Over the past decade, metabolomics has developed into a major tool for studying the metabolism of organisms and cells, and through this approach much has been learned about metabolic networks and the reactions of organisms to various external conditions (Lay et al., 2006). Most of this work involves a number of chemometric methods to identify markers in the metabolomic data for various events. But in fact, little work has been done on understanding the meaning of the metabolomic data itself and the role of the total of the compounds observed. Is there any logic in the studying the combination of compounds itself, instead of looking at the correlations between the compounds observed and any disease or applied experimental conditions? NMR-based metabolomics in particular give a clear view of the major compounds present in an organism or cell and enable the direct quantitative comparison of all major compounds. Considering all the information we have collected in recent years using our protocol for NMR-based metabolomics (Kim et al., 2010), we asked ourselves why a few very simple molecules are always present in considerable amounts in all microbial, mammalian, and plant cells. It seems that these compounds must serve some basic function in living cells and organisms. These compounds include sugars, some amino acids, choline, and some organic acids such as malic acid, citric acid, lactic acid, and succinic acid. With the exception of sugars, which may serve as storage products and a source of energy, the other compounds are present in such large amounts that it does not make sense to consider them as only intermediates in metabolic pathways.
Here, we develop a novel theory about the role of these compounds, which may explain many questions in the biochemistry of cells and organisms. The theory is based on analogy with green chemistry, where in past years various synthetic ionic liquids (ILs) have been developed for chemical and enzymatic reactions as well as for the extraction of natural products.
The field of ILs began in 1914, when Paul Walden (Plechkova and Seddon, 2008) reported on the physical properties of ethylammonium nitrate. But it is only in recent years that ILs and deep eutectic solvents (DES) have been revisited by chemical engineering, because such solvents can replace conventional organic solvents. Mixing salts and/or organic compounds may cause a considerable reduction of the melting point, turning them into liquids even at very low temperatures. Using the liquids made from synthetic chemicals, ILs and DES now have many different applications such as dissolving polymers and metals and as media for biotransformation (Welton, 1999; Wasserscheid and Keim, 2000; Abbott et al., 2004; Gorke et al., 2008). In fact, many of the synthetic ILs contain choline and in some cases also natural organic acids.
In analogy with the synthetic ILs, we hypothesized that the metabolites that occur in large amounts in cells may form a third type of liquid, one separate from water and lipids. Taking the plant metabolomics data we have collected over recent years into consideration, we saw a clear parallel with the synthetic ILs. The above-mentioned major cellular constituents seemed perfect candidates for making ILs and DES. As the first step, we made various combinations of these candidates, thereby discovering more than 30 combinations that form viscous liquids (Table I). Here, we will use “natural deep eutectic solvents” (NADES) as a common term for these mixtures. The preparation of NADES and NMR measurements are described in Supplemental Materials and Methods S1.
Table I. List of natural ILs and DES.
| Combination | Molar Ratio |
| Citric acid:choline chloride | 1:2, 1:3 |
| Malic acid:choline chloride | 1:1, 1:2, 1:3 |
| Maleic acid:choline chloride | 1:1, 1:2, 1:3 |
| Aconitic acid:choline chloride | 1:1 |
| Glc:choline chloride:water | 1:1:1 |
| Fru:choline chloride:water | 1:1:1 |
| Suc:choline chloride:water | 1:1:1 |
| Citric acid:Pro | 1:1, 1:2, 1:3 |
| Malic acid:Glc | 1:1 |
| Malic acid:Fru | 1:1 |
| Malic acid:Suc | 1:1 |
| Citric acid:Glc | 2:1 |
| Citric acid:trehalose | 2:1 |
| Citric acid:Suc | 1:1 |
| Maleic acid:Glc | 4:1 |
| Maleic acid:Suc | 1:1 |
| Glc:Fru | 1:1 |
| Fru:Suc | 1:1 |
| Glc:Suc | 1:1 |
| Suc:Glc:Fru | 1:1:1 |
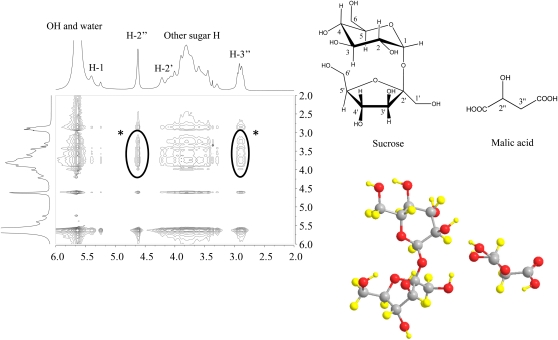
In a 1H-1H-nuclear Overhauser enhancement spectroscopy spectrum of the Suc and malic acid mixture, some of the protons showed intermolecular interaction, implying that the molecules of these compounds in the liquid are aggregated into larger structures, as in liquid crystals (Fig. 1). In some mixtures, such as different sugars with choline chloride, water can be present as part of the solvent (1:1:1 molar ratio, corresponding to approximately 6% water; Table I). This water is strongly retained in the liquid and cannot be evaporated.
Figure 1.
1H-1H-Nuclear Overhauser enhancement spectroscopy correlation between Suc and malic acid. The asterisks indicate molecular correlation between Suc and malic acid.
Having shown that the combination of ubiquitous natural compounds present at high concentrations in all living cells forms liquids, the next step is to ask what the role of the NADES could be. To date, cells have been considered to contain two immiscible liquid phases (i.e. water and lipids [membranes]). However, cells do contain numerous compounds of intermediate polarity in high concentrations that neither dissolve in the lipid nor the water phase. This raises the question of how these compounds are biosynthesized and stored. For example, the flowers of Sophora species contain between 10% and 30% dry mass of the sparsely water-soluble flavonoid rutin (Paniwnyk et al., 2001). In biosynthesis, it is generally thought that the enzyme-mediated reactions in cells occur in water. However, this raises questions of how these reactions function with substrates and products that are poorly soluble in water. In addition, the biosynthesis of water-insoluble polymers such as cellulose, amylose, and lignins probably needs a stage of the macromolecule being dissolved to enable the further addition of building blocks.
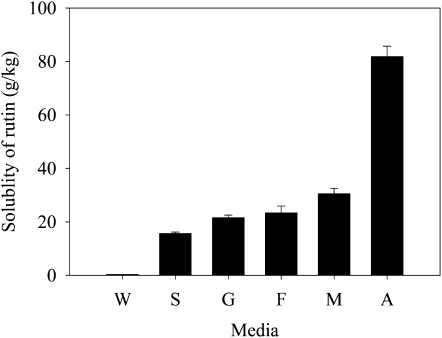
To assess the possibility of NADES being the third liquid phase in organisms in which certain biosynthetic steps or storage of products may occur, the solubility of some common natural products in NADES was measured. For example, we found that the solubility of the flavonoid rutin in various NADES was 50 to 100 times higher than in water (Fig. 2). Moreover, the completely water-insoluble paclitaxel and ginkgolide B showed high solubility: 0.81 and 5.85 mg mL−1, respectively, in Glc-choline chloride. Considering macromolecules, DNA (from male salmon), albumin, and amylase did show good solubility in some of the NADES tested. The solubility of the salmon DNA was shown to be 39.4 mg mL−1 in malic acid:Pro (1:1) compared with 26.9 mg mL−1 in water. The albumin solubility was 30.6 mg mL−1 in Fru:Glc:Suc (1:1:1) and 235.0 mg mL−1 in water. Even the non-water-soluble polysaccharide, starch, showed a solubility of 17.2 mg mL−1 in Glc:choline chloride (1:1).
Figure 2.
Comparison of rutin solubility (g kg−1) in water (W), Suc-choline chloride (S), Glc-choline chloride (G), Fru-choline chloride (F), malic acid-choline chloride (M), and aconitic acid-choline chloride (A).
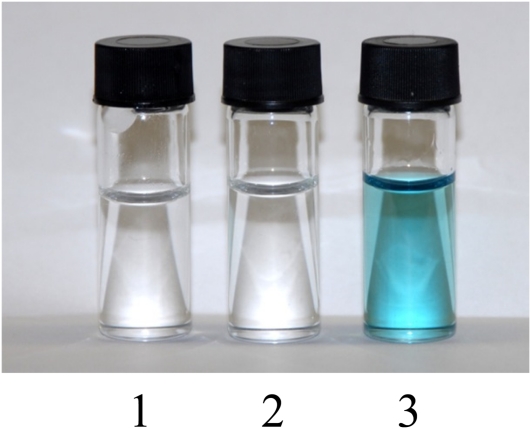
In green chemistry using synthetic ILs and DES, it has already been shown that enzymes are quite stable and capable of catalyzing various chemical reactions (Gorke et al., 2008). We found that laccase completely dissolved in a NADES but was inactive. However, after adding water, the enzyme became active (Fig. 3). This interesting observation obviously links the NADES to phenomena such as cryoprotection, drought resistance, and germination. In fact, the major cryoprotectants used in cryopreservation are all compounds we found to be able to form NADES (Mustafa et al., 2011). It has been reported that in wheat (Triticum aestivum), freezing tolerance correlates with a high Pro level (Kovács et al., 2011). This confirms our results in which Pro was seen to be a good candidate to form liquids with organic acids or sugars.
Figure 3.
Laccase activity in malic acid:choline chloride (1:1) with the addition of water. Container 1, 0% water; container 2, 25% water; container 3, 50% water.
One of the most interesting mysteries in nature is how plants can survive dehydration (anhydrobiosis). There are two types of tolerance to dehydration, drought and desiccation tolerance (Hoekstra et al., 2001). Desiccation tolerance is for more severe conditions in which water content is lower than 0.3 g water g−1 dry weight and widespread in ferns, mosses, pollen, and seeds (Oliver and Bewley, 1996; Hoekstra et al., 2001). We may hypothesize that in extreme drought conditions, plants should have a replacement of water to maintain their life. Interestingly, the drought tolerance was found to correlate with the induction of sugars, including disaccharides and oligosaccharides, and some amino acids like Pro, Glu, and Gly-betaine (Hoekstra et al., 2001); the exact roles of the induced compounds are not clear yet, but glass formation by the sugars (amorphous solid of high viscosity, with highly restricted mobility of the components) is thought to be a key factor. However, several authors mention that the sugars alone are not sufficient for protecting the cells in anhydrobiosis (Tunnacliffe and Lapinski, 2003; Buitink and Leprince, 2004; Vicre et al., 2004; Pandey et al., 2010). Many of the induced compounds are proved to act as NADES in our research. NMR-based metabolomics of dry resurrection plants that are capable of becoming photosynthetically active in a very short time after water is added to the plant show the presence of all the ingredients necessary for a NADES. “Glass formation,” which is generally thought to be due to the action of sugars under drought conditions (Buitink and Leprince, 2004), could in fact be the formation of a NADES consisting of sugar(s), choline, Pro, and organic acids, which are observed in the metabolome on NMR measurement. In fact, there might be various different NADES in individual cellular compartments, adapted to the kind of compounds and proteins that should need to be conserved when dissolved in the NADES. Similarly, when we analyzed the metabolome of the tissue (aleurone) of barley (Hordeum vulgare) seeds from where germination starts (Schuurink et al., 1992), the major ingredients like Suc and choline did form NADES, with a molar ratio of 1:1. Under drought conditions at high or low temperature, living cells may survive by forming NADES in which the membranes, enzymes, and metabolites remain stable and in which the last water is strongly retained and freezing is prohibited by the very low melting point of the deep eutectic mixture.
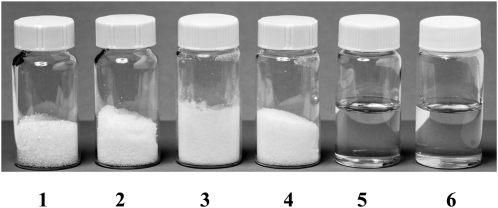
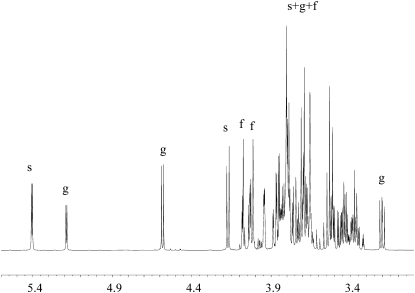
All this strong circumstantial evidence that NADES have an important role to play in cellular metabolism then raises the question of whether they really occur in nature. Accordingly, we went on to investigate the composition of some plant saps. Maple (Acer spp.) syrup is composed mostly of Suc and minor amounts of other sugars, including Glc and malic acid. The individual components are solid at room temperature (melting point above 130°C). However, all combinations of these compounds remained liquid even after all residual water had evaporated (less than 1% [w/w] water; Fig. 4). Particularly, the most stable composition was found to be 1:1, for the molar ratio between Glc and malic acid. Measuring the metabolome of Cleome hasselorana nectar, which remains liquid even after freeze drying, showed that it is made up mostly of sugars (Fig. 5).
Figure 4.
Typical natural eutectic solvents. Container 1, Suc; container 2, Fru; container 3, Glc; container 4, malic acid; container 5, Suc:Fru:Glc (1:1:1, molar ratio); container 6, Suc:malic acid (1:1, molar ratio).
Figure 5.
1H NMR spectrum of C. hasselorana. f, Fru; g, Glc; s, Suc.
Tunnacliffe and Lapinski (2003) reported that the metabolic profiles (gas chromatography, NMR) of dehydrated rotifers were similar to those in the hydrated stage, whereas Pandey et al. (2010), in the resurrection plant Selaginella bryopteris, observed an increase of Pro, reaching a similar molar concentration as Suc that showed a slight decrease in concentration. Apparently, all the ingredients for making natural NADES can be found in cells, but where are these ingredients located? Studies on the localization of anthocyanins in cells (Andersen and Jordheim, 2006) provide some interesting clues. Anthocyanoplasts and anthocyanic vacuolar inclusions might very well consist of NADES, as the level of anthocyanins has been found to be higher than the solubility in water would allow (Markham et al., 2000). As the biosynthesis of anthocyanins is thought to occur on the outside of the endoplasmic reticulum (ER; Hrazdina and Jensen, 1992), this would fit a model in which we envisage finding different types of NADES not only in different organelles such as plastids and vesicles but also attached to protein aggregates or cellular membranes. In fact, the ER might be a NADES with dissolved enzymes in which the usually medium polar substrates are preferentially dissolved; thus, the ER extracts these from the aqueous phase of the cytoplasm. This would fit with the dynamic model of a metabolon (Møller, 2010) and may explain the close interaction between enzymes without the need to be really attached to each other. It may also explain the easy transfer of substrates and products between the enzymes. In membranes, the positively charged choline function of membrane lipids could bind bicarboxylic acids or amino acids, which by associating with sugars and more acids and bases could form a dynamic sheet of NADES inside or around the vacuolar membrane or inside or around other cellular compartments and membranes. This concept is supported by the observations of Crow (2002) that to preserve liposomes under dehydration conditions and to allow rehydration, sugars are required on both sides of the lipid bilayer membrane. To further protect the membranes and the contents of the various cellular compartments, antioxidants like ascorbate, glutathione, flavonoids, and anthocyanins could concentrate at high levels in such a sheet of NADES and protect against oxidation and light damage. This view is supported by the study of Georgieva et al. (2010). They reported that in the resurrection plant Haberlea rhodopensis in the thylakoid lumen, a dense lumenal substance of unknown chemical composition is formed during dehydration. This substance is thought to protect the thylakoid membrane. From previous studies, the dense lumenal substance was thought to have a phenolic character (Van Steveninck and Van Steveninck, 1980), which in fact might be a NADES with a dissolved phenolic compound. The occurrence of NADES in the cell may explain the occurrence of compounds like flavonoids and anthocyanins in cells at much higher levels than what is soluble in water.
The existence of the NADES as alternative solvents in organisms should be further investigated, particularly considering the cellular localization but also other combinations of compounds such as in synthetic ILs. Long before the advent of green chemistry using ILs, nature may have engineered all kinds of complex combinations of compounds into NADES to serve as solvents in various biochemical reactions and physiological functions. This includes combinations that might be considered as self-organizing liquid crystals.
Taken together, our findings strongly suggest that water and lipids are indeed not the only solvents present in living organisms. Apart from implying a paradigmatic change in cellular biochemistry and physiological mechanisms, NADES have an enormous potential for applications, as they are nontoxic, sustainable, and environmentally friendly. They are thus very interesting alternatives to the currently used toxic synthetic ILs.
Potential uses could be as truly green solvents for extractions (e.g. food flavors, fragrances and dyes, medicines, cosmetics, agrochemicals), synthetic organic chemistry, and enzymatic reactions. NADES could act as solvents encapsulated in liposomes for non-water-soluble drugs (e.g. paclitaxel) or for compounds that are unstable in an aqueous medium and as a medium for chemical and biochemical reactions.
We have discovered a completely novel set of ILs and DES: the NADES. These NADES may play a role in all kinds of cellular processes, explaining mechanisms and phenomena that are otherwise difficult to understand, such as the biosynthesis of non-water-soluble small molecules and macromolecules. Green chemistry based on NADES probably evolved very early in the history of living organisms and may reflect a fundamental component of the chemistry of life on earth, even allowing living cells to survive extreme environmental conditions such as drought, salt stress, and high and low temperatures.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Materials and Methods S1. Preparation of NADES and NMR measurement.
References
- Abbott AP, Capper G, Davies DL, Rasheed RK. (2004) Ionic liquids based upon metal halide/substituted quaternary ammonium salt mixtures. Inorg Chem 43: 3447–3452 [DOI] [PubMed] [Google Scholar]
- Andersen ØM, Jordheim M. (2006) The anthocyanins. Andersen ØM, Markham KR, , Flavonoids: Chemistry, Biochemistry and Applications. CRC Press/Taylor & Francis, Boca Raton, FL, pp 471–551 [Google Scholar]
- Buitink J, Leprince O. (2004) Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 48: 215–228 [DOI] [PubMed] [Google Scholar]
- Crow L. (2002) Lessons from nature: the role of sugars in anhydrobiosis. Comp Biochem Physiol A 131: 505–513 [DOI] [PubMed] [Google Scholar]
- Georgieva K, Sarvari E, Keresztes A. (2010) Protection of thylakoids against combined light and drought by a luminal substance in the resurrection plant Haberlea rhodopensis. Ann Bot (Lond) 105: 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorke JT, Srienc F, Kazlauskas RJ. (2008) Hydrolase-catalyzed biotransformation in deep eutectic solvents. Chem Commun (Camb) 2008: 1235–1237 [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6: 431–438 [DOI] [PubMed] [Google Scholar]
- Hrazdina G, Jensen RA. (1992) Spatial organization of enzymes in plant metabolic pathways. Annu Rev Plant Physiol Plant Mol Biol 43: 241–267 [Google Scholar]
- Kim HK, Choi YH, Verpoorte R. (2010) NMR-based metabolomic analysis of plants. Nat Protoc 5: 536–549 [DOI] [PubMed] [Google Scholar]
- Kovács Z, Simon-Sarkadi L, Sovány C, Kirsch K, Galiba G, Kocsy G. (2011) Differential effects of cold acclimation and abscisic acid on free amino acid composition in wheat. Plant Sci 180: 61–68 [DOI] [PubMed] [Google Scholar]
- Lay JO, Jr, Borgmann S, Liyanage R, Wilkins CL. (2006) Problems with the “omics”. Trends Analyt Chem 25: 1046–1056 [Google Scholar]
- Markham KR, Gould KS, Winefield CS, Mitchell KA, Bloor SJ, Boase MR. (2000) Anthocyanic vacuolar inclusions: their nature and significance in flower colouration. Phytochemistry 55: 327–336 [DOI] [PubMed] [Google Scholar]
- Møller BL. (2010) Plant science: dynamic metabolons. Science 330: 1328–1329 [DOI] [PubMed] [Google Scholar]
- Mustafa NR, de Winter W, van Iren F, Verpoorte R. (2011) Initiation, growth and cryopreservation of plant cell suspension cultures. Nat Protoc 6: 715–742 [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Bewley JD. (1996) Desiccation tolerance of plant tissues: a mechanistic overview. Hortic Rev (Am Soc Hortic Sci) 18: 171–213 [Google Scholar]
- Pandey V, Ranjan S, Deeba F, Pandey AK, Singh R, Shirke PA, Pathre UV. (2010) Desiccation-induced physiological and biochemical changes in resurrection plant, Selaginella bryopteris. J Plant Physiol 167: 1351–1359 [DOI] [PubMed] [Google Scholar]
- Paniwnyk L, Beaufoy E, Lorimer JP, Mason TJ. (2001) The extraction of rutin from flower buds of Sophora japonica. Ultrason Sonochem 8: 299–301 [DOI] [PubMed] [Google Scholar]
- Plechkova NV, Seddon KR. (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37: 123–150 [DOI] [PubMed] [Google Scholar]
- Schuurink RC, Sedee NJA, Wang M. (1992) Dormancy of the barley grain is correlated with gibberellic acid responsiveness of the isolated aleurone layer. Plant Physiol 100: 1834–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnacliffe A, Lapinski J. (2003) Resurrecting Van Leeuwenhoek’s rotifers: a reappraisal of the role of disaccharides in anhydrobiosis. Philos Trans R Soc Lond B Biol Sci 358: 1755–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicre M, Farrant JM, Driouich A. (2004) Insights into the cellular mechanisms of desiccation tolerance among angiosperm resurrection plant species. Plant Cell Environ 27: 1329–1340 [Google Scholar]
- Van Steveninck ME, Van Steveninck RFM. (1980) Plastids with densely staining thylakoid contents in Nymphoides indica. II. Characterization of stainable substance. Protoplasma 103: 343–360 [Google Scholar]
- Wasserscheid P, Keim W. (2000) Ionic liquids: new “solutions” for transition metal catalysis. Angew Chem Int Ed Engl 39: 3772–3789 [DOI] [PubMed] [Google Scholar]
- Welton T. (1999) Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem Rev 99: 2071–2084 [DOI] [PubMed] [Google Scholar]