, Carroll AJ, Delannoy E, Vallet C, Day DA, Small ID, Millar AH. (2008) Complex I dysfunction redirects cellular and mitochondrial metabolism in Arabidopsis. Plant Physiol 148: 1324–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M. (2000) Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol Chem 381: 723–740 [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30: 1–47 [DOI] [PubMed] [Google Scholar]
- Gibbs J, Morrell S, Valdez A, Setter TL, Greenway H. (2000) Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. J Exp Bot 51: 785–796 [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M. (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221–235 [DOI] [PubMed] [Google Scholar]
- Good AG, Crosby WL. (1989) Anaerobic induction of alanine aminotransferase in barley root tissue. Plant Physiol 90: 1305–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Perata P, Alpi A. (1995) Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiol 108: 735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou WC, Lin YH. (1997) Dehydroascorbate reductase and monodehydroascorbate reductase activities of trypsin inhibitors, the major sweet potato (Ipomoea batatas [L] Lam) root storage protein. Plant Sci 128: 151–158 [Google Scholar]
- Hou WC, Wang YT, Lin YH, Hsiao LJ, Chen TE, Wang CW, Dai H. (2000) A complex containing both trypsin inhibitor and dehydroascorbate reductase activities isolated from mitochondria of etiolated mung bean (Vigna radiata L. (Wilczek) cv. Tainan no. 5) seedlings. J Exp Bot 51: 713–719 [PubMed] [Google Scholar]
- Howell KA, Cheng K, Murcha MW, Jenkin LE, Millar AH, Whelan J. (2007) Oxygen initiation of respiration and mitochondrial biogenesis in rice. J Biol Chem 282: 15619–15631 [DOI] [PubMed] [Google Scholar]
- Howell KA, Narsai R, Carroll A, Ivanova A, Lohse M, Usadel B, Millar AH, Whelan J. (2009) Mapping metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Physiol 149: 961–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Greenway H, Colmer TD. (2003) Anoxia tolerance in rice seedlings: exogenous glucose improves growth of an anoxia-‘intolerant’, but not of a ‘tolerant’ genotype. J Exp Bot 54: 2363–2373 [DOI] [PubMed] [Google Scholar]
- Huang S, Greenway H, Colmer TD, Millar AH. (2005) Protein synthesis by rice coleoptiles during prolonged anoxia: implications for glycolysis, growth and energy utilization. Ann Bot (Lond) 96: 703–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Noguchi H, Ohashi C. (2006) Effects of anoxia on amino acid levels in rice coleoptiles. Plant Prod Sci 9: 383–387 [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES. (2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14: 2481–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordan HA. (1974) The rice shoot in relation to oxygen supply and root growth in seedlings germinating under water. New Phytol 73: 695–697 [Google Scholar]
- Kreuzwieser J, Hauberg J, Howell KA, Carroll A, Rennenberg H, Millar AH, Whelan J. (2009) Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiol 149: 461–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasanthi-Kudahettige R, Magneschi L, Loreti E, Gonzali S, Licausi F, Novi G, Beretta O, Vitulli F, Alpi A, Perata P. (2007) Transcript profiling of the anoxic rice coleoptile. Plant Physiol 144: 218–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CP, Eubel H, O’Toole N, Millar AH. (2008) Heterogeneity of the mitochondrial proteome for photosynthetic and non-photosynthetic Arabidopsis metabolism. Mol Cell Proteomics 7: 1297–1316 [DOI] [PubMed] [Google Scholar]
- Liu FL, Vantoai T, Moy LP, Bock G, Linford LD, Quackenbush J. (2005) Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol 137: 1115–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti E, Poggi A, Novi G, Alpi A, Perata P. (2005) A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol 137: 1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magneschi L, Kudahettige RL, Alpi A, Perata P. (2009) Comparative analysis of anoxic coleoptile elongation in rice varieties: relationship between coleoptile length and carbohydrate levels, fermentative metabolism and anaerobic gene expression. Plant Biol (Stuttg) 11: 561–573 [DOI] [PubMed] [Google Scholar]
- Mapelli S, Locatelli F. (1995) The relation of rice coleoptiles, auxin-binding protein, and protein-biosynthesis to anoxia and indoleacetic-acid. Russ J Plant Physiol 42: 624–629 [Google Scholar]
- Menegus F, Cattaruzza L, Chersi A, Fronza G. (1989) Differences in the anaerobic lactate-succinate production and in the changes of cell sap pH for plants with high and low resistance to anoxia. Plant Physiol 90: 29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E. (1991) Response to anoxia in rice and wheat seedlings: changes in the pH of intracellular compartments, glucose-6-phosphate level, and metabolic rate. Plant Physiol 95: 760–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz V, Howell KS, Gentina S, Epstein S, Riezman I, Fornallaz-Mulhauser M, Hengartner MO, Gomez M, Riezman H, Martinou J-C. (2009) Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science 324: 381–384 [DOI] [PubMed] [Google Scholar]
- Millar AH, Trend AE, Heazlewood JL. (2004) Changes in the mitochondrial proteome during the anoxia to air transition in rice focus around cytochrome-containing respiratory complexes. J Biol Chem 279: 39471–39478 [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Dolferus R, Ismond KP, Good AG. (2007) Alanine aminotransferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. Plant J 49: 1108–1121 [DOI] [PubMed] [Google Scholar]
- Mocquot B, Prat C, Mouches C, Pradet A. (1981) Effect of anoxia on energy charge and protein synthesis in rice embryo. Plant Physiol 68: 636–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J. (2010) Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol 152: 1484–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Makino A. (2009) Differences between rice and wheat in temperature responses of photosynthesis and plant growth. Plant Cell Physiol 50: 744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R, Howell KA, Carroll A, Ivanova A, Millar AH, Whelan J. (2009) Defining core metabolic and transcriptomic responses to oxygen availability in rice embryos and young seedlings. Plant Physiol 151: 306–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R, Rocha M, Geigenberger P, Whelan J, van Dongen JT. (2011) Comparative analysis between plant species of transcriptional and metabolic responses to hypoxia. New Phytol 190: 472–487 [DOI] [PubMed] [Google Scholar]
- Öpik H. (1973) Effect of anaerobiosis on respiratory rate, cytochrome oxidase activity and mitochondrial structures in coleoptiles of rice (Oryza sativa L.). J Cell Sci 12: 725–739 [DOI] [PubMed] [Google Scholar]
- Pegoraro R, Mapelli S, Torti G, Bertani A. (1988) Indole-3-acetic acid and rice coleoptile elongation under anoxia. J Plant Growth Regul 7: 85–94 [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. (1997) Mobilization of endosperm reserves in cereal seeds under anoxia. Ann Bot (Lond) 79: 49–56 [Google Scholar]
- Perata P, Pozueta-Romero J, Akazawa T, Yamagushi J. (1992) Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 188: 611–618 [DOI] [PubMed] [Google Scholar]
- Raymond P, Al-Ani A, Pradet A. (1985) ATP production by respiration and fermentation, energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiol 79: 879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B, Pradet A. (1989) Anaerobic protein synthesis in different organs of germinating rice seeds. Plant Physiol Biochem 27: 761–768 [Google Scholar]
- Ricard B, Rivoal J, Spiteri A, Pradet A. (1991) Anaerobic stress induces the transcription and translation of sucrose synthase in rice. Plant Physiol 95: 669–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M, Licausi F, Araújo WL, Nunes-Nesi A, Sodek L, Fernie AR, van Dongen JT. (2010) Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol 152: 1501–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M, Subaiah C, Saab I. (1996) Anaerobic gene expression and flooding tolerance in maize. J Exp Bot 47: 1–15 [Google Scholar]
- Setter TL, Waters I. (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253: 1–34 [Google Scholar]
- Siddiqui NU, Chung H-J, Thomas TL, Drew MC. (1998) Abscisic acid-dependent and -independent expression of the carrot late-embryogenesis-abundant-class gene Dc3 in transgenic tobacco seedlings. Plant Physiol 118: 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NL, Heazlewood JL, Day DA, Millar AH. (2005) Differential impact of environmental stresses on the pea mitochondrial proteome. Mol Cell Proteomics 4: 1122–1133 [DOI] [PubMed] [Google Scholar]
- Tijsen MJH, Peters SMA, Bindels RJM, van Os CH, Wetzels JFM. (1997) Glycine protection against hypoxic injury in isolated rat proximal tubules: the role of proteases. Nephrol Dial Transplant 12: 2549–2556 [DOI] [PubMed] [Google Scholar]
- Timmins M, Zhou W, Rupprecht J, Lim L, Thomas-Hall SR, Doebbe A, Kruse O, Hankamer B, Marx UC, Smith SM, et al. (2009) The metabolome of Chlamydomonas reinhardtii following induction of anaerobic H2 production by sulfur depletion. J Biol Chem 284: 23415–23425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan WE, Harrison JS. (1952) Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J 50: 298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Fröhlich A, Ramírez-Aguilar SJ, Schauer N, Fernie AR, Erban A, Kopka J, Clark J, Langer A, Geigenberger P. (2009) Transcript and metabolite profiling of the adaptive response to mild decreases in oxygen concentration in the roots of Arabidopsis plants. Ann Bot (Lond) 103: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Schurr U, Pfister M, Geigenberger P. (2003) Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol 131: 1529–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-H, Jiang Z-L, Chen Z-Q, Li X, Peng L-L. (2010) Neuroprotective effect of L-serine against temporary cerebral ischemia in rats. J Neurosci Res 88: 2035–2045 [DOI] [PubMed] [Google Scholar]
- Waters I, Morrels S, Greenway H, Colmer T. (1991) Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. J Exp Bot 42: 1437–1447 [Google Scholar]
- Weinberg JM, Nissim I, Roeser NF, Davis JA, Schultz S, Nissim I. (1991) Relationships between intracellular amino acid levels and protection against injury to isolated proximal tubules. Am J Physiol 260: F410–F419 [DOI] [PubMed] [Google Scholar]
- Went FW. (1942) Growth, auxin and tropisms in decapitated Avena coleoptiles. Plant Physiol 17: 236–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D, Flügge UI. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138: 141–143 [DOI] [PubMed] [Google Scholar]
- Wu WW, Wang G, Baek SJ, Shen R-F. (2006) Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel- or LC-MALDI TOF/TOF. J Proteome Res 5: 651–658 [DOI] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Yan B, Dai Q, Liu X, Huang S, Wang Z. (1996) Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179: 261–268 [Google Scholar]
- Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, et al. (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79–92 [DOI] [PubMed] [Google Scholar]