Abstract
Changes in cell wall polysaccharides, transcript abundance, metabolite profiles, and hormone concentrations were monitored in the upper and lower regions of maize (Zea mays) pulvini in response to gravistimulation, during which maize plants placed in a horizontal position returned to the vertical orientation. Heteroxylan levels increased in the lower regions of the pulvini, together with lignin, but xyloglucans and heteromannan contents decreased. The degree of substitution of heteroxylan with arabinofuranosyl residues decreased in the lower pulvini, which exhibited increased mechanical strength as the plants returned to the vertical position. Few or no changes in noncellulosic wall polysaccharides could be detected on the upper side of the pulvinus, and crystalline cellulose content remained essentially constant in both the upper and lower pulvinus. Microarray analyses showed that spatial and temporal changes in transcript profiles were consistent with the changes in wall composition that were observed in the lower regions of the pulvinus. In addition, the microarray analyses indicated that metabolic pathways leading to the biosynthesis of phytohormones were differentially activated in the upper and lower regions of the pulvinus in response to gravistimulation. Metabolite profiles and measured hormone concentrations were consistent with the microarray data, insofar as auxin, physiologically active gibberellic acid, and metabolites potentially involved in lignin biosynthesis increased in the elongating cells of the lower pulvinus.
Midseason root lodging in cereals can cause considerable reductions in grain yield. Depending upon the stage of development, the lodged plants may or may not attain a vertical orientation, which is important in mitigating yield loss. Even partial recovery to the upright orientation, referred to as goose necking in plant breeding, can help minimize yield losses (Dhugga, 2007). In the work reported here, we examined changes that occur when maize (Zea mays) plants are subjected to gravitational stress through placing them in a horizontal orientation.
Alterations in cell wall polysaccharide composition and architecture in response to such gravitational stresses have been studied in woody plants, where responses vary between species. In gymnosperms, gravitational forces increase cell growth in the lower region of a tree branch and result in the formation of compression wood in that region. In angiosperms gravitational responses include the formation of tension wood in the upper regions of branches. Compression wood typically has thickened secondary walls in its tracheids, and higher cellulose microfibril angles and lignin contents (Wilson and Archer, 1977). In contrast, tension wood in angiosperms is characterized by a gelatinous layer in the secondary wall, in which cellulose content increases, but cellulose microfibril angle and lignin content decrease (Wilson and Archer, 1977; Andersson-Gunnerås et al., 2006).
Information on changes that occur in cell wall polysaccharides in the grasses in response to gravistimulation is more limited. Gravitropic responses are initiated in leaf sheath pulvini in festucoid grasses such as barley (Hordeum vulgare), wheat (Triticum aestivum), and oats (Avena sativa), and in both leaf sheath pulvini and culm pulvini in panicoid grasses such as maize (Brown et al., 1959). Collings et al. (1998) examined the growth dynamics and cytoskeleton organization when maize shoots were subjected to gravitational stimulation by placing them in a horizontal position. These authors reported the induction of a gradient of cell elongation in different regions of stem nodes, where maximal elongation was observed in the cells of the lowermost pulvini but where negligible cell elongation was detected in the cells of the uppermost pulvini. Differential cell elongation in the lower and upper zones of the pulvinus causes the shoots to return to the upright orientation (Kaufman et al., 1987; Collings et al., 1998).
At the polysaccharide level, Montague (1995) reported that [14C]Glc was incorporated into cell wall fractions in whole oat pulvini in response either to gravistimulation or upon treatment with the phytohormone, auxin. Biosynthesis of both cellulose and wall matrix polysaccharides increased in the pulvini. Furthermore, Gibeaut et al. (1990) noted that (1,3;1,4)-β-glucan content was higher in the lower pulvini than in the upper regions of oat pulvini, which was consistent with their observations that (1,3;1,4)-β-glucan synthase activities increased in the lower regions of the pulvini.
If it is accepted that cells in the lower region of the pulvinus elongate at a higher rate than those in the upper pulvinus during the reorientation of plant growth from the horizontal to the vertical position (Kaufman et al., 1987; Collings et al., 1998), then one might anticipate that differential stimulation of the upper and lower regions by auxin would be involved in this process. Long et al. (2002) suggested that there is a transient gradient of indole-3-acetic acid (IAA) across maize pulvinal tissue shortly after gravitational stimulation, which would support this suggestion. Auxin-induced cell elongation is known to stimulate wall loosening agents such as β-expansins, (1,4)-β-glucanases, and xyloglucan endotransglycosylases/hydrolases (XTHs; Catalá et al., 1997; Cosgrove, 2000). The β-expansins are believed to bind to noncrystalline regions of cellulose microfibrils, where they promote cell elongation by disrupting hydrogen bonding between cellulose molecules and between cellulose and other wall polysaccharides (McQueen-Mason and Cosgrove, 1995). The (1,4)-β-glucan endohydrolases catalyze the hydrolysis of cellulosic polysaccharides, while XTHs are believed to facilitate wall expansion through their action on xyloglucans (Fry et al., 1992; Nishitani and Tominaga, 1992; Rose et al., 2002). More recently it has been proposed that XTHs might not only promote wall elongation through their action on xyloglucans, but that they might also be involved in subsequent cell wall immobilization through the covalent cross linkage of xyloglucans, cellulose, and (1,3;1,4)-β-glucans (Hrmova et al., 2007, 2009; Fry et al., 2008). Roles for (1,4)-β-glucan endohydrolases and XTHs in auxin-induced cell elongation are consistent with observations that turnover rates of (1,3;1,4)-β-glucans in grasses and of xyloglucans in dicotyledons increase in response to auxin stimulation (Cosgrove, 2000).
Here, changes that occur in the pulvinus during the reorientation of maize plants from the horizontal to the vertical position have been defined. Detailed temporal and spatial changes in wall composition have been monitored using linkage analysis of wall polysaccharides and chemical analyses of lignin contents. Transcriptional activities of approximately 45,000 maize genes in the upper and lower regions of the pulvinus have been monitored through microarray analyses and changes in transcript levels were consistent with changes observed in the amounts and fine structures of major wall components. The transcriptional patterns of genes involved in the interconversion of sugar nucleotides, together with metabolite profiles, could also be reconciled with changes in the relative proportions of heteroxylans and Glc-containing wall polysaccharides, and lignin. The microarray data further suggested that the transcription of genes encoding enzymes involved in phytohormone biosynthesis differentially changed in the upper and lower regions of the pulvinus, and in the cases of auxin, GAs, and abscisic acid (ABA), the transcript profiles of the biosynthetic genes were shown to correspond with the actual levels of these hormones measured in tissue extracts and with the expected biological functions of the hormones.
RESULTS
Cells in the Lower Pulvinus Elongate during Gravitational Stimulation
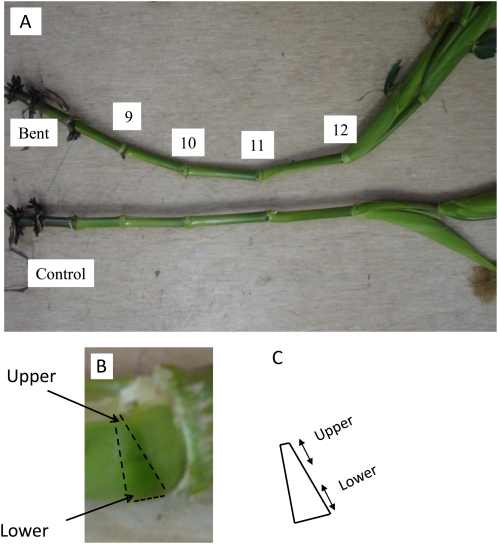
The bending capacity of maize pulvinus during the gravitropic responses observed here varied depending on the position of the pulvinus in the plant and on the age of the plant. Thus, pulvini from node 9 to node 13, where node 1 was the lowest on the plant, were responsive to gravitational stimulation in 60- to 65-d-old maize plants (Fig. 1A; Supplemental Fig. S1), while pulvini from node 8 and below did not show signs of curvature. The thickness of the pulvinus from node 10 was 9 to 11 mm on the lowermost surface in the gravitationally stimulated maize plants, but only 3 to 4 mm on the uppermost surface; the latter was the same thickness as that measured in control plants (Fig. 1B).
Figure 1.
Gravitropic response of maize stems. A Maize plant (65-d-old) was placed on the floor at an angle of about 20° for 14 d. Pulvini from nodes 9 to 13 were responsive to gravistimulation, but pulvini below node 9 did not show signs of curvature (A). Leaves have been removed to show stem curvature more clearly. The outline of a single pulvinus from node 10 is indicated by dashed lines (B). When excised, the pulvinus is a wedge-shaped disk with thin tissue on the upper region and thick tissue on the lower region. Approximately one-third of the upper region and one-third of the lower region were collected, designated as upper and lower pulvinus, and used for cell wall polysaccharide and gene expression analysis (C). The middle part of the pulvinus was discarded.
The thicknesses of the upper and lower sides of stem pulvini from node 10 were correlated with the lengths of cells in these regions. Although the lengths of cells in the pulvini varied considerably, in the lower regions of pulvini in gravistimulated maize plants most sclerenchyma cells were 400 μm or longer, while in control plants most of the sclerenchyma cells in pulvini were in the range of 150 to 250 μm in length (Supplemental Table S1). Overall, the average lengths of sclerenchyma cells from node 10 were 200, 220, and 498 μm in pulvini of controls, upper regions, and lower regions, respectively. Parenchyma cells were about 3.5-fold longer in the lower pulvini than in control pulvini (Supplemental Table S1). Thus, there was a 2.5- to 3.5-fold increase in the length of these cell types, which was consistent with the differences in length of the pulvini overall, as described above. Cell elongation in leaf sheath pulvini followed the same pattern as in stem pulvini (data not shown).
Mechanical Strength Increased in the Lower Pulvinus
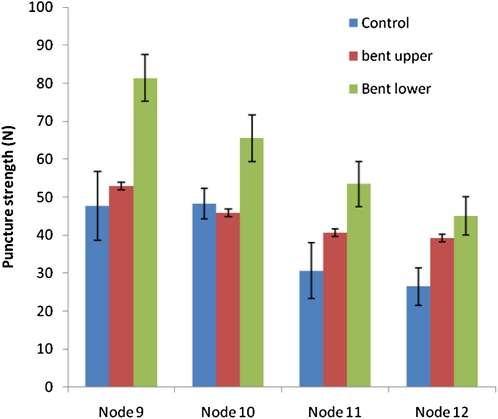
In both control and gravistimulated plants, pulvini from the lower nodes on the plant had a higher resistance to puncture penetration than pulvini from the top nodes (Fig. 2). In addition, the lower pulvini in the gravistimulated plants were mechanically stronger than upper pulvini, as measured by resistance to the puncture probe (Fig. 2). The upper pulvini from nodes 9 and 10 of the gravistimulated plants showed resistance to the puncture probe that was similar to control pulvini. The increase in puncture resistance in the lower pulvini was up to 30% or more in nodes 9 and 10, compared with controls (Fig. 2). In nodes 11 and 12 puncture resistance was higher in both the lower and upper pulvini compared with the controls and it may be that these nodes, being younger and weaker than nodes 9 and 10, would need to increase strength on both sides to sustain a vertical orientation in a bent plant. The increased strength might be achieved simply through the changing shape of the pulvini or through reinforcement of their cell walls.
Figure 2.
Puncture resistance of maize stem pulvini. Maize plants (65-d-old) were placed at an angle of 20° to the horizontal for 2 weeks. Puncture resistance was tested on fresh pulvini from nodes 9 to 12, using a puncture probe. Pulvini from control plants that had not been subjected to gravistimulation were also tested.
Lignin Contents of Walls Increased in the Lower Pulvinus
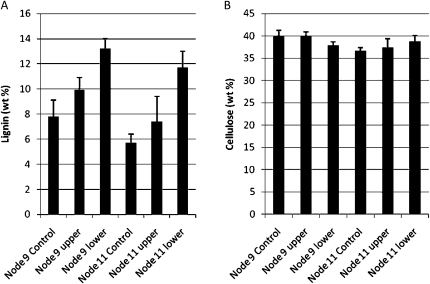
There was a substantial, statistically significant increase in lignin content of approximately 40% in the lower pulvinus of nodes 9 and 11 in gravitationally stimulated maize stalks (Fig. 3A), compared with the upper pulvinus. The increase in lignin on the upper side relative to the control was not statistically significant. There was also a significant increase in ester-linked ferulic acid and p-coumaric acid on the lower side of the pulvinus (data not shown), but whether these phenolic acids were linked with lignin or polysaccharides such as heteroxylans is not known.
Figure 3.
Lignin and crystalline cellulose contents in maize stem pulvini. Maize plants were subjected to gravitropic stimulation for 2 weeks. Cell walls were prepared for the determination of lignin (A) and crystalline cellulose (B) contents. Lignin was assayed by the modified Klason method, while crystalline cellulose was measured by acetic/nitric acid digestion. Cellulose content deduced from the linkage analysis (Table II) produced compatible results with this acid digestion method.
Heteroxylan Levels and Fine Structures Change in Walls from Lower Pulvini
Monosaccharide linkage analyses showed no significant difference in cellulose content in control, upper, and lower stem pulvini, as determined by levels of (1,4)-glucosyl residues in the cell wall preparations (Tables I and II), and this was confirmed using acetic acid/nitric acid procedures (Updegraff, 1969; Fig. 3B). Similarly, no significant differences were detected in the levels of (1,3;1,4)-β-glucan, pectic arabinan, or type II arabinogalactan (Table II).
Table I. Cell wall monosaccharide linkage composition in maize pulvini.
Maize plants were subjected to gravistimulation for 2 weeks. Pulvini from nodes 9 and 11 were harvested for determination of monosaccharide linkages (mol%). Results represent five biological replicates, each analyzed in duplicate. Values in bold are statistically significant (P < 0.05) using a Student’s t test. Ara(f), Arabinofuranose; Xyl(p), xylopyranose; Man(p), mannopyranose; Gal(p), galactopyranose; Glc(p), glucopyranose; Ara + Xyl, sum of Ara(f) and Xyl(p); Xyl sub, sum of substituted 4-Xyl(p); Xyl Uns, unsubstituted 4-Xyl(p); us/s, ratio of unsubstituted over substituted xylans. Trace amounts indicated by tr.
| Monosaccharide | Node 9 |
Node 11 |
|||||
| Deduced Linkage | Control | Upper | Lower | Control | Upper | Lower | |
| Ara(f) | Terminal | 5.3 | 4.3 | 3.9 | 6.8 | 6.2 | 4.7 |
| 2- | tr | tr | tr | tr | tr | tr | |
| 3- | tr | tr | 0.5 | tr | tr | 0.5 | |
| 5- | 0.6 | 0.8 | 0.9 | 1.0 | 1.3 | 0.8 | |
| 3,5- | tr | tr | tr | tr | tr | tr | |
| Total | 6.2 | 5.4 | 5.4 | 8.2 | 7.9 | 6.2 | |
| Xyl(p) | Terminal | 1.4 | 1.2 | 1.3 | 1.7 | 1.7 | 1.4 |
| 3- | tr | tr | tr | tr | tr | Tr | |
| 4- | 10.9 | 14.6 | 15.6 | 7.7 | 10.3 | 17.0 | |
| 2,4- | 0.9 | 1.1 | 1.3 | 1.0 | 1.0 | 1.4 | |
| 3,4- | 3.8 | 3.4 | 3.2 | 5.2 | 3.9 | 3.6 | |
| 2,3,4- | 0.5 | 1.2 | 1.1 | 0.5 | 0.5 | 0.8 | |
| Total | 17.6 | 21.4 | 22.6 | 16.2 | 17.3 | 24.4 | |
| Man(p) | Terminal | tr | tr | tr | tr | tr | tr |
| 4- | 4.0 | 2.8 | 2.4 | 4.8 | 4.0 | 2.3 | |
| Gal(p) | Terminal | 0.9 | 0.7 | 0.9 | 1.2 | 1.3 | 1.0 |
| 2- | tr | tr | tr | 0.6 | 0.4 | tr | |
| 3,6- | tr | tr | tr | tr | tr | tr | |
| Glc(p) | Terminal | 0.8 | 0.8 | 1.0 | 0.7 | 0.9 | 0.8 |
| 3- | 2.0 | 2.0 | 2.0 | 2.2 | 1.7 | 1.7 | |
| 4- | 65.5 | 63.8 | 62.9 | 62.3 | 63.3 | 60.5 | |
| 2,4- | tr | tr | tr | tr | tr | tr | |
| 3,4- | tr | tr | tr | tr | tr | 0.5 | |
| 4,6- | 2.1 | 1.9 | 1.7 | 2.9 | 2.2 | 1.8 | |
| Total | 70.7 | 69.1 | 68.1 | 68.5 | 68.4 | 65.4 | |
| Ara + Xyl | 23.8 | 26.9 | 28.0 | 24.4 | 25.3 | 30.6 | |
| 4-Xyl Sub | 5.3 | 5.6 | 5.6 | 6.8 | 5.3 | 5.8 | |
| 4-Xyl Uns | 10.9 | 14.6 | 15.6 | 7.7 | 10.3 | 17.0 | |
| us/s | 2.1 | 2.6 | 2.8 | 1.1 | 1.9 | 2.9 | |
Table II. Deduced cell wall polysaccharide contents in maize pulvini.
Monosaccharide linkages were summarized according to their representation of individual polysaccharides as described by Shea et al. (1989) and Zhu et al. (2005). Values in bold are statistically significant (P < 0.05) using a Student’s t test. AG, Arabinogalactan.
| Polysaccharide | Node 9 |
Node 11 |
||||
| Control | Upper | Lower | Control | Upper | Lower | |
| Arabinan | 0.6 | 0.8 | 0.9 | 0.9 | 1.3 | 0.8 |
| Type II AG | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 |
| Arabinoxylan | 22.3 | 27.4 | 28.6 | 22.1 | 21.9 | 30.3 |
| (1,3;1,4)-β-Glucan | 6.6 | 6.8 | 6.6 | 7.3 | 5.6 | 5.6 |
| Xyloglucan | 5.5 | 5.0 | 4.6 | 7.6 | 6.1 | 4.9 |
| Heteromannan | 7.9 | 5.6 | 4.9 | 9.7 | 8.0 | 4.7 |
| Cellulose | 54.8 | 54.3 | 54.2 | 49.4 | 53.2 | 52.5 |
However, the linkage analyses indicated that the arabinoxylan content of walls increased in the lower pulvini of both nodes, but more particularly in node 11 (Tables I and II), where increases of about 50% were observed in total xylosyl residues in the lower pulvinus compared with values obtained for controls and upper pulvini from node 11 (Table I). This was mainly attributable to an increase in (1,4)-xylosyl residues, which represent unsubstituted backbone xylosyl residues in the polysaccharide. In pulvini from node 11, levels of both total xylosyl residues and (1,4)-linked xylosyl residues were significantly higher in the lower pulvini, compared with the upper pulvini; increases were less pronounced in node 9 where increases in both the upper and lower pulvinus were observed (Table I). Consistent with the increases in (1,4)-linked xylosyl residues, total arabinosyl residues decreased in lower pulvini from node 11 and this was at least partly attributable to a reduction in terminal arabinosyl residues (Table I). This resulted in an increase in ratio of xylosyl to arabinosyl residues from about 2.0:1 to about 3.9:1 in the lower pulvini from node 11 (Table I). As noted above, the changes in pentose sugar contents in pulvini from node 9 were not as pronounced as in pulvini from node 11, but they nevertheless moved in the same direction. It is important to note that the amounts of terminal arabinofuranosyl derivatives were generally close to the total amounts of substituted xylopyranosyl derivatives, namely the levels of (2,4)-, (3,4)-, and (2,3,4)-xylopyranosyl derivatives (Table I). The latter correspond to 2-, 3-, and (2,3)-substituted xylosyl residues of the (1,4)-xylan backbone of the polysaccharide. This indicated that most of the substituents on the xylan chain were arabinosyl residues but we acknowledge that some glucuronosyl substituents might be present.
Mannosyl residues decreased in the lower pulvini from both nodes, and the analyses indicated that (1,4)-linked mannosyl residues were largely responsible for these decreases (Table I).
When the linkage analysis data were used to estimate the relative abundance of different classes of polysaccharides in the walls of the upper and lower pulvini, the results indicated that levels of heteroxylans increased by about 35% in the lower regions of pulvini in node 11, and that galactoglucomannans and xyloglucans decreased by about 50% and 30%, respectively, in lower pulvini of node 11; it should be noted that the levels of galactoglucomannans and xyloglucans in the walls were relatively low (Table II). The (1,3;1,4)-β-glucan contents of the walls were also low and no significant changes occurred in the levels of this polysaccharide (Table II).
In the interpretations suggested above for the data presented in Tables I and II, it is important to emphasize that only values that are statistically significant to the P < 0.05 level, which are indicated in bold font in the tables, were considered.
Transcript Profiles of Genes for Sugar Nucleotide Interconverting Enzymes Were Consistent with Increases in Pentose-Containing Polysaccharides
As noted above and shown in Table II, the major change observed in cell wall composition in the pulvini of gravistimulated maize stalks was an increase in arabinoxylan content on the lower side of the pulvini. Biosynthesis of heteroxylans requires at least three activated sugar nucleotides, namely UDP-d-glucuronate, UDP-l-arabinose (UDP-Ara), and UDP-d-Xyl (UDP-Xyl). Key enzymes in the synthesis and interconversion of sugar nucleotides for cell wall biosynthesis are UDP-Glc pyrophosphorylase (UGPP), UDP-Glc epimerase (UGE), UDP-Glc dehydrogenase, UDP-d-glucuronate epimerase (UGAE), UDP-Xyl synthase (UXS), and UDP-Xyl epimerase (Zhang et al., 2005; Sharples and Fry, 2007; Reiter, 2008).
Microarray data analysis showed that UGPP, UGE, UGAE, and UXS genes were all up-regulated by more than 2-fold in the lower pulvini 24 h after the initiation of gravistimulation, based on the relative abundance of specific mRNAs (Table III). Eight probes on the microarray corresponded to UGPP genes. One of the UGPP genes (probe 4201585) showed a significant increase in mRNA levels in lower pulvini from both stems and leaves at 24 h (Table III; data for leaves not shown).
Table III. Fold changes in transcript levels of genes that mediate sugar nucleotide interconversions in stem pulvini.
The values are fold changes compared with control. Significant changes (>2-fold and P < 0.05) are indicated in bold. Functions of genes were analyzed according to Hayes et al. (2010) and are shown in the Annotation column with GeneBank numbers in parentheses. Note that the value of 28.8-fold for the increase in UGE transcripts in the lower pulvinus at 6 h was not significant because of large variation between replicates.
One of the seven microarray probes for UXS genes (probe 4241027) detected increases of 2.2-fold and 4.1-fold in mRNA levels for the gene in lower stem pulvini at 6 and 24 h, respectively (Table III). Although there was an increase in transcript level of this gene in upper pulvini at 1 h, this had returned to control levels by 24 h (Table III). The increases in abundance of UXS gene transcripts implied increased production of UDP-Xyl and UDP-Ara.
Microarray Analyses of Transcription of Glycosyltransferase Genes
The microarray contained approximately 40 probes for cellulose synthase (CesA) genes, including ZmCesA1, ZmCesA2, ZmCesA4, ZmCesA5, ZmCesA6, ZmCesA8, ZmCesA9, ZmCesA11, and ZmCesA12. No significant differences between transcript levels in upper and lower pulvini could be detected for any of the ZmCesA genes (Supplemental Fig. S2), and this was consistent with the similar levels of cellulose in walls of the upper and lower pulvini (Table II; Fig. 3B). There were two probes on the microarray for the cellulose synthase-like CslF genes that have been implicated in (1,3;1,4)-β-glucan biosynthesis (Burton et al., 2006). Transcript levels for these CslF genes were very low and did not change significantly in any of the samples analyzed, which was consistent with the low and essentially unchanged levels of (1,3;1,4)-β-glucan in the walls (Table II). There was no probe for the CslH gene on the microarray; the CslH gene has also been implicated in (1,3;1,4)-β-glucan synthesis (Doblin et al., 2009). The CesA, CslF, and CslH genes are all members of the family glycosyltransferase2 (GT2) group of GTs (Cantarel et al., 2009; http://www.cazy.org/).
More than 36 GT probes were included on the microarray and are named here according to the GT family to which they belong (Cantarel et al., 2009; http://www.cazy.org/). A family GT8 GT gene was transcribed at significantly higher levels than controls in both the upper and lower pulvini, and at different times after the imposition of the gravitational stress. In the upper pulvinus, levels of the GT8 gene transcripts decreased after 6 h (Table IV) but returned to control levels at 24 h. Three family GT61 GT genes, some of which are believed to encode β-1,2-xylosyltransferases, showed quite different changes in transcript patterns. One (probe 4304503) showed increased transcription of the corresponding gene after 24 h in both the upper and lower pulvinus, while another (probe 4308259) showed increases only in the lower pulvinus after 24 h, and after 1 h in the upper pulvinus (Table IV). Transcripts for the third family GT61 gene (probe 4285578) increased 2-fold in the lower pulvinus, but remained unchanged in the upper pulvinus (Table IV). Finally, a gene encoding a GT34 enzyme was transcribed at high levels after 1 h only, but in both the upper and lower pulvinus (Table IV).
Table IV. Changes in transcript levels of GT genes in stem pulvini.
The values are fold changes compared with control. Significant changes (>2-fold and P < 0.05) are indicated in bold.
| Probe | Family | Lower |
Upper |
Annotation | ||||
| 1 h | 6 h | 24 h | 1 h | 6 h | 24 h | |||
| 4262796 | GT34 | 4.9 | 1.3 | 1.1 | 5.5 | 1.0 | 1.1 | Putative GT (Q2P9N4) |
| 4285578 | GT61 | 1.1 | 1.9 | 2.3 | 1.2 | 1.6 | 1.4 | Putative β-1,2-xylosyltransferase (Q2UVB3) |
| 4293499 | GT8 | 3.4 | 2.6 | 4.3 | 5.0 | 2.8 | 1.8 | Putative GT (Q75IZ5) |
| 4304503 | GT61 | 1.2 | 0.9 | 4.1 | 0.7 | 1.3 | 3.9 | Putative GT (Q8RYJ1) |
| 4308259 | GT61 | 1.6 | 1.7 | 6.9 | 3.4 | 1.3 | 1.9 | GT (Q5QPZ0) |
Altered Transcription of Genes Involved in Heteroxylan Modification
As discussed above, the degree of substitution of heteroxylans with arabinofuranosyl residues decreased in walls of the lower pulvinus following gravistimulation. Arabinofuranosidases from several families of glycosyl hydrolases (GHs) catalyze the removal of α-arabinofuranosyl groups from the (1,4)-β-xylan backbone of arabinoxylans (Cantarel et al., 2009). Three probes on the microarray were annotated as arabinofuranosidase genes. Transcriptional activities of two of these genes (probes 4295907 and 4292768), both of which belong to family GH51 and are likely to be arabinoxylan arabinofuranohydrolases (Lee et al., 2001), did not change significantly in either the upper or lower pulvini (Table V). However, mRNA levels of the other gene (probe 4211175), which encodes a GH3 family enzyme, increased substantially in the lower pulvinus at 24 h (Table V). Thus, the changes in this transcript are consistent with the decreased arabinosyl content of the arabinoxylan in the lower pulvinus (Tables I and II).
Table V. Transcript levels of glucuronoarabinoxylan degradation genes in stem pulvini.
The values are fold changes compared with control. Significant changes (>2-fold and P < 0.05) are indicated in bold.
| Probe | Glycoside Hydrolase Family | Lower |
Upper |
Annotation | ||||
| 1 h | 6 h | 24 h | 1 h | 6 h | 24 h | |||
| Arabinofuranosidases | ||||||||
| 4195907 | GH51 | 0.7 | 0.6 | 0.4 | 0.7 | 0.7 | 1.0 | α-l-Arabino-furanosidase C terminus family protein (Q2RAZ7) |
| 4211175 | GH3 | 1.4 | 0.9 | 7.2 | 2.0 | 1.0 | 1.5 | Putative α-l-arabinofuranosidase (Q94IY5) |
| 4292768 | GH51 | 0.4 | 0.3 | 0.3 | 0.4 | 0.5 | 0.3 | α-l-Arabinofuranosidase C terminus family protein (Q2QY88) |
| Xylanases and xylanase inhibitors | ||||||||
| 4217504 | GH18 | 1.5 | 4.4 | 1.8 | 2.2 | 3.7 | 3.2 | Xylanase inhibitor XIP-III (Q4W6G2) |
| 4280469 | GH3 | 2.2 | 3.9 | 0.5 | 2.5 | 5.5 | 3.7 | Putative β-d-xylosidase (Q6Z8I7) |
| 4291762 | GH18 | 12.5 | 54.0 | 3.8 | 38.3 | 43.1 | 3.7 | Xylanase inhibitor protein 1 precursor (Q8L5C6) |
Ten features on the microarray were identified as (1,4)-β-xylan endohydrolase inhibitor genes. The mRNA level of one of these genes (probe 4291762) increased substantially in both lower and upper stem pulvini (Table V). Transcript levels of another xylanase inhibitor gene (probe 4217504) were significantly higher in the lower pulvinus at 6 h (Table V). Both genes encode xylanase inhibitors of the XIP class and belong to the GH18 family (Cantarel et al., 2009).
Increased Transcriptional Activities of Other Genes Involved in Wall Loosening in the Lower Pulvinus
Enzymes and nonenzymic proteins such as polysaccharide endo- and exohydrolases, XTHs, and expansins have been implicated in cell wall loosening during cell growth in plants (Catalá et al., 1997; Cosgrove, 2000; Hrmova et al., 2009). Thirteen probes were annotated as either endo- or exo-β-d-glucanase genes on the microarray. The mRNA level of a family GH9 gene (probe 4314428) increased significantly in lower stem pulvini, but not in the upper pulvinus (Supplemental Table S2). A family GH3, putative β-glucosidase gene (probe 4250320) also showed increased transcriptional activity in the lower pulvinus, but not in the upper pulvinus (Supplemental Table S2). Eleven probes were identified as GH17 (1,3;1,4)-β-glucanase genes on the microarray. One of these (probe 4297356) showed a significant increase in mRNA levels in the lower pulvinus (Supplemental Table S2). Thirty-four probes were annotated as encoding (1,3)-β-glucanase genes of the GH17 family. Two of these (probes 4240483 and 4218229) showed a significant increase in transcript levels in the lower pulvinus (Supplemental Table S2). Another two (probes 4279302 and 4302273) showed increased transcription in both lower and upper pulvini (Supplemental Table S2).
Forty-one microarray probes were annotated as β-expansin genes. Four (probes 4224980, 4258316, 4259490, and 4271529) showed more than 4-fold increases in transcript levels in the lower pulvinus (Supplemental Table S3). An additional two probes with the same accession number as probe 4259490 also indicated increases in β-expansin transcripts in lower pulvini (data not shown). Another two of the putative β-expansin genes (probes 4222177 and 4280874) showed increased transcriptional activities in both lower and upper pulvini. One putative β-expansin gene (probe 4307195) showed decreased transcription activity in both the upper and lower pulvini (Supplemental Table S3).
Nineteen of the microarray features encoded family GH16 XTH genes. One (probe 4278710) showed a significant increase in transcript levels in the lower pulvinus (Supplemental Table S4), while another (probe 4265553) showed increased transcriptional activity in both the lower and upper pulvini. Two probes with the same GenBank accession number were annotated as a family GH16 xyloglucan (1,4)-β-glucan endohydrolases (probe 4238076). Transcripts of this gene increased in both the lower (6 h) and upper (1 h) pulvinus (Supplemental Table S4).
Transcript Levels of Genes Involved in Lignin Metabolism
Microarray analyses showed that of more than 50 probes representing lignin biosynthetic genes (Supplemental Table S5), levels of mRNA for a cinnamoyl-CoA reductase (CCR) gene (probe 4245740) increased significantly (P < 0.05) 1 h after the commencement of gravistimulation, in both the lower and upper pulvini (Supplemental Table S5). Transcripts for this gene remained 2.4-fold higher in the lower pulvinus at 24 h, compared with control plants, but returned to control levels in the upper pulvini during this time. Transcripts of a cinnamate-4-hydroxylase (C4H) gene (probe 4268958) increased substantially after 1 h in upper pulvini. Similarly, putative hydroxycinnamoyl transferase gene transcripts appeared to increase early after gravistimulation (probe 4229300). In each of these three cases, increases in transcript abundance was higher in the upper pulvinus (Supplemental Table S5), which can be contrasted to the actual measurements of lignin content, which showed higher levels in the lower pulvinus (Fig. 3A). Consistent with this, transcript levels for three peroxidase genes (probes 4267626, 4213200, and 4225259) increased in the lower pulvinus, usually 6 to 24 h after the initiation of gravistimulation (Supplemental Table S5).
Transcript Levels of Genes Involved in Hormone Metabolism Changed in the Pulvinus
Among the genes involved in auxin biosynthesis, mRNA levels for anthranilate 5-phosphoribosyltransferase (probe 4250382) increased about 2.7-fold in lower stem pulvini at 6 h and remained high at 24 h, but did not change in the upper pulvinus (Table VI). No changes in transcription levels were found for other genes involved in auxin biosynthesis. The transcript levels of an auxin conjugate hydrolase gene (probe 4282474) increased 12- to 40-fold in both lower and upper pulvini at 1 h, but returned to control levels at 6 h (Table VI).
Table VI. Changes in transcript levels of phytohormone genes in stem pulvini.
The values are fold changes compared with control. Significant changes (>2-fold and P < 0.05) are indicated in bold.
| Probe | Lower |
Upper |
Top Hits | ||||
| 1 h | 6 h | 24 h | 1 h | 6 h | 24 h | ||
| 4205461 | 131.5 | 72.1 | 18.4 | 12.7 | 22.1 | 0.7 | ACC synthase (Q6JN57) |
| 4218360 | 3.3 | 4.7 | 3.0 | 2.9 | 2.5 | 1.1 | Gibberellin 20-oxidase (Q0PWF8) |
| 4235513 | 13.4 | 6.8 | 2.4 | 18.5 | 6.8 | 0.7 | Gibberellin 2-oxidase (Q8S0S6) |
| 4250382 | 1.8 | 2.7 | 2.2 | 1.4 | 1.3 | 1.2 | Putative anthranilate phosphoribosyltransferase (Q5TKJ0) |
| 4266528 | 12.0 | 1.1 | 1.0 | 135.2 | 2.3 | 4.5 | ACC oxidase (Q6JN55) |
| 4282474 | 12.6 | 0.7 | 1.0 | 41.6 | 1.0 | 0.7 | Putative auxin conjugate hydrolase (Q5N8F2) |
| 4286393 | 6.7 | 1.4 | 1.6 | 46.2 | 2.8 | 1.7 | ACC synthase (Q6JN58) |
| 4291681 | 3.4 | 1.1 | 1.0 | 34.5 | 2.3 | 0.8 | ACC oxidase (Q6JN56) |
| 4291827 | 1.7 | 7.5 | 2.6 | 0.9 | 3.5 | 1.5 | CK oxidase 3 (Q709Q3) |
| 4296711 | 1.9 | 9.2 | 2.9 | 1.0 | 4.0 | 1.6 | CK oxidase 2 (Q709Q5) |
| 4308055 | 1.8 | 3.9 | 4.4 | 1.7 | 4.4 | 2.9 | Kaurene synthase A (Q41771) |
| 4316583 | 10.0 | 4.1 | 2.7 | 10.0 | 3.3 | 2.6 | (+)-ABA 8-hydroxylase (Q05JG2) |
Transcripts of an ent-kaurene synthase gene (probe no. 4308055), which encodes an enzyme that catalyzes the formation of ent-kaurene from geranylgeranyl diphosphate during GA biosynthesis (Hedden and Kamiya, 1997), increased 3- to 4-fold in both upper and lower stem pulvini 6 h after gravistimulation (Table VI). Transcript levels of a GA 20-oxidase gene (probe 4218360) increased more than 3-fold at 1 h and remained high at 24 h in the lower pulvinus (Table VI). Although the mRNA level of the GA 20-oxidase gene also increased at 1 and 6 h, it had decreased to control levels by 24 h. The increase in transcription of a GA 2-oxidase gene (probe 4235513) was more than 13-fold at 1 h and remained high at 6 h in both upper and lower stem pulvini (Table VI). However, the mRNA level decreased to control levels in the upper pulvinus but remained high in the lower pulvinus at 24 h.
The microarray included probes of putative violaxanthin deepoxidase, molybdenum cofactor sulfurase, and 9-cis-epoxycarotenoid dioxygenase, which are enzymes involved in the ABA biosynthetic pathway. In addition, genes encoding ABA 8-hydroxylase and cytochrome 450 monooxygenase, which are involved in ABA catabolism, were represented on the microarray. Of these genes, mRNA of an ABA hydroxylase gene (probe 4316583) increased substantially in both upper and lower pulvini (Table VI).
The transcripts of a cytokinin (CK) oxidase gene (probe 4296711) increased by more than 4-fold in both lower and upper pulvini at 6 h (Table VI), remained high in the lower pulvinus, but returned to control levels in the upper pulvinus at 24 h. Another CK oxidase gene (probe 4291827) showed increased transcription, but only in the lower pulvinus. There were no changes in transcripts of CK biosynthetic genes.
As an indication of ethylene metabolism in the gravitationally stimulated pulvini, transcript levels of two 1-aminocyclopropane-1-carboxylic acid (ACC) synthase genes were monitored with the microarray. The mRNA levels of one of these genes (probe 4286393) was about 7-fold higher in upper than lower pulvini at 1 h (Table VI), while the mRNA level of the other ACC synthase gene (probe 4205461) was higher in lower pulvini (Table VI). When mRNA levels of two ACC oxidase genes (probes 4291681 and 4266528) were measured, both were 10-fold higher in the upper pulvinus than in the lower pulvinus (Table VI).
Microarray Data Confirmed by Quantitative-PCR
To confirm the microarray analyses, changes in transcript levels of selected genes were monitored in upper, lower, and control pulvini by quantitative-PCR, using experimental and data normalization procedures described by Burton et al. (2004). The direction and approximate quanta of changes in transcript levels were confirmed for all genes analyzed, and some examples are presented in Supplemental Figure S2.
Hormones Were Measured in Extracts of the Pulvinus
The changes in mRNA levels described above for genes that have been implicated in the synthesis and degradation of various phytohormones suggested that temporal and spatial changes were occurring in hormone concentrations. To check this, the actual concentrations of a range of auxins, CKs, ABA, and GA were measured in tissue extracts, together with related metabolic intermediates and conjugated forms of these hormones. Ethylene was not measured in the experiments described here.
Auxin
Pulvinus from control maize plants contained 100 to 130 ng/g dry weight IAA, but this was almost halved after 1 h gravistimulation. However, IAA levels increased to 300 and 460 ng/g dry weight at 6 h after gravistimulation in upper and lower pulvini, respectively (Table VII). Levels of IAA conjugates including IAA-Ala, IAA-Asp, IAA-Leu, and IAA-Glu were very low, with less than 10 ng/g dry weight in the maize pulvinus (Table VII; data not shown except IAA-Glu).
Table VII. Phytohormone concentrations in maize pulvini.
Hormone concentrations (ng/g DW) were measured in control (con), upper (Upp), and lower (low) pulvini at 0, 1, and 6 h. IAA-Glu, N-(indole-3-yl-acetyl)-Glu; t-ZOG, (trans) zeatin-O-glucoside; c-ZOG, (cis) zeatin-O-glucoside; nd, not detectable.
| Compound | Con 0 h | Con 1h | Upp 1 h | low 1 h | Con 6 h | Upp 6 h | Low 6 h |
| IAA | 104 ± 13 | 130 ± 5 | 58 ± 17 | 60 ± 9 | 110 ± 34 | 300 ± 59 | 466 ± 91 |
| IAA-Glu | 6 ± 2 | 7 ± 2 | nd | nd | nd | nd | 5 ± 1 |
| ABA | 153 ± 39 | 212 ± 73 | 95 ± 43 | 147 ± 24 | 124 ± 10 | 306 ± 75 | 828 ± 234 |
| ABA-GE | 114 ± 13 | 123 ± 12 | 96 ± 23 | 97 ± 25 | 88 ± 20 | 84 ± 21 | 106 ± 12 |
| DPA | 911 ± 193 | 701 ± 186 | 711 ± 307 | 709 ± 346 | 983 ± 197 | 1,078 ± 193 | 1,632 ± 355 |
| PA | 87 ± 63 | 167 ± 41 | 254 ± 49 | 241 ± 95 | 133 ± 107 | 262 ± 57 | 936 ± 225 |
| t-ZOG | 34 ± 8 | 42 ± 11 | 39 ± 6 | 32 ± 4 | 31 ± 5 | 26 ± 5 | 24 ± 6 |
| c-ZOG | 244 ± 56 | 315 ± 70 | 286 ± 57 | 278 ± 37 | 231 ± 60 | 236 ± 31 | 251 ± 40 |
| GA19 | 48 ± 10 | 40 ± 15 | 62 ± 45 | 59 ± 29 | 79 ± 23 | 81 ± 30 | 41 ± 15 |
| GA44 | 23 ± 12 | 19 ± 10 | 31 ± 23 | 34 ± 13 | 58 ± 34 | 42 ± 16 | 59 ± 22 |
| GA53 | 56 ± 30 | 36 ± 20 | 71 ± 68 | 71 ± 62 | 151 ± 71 | 379 ± 131 | 30 ± 17 |
| GA4 | nd | nd | nd | nd | nd | nd | 17 ± 4 |
| GA9 | nd | nd | nd | nd | nd | nd | 47 ± 22 |
| GA20 | nd | nd | nd | nd | nd | nd | 37 ± 30 |
ABA
Maize pulvini contained 150 to 200 ng/g dry weight ABA in control plants, while in response to gravistimulation, ABA levels decreased slightly at 1 h in the upper pulvinus and increased by 2- and 6-fold at 6 h in upper and lower pulvini, respectively (Table VII). However, high levels of ABA degradation products were also detected at 6 h in the lower pulvinus. Dihydrophaseic acid (DPA) was the major ABA metabolite in the pulvinus (Table VII). Its level was 7- to 9-fold higher than other ABA metabolites (Table VII). The levels of 7’-hydroxy ABA and 9’-hydroxy ABA were extremely low.
GA
GA4 and GA7 are active hormones derived via the non-13-hydroxylation pathway from metabolites of GA12, GA15, GA24, and GA9, while GA1 and GA3 are also active hormones, but are derived from the early 13-hydroxylation pathway in which GA53, GA44, GA19, and GA20 are intermediate metabolites (Hedden and Kamiya, 1997; Hedden and Phillips, 2000). Only GA9 and GA4 from the non-13-hydroxylation pathway were quantifiable by the hormone profiling method. Gravistimulation increased GA4 and GA9 from undetected levels to about 17 and 50 ng/g dry weight in the lower pulvinus at 6 h, respectively. All four metabolites from the early 13-hydroxylation pathway were quantified. The GA53 metabolite, which initiates the early 13-hydroxylation pathway (Hedden and Kamiya, 1997), increased in upper pulvini 6 h after gravistimulation. However, it decreased sharply in the lower pulvinus at 6 h (Table VII). Further, GA20 was undetectable in control and upper pulvini but increased to about 40 ng/g dry weight in the lower pulvinus. The levels of GA44 and GA19 did not change in the lower pulvinus after gravistimulation.
CK
The maize extracts contained substantial amounts of cis-zeatin (cZ; Veach et al., 2003), which is converted to cZ-O-glucoside (cZOG) as a storage conjugate by cZ-O-GT. The cZOG was most abundant in maize pulvini, while the level of transzeatin-O-glucoside (tZOG) was about 8-fold lower than cZOG in maize pulvini (Table VII). There were no differences in levels of tZOG and cZOG in control, upper, and the lower pulvinus. Isopentenyladenosine (iPA) and transzeatin riboside levels were very low in control, upper, and lower pulvini. Active cZ was not detected by the hormone profiling method used here.
Metabolite Profiles Were Consistent with Wall Changes in Lower Pulvini
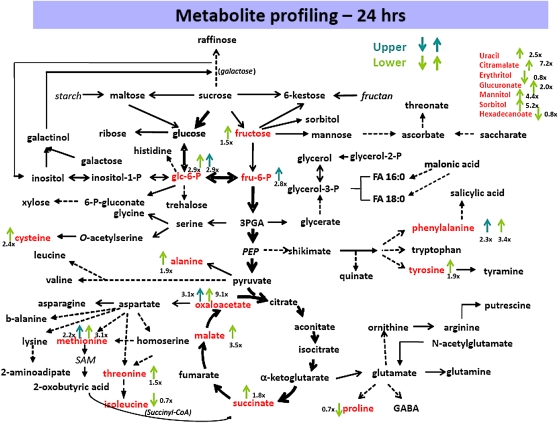
Metabolite profiles were compared in the upper and lower regions of pulvini from node 10 at 12 and 24 h after the plants were placed in the horizontal position and a summary of changes observed at 24 h is presented in Figure 4. A relatively small number of metabolites changed significantly after 12 h, including phosphorylated sugars of central metabolic pathways, Phe, and ethanolamine (Supplemental Table S6; Supplemental Fig. S3). More pronounced changes in metabolites occurred 24 h after gravistimulation, when substantial increases in Glc-6-phosphate, Fru, Tyr, Phe, Met, Cys, Ala, oxaloacetate, malate, succinate, citramalate, mannitol, and uracil were detected in lower pulvini (Fig. 4; Supplemental Table S6; Supplemental Fig. S3). These changes are consistent with, but not necessarily indicative of, increased sugar metabolism through the tricarboxylic acid cycle, increased protein biosynthesis, and increased synthesis of monolignols. It is possible that increased tricarboxylic acid cycle activity and protein synthesis would be required for the increased cell elongation observed in the lower pulvini, and that the increased Phe and Tyr levels were related to the increased lignin content in lower pulvini (Fig. 3A). The increase in inositol at 12 but not 24 h is consistent with reports by Perera et al. (1999), who showed that an initial increase in inositol 1,4,5-trisphosphate returned to base levels by 30 h after gravistimulation.
Figure 4.
Changes in metabolic levels in maize stem pulvini. Maize plants were subjected to gravistimulation for 24 h. Pulvini were collected and ground on liquid nitrogen. The metabolites were extracted and analyzed by GC-MS.
DISCUSSION
In the experiments described here, maize plants in pots were subjected to gravitational stress by placing them in an essentially horizontal position. Responses to this stress were monitored for 2 weeks and the kinetics of the plant’s overall growth responses were similar to those described by Collings et al. (1998). After 2 weeks the tops of the plants had returned to the vertical orientation through differential growth of the stalk pulvini of the upper four nodes (Fig. 1, A and B). Measurements of cell length in the pulvini indicated that realignment of the plant to the vertical orientation was attributable mainly to enhanced cell elongation in the lower pulvinus, rather than to higher rates of cell division. For a more comprehensive description of the molecular changes underpinning this process, approximately one-third of the upper and lower pulvini were excised as shown in Figure 1 for analyses of cell wall polysaccharide composition, gene transcriptional patterns, metabolite profiling, and for the quantitation of phytohormone levels.
Gravistimulated cell elongation in the lower region of maize pulvinus was accompanied by increased mechanical strength (Fig. 2) and substantial modifications of some cell wall components. The most noticeable changes were the increases in lignin (Fig. 3A) and arabinoxylans (Table II) in walls of the lower pulvinus. The increases in lignin content of 50% or more in the lower pulvinus (Fig. 3A) were consistent with its potential role in increasing the compressive strength of plant walls (Gindl and Teischinger, 2002; Bonawitz and Chapple, 2010), as observed in the compression wood of gymnosperms (Wilson and Archer, 1977). Heteroxylans play key structural roles in the matrix phase of cell walls from both dicots and monocots, but are especially abundant in walls of the Poaceae (Carpita, 1996; Fincher, 2009; Scheller and Ulvskov, 2010). Levels of heteroxylans increased about 35% in the lower pulvinus of gravistimulated maize plants (Table II). Cellulose, which together with arabinoxylan accounts for 70% to 80% by weight of the walls (Table II), did not change significantly in either the upper or lower pulvini of the gravistimulated maize stalks and this is consistent with the observations of Lu et al. (1992) in gravistimulated pulvini of oats. It is noteworthy that the absolute levels of cellulose assayed via the acetic acid/nitric acid method are in the range 35% to 40% by weight (Fig. 3B), while those deduced from the linkage analyses are in the range 50% to 55% by weight (Table II). The discrepancy between these values is probably attributable, in part at least, to differences in the units of expression of cellulose content. In the acetic acid/nitric acid method, cellulose is expressed as a percentage of total cell wall weight (the ethanol insoluble residue), which contains lignin, while in the linkage analysis, it is expressed as a percentage of measured monosaccharides, not including lignin. The discrepancy could also be partly attributable to different forms of cellulose. Crystalline cellulose is measured by the acetic acid/nitric acid method, while both crystalline and noncrystalline cellulose will be measured in the linkage analyses (Burton et al., 2010b). Levels of the minor components of the walls, namely the heteromannans, xyloglucans, and (1,3;1,4)-β-glucans, appeared to be similar or slightly lower in the walls of the lower pulvini of the plants (Table II).
Although the genes that mediate arabinoxylan biosynthesis have not been defined unequivocally, comparative bioinformatics analyses led Mitchell et al. (2007) to conclude that genes encoding enzymes in the GT43 family encode (1,4)-β-xylan synthases that are responsible for the synthesis of the (1,4)-β-xylan backbone of the polysaccharide, GT47 family genes encode the (1,2)-α- or (1,3)-α-l-arabinosyl transferases that add arabinosyl residues to the backbone, and GT61 family genes encode feruloyl-arabinoxylan (1,2)-β-xylosyl transferases. Mortimer et al. (2010) have recently shown that the glucuronyl transferases that add α-glucuronosyl residues to the backbone of (1,4)-β-xylans are members of the GT8 family of glycosyl transferases. In the microarray analyses performed here on the straightening maize stalks, transcript abundance of genes from the GT8, GT34, and GT61 families increased in the lower pulvini, where arabinoxylan contents increased (Table IV). The GT8 family includes, in addition to the xylan α-glucuronyltransferase mentioned above, a large number of galactosyl and glucosyl transferases (http://www.cazy.org/; Cantarel et al., 2009). Similarly, the GT34 family includes galactomannan α-1,6-galactosyltransferases and xyloglucan α-1,6-xylosyltransferases (http://www.cazy.org/; Cantarel et al., 2009). Whether these particular enzymes are involved in arabinoxylan synthesis in the lower pulvini of gravistimulated maize plants is not known at this stage. It is also possible that the increases in arabinoxylan content in walls of the lower pulvinus result from decreased (1,4)-β-xylan endohydrolase activity. Although we did not detect changes in mRNAs for (1,4)-β-xylanase genes, enzyme activity was not measured but might be modulated by GH18 (1,4)-β-xylanase inhibitors of the XIP group. The transcript profiles did indicate that levels of (1,4)-β-xylanase inhibitors increased in the lower pulvinus (Table V).
Perhaps the most important observations with respect to the composition of the cell walls in the pulvini were that the fine structure of the arabinoxylan changed and that the changes could be reconciled with the increased strength of the lower pulvini. More specifically, the degree of substitution of the arabinoxylan with arabinofuranosyl residues decreases markedly in the lower pulvinus, particularly in node 11 (Table I), which appears to have the highest angle of curvature (Fig. 1A). This is reflected in higher levels of (1,4)-xylosyl residues, fewer terminal arabinofuranosyl residues, and a higher Xyl:Ara ratio (Table I). There is evidence in the literature that the arabinoxylans of the Poaceae are initially synthesized in a highly substituted form, in which almost all of the backbone xylosyl residues are substituted with arabinofuranosyl residues (Reid and Wilkie, 1969; Gibeaut and Carpita, 1991; Scobbie et al., 1993; Gibeaut et al., 2005; Jung and Casler, 2006; Burton et al., 2010a). Following deposition into the wall, arabinfuranosyl residues are removed enzymatically in a manner that leaves some open, unsubstituted regions of the (1,4)-β-xylan backbone and other regions with various patterns of arabinofuranosyl substitution (Viëtor et al., 1992; Burton et al., 2010b). Enzymes that have been implicated in the removal of the arabinofuranosyl residues include family GH3 α-arabinofuranosidases and family GH51 α-arabinofuranosidases; the latter are sometimes referred to as arabinoxylan arabinofuranohydrolases (Harvey et al., 2000; Lee et al., 2001, 2003). Micorarray analyses confirmed that transcription of one member of the GH3 α-arabinofuranosidase gene family increased markedly in the lower pulvinus during gravitational stimulus (Table V).
The removal of arabinosyl substituents from the arabinoxylan will alter the physicochemical properties of the polysaccharide and its behavior within the wall through increasing the abundance and length of unsubstituted regions of the (1,4)-β-xylan backbone. These unsubstituted regions of (1,4)-β-xylan will facilitate hydrogen bonding between neighboring arabinoxylan chains and between arabinoxylans and other wall polysaccharides, such as cellulose, (1,3;1,4)-β-glucans, and xyloglucans (Fincher, 2009). On this basis one would expect the changes observed in the fine structure of the arabinoxylan to increase the intermolecular binding in the wall and hence mechanical strength of the lower pulvinus, consistent with the puncture resistance data (Fig. 2). One might further speculate that the changes in the amount of arabinoxylan and its structure might contribute positively to the compressive strength of the wall, given that the changes occur on the lower side of the gravistimulated pulvinus and that increases in lignin are also observed in the lower pulvinus. Despite the fact that maize stalk strength is correlated with cellulose rather than lignin content (Appenzeller et al., 2004) and that cellulose-enriched tension wood is found on the upper side of tree branches (Andersson-Gunnerås et al., 2006), it was noteworthy that cellulose concentrations in walls did not change significantly in any part of the pulvinus (Fig. 3B) and no significant changes in CesA transcript levels were detected.
The transcript analyses revealed changes in the activity of a number of other genes that are involved in cell wall synthesis, wall loosening and remodeling, and wall degradation. First, the microarray data suggest that transcript levels of genes encoding sugar nucleotide interconverting enzymes altered in a manner consistent with the channelling of more carbon through the sugar nucleotide pathway overall via increased transcription of the UGPP gene, and the nonreversible commitment of carbon to UDP-Xyl and UDP-Ara formation through the action of UXS. In each case these changes can be rationalized with the increases observed in the increased arabinoxylan contents in the cell walls of the lower pulvinus (Table II).
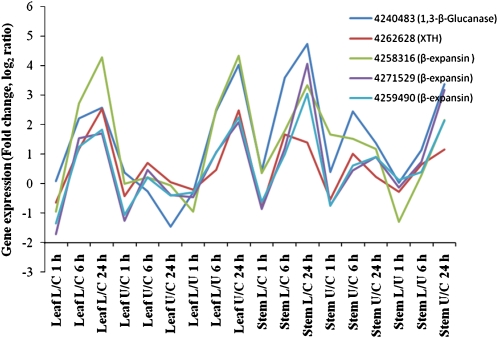
Second, levels of mRNA for several β-expansin genes increased from 4- to 27-fold in the lower pulvinus (Supplemental Table S3), while mRNA for two XTH genes of the GH16 family increased 5- to 11-fold in the lower pulvinus (Supplemental Table S4). Both β-expansins and XTHs have been implicated in cell wall loosening, which would be required for differential elongation of the cells on the lower side for the plant to achieve an upright configuration (Fry et al., 1992; McQueen-Mason and Cosgrove, 1995). Changes in the degree of polymerization of wall polysaccharides have been observed in auxin-treated pea (Pisum sativum) stem (Talbott and Ray, 1992). The XTHs might also catalyze the formation of covalent linkages between different wall polysaccharides (Hrmova et al., 2007, 2009; Fry et al., 2008) and, if so, this could contribute to the increased mechanical strength of walls in the lower pulvinus, as discussed above. Levels of mRNA encoding several GH17 (1,3)-β-glucanase genes also increased, in some cases up to 27-fold, in the lower pulvinus of the gravistimulated maize plants (Supplemental Table S2). These enzymes, which are encoded by large gene families in the Poaceae (Xu et al., 1992), have several functions, including roles in wound responses and plant-pathogen interactions, but the reason these genes are up-regulated to such an extent in the lower pulvinus is not yet clear. It is of interest to note that certain β-expansin, XTH, and (1,3)-β-glucanase genes appeared to be transcribed in a highly coordinate manner in response to gravitational stress (Fig. 5).
Figure 5.
Coordinate expression of genes involved in cell wall loosening. Gene expression was analyzed using the microarray. The numbers indicate probe numbers and gene annotations are shown in parentheses. The data are expressed as the ratio of signal strength in different tissues: L/C, Lower pulvinus over control pulvinus; U/C, upper pulvus over control; L/U, lower pulvinus over upper pulvinus. Expression of all these genes were higher in lower pulvini than in upper pulvini, from both leaf sheath and stem at 24 h.
The increased abundance of mRNA for GH17 (1,3;1,4)-β-glucan endohydrolases in the lower pulvinus (Supplemental Table S2) might be responsible for the decrease in (1,3;1,4)-β-glucan in the walls of the lower pulvinus, although the decrease is small in absolute terms (Table II). These enzymes are active in vegetative tissues of barley and appear to be under hormonal control (Slakeski and Fincher, 1992). The small but significant decreases in (1,3;1,4)-β-glucan content in the walls of the lower maize stalk pulvinus can be contrasted to increases in (1,3;1,4)-β-glucan content of walls in the lower leaf sheath pulvini of detached segments of oat stems (Gibeaut et al., 1990). These differences are probably attributable to significant differences in the experimental procedures used to impose the gravitational stress.
The microarray results for genes involved in lignin metabolism (Supplemental Table S5) are not easily explained, because the levels of transcripts for genes encoding certain CCR and C4H enzymes increase significantly in the upper pulvinus, where lignin levels are essentially unchanged. These enzymes are involved in the synthesis of monolignol precursors of lignin and increases in their transcripts might be expected to lead to increases in lignin levels or to changes in lignin composition (Bonawitz and Chapple, 2010). Metabolite profiles do not clarify the situation, because increases in Phe are observed in both the upper and lower pulvinus, while Tyr increased only in the lower pulvinus (Fig. 4); both amino acids would be expected to feed more carbon into the monolignol biosynthetic pathways. Lignin levels increased in the lower pulvini after 2 weeks of gravistimulation (Fig. 3A) but we did not monitor lignin levels during the first 2 weeks of gravistimulation. Collings et al. (1998) reported that wall lignification does not occur in the lower stem pulvinus of gravity-induced stalk bending in maize until the pulvinus loses its capacity to bend further. Thus, actual lignin deposition may have occurred sometime after the observed changes in transcripts and metabolites discussed above.
The microarray data proved to be useful as an indicator of changes in hormone concentrations in the pulvini of the maize plants examined here. Changes were observed in mRNA levels for genes that mediate phytohormones synthesis, release from conjugated forms, and degradation. When these data were cross checked against the actual levels of common hormones extracted from the upper, lower, and control pulvini, changes in transcript abundance could usually be reconciled with changes in hormone concentrations, although ethylene could not be measured in the plant material used for the microarray analyses (Tables VI and VII).
Auxin (IAA) is generally associated with cell elongation (Bartel, 1997) and would therefore be expected to mediate the elongation of cells observed in the lower pulvinus. Although there were few changes in genes involved in auxin synthesis at 1 h, hydrolases that have been implicated in IAA conjugate hydrolysis (Long et al., 2002) increased substantially in both the upper and lower pulvini at 1 h, but returned to control levels at 6 h (Table VI). Maize vegetative tissues contain up to 13-times higher concentrations of esterified IAA conjugates than free IAA (Bandurski and Schulze, 1977) and the increase in IAA concentration in the lower pulvinus at 6 h presumably resulted from the action of the auxin conjugate hydrolases (Table VII). It appears there might have been a pause in auxin synthesis or redistribution in the first hour, perhaps to adapt to stress, but auxin subsequently increased as expected, more so on the lower side than the upper side of the pulvinus. One might speculate that this reflects the redistribution of auxin efflux carriers (PIN proteins) after the plants were placed in a horizontal position (Noh et al., 2003; Petrásek et al., 2006). No changes were observed in mRNA levels for the 10 PIN genes measured by the microarray.
Transcript levels of the anthranilate phosphoribosyltransferase gene, which is involved in auxin biosynthesis, increased about 2.7-fold in the lower pulvinus but remained essentially unchanged in the upper pulvinus (Table VI). Similarly, mRNA for two auxin response factors increased more than 2-fold in the lower pulvinus, while mRNA for the Aux/IAA protein, which suppresses the auxin response, decreased at 6 h in the lower pulvinus. Based on these data, auxin levels would be expected to be higher in the lower pulvinus at 6 h, and this was indeed observed (Table VI).
In the case of ABA, which normally functions as a general growth inhibitor (Nambara and Marion-Poll, 2005), the increase in transcription of the 9 cis-epoxycarotenoid dioxygenase gene observed in lower pulvini at 1 h would be expected to increase ABA levels. However, the increase in cytochrome P450 monooxygenase transcription in lower pulvini at 6 h would result in an increase in ABA degradation, as would the high levels of ABA 8’-hydroxylase mRNA in upper pulvini. ABA is inactivated by 7’-, 8’-, and 9’-hydroxylation pathways. The 8-hydroxylation reaction is believed to be the most important regulatory step in physiological responses to ABA, and phaseic acid (PA) and DPA are usually the most abundant ABA catabolites (Nambara and Marion-Poll, 2005). This is supported by this work, where it is clear that levels of the ABA conjugate, ABA-Glc ester (GE), remained at similar levels in all the samples (Table VII), indicating that there was little conjugation of ABA occurring in ABA turnover, and that the majority of ABA was metabolized through 8’-hydroxylation.
Although there were few changes in ABA and ABA metabolites at 1 h, measured levels of ABA, PA, and DPA all increased at 6 h, especially in the lower pulvinus (Table VII). Given the inhibitory functions of ABA in cell elongation, together with the fact that ABA levels are determined by a precise balance between biosynthesis and catabolism of the hormone (Nambara and Marion-Poll, 2005), it is difficult to interpret the increased levels of ABA in the lower pulvinus (Table VII), where cell elongation is occurring at a rapid rate. One explanation of this observation relates to the conventional dogma that ABA functions as a growth inhibitor, which is not always the case. In seed development, ABA promotes deposition of storage products and Sharp and LeNoble (2002) have shown that ABA plays an important role in limiting ethylene production and further suggest that ABA might promote growth of corn seedlings through an involvement with ethylene signaling.
It is also possible that the ABA concentrations observed in tissue extracts here are related to GA levels, given that ABA and GA often act as antagonists. The functions of GA are extensive, but include the promotion of stem elongation (Hedden and Kamiya, 1997). On this basis one might expect active GA levels to be higher in the elongating cells of the lower pulvinus. Transcript levels of ent-kaurene synthase, which is involved in GA12-aldehyde biosynthesis, increased in both the upper and lower pulvini, as did the levels of mRNA for a number of GA oxidases (Table VI). The GA 20-oxidase enzyme catalyzes successive oxidations of C-20 methyl groups of GAs in non-13-hydroxylation and early 13-hydroxylation pathways (Hedden and Kamiya, 1997). The non-13-hydroxylation pathway generates active GA1 and GA3, while the early 13-hydroxylation pathway produces active GA4 and GA7. However, there were also substantial increases in GA intermediates such as GA53 at 6 h (Table VII) in the upper pulvinus, but GA53 decreased significantly in the lower pulvinus. This indicated a clear difference in GA metabolism in the two regions of the straightening maize pulvini. Overall, the GA forms that increased in the lower pulvinus were GA4, GA9, and GA20 and, although these were not the most abundant forms of GA, they might be the active forms or the immediate precursors of active forms in the elongating cells of the lower pulvinus (Table VII).
CKs, which generally promote cell division rather than cell elongation, were also measured in the pulvini of the maize plants. Active CKs include iPA, tZOG, cZOG, and dihydrozeatin (Sakakibara, 2006). These are synthesized by a chain of reactions catalyzed by phosphatases, adenosine kinases, and adenine phosphoribosyltransferases. CKs are inactivated by cleavage of side chains with CK oxidases and CK dehydrogenases. Given that we did not detect high levels of cell division in either the upper or lower pulvini, one might expect either decreased synthesis of the hormone or its more rapid degradation. At the transcript level, the microarray analyses showed that mRNAs for CK oxidases 2 and 3, which remove CKs, were significantly elevated in the lower pulvini at 6 h (Table VI). This would suggest a reduction on active CK levels in the lower pulvini, consistent with cell elongation without associated cell division in these regions of the gravistimulated maize stalks. Transcription of genes encoding enzymes involved in CK biosynthesis, such as adenine phosphoribosyl transferase, did not appear to change significantly in the upper or lower pulvini. Despite the apparent increases in transcriptional activity of genes involved in CK degradation in the lower pulvinus (Table VI), we could not detect any significant changes in active CK levels measured in tissue extracts (Table VII).
Finally, changes were detected in transcripts of ACC synthase and ACC oxidase genes normally associated with ethylene biosynthesis (Table VI), although we were not able to measure ethylene concentrations in extracts of the maize pulvini. We conclude from these data that ethylene levels might be elevated in both regions of the pulvinus, but particularly in the upper pulvinus (Peck et al., 1998). It is clear that the changes in transcript levels of these two genes occur much earlier after gravistimulation than do those associated with ABA, GA, and auxin metabolism. Ethylene has been implicated in many biological functions, including gravitropic responses and growth inhibition (Peck et al., 1998; Visser and Pierik, 2007), but its precise mode of action in the pulvinus of gravitationally stressed maize stalks cannot yet be defined in detail, although it appears it might initiate a stress response in the affected zone of the stalk. The 4- to 5-fold increase in mannitol and sorbitol is consistent with this stress on the lower side of the pulvinus.
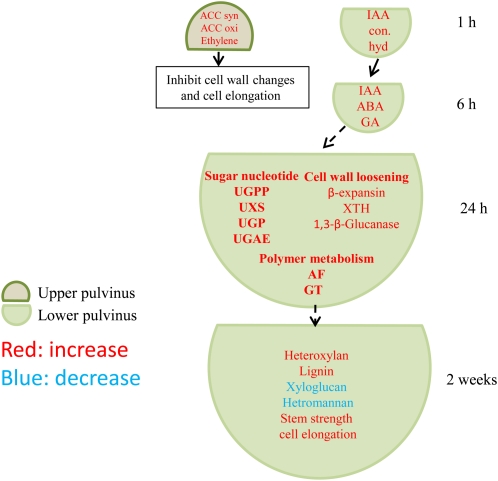
In summary, we have quantitated a significant number of changes that occur in the pulvinus of maize plants subjected to gravitational stress, and have summarized the major changes diagrammatically in Figure 6. Important cell wall modifications occur during gravistimulated cell elongation in the lower regions of stem pulvini in gravistimulated maize plants. Fine structural changes in wall arabinoxylans can be correlated with observed increases in tissue strength in the lower pulvinus, as can increases in lignin content. Microarray analyses of RNA preparations from the upper and lower pulvinus, and from control pulvini, were largely consistent with changes in wall composition. The microarray data also suggested that changes in the levels of several phytohormones were responsible for these changes in the wall, but the actual measurement of hormone levels in extracts of pulvini demonstrated the complexity of the hormone biosynthetic and degradative pathways. Thus, the assays supported previous work on the extensive cross talk that occurs within and between the complex signaling networks, and underscored the fine balance of phytohormone levels and distribution within the gravistimulated pulvinus.
Figure 6.
Summary of changes occurring in maize pulvini during gravitational stress. Hormones mediate changes in cell wall polysaccharide biosynthesis and increases in stem strength in the lower maize pulvinus. Maize stems contained abundant IAA ester conjugates. An increase of IAA conjugate hydrolase (IAA con hyd) mRNA at 1 h indicated that storage IAA was mobilized upon gravistimulation, which subsequently mediated changes in several classes of cell wall metabolic gene expression. These included nucleotide sugar interconversion genes, cell wall loosening genes, GTs, and arabinofuranosidases (AF). A consequence was an increase in unsubstituted heteroxylan, lignin, and eventually stem strength. However, ethylene or the balance of ethylene with other phytohormones might play a role in an inhibition of cell wall changes and cell elongation in upper pulvinus, as indicated by the substantial increases in expression of ACC synthase (ACC syn) and ACC oxidase (ACC oxi) genes in both upper and lower pulvinus at early gravistimulation.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Maize (Zea mays) plants (O9B) were grown in a glasshouse at 17°C/27°C under natural light conditions and were subjected to gravistimulation when the tip of primary cob was first visible (60–65 d from sowing) by placing on floors at approximately 20° angle. The maize plants were placed on the floor with leaves protruding from both sides. Stem pulvini were dissected and frozen in liquid nitrogen immediately for RNA, metabolite, and cell wall preparation. One-third of pulvinus from both the top and bottom regions was excised and designated as upper and lower pulvini, respectively (Fig. 1B). The central part of the pulvinus was discarded. For control plants, which were not subjected to gravistimulation, one-third of pulvinus was collected from either side of pulvinus and pooled. The central part of the disk was also discarded.
Cell Wall Preparation and Analysis
Pulvini were ground on liquid nitrogen and extracted with 80% ethanol five to seven times. The ethanol-insoluble residues were extracted with acetone and methanol once each and dried in a dessicator with dried silica gel. The ethanol-insoluble residues were used for cellulose, lignin content, and polysaccharide linkage analysis. Cellulose was determined according to Updegraff (1969) with modifications as described in Burton et al. (2010b).
Klason lignin was determined as described by Theander and Westerlund (1986) with the following modifications. Approximately 20 mg of the ethanol-insoluble residues of cell wall preparations (in duplicate) was hydrolyzed with 0.2 mL 12 m sulphuric acid for 1 h at 35°C, which was then diluted to 2 m and the sample heated at 121°C for 1 h. The hot acid-insoluble residue was collected on preweighed 25-mm 542 Whatman filter paper discs using a Whatman 1225 sampling manifold (Whatman Asia Pacific). The filters were washed in place with water and dried in a vacuum oven (40°C) overnight before weighing.
Monosaccharide linkage analysis was performed on five biological replicates by methylation with methyl iodide in sodium hydroxide and dimethyl sulfoxide as described by Ciucanu and Kerek (1984) followed by acid hydrolysis, reduction, and acetylation as described in Sims and Bacic (1995). Monosaccharide linkages and relative polysaccharide proportions were deduced from the partially methylated alditol acetates that were separated and analyzed by gas chromatography-mass spectrometry (GC-MS) as described by Zhu et al. (2005).
Measurement of Cell Length by Light Microscopy
Pulvini from the uppermost and lowermost surfaces of maize stems were sectioned longitudinally and examined under a light microscope. Cell length was measured using Image-Pro Plus software (MediaCybernetics, Inc.) after calibrating with a cytometer.
Metabolite Profiling
Maize pulvini from node 10 were ground in liquid nitrogen. Metabolites from five biological replicates were extracted and derivatized with N-methyl-N-(tert-butyldimethylsilyl)trifluoroaceteamide + 1% tert-butyldimethylchlorosilane and N-methyl-N-(trimethylsilyl)trifluoroacetamide and analyzed by GC-MS according to Jacobs et al. (2007).
The value of each compound was calculated on a weight basis relative to internal standards. The fold differences between control and upper or between control and lower pulvini were calculated relative to the appropriate time point control. Only those metabolites that were statistically significant (P < 0.05) are listed in Supplemental Table S6.
Measurement of Puncture Resistance
Maize plants were harvested 2 weeks after the imposition of the gravitropic stress and the leaf sheath was pealed to reveal the pulvinus. A small punctual probe was mounted on the Instron 5543 single column testing system (Instron) and flexure stress at maximum load was determined with a flat-faced, tungsten puncture probe of 1-mm2 surface area and an anvil movement rate of 100 mm/min. The penetration was set to stop when the peak penetration load dropped by 30%.
Plant Hormone Profiling
Maize pulvini were freeze dried and homogenized. Qualification of auxin, ABA, GA, and cytokine metabolites in maize pulvini was conducted at the Plant Biotechnology Institute of the National Research Council of Canada by ultra-performance liquid chromatography coupled with a tandem quadrupole mass spectrometer equipped with an electrospray interface (http://www.nrc-cnrc.gc.ca/eng/facilities/pbi/plant-hormone.html). Deuterated forms of the hormones were used as internal standards. These included d3-DPA, d5-ABA-GE, d3-PA, d4-7′-OH-ABA, d3-neoPA, d4-ABA, d4-trans-ABA, d3-IAA-Leu, d3-IAA-Ala, d3-IAA-Asp, d3-IAA-Glu, d5-IAA, d3-dhZ, d3-dhZR, d5-Z-O-Glu, d6-iPA, and d6-2iP, and d2-GAs 1, 3, 4, 7, 8, 9, 19, 20, 24, 29, 34, 44, 51, and 53. They were either synthesized according to Abrams et al. (2003) and Zaharia et al. (2005) or purchased from Cambridge Isotope Laboratories, Olchemim Ltd., and Research School of Chemistry, Australian National University. Hormones were extracted with isopropanol/water/glacial acetic acid (80:19:1, v/v), purified, and quantified according to Abrams et al. (2003).
Transcript Profiling Using Maize Microarrays
Maize pulvini were ground under liquid nitrogen and total RNA extracted with the TRIZOL reagent (Invitrogen) according to the manufacturer’s instructions. Polyadenylated mRNA was purified using the Illustra mRNA purification kit (GE Biosciences). Sample quality and RNA concentration were assessed using an Agilent Bioanalyzer. The mRNA was transcribed into double-stranded cDNA, which was amplified and labeled with Cy3 or Cy5 fluorescent dye, all using the Agilent Low RNA linear amp kit. Biological replicates were labeled alternately using Cy3 or Cy5 to guard against dye bias. The cRNA was hybridized to Pioneer’s custom 105 k microarrays that contained 103,685 oligonucleotides for about 45,000 maize genes (Hayes et al., 2010) and the microarrays were washed according to Agilent standard protocols. The microarray chips were scanned with an Agilent G2505B DNA microarray scanner at two laser power settings (100% and 10%). The images were inspected visually for image artifacts, and feature intensities were extracted, filtered, and normalized with Agilent feature extraction software (v 9.5.1). The data were normalized using quantile normalization (BOLSTAD, http://www.ncbi.nlm.nih.gov/pubmed/12538238). The normalized data were used to compare expression levels of genes related to cell wall and phytohormone metabolism. The microarray data have been submitted to Gene Expression Omnibus under series accession number GSE26071 (http://www.ncbi.nlm.nih.gov/geo/) and platform accession number GPL10837 (Hayes et al., 2010). Of the approximately 45,000 gene probes on the microarray, about 4.6% of those with functional annotations were related to metabolic pathways leading to sugar nucleotides, to wall polysaccharide synthesis, modification and degradation, to lignin metabolism, and to other cell wall components.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S2. Confirmation of microarray data by QPCR.
Supplemental Figure S3. Changes in metabolite levels in maize pulvini after 12 h.
Supplemental Table S1. Cell elongation in control, upper, and lower pulvini.
Supplemental Table S2. Transcript levels of β-glucan degradation genes in stem pulvini.
Supplemental Table S3. Transcript levels of expansin genes in stem pulvini.
Acknowledgments
Metabolite data were generated by Metabolomics Australia, a National Collaborative Research Infrastructure Scheme facility.
References
- Abrams SR, Nelson K, Ambrose SJ. (2003) Deuterated abscisic acid analogs for mass spectrometry and metabolism studies. J Labelled Comp Radiopharm 46: 273–283 [Google Scholar]
- Andersson-Gunnerås S, Mellerowicz EJ, Love J, Segerman B, Ohmiya Y, Coutinho PM, Nilsson P, Henrissat B, Moritz T, Sundberg B. (2006) Biosynthesis of cellulose-enriched tension wood in Populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J 45: 144–165 [DOI] [PubMed] [Google Scholar]
- Appenzeller L, Doblin M, Barreiro R, Wang H, Niu X, Kollipara K, Carrigan L, Tomes D, Chapman M, Dhugga KS. (2004) Cellulose synthesis in maize: isolation and expression analysis of the cellulose synthase (CesA) gene family. Cellulose 11: 287–299 [Google Scholar]
- Bandurski RS, Schulze A. (1977) Concentration of indole-3-acetic acid and its derivatives in plants. Plant Physiol 60: 211–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel B. (1997) Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 48: 51–66 [DOI] [PubMed] [Google Scholar]
- Bonawitz ND, Chapple C. (2010) The genetics of lignin biosynthesis: connecting genotype to phenotype. Annu Rev Genet 44: 337–363 [DOI] [PubMed] [Google Scholar]
- Brown WV, Pratt GA, Mobley HM. (1959) Grass morphology and systematics. II. The nodal pulvinus. Southwest Nat 4: 126–130 [Google Scholar]
- Burton RA, Collins HM, Fincher GB. (2010a) The role of endosperm cell walls in barley malting quality. In Zhang G, Li C, eds, Genetics and Improvement of Barley Malt Quality. Springer, Berlin, pp 190–237 [Google Scholar]
- Burton RA, Ma G, Baumann U, Harvey AJ, Shirley NJ, Taylor J, Pettolino F, Bacic A, Beatty M, Simmons CR, et al. (2010b) A customized gene expression microarray reveals that the brittle stem phenotype fs2 of barley is attributable to a retroelement in the HvCesA4 cellulose synthase gene. Plant Physiol 153: 1716–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Shirley NJ, King BJ, Harvey AJ, Fincher GB. (2004) The CesA gene family of barley: quantitative analysis of transcripts reveals two groups of co-expressed genes. Plant Physiol 134: 224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, Stone BA, Newbigin EJ, Bacic A, Fincher GB. (2006) Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-D-glucans. Science 311: 1940–1942 [DOI] [PubMed] [Google Scholar]
- Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res (Database issue) 37: D233–D238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC. (1996) Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol 47: 445–476 [DOI] [PubMed] [Google Scholar]
- Catalá C, Rose JK, Bennett AB. (1997) Auxin regulation and spatial localization of an endo-1,4-beta-D-glucanase and a xyloglucan endotransglycosylase in expanding tomato hypocotyls. Plant J 12: 417–426 [DOI] [PubMed] [Google Scholar]
- Ciucanu I, Kerek F. (1984) A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res 131: 209–217 [Google Scholar]
- Collings DA, Winter H, Wyatt SE, Allen NS. (1998) Growth dynamics and cytoskeleton organization during stem maturation and gravity-induced stem bending in Zea mays L. Planta 207: 246–258 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38: 109–124 [DOI] [PubMed] [Google Scholar]
- Dhugga KS. (2007) Maize biomass yield and composition for biofuels. Crop Sci 47: 2211–2227 [Google Scholar]
- Doblin MS, Pettolino FA, Wilson SM, Campbell R, Burton RA, Fincher GB, Newbigin E, Bacic A. (2009) A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-D-glucan synthesis in transgenic Arabidopsis. Proc Natl Acad Sci USA 106: 5996–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GB. (2009) Revolutionary times in our understanding of cell wall biosynthesis and remodeling in the grasses. Plant Physiol 149: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Mohler KE, Nesselrode BH, Franková L. (2008) Mixed-linkage beta-glucan:xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. Plant J 55: 240–252 [DOI] [PubMed] [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. (1992) Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J 282: 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Carpita NC. (1991) Tracing cell wall biogenesis in intact cells and plants: selective turnover and alteration of soluble and cell wall polysaccharides in grasses. Plant Physiol 97: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Karuppiah N, Chang S-R, Brock TG, Vadlamudi B, Kim D, Ghosheh NS, Rayle DL, Carpita NC, Kaufman PB. (1990) Cell wall and enzyme changes during the graviresponse of the leaf-sheath pulvinus of oat (Avena sativa). Plant Physiol 94: 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Pauly M, Bacic A, Fincher GB. (2005) Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta 221: 729–738 [DOI] [PubMed] [Google Scholar]
- Gindl W, Teischinger A. (2002) Axial compression strength of Norway spruce related to structural variability and lignin content. Compos, Part A Appl Sci Manuf 33: 1623–1628 [Google Scholar]
- Harvey AJ, Hrmova M, De Gori R, Varghese JN, Fincher GB. (2000) Comparative modeling of the three dimensional structures of family 3 glycoside hydrolases. Proteins 41: 257–269 [DOI] [PubMed] [Google Scholar]
- Hayes KR, Beatty M, Meng X, Simmons CR, Habben JE, Danilevskaya ON. (2010) Maize global transcriptomics reveals pervasive leaf diurnal rhythms but rhythms in developing ears are largely limited to the core oscillator. PLoS ONE 5: e12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Kamiya Y. (1997) GIBBERELLIN BIOSYNTHESIS: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol 48: 431–460 [DOI] [PubMed] [Google Scholar]
- Hedden P, Phillips AL. (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5: 523–530 [DOI] [PubMed] [Google Scholar]
- Hrmova M, Farkas V, Harvey AJ, Lahnstein J, Wischmann B, Kaewthai N, Ezcurra I, Teeri TT, Fincher GB. (2009) Substrate specificity and catalytic mechanism of a xyloglucan xyloglucosyl transferase HvXET6 from barley (Hordeum vulgare L.). FEBS J 276: 437–456 [DOI] [PubMed] [Google Scholar]
- Hrmova M, Farkas V, Lahnstein J, Fincher GB. (2007) A Barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-beta-D-glucans. J Biol Chem 282: 12951–12962 [DOI] [PubMed] [Google Scholar]
- Jacobs A, Lunde C, Bacic A, Tester M, Roessner U. (2007) The impact of constitutive heterologous expression of a moss Na+ transporter on the metabolomes of rice and barley. Metabolomics 3: 307–317 [Google Scholar]
- Jung HG, Casler MD. (2006) Maize stem tissues: cell wall concentration and composition during development. Crop Sci 46: 1793–1800 [Google Scholar]
- Kaufman PB, Brock TG, Song I, Rho YB, Ghosheh NS. (1987) How cereal grass shoots perceive and respond to gravity. Am J Bot 74: 1446–1457 [PubMed] [Google Scholar]
- Lee RC, Burton RA, Hrmova M, Fincher GB. (2001) Barley arabinoxylan arabinofuranohydrolases: purification, characterization and determination of primary structures from cDNA clones. Biochem J 356: 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Hrmova M, Burton RA, Lahnstein J, Fincher GB. (2003) Bifunctional family 3 glycoside hydrolases from barley with α-L-arabinofuranosidase and β-D-xylosidase activity: characterization, primary structures, and COOH-terminal processing. J Biol Chem 278: 5377–5387 [DOI] [PubMed] [Google Scholar]
- Long JC, Zhao W, Rashotte AM, Muday GK, Huber SC. (2002) Gravity-stimulated changes in auxin and invertase gene expression in maize pulvinal cells. Plant Physiol 128: 591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CR, Kim D, Kaufman PB. (1992) Changes in the ultrastructure of cell walls, cellulose synthesis, and glucan synthase activity from gravistimulated pulvini of oat (Avena sativa). Int J Plant Sci 153: 164–170 [DOI] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ. (1995) Expansin mode of action on cell walls: analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol 107: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RA, Dupree P, Shewry PR. (2007) A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol 144: 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague MJ. (1995) Hormonal and gravitropic specificity in the regulation of growth and cell wall synthesis in pulvini and internodes from shoots of Avena sativa L. (oat). Plant Physiol 107: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Miles GP, Brown DM, Zhang Z, Segura MP, Weimar T, Yu X, Seffen KA, Stephens E, Turner SR, et al. (2010) Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci USA 107: 17409–17414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R. (1992) Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J Biol Chem 267: 21058–21064 [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS. (2003) Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Peck SC, Pawlowski K, Kende H. (1998) Asymmetric responsiveness to ethylene mediates cell elongation in the apical hook of peas. Plant Cell 10: 713–720 [Google Scholar]
- Perera IY, Heilmann I, Boss WF. (1999) Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA 96: 5838–5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, et al. (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Reid J, Wilkie K. (1969) Total hemicelluloses from oat plants at different stages of growth. Phytochem 8: 2059–2065 [Google Scholar]
- Reiter WD. (2008) Biochemical genetics of nucleotide sugar interconversion reactions. Curr Opin Plant Biol 11: 236–243 [DOI] [PubMed] [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. (2002) The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol 43: 1421–1435 [DOI] [PubMed] [Google Scholar]
- Sakakibara H. (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57: 431–449 [DOI] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P. (2010) Hemicelluloses. Plant Biol 61: 263–289 [DOI] [PubMed] [Google Scholar]
- Scobbie L, Russell W, Provan G, Chesson A. (1993) The newly extended maize internode: a model for the study of secondary cell wall formation and consequences for digestibility. J Sci Food Agric 61: 217–225 [Google Scholar]
- Sharp RE, LeNoble ME. (2002) ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 53: 33–37 [PubMed] [Google Scholar]
- Sharples SC, Fry SC. (2007) Radioisotope ratios discriminate between competing pathways of cell wall polysaccharide and RNA biosynthesis in living plant cells. Plant J 52: 252–262 [DOI] [PubMed] [Google Scholar]
- Shea E, Gibeaut D, Carpita N. (1989) Structural analysis of the cell walls regenerated by carrot protoplasts. Planta 179: 293–308 [DOI] [PubMed] [Google Scholar]
- Sims I, Bacic A. (1995) Extracellular polysaccharides from suspension cultures of Nicotiana plumbaginifolia. Phytochem 38: 1397–1405 [Google Scholar]
- Slakeski N, Fincher GB. (1992) Developmental regulation of (1—>3, 1—>4)-beta-glucanase gene expression in barley: tissue-specific expression of individual isoenzymes. Plant Physiol 99: 1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Ray PM. (1992) Changes in molecular size of previously deposited and newly synthesized pea cell wall matrix polysaccharides: effects of auxin and turgor. Plant Physiol 98: 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theander O, Westerlund E. (1986) Studies on dietary fiber. 3. Improved procedures for analysis of dietary fiber. J Agric Food Chem 34: 330–336 [Google Scholar]
- Updegraff DM. (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32: 420–424 [DOI] [PubMed] [Google Scholar]
- Veach YK, Martin RC, Mok DW, Malbeck J, Vankova R, Mok MC. (2003) O-glucosylation of cis-zeatin in maize: characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol 131: 1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viëtor R, Angelino S, Voragen A. (1992) Structural features of arabinoxylans from barley and malt cell wall material. J Cereal Sci 15: 213–222 [Google Scholar]
- Visser EJ, Pierik R. (2007) Inhibition of root elongation by ethylene in wetland and non-wetland plant species and the impact of longitudinal ventilation. Plant Cell Environ 30: 31–38 [DOI] [PubMed] [Google Scholar]
- Wilson BF, Archer RR. (1977) Reaction wood: induction and mechanical action. Annu Rev Plant Physiol Plant Mol Biol 28: 23–43 [Google Scholar]
- Xu P, Wang J, Fincher GB. (1992) Evolution and differential expression of the (1—>3)-β-glucan endohydrolase-encoding gene family in barley, Hordeum vulgare. Gene 120: 157–165 [DOI] [PubMed] [Google Scholar]
- Zaharia LI, Galka MM, Ambrose SJ, Abrams SR. (2005) Preparation of deuterated abscisic acid metabolites for use in mass spectrometry and feeding studies. J Labelled Comp Radiopharm 48: 435–445 [Google Scholar]
- Zhang Q, Shirley N, Lahnstein J, Fincher GB. (2005) Characterization and expression patterns of UDP-D-glucuronate decarboxylase genes in barley. Plant Physiol 138: 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pettolino F, Mau S-L, Bacic A. (2005) Characterization of cell wall polysaccharides from the medicinal plant Panax notoginseng. Phytochemistry 66: 1067–1076 [DOI] [PubMed] [Google Scholar]