Abstract
The surface of peach (Prunus persica ‘Calrico’) is covered by a dense indumentum, which may serve various protective purposes. With the aim of relating structure to function, the chemical composition, morphology, and hydrophobicity of the peach skin was assessed as a model for a pubescent plant surface. Distinct physicochemical features were observed for trichomes versus isolated cuticles. Peach cuticles were composed of 53% cutan, 27% waxes, 23% cutin, and 1% hydroxycinnamic acid derivatives (mainly ferulic and p-coumaric acids). Trichomes were covered by a thin cuticular layer containing 15% waxes and 19% cutin and were filled by polysaccharide material (63%) containing hydroxycinnamic acid derivatives and flavonoids. The surface free energy, polarity, and work of adhesion of intact and shaved peach surfaces were calculated from contact angle measurements of water, glycerol, and diiodomethane. The removal of the trichomes from the surface increased polarity from 3.8% (intact surface) to 23.6% and decreased the total surface free energy chiefly due to a decrease on its nonpolar component. The extraction of waxes and the removal of trichomes led to higher fruit dehydration rates. However, trichomes were found to have a higher water sorption capacity as compared with isolated cuticles. The results show that the peach surface is composed of two different materials that establish a polarity gradient: the trichome network, which has a higher surface free energy and a higher dispersive component, and the cuticle underneath, which has a lower surface free energy and higher surface polarity. The significance of the data concerning water-plant surface interactions is discussed within a physiological context.
Plant surfaces have a key role in the protection against abiotic stress factors, such as water losses, high densities of UV and visible radiation, or temperature extremes, but they are also crucial as a defense barrier against biotic threats, such as the attack of pathogens or herbivores (Jeffree, 2006; Stavrianakou et al., 2010; Xia et al., 2010).
The cuticle can be considered a cutinized cell wall, emphasizing the composite and heterogeneous nature of this layer and the physiologically crucial interaction between it and the cell wall underneath (Domínguez et al., 2011). This extracellular layer is composed of a polymer matrix with waxes embedded into (intracuticular) or deposited onto (epicuticular) the surface (Heredia, 2003). On the inner side of the cuticle, cutin is mixed with polysaccharide material from the epidermal cell wall (Domínguez et al., 2011). The cuticle matrix is commonly made of a biopolyester known as cutin, which is constituted by a network of cross-esterified, hydroxy C16 and/or C18 fatty acids (Kolattukudy, 1980; Domínguez et al., 2011). Cuticles from some species may contain an alternative nonsaponifiable and nonextractable polymer known as cutan, which yields a highly characteristic series of long chain n-alkenes and n-alkanes upon flash pyrolysis (Villena et al., 1999; Jeffree, 2006).
Cuticular waxes are generally mixtures of long-chain aliphatic molecules (mainly C20–C40 n-alcohols, n-aldehydes, very long-chain fatty acids, and n-alkanes) and of aromatic compounds (Jetter and Schäffer, 2001; Suh et al., 2005; Leide et al., 2007; Kosma et al., 2009). Apart from the polymer matrix and the waxes, a variable amount of phenolics may be present in the cuticle in free form, trapped in the matrix, or chemically bound to cutin or waxes by ester or ether bonds (Karabourniotis and Liakopoulos, 2005; Domínguez et al., 2009).
According to Werker (2000), trichomes are defined as unicellular or multicellular appendages that originate from epidermal cells only and develop outward on the surface of various plant organs. Trichomes can grow in all plant parts and are chiefly classified as “glandular” or “nonglandular.” While nonglandular trichomes are distinguished by their morphology, different kinds of glandular trichomes are established by the secretory materials they excrete, accumulate, or absorb (Johnson, 1975; Werker, 2000; Wagner et al., 2004). Nonglandular trichomes exhibit major variabilities in size, morphology, and function. They often occur in plants thriving in dry habitats and are abundant in young organs (Fahn, 1986; Karabourniotis et al., 1995).
The effect of the topography of plant surfaces on the deposition of water and pollutants has been largely studied in association with glabrous, waxy surfaces (Holloway, 1969; Schreiber and Schönherr, 1993; Barthlott and Neinhuis, 1997; Wagner et al., 2003; Brewer and Nuñez, 2007; Koch and Ensikat, 2008). However, assessment of liquid-solid interactions following a strict physicochemical approach, as implemented in membrane science (Khayet et al., 2003, 2007), has not been attempted within a plant physiological context.
In this study, we aimed at characterizing, to our knowledge for the first time, the physical properties of a model pubescent plant surface, taking into account the structure and function of the indumentum. We selected a highly pubescent plant surface to address the following questions. What is the structure of the peach (Prunus persica) surface and of the epidermis underneath? Is the surface a composite material formed by the trichomes and the cuticle, and what is the chemical composition of both surface constituents? And what effect has the trichome layer on the surface free energy, polarity, work of adhesion, and rate of water loss by the fruit?
RESULTS
Topography and Structure of the Peach Epidermis
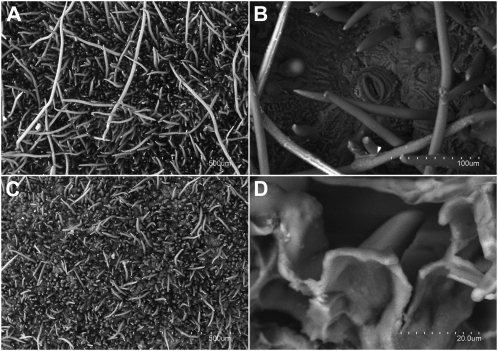
The intact peach surface is covered by a dense indumentum (0.4–1 mm thick) constituted by trichomes of different lengths (from 100 to 1,000 μm; Fig. 1A). When cuticles were enzymatically isolated, most of the longest trichomes fell out (Fig. 1C), reducing the thickness and density of the trichome layer. A few stomata (approximately 3 mm−2) occurred in the epidermis underneath. The enzymatic removal of polysaccharides led to the isolation of a sinuous and continuous cuticle that fully covered the small trichomes (approximately 150 μm long; Fig. 1D). The mechanical removal of trichomes did not induce any visible damage on the fruit epidermis, as observed with the naked eye and by microscopy. The remaining shaved peach surface preserved the small trichomes (for an example, see Fig. 6, B, D, and F) and had a similar topography to the one observed on enzymatically isolated cuticular membranes.
Figure 1.
Scanning electron micrographs of peach intact surfaces (A and B) and isolated cuticles (C and D). A, Intact peach surface (×100). B, Stoma observed on an intact surface after the mechanical removal of trichomes (×450). C, Upper surface of an isolated cuticle (×100). D, Lower surface of an isolated cuticle (×2,000).
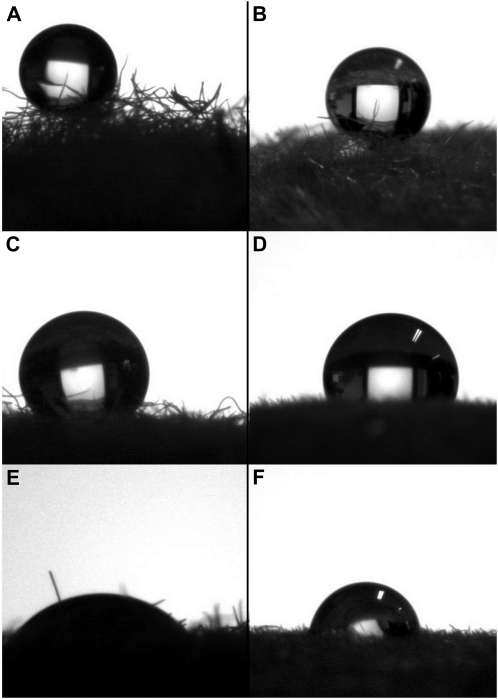
Figure 6.
Contact angles of intact peach surfaces and water (A), glycerol (C), and diiodomethane (E) and of shaved peach surfaces and water (B), glycerol (D), and diiodomethane (F).
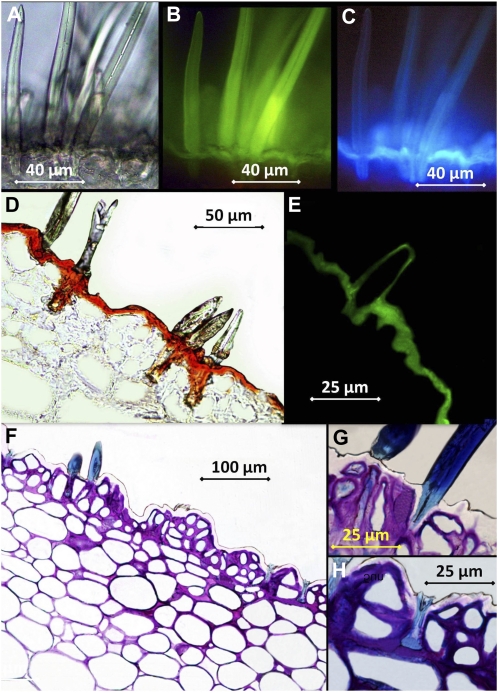
Examination of hand-cut, intact peach sections (Fig. 2, A–C) by light, transmission, and fluorescence microscopy indicated that the trichomes were nonglandular and unicellular. Trichomes were deeply rooted into the epidermis and had a thin lumen and thick cell walls. Only a few trichomes darkened during examination, suggesting that the majority of them were dead cells at the stage of ripening when fruits were investigated (data not shown). Observation of intact tissues after the application of 10% KOH as an inducer led to the green-yellow fluorescence of the flavonoids present in the trichomes (blue-light excitation; Fig. 2B) and to the light blue fluorescence of the simple phenols occurring in the cuticle underneath (UV light excitation; Fig. 2C).
Figure 2.
Micrographs of transverse peach fruit sections. A, Intact tissue observed by light transmission. B, Intact tissue treated with the inducer and observed under blue light excitation. C, Intact tissue treated with the inducer and observed under UV light excitation. D, Embedded tissue stained with Sudan IV. E, Embedded tissue stained with Auramine O and observed under UV light. F to H, Embedded tissue stained with Toluidine blue.
Thin sections of peach tissues (Fig. 2, D–H) were observed by optical microscopy in combination with different dyes. Tissue treatment with Sudan IV (Fig. 2D) led to the red staining of the cuticle and of the base of trichomes, which appeared to be strongly cutinized. Peach transversal sections stained with Auramine O and observed with long exposure times revealed that both the epidermis and the trichomes were covered by a lipidic layer giving green-yellow fluorescence when examined under UV light (Fig. 2E). The peach epidermis was found to be sinuous and uneven, having concave (valleys) and convex (peaks) epidermal areas. A disorganized, multiseariate epidermis of three to four layers of epidermal cells occurred above one or two layers of hypodermis and the large parenchyma cells (Fig. 2F). Trichomes stained in blue (Fig. 2, F–H) and initially developed as elongated epidermal cells.
Chemical Composition of Trichomes and Isolated Cuticles
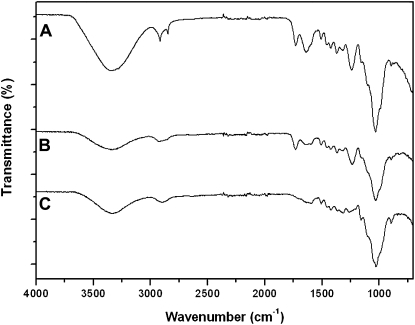
The proportion of the chemical constituents of isolated cuticles and trichomes was assessed by Fourier transform infrared spectroscopy (ATR-FTIR). Intact tissues were first analyzed and then subjected to the removal of waxes followed by a process of cutin depolymerization.
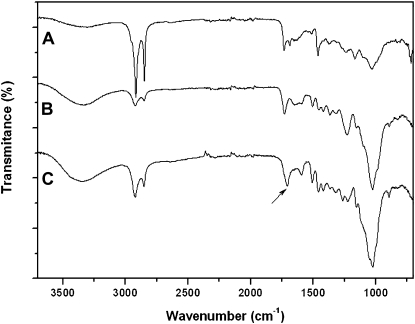
The ATR-FTIR spectra of peach cuticles and their corresponding isolates after controlled chemical treatment are shown in Figure 3. The spectrum of the peach fruit cuticle (Fig. 3A) presented strong features of long-chain aliphatic compounds (i.e. bands assigned to asymmetric and symmetric CH2 stretching at 2,918 and 2,849 cm−1 and CH2 bending at 1,462 cm−1). Additionally, the presence of ester functional groups assigned to cutin was revealed by the 1,732-cm−1 weak band and by the partially masked vibrations at 1,159 and 1,104 cm−1 (asymmetrical and symmetrical C-O-C stretching, respectively). The band at 1,034 cm−1 (medium intensity) was assigned to glycosidic bonds typical of polysaccharides. The band appearing at approximately 1,688 cm−1 was associated with free carboxylic acid functional groups. Vibrations around 1,640 and 1,515 cm−1 were assigned to the stretching of C=C bonds and the stretching of aromatic rings, respectively. More details about the assignments described above can be found in the literature (Ramírez et al., 1992; Luque et al., 1995; Villena et al., 2000). Wax extraction from isolated cuticles (Fig. 3B) induced a severe reduction of the aliphatic character and an increase of ester and polysaccharide bands. Finally, the ATR-FTIR spectrum of the residue resulting after cutin depolymerization (Fig. 3C) indicated a strong polysaccharide character (bands around 1,100–1,000 cm−1) of the remaining material. Nevertheless, the shift from bands corresponding to ester groups to the spectral region of carboxylate groups indicated the presence of significant amounts of the biopolymer cutan (Villena et al., 1999). The chemical composition of isolated peach fruit cuticles corresponded to 27% waxes, 20% cutin, and 53% insoluble residue consisting of a mixture of polysaccharides and cutan.
Figure 3.
ATR-FTIR spectra of isolated peach fruit cuticles (A), isolated cuticles without waxes (B), and residue after chemical depolymerization of cutin (C). Spectra B and C show significant losses of aliphatic components and a higher presence of polysaccharides. The arrow in C indicates a shift from ester to carboxylate groups, showing the presence of cutan.
In contrast to the cuticle, the ATR-FTIR spectrum of intact trichomes (Fig. 4A) presented typical cell wall characteristics, with a small contribution of aliphatic compounds and esterified material that disappeared after progressive wax and cutin removal (Fig. 4, B and C, respectively). Thus, the trichomes were found to be chiefly made of polysaccharide material (66%), with a lower proportion of waxes (15%) and cutin (19%).
Figure 4.
ATR-FTIR spectra of isolated peach fruit trichomes (A), isolated trichomes without waxes (B), and residue after depolymerization of cutin (C). The overall spectra have a strong polysaccharide character, typical of cell wall isolates.
Cuticular waxes were extracted from isolated trichomes and cuticles, and in both cases the predominant compounds were n-alkanes. Trichome waxes contained 92% n-alkanes, the most abundant compounds being unbranched C22 to C34 alkanes. The waxes extracted from isolated peach cuticles also had a high n-alkane fraction (76%), but the most abundant compounds were C23 to C29 unbranched and methylated alkanes. An array of fatty acids and only a few primary alcohols were determined as minor constituents of the waxes extracted from trichomes and isolated cuticles.
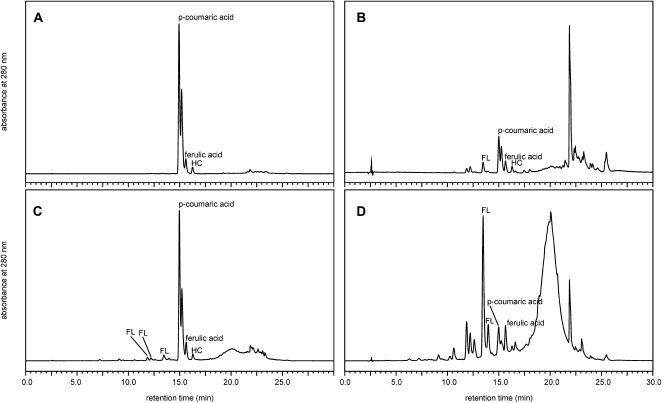
Phenolic compounds released after alkaline hydrolysis from different subfractions of peach trichomes or isolated cuticles are shown in the HPLC results (Fig. 5). These hydrolysates revealed a very specific phenolic compound composition that was almost identical between the two fractions (Fig. 5, A and C). Three major cinnamic acid derivatives were determined in both fractions, two of them being p-coumaric and ferulic acids, while the latter fraction also contained a number of minor flavonoids (Fig. 5C). The above hydroxycinnamic acid derivatives were also found in the corresponding fractions of the trichomes as part of a more complex profile (Fig. 5, B and D), although they failed to be the dominant compounds.
Figure 5.
Chromatograms. A, Hydroxycinnamic acid derivatives (p-coumaric acid, ferulic acid, and unidentified HC peak) released from alkaline hydrolysis of chloroform-isolated cuticular waxes. B, Hydroxycinnamic acid derivatives and flavonoid (unidentified FL peak) released from alkaline hydrolysis of chloroform-isolated trichome waxes. C, Hydroxycinnamic acid derivatives and flavonoids (unidentified FL peaks) released from alkaline hydrolysis of STR. D, Hydroxycinnamic acid derivatives and flavonoids released from alkaline hydrolysis of the trichome STR. Absorbance axes are scaled to include the largest peak in each chromatogram and are not quantitatively comparable between samples.
The isolated cuticles were 9-fold richer in chloroform-extractable wax per unit of mass compared with the trichomes. In particular, chloroform-isolated wax accounted for 20.2% of the cuticle, while only 2.19% of the trichome mass was recovered as chloroform-extractable wax. All cuticular fractions were much richer in phenolic compounds compared with the corresponding trichome fractions (Table I). Total phenolics accounted for 2.31% of the cuticular wax, a much higher amount as compared with the trichome wax layer (0.62% of the trichome wax; data not shown). Isolated cuticles contained a 236-fold higher concentration of wax-bound p-coumaric acid and an 89-fold higher concentration of ferulic acid compared with the trichomes. Phenolic compounds bound to the solid residue were also much more abundant in the cuticles as compared with the trichomes, since the former afforded a 34-fold higher amount of p-coumaric acid and a 6-fold higher amount of ferulic acid when subjected to alkaline hydrolysis as compared with the hairs (Table I).
Table I. Concentrations of major phenolic compounds released after alkaline hydrolysis from different fractions extracted from trichomes and isolated cuticles.
| Material | Fraction | Compound | Concentration |
| μg g−1 material | |||
| Trichomes | Chloroform-isolated wax | p-Coumaric acid | 8.97 |
| Ferulic acid | 6.37 | ||
| Solid residue | p-Coumaric acid | 27.9 | |
| Ferulic acid | 46.5 | ||
| Isolated cuticles | Chloroform-isolated wax | p-Coumaric acid | 2,120 |
| Ferulic acid | 567 | ||
| Solid residue | p-Coumaric acid | 957 | |
| Ferulic acid | 278 |
Contact Angle Measurements
Examples of the contact angles obtained for drops of the three liquids in contact with either an intact or shaved peach surface are provided in Figure 6. The average values of the measured contact angles together with their sd are summarized in Table II. For the two surfaces, the higher contact angle value was obtained for water, followed by that of glycerol and then diiodomethane. The water contact angles of both samples were similar, whereas differences were detected with regard to glycerol and diiodomethane. These results reflect the hydrophobicity of the intact and shaved peach fruit skin, indicating the hydrophobic character of the material covering the surface of both tissues.
Table II. Contact angles of water (θw), glycerol (θg), and diiodomethane (θd) on intact and shaved peach fruit surfaces.
| Sample | θw | θg | θd |
| ° | |||
| Intact | 134.2 ± 7.0 | 130.9 ± 7.0 | 55.7 ± 3.9 |
| Shaved | 134.5 ± 7.0 | 117.9 ± 4.9 | 80.3 ± 7.5 |
The surface free energy of both peach tissues was determined according to relations 1 to 3, which are based on the contact angle measurements and on the physical properties of the three liquids (Table III). The total surface free energy per unit of area of the shaved peach surface is lower than the value determined for the intact skin. This indicates that the morphology and chemistry of the peach surface are changed after the mechanical removal of the trichome layer.
Table III. Surface free energy per unit of area.
Studied components were as follows: Lifshitz-van der Waals component (γLW) and acid-base component (γAB) with the contribution of electron donor (γ−) and electron acceptor (γ+) interactions, total surface free energy (γ), and surface polarity (γAB γ−1) for intact and shaved peach fruit surfaces.
| Sample | γLW | γ− | γ+ | γAB | γ | γAB γ−1 |
| mJ m−2 | % | |||||
| Intact | 31.06 | 0.04 | 10.03 | 1.22 | 32.28 | 3.8 |
| Shaved | 17.37 | 0.99 | 7.26 | 5.37 | 22.73 | 23.6 |
The degree of surface polarity was calculated as the ratio of the nondispersive surface energy to the total surface energy (γAB γ−1). The obtained values are 3.8% and 23.6% for intact and shaved peach surfaces, respectively. The shaved peach surface has a relatively high nondispersive (polar) component and lower dispersive (nonpolar) component in comparison with the intact peach skin. By removing the trichome layer, the total surface energy decreased because of the decrease in dispersive surface energy and the increase in nondispersive surface energy (i.e. the increase of polar groups at the surface of the shaved peach skin).
The work of adhesion for the three liquids was calculated using Equation 3, as shown in Table IV. Both peach surfaces exhibit higher adhesion to diiodomethane, followed by that of glycerol and then water. This indicates that the interactions between phases are mainly dispersive in nature.
Table IV. Work of adhesion of intact and shaved peach surfaces for water (Ww), glycerol (Wg), and diiodomethane (Wd).
| Sample | Wa,w | Wa,g | Wa,d |
| mJ m−2 | |||
| Intact | 22.0 | 22.1 | 79.4 |
| Shaved | 21.8 | 34.0 | 59.40 |
Rate of Fruit Dehydration and Material Swelling
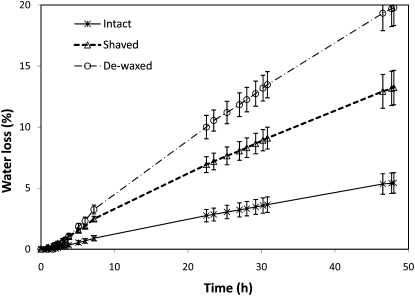
The effect of removing surface waxes and trichomes in relation to the loss of water by the intact fruit is shown in Figure 7. The highest rates of water loss were determined for dewaxed peaches (20% loss after 2 d) followed by shaved ones (13%), while intact fruits only lost 5% of water over the experimental period.
Figure 7.
Water loss of intact versus dewaxed and shaved peaches (2 d at 24°C and 40% relative humidity). Data are means ± sd.
After a period of 24 h of storage at 95% relative humidity, water sorption capacities of 19.2% ± 2.5% and 9.7% ± 0.6% were recorded for trichomes and isolated cuticles, respectively. The water sorption capacity of the trichomes was found to be twice as high as that of the isolated cuticles. This can be explained by the high proportion of polysaccharides present in the trichomes, which have a higher water sorption capacity as compared with lipids, which are the most abundant fraction of compounds determined in the cuticles (Figs. 3 and 4).
DISCUSSION
The surface of the highly pubescent peach fruit cv Calrico was investigated as a model to assess the relationship between surface chemistry and structure with regard to the hydrophobicity of the material. To our knowledge, this is the first report in which the surface free energy, polarity, and work of adhesion of two different plant materials have been calculated within a physiological plant science context. The significance of the obtained physical parameters has been complemented with structural and chemical determinations of the outer surfaces to help us understand the trichome layer in ecophysiological terms. This innovative approach provides an array of new opportunities to improve our understanding of plant surface-related phenomena.
In commercial peach production, there is a growing fashion to clear the trichomes off of the surface of peaches via a brushing process that is applied immediately after harvest, which causes no visible damage to the fruit epidermis. Taking into account that the peach epidermis is covered by two distinct materials, namely the trichome layer and the cuticle underneath, it is suggested that the properties of the fruit surface are governed by the combined effects of the above-mentioned layers. Thereby, to evaluate the contribution of each material on the physicochemical properties of the surface, analyses were carried out on enzymatically isolated peach cuticles, mechanically isolated trichomes, and intact and shaved peach fruits.
Structure and Topography of the Peach Epidermis
The trichomes covering the surface were found to be unicellular and nonglandular. Histological studies revealed that the entire peach surface including the trichomes was covered by a cuticle and that the base of the trichomes was strongly cutinized, as described to occur in leaves of xeromorphic plants (Fahn, 1986). Furthermore, a disorganized multiseriate epidermis was observed underneath the cuticle, as reported for the pomaceous fruit of Mespilus germanica (Miller, 1984) and for the peach cv O’Henry (Crisosto et al., 1994).
Chemical Composition of the Peach Surface
Concerning the chemical constituents of the cuticular membranes, 76% of the material was associated with polymer matrix components, containing a strikingly large proportion of cutan. The occurrence of cutan in apple (Malus domestica), pepper (Capsicum annuum), and berry fruit cuticles has been recently reported by Johnson et al. (2007) and Järvinen et al. (2010). While the significance of this insoluble and more hydrophobic biopolymer remains unclear both in paleobotanical and ecophysiological terms (Deshmukh et al., 2005; Gupta et al., 2006), it has been suggested that it may be a preserved compound in plants growing in xeromorphic environments (Boom et al., 2005).
In contrast, trichomes were largely composed of polysaccharide material and were covered by a thin cuticular layer containing only cutin as a matrix. A higher proportion of wax was extracted from the cuticles as compared with the trichomes. The most abundant compounds in both samples corresponded to n-alkanes, as observed in other plant species (Jetter et al., 2006). However, longer chain n-alkanes were detected in trichome wax extracts as compared with the cuticles. As minor wax constituents, an array of fatty acids was detected, with a predominance of palmitic and stearic acids in the trichomes and palmitic, arachidic, and linoleic acids in the isolated cuticles. In the case of cuticular isolates, the presence of such compounds may be due to contamination during the process of cuticle isolation, since they are precursors of the structural cuticular biopolymers that are synthesized and accumulated in the epidermal tissue. Minor fatty acid amounts were recovered in the wax extracted from trichomes as compared with the cuticles. The presence of fatty acids in wax extracts has been described in various studies (Jetter et al., 2006), but there is currently no direct evidence that they are part of the wax fraction; it is more likely that they occur due to contamination from the cells underneath.
Three hydroxycinnamic acid derivatives were the dominant compounds extracted from the cuticular waxes. In particular, p-coumaric acid and ferulic acid have been characterized as the primary phenylpropanoids responsible for the characteristic UV-induced blue fluorescence of surface tissues of several plant species (Lichtenthaler and Schweiger, 1998; Karabourniotis et al., 2001; Liakopoulos et al., 2001). Similar to the results reported for other species, these compounds are not part of the pool of tissue-soluble phenolic compounds of peach fruits (Tomás-Barberán et al., 2001) but, instead, are often found covalently bound to plant biopolymers (Riley and Kolattukudy, 1975; Kroon and Williamson, 1999). Our results indicate that the majority of phenolic compounds determined were bound to the plant biopolymers, in contrast to the amounts extracted either in chloroform or methanol (data not shown).
The same three hydroxycinnamic acid derivatives were also part of a more complex profile determined in trichome hydrolysates. The numerous compounds released in the trichome fractions can be ascribed to the fact that trichomes are more complex than isolated cuticles alone. It is most probable that part of the HPLC profile of both fractions of the trichomes may also originate from extractable compounds deposited in the cell walls (Skaltsa et al., 1994; Karabourniotis et al., 1998; Liakopoulos et al., 2006).
Apart from having an effect on pathogen quiescence (Lee and Bostock, 2007), the waxes and phenols present in nonglandular trichomes and cuticles will act as optical filters of excess solar radiation (Reicosky and Hanover, 1978; Karabourniotis and Bornman, 1999; Pfündel et al., 2006).
Hydrophobicity of the Surface within an Ecophysiological Context
The interactions of plant surfaces with water and solutes have been a matter of scientific interest since long ago (Stone, 1963; Fernández and Eichert, 2009). The effect of surface wetness on plant physiology due to natural phenomena such as dew, fog, and mist has been addressed in some investigations (Brewer et al., 1991; Brewer and Smith, 1997; Pandey and Nagar, 2003; Hanba et al., 2004; Dietz et al., 2007) and is a topic of growing interest for plant ecophysiology (Limm et al., 2009; Aryal and Neuner, 2010; Johnstone and Dawson, 2010; Limm and Dawson, 2010).
By measuring the contact angle and retention of water drops, Brewer et al. (1991) found three different patterns of wettability of pubescent surfaces on 38 species investigated. With the determination of the contact angle of the three liquids and the calculation of the surface free energy, polarity, and work of adhesion of the intact and shaved peach surface, we could go a step further in our understanding of the liquid-solid properties of the indumentum. The surface free energy is a parameter specific for each material, and different values were obtained for intact and shaved peach surfaces. The higher Lifshitz-van der Waals surface energy component of the natural surface indicated the more dispersive (nonpolar) character of the trichome surface as compared with the cuticle. This result is supported by the data we obtained that confirmed the presence of longer chain n-alkanes in the waxes extracted from the trichomes as compared with those obtained from isolated cuticles. As a consequence, the surface polarity of the intact peach skin was much lower than after the removal of the trichomes (3.8% versus 23.6%), which indicates that the intact surface has a predominant dispersive component and a lower nondispersive component. By removing the trichomes from the surface, as is commonly done with commercial peaches prior to their storage and distribution to the market, the total surface free energy is decreased due to the decrease in the Lifshitz-van der Waals component and the increase in the acid-base component. This would imply that the trichomes confer a more nonpolar character to the surface and that their removal leaves the surface more polar and, therefore, more susceptible to the occurrence of interactions with water and water-soluble compounds and contaminants.
The peach skins analyzed are not superhydrophobic (contact angle is not above 150°; Nosonovsky and Bhushan, 2009) but had high contact angles with water due to the presence of air, to the microrugosity and nanorugosity of the surface, and to its chemical composition. The trichome layer will increase the roughness and surface area of the fruit. However, after the mechanical removal of the long trichomes, a rough surface persisted (Fig. 6), and the occurrence of air pockets can also be expected. The occurrence of air chambers and their effect on surface water repellency have been modeled for various synthetic and biological surfaces (Nosonovsky and Bhushan, 2009; Xue et al., 2010).
Our results suggest that the peach surface counts on a double hydrophobic protection: on the one hand, the trichome layer, covered by longer chain n-alkanes and a lower wax proportion; and on the other hand, the cuticle, which presents a high amount of more polar waxes and the hydrophobic cutan as a major matrix polymer.
When trying to clarify the major role of the trichome indumentum covering the peach surface with regard to the bidirectional exchange of water, we observed that the removal of waxes and trichomes led to significant water losses over time. Several characters reported for xeromorphic plant tissues, namely, the occurrence of a highly pubescent surface, the multiseriate epidermis, the markedly cutinized base of the trichomes, and the presence of cutan as a major constituent of the cuticle matrix, made us think that the selected Calanda peach cultivar may be adapted to the prevailing semiarid conditions in northeast Spain, which are especially hot and dry during the season of fruit growth and development. Such yellow-flesh peach traits may have been developed and selected in China during the many centuries of cultivation of this fruit species in a potentially similar climatic zone (Li, 1970; Lirong, 2005).
The dense indumentum covering the surface up to 1 mm above can affect the boundary layer surrounding the fruit but may not be the only factor responsible for the increased transpiration rate of shaved peaches. Some studies performed with leaves of Olea europaea, Tillandsia species, and Mallotus macrostachyus failed to find a clear relationship between trichome layers and transpiration (Grammatikopoulos et al., 1994; Benz and Martin, 2006; Kenzo et al., 2008).
Despite the hydrophobic character of the surface of trichomes, we showed that they had a high water sorption capacity due to the presence of polysaccharides, which might lead to the absorption of water under certain environmental conditions, as shown by Grammatikopoulos and Manetas (1994). However, the mechanisms of water absorption by plant surfaces including trichomes are currently not fully understood (Fernández and Eichert, 2009) and should be further elucidated in the future.
In summary, the surface of the peach fruit cv Calrico is covered by a dense layer of trichomes and a cuticle underneath that protects it against an array of potential biotic and abiotic stress factors. The two materials offer a dual protection against the entry and chiefly the loss of water by the fruit. On the other hand, the occurrence of a dense indumentum and the presence of a considerable amount of phenols and waxes on the surface will contribute to limit the attack of pathogens and to attenuate excess radiation. The hydrophobic properties of the peach surface may also influence the bidirectional diffusion of gases and will determine the contact phenomena of the surface with water, contaminants, and pathogens.
MATERIALS AND METHODS
Plant Material
All materials analyzed corresponded to ripe, undamaged peaches (Prunus persica ‘Calrico’) harvested by mid September (2009 and 2010) from an experimental orchard located in the Bajo Aragón area. The selected cv Calanda is classified as a late-maturing, nonmelting, yellow skin and flesh, cling-stone peach variety.
Cuticles were isolated in a citrate buffer solution (pH 3.5) containing 4% cellulase and 4% pectinase (Novozymes) plus 1 mm NaN3 (8-d extraction period; solution changed twice). Trichomes were isolated by gently scraping the peach surface with a sharp knife.
The dehydration rate of intact versus mechanically shaved and dewaxed (1 min in 2:1 chloroform:methanol, v/v) peaches was determined gravimetrically by storing the fruits at 24°C and 50% relative humidity for 2 d.
Microscopy
Thin, hand-cut cross-sections of intact peach surfaces were observed with a Zeiss Axiolab fluorescence microscope. Transverse sections were examined first by light transmission and then under blue (emission of green fluorescence by flavonoids) and UV (emission of blue fluorescence by simple phenols) excitation after immersion in a 10% (w/v) solution of KOH for 2 min followed by a thorough distilled water rinse. Filter combinations (exciter filter/chromatic beam splitter/barrier filter) were G365/FT395/LP420 (UV 365-nm excitation) and BP450-490/FT510/LP520 (blue light excitation; Carl Zeiss). Microphotographs were taken using a Cybershot DSCS75 digital camera (Sony).
Approximately 2-mm-thick peach surface pieces were fixed in a 90% ethanol-water, 5% formol, and 5% acetic acid solution, dehydrated, and embedded in Historesin (Leica). Transverse sections were cut with a microtome and were stained with Toluidine blue, Auramine O, and Sudan IV prior to microscopic examination (Nikon E 800).
Fresh intact and shaved peach surfaces and isolated cuticles were directly examined with a variable pressure scanning electron microscope (Hitachi S-3400 N; acceleration potential, 15 kV; working distance, 10–11 mm).
The density of stomata and length of trichomes were assessed by image analysis of scanning electron micrographs (Image-Pro Plus 6).
Quantitative and Qualitative Estimation of Chemical Components by ATR-FTIR
Waxes from isolated cuticles and trichomes were extracted by refluxing in chloroform:methanol (2:1, v/v) for 4 h. The remaining residue was depolymerized by saponification in 2% NaOH for 24 h under reflux conditions. The residual material was weighed. Percentages were calculated according to the weight loss after extraction.
Infrared spectra of isolated cuticles and trichomes, wax extracts, and the residues remaining after alkaline hydrolysis were obtained with an ATR accessory (MIRacle ATR; PIKE Technologies) coupled to a FTIR spectrometer (FT/IR-4100; JASCO). All spectra were recorded in the 4,000- to 700-cm−1 range at 4 cm−1 resolution, and 25 scans were accumulated.
Extraction and Determination of Cuticular Waxes
Dehydrated cuticles and trichomes (250 mg of tissue with two replications) were extracted for 5 min in 15 mL of chloroform:methanol (2:1, v/v) using an ultrasonic bath. Samples were subsequently homogenized and evaporated to dryness with a rotary evaporator. Then, 5 mL of a methanolic NaOH solution (0.5 m) was added to the plant solid residue, the mixture being boiled for 10 min using a Vigreux column. When samples were cool, 5 mL of BF3-methanol (14% [w/w], diluted with water-free methanol) was added and the mixture was boiled for 2 min prior to the addition of 4 mL of n-heptane. When the samples cooled down again, 15 mL of saturated NaCl (2.5 g L−1) dissolved in ultrapure water (MilliQ Plus 185; Millipore) was added, and the solutions were homogenized for 15 s. The organic phase was collected (99% n-heptane; HPLC grade; Scharlau) and filtered. The composition of the samples was determined by gas chromatography-mass spectrometry (Hewlett Packard HP-6890 gas chromatograph equipped with a Combipal autosampler and an HP 5973 quadrupole mass spectrometer). The chromatographic conditions were as follows (86 min per run): the injection volume was 1 μL (splitless mode), helium was the carrier gas (1 mL min−1), and the injector and detector temperatures were set to 250°C. The column (J&W 122-5532 DB-5ms; Agilent Technologies) was set to 55°C isothermal for 4 min, increased to 155°C at a rate of 5°C min−1 and held isothermal for 2 min, and then raised to 320°C at a rate of 3°C min−1 and held isothermal for 5 min. The mass spectrometry conditions were as follows: 70 eV ionization voltage, 230°C ion source temperature, 50 to 650 units of mass scan range, and 5-min wait time. The compounds were identified by comparing their mass spectra with National Institute of Standards and Technology and WILEY275 library spectra, confirming the results by the Kovats index. All standards used were from Sigma-Aldrich.
Extraction and Determination of Phenolic Compounds
Cuticular and trichome waxes from 1 g of tissue were extracted in chloroform (5 min) and subjected to alkaline hydrolysis (4 m NaOH, 1 h at 60°C under a N2 stream) as described by Liakopoulos et al. (2001). After acidification of the solutions with HCl (pH 1), samples were extracted three times in ethyl acetate, and the combined extracts were extracted with water to remove acid and concentrated in a rotary evaporator at 30°C. The solid tissue residue after wax removal (STR) was subsequently extracted in methanol (1 mL per 10 mg of material, 1 h in an ultrasonic bath), and methanolic extracts were evaporated to dryness. The remaining STR was subjected to alkaline hydrolysis, acidification, and concentration to dryness as described above. All dry residues were rediluted in 4 or 8 mL of 50% methanol and injected onto a Zorbax Stablebond SB-C18 column (5 μm particle size, 250 × 4.6 mm; Agilent Technologies) via a 20-μL loop connected to a Prominence HPLC apparatus equipped with a photodiode array detector operating at 200 to 800 nm (Shimadzu). The column was eluted at 30°C using the following linear gradient: initially, A (1% H3PO4):B (methanol) 75:25; gradient to 70:30 in 10 min; gradient to 65:35 in 7 min; gradient to 0:100 in 3 min; flow rate 1 mL min−1. Chromatograms were captured using LC Solution version 1.23 SP1. Phenolic compounds were identified by comparison with pure standards (Extrasynthese). The quantitative determination of p-coumaric acid and ferulic acid was based on reference curves at 280 nm.
Water Sorption of Cuticles and Trichomes
The water sorption capacity of trichomes and isolated cuticles was measured gravimetrically. Tissues (55 and 65 mg) were dried for 24 h in a desiccator at very low relative humidity (silica gel). The samples were subsequently kept in a closed chamber for 24 h at 95% relative humidity, which was achieved by exposure to a supersaturated solution of Pb(NO3)2 at 25°C. The water sorption capacity was calculated by measuring the weight increment of dehydrated and water-saturated tissues.
Contact Angle Measurement and Estimation of Surface Free Energy
The advancing contact angles of three liquids, double-distilled water, glycerol (99% purity; Sigma-Aldrich), and diiodomethane (99% purity; Sigma-Aldrich), were measured at ambient temperature (25°C) using a DSA 100 Drop Shape Analysis System (Krüss).
Contact angles were determined on intact and shaved peach surfaces (30 repetitions) by placing the baseline tangent to the area of touch between the solid and the liquid as enabled by the measuring device software. In the latter case, trichomes were removed by gently scraping the peach surface with a sharp knife. Skin sections of approximately 1 × 0.5 cm2 and 1 mm thickness were cut with a scalpel. Drops of the different liquids were deposited onto the surface using a manual dosing system with a 3-mL syringe and a 0.5-mm-diameter needle. Side-view images of the drops were captured at a rate of 10 frames s−1. The contact angles were automatically calculated by fitting the captured drop shape to that calculated from the Young-Laplace equation.
Theoretical Background and Calculations Based on Contact Angle Determinations
Several data based on contact angle measurements of the three liquids with intact skins or after the removal of the trichomes were obtained by means of Equations 1 to 3. The film surface free energy (or surface tension [γ]) components were determined from contact angle measurements using the Lifshitz-van der Waals method, also known as the acid-base (AB) approach or the van Oss, Good, and Chaudhury method (van Oss et al., 1987, 1988). The theory behind this method of estimating the solid surface free energy (γ) and its components has been extensively described elsewhere (Owens and Wendt, 1969; Mittal, 1993). van Oss et al. (1987, 1988) divided γ into different components, i.e. the Lifshitz-van der Waals (LW), acid (+), and base (−) components:
where i denotes either the solid or the liquid phase. The acid-base component (γiAB) takes into account the electron-donor (γi−) and the electron-acceptor (γi+) interactions. The following expression was given for solid-liquid systems (van Oss et al., 1987, 1988):
where the three components of the surface free energy of the solid (γsLW, γs+, and γs−) can be determined from the contact angle measurements of three testing liquids with known surface tension components (i.e. for water, γsLW = 21.8 mJ m−2, γs+ = γs− = 25.5 mJ m−2; for glycerol, γsLW = 34.0 mJ m−2, γs+ = 3.92 mJ m−2, γs− = 57.4 mJ m−2; and for diiodomethane, γsLW = 50.8 mJ m−2, γs+ = γs− = 0 mJ m−2).
In addition, the degree of surface polarity of intact and shaved peach surfaces was calculated as the ratio of the nondispersive surface energy to the total surface energy (γAB γ−1).
Finally, to discuss liquid-solid interactions, the total work of adhesion (Wa; Kwok and Neumann, 1999) was determined for each liquid and type of peach surface following the equation:
where γs is the surface free energy of the solid, γlv is the interfacial tension of the liquid, and γsl corresponds to the interfacial tension between the solid and the liquid.
Acknowledgments
We thank Drs. M.J. Rubio, A. Wünsch, and J.M. Alonso (Centro de Investigación y Tecnología Agroalimentaria de Aragón), Dr. M.J. Aranzana (Institut de Recerca i Tecnologia Agroalimentàries), Dr. G. Reighard (Clemson University), and J.L. Espada (Centro de Transferencia Agroalimentaria del Gobierno de Aragón) for providing information about the origins of peaches and on the characteristics of Calanda peaches.
References
- Aryal B, Neuner G. (2010) Leaf wettability decreases along an extreme altitudinal gradient. Oecologia 162: 1–9 [DOI] [PubMed] [Google Scholar]
- Barthlott W, Neinhuis C. (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202: 1–8 [Google Scholar]
- Benz BW, Martin CE. (2006) Foliar trichomes, boundary layers, and gas exchange in 12 species of epiphytic Tillandsia (Bromeliaceae). J Plant Physiol 163: 648–656 [DOI] [PubMed] [Google Scholar]
- Boom A, Sinninghe Damsté JS, de Leeuw JW. (2005) Cutan, a common aliphatic biopolymer in cuticles of drought-adapted plants. Org Geochem 36: 595–601 [Google Scholar]
- Brewer CA, Nuñez CI. (2007) Patterns of leaf wettability along an extreme moisture gradient in western Patagonia, Argentina. Int J Plant Sci 168: 555–562 [Google Scholar]
- Brewer CA, Smith WK. (1997) Patterns of leaf surface wetness for montane and subalpine plants. Plant Cell Environ 20: 1–11 [Google Scholar]
- Brewer CA, Smith WK, Vogelmann TC. (1991) Functional interaction between leaf trichomes, leaf wettability and the optical properties of water droplets. Plant Cell Environ 14: 955–962 [Google Scholar]
- Crisosto CH, Johnson RS, Luza JG, Crisosto GM. (1994) Irrigation regimes affect fruit soluble solids concentration and rate of water loss of ‘O’Henry’ peaches. HortSci 29: 1169–1171 [Google Scholar]
- Deshmukh AP, Simpson AJ, Hadad CM, Hatcher PG. (2005) Insights into the structure of cutin and cutan from Agave americana leaf cuticle using HRMAS NMR spectroscopy. Org Geochem 36: 1072–1085 [Google Scholar]
- Dietz J, Leuschner C, Hölscher D, Kreilein H. (2007) Vertical patterns and duration of surface wetness in an old-growth tropical montane forest, Indonesia. Flora 202: 111–117 [Google Scholar]
- Domínguez E, Heredia-Guerrero JA, Heredia A. (2011) The biophysical design of plant cuticles: an overview. New Phytol 189: 938–949 [DOI] [PubMed] [Google Scholar]
- Domínguez E, Luque P, Heredia A. (2009) Sorption and interaction of the flavonoid naringenin on tomato fruit cuticles. J Agric Food Chem 57: 7560–7564 [DOI] [PubMed] [Google Scholar]
- Fahn A. (1986) Structural and functional properties of trichomes of xeromorphic leaves. Ann Bot (Lond) 57: 631–637 [Google Scholar]
- Fernández V, Eichert T. (2009) Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Crit Rev Plant Sci 28: 36–68 [Google Scholar]
- Grammatikopoulos G, Karabourniotis G, Kyparissis A, Petropoulou Y, Manetas Y. (1994) Leaf hairs of olive (Olea europaea) prevent stomatal closure by ultraviolet-B radiation. Aust J Plant Physiol 21: 293–301 [Google Scholar]
- Grammatikopoulos G, Manetas Y. (1994) Direct absorption of water by hairy leaves of Phlomis fruticosa and its contribution to drought avoidance. Can J Bot 72: 1805–1811 [Google Scholar]
- Gupta NS, Collinson ME, Briggs EG, Evershed RP, Pancost RD. (2006) Reinvestigation of the occurrence of cutan in plants: implications for the leaf fossil record. Paleobiology 32: 432–449 [Google Scholar]
- Hanba YT, Moriya A, Kimura K. (2004) Effect of leaf surface wetness and wettability on photosynthesis in bean and pea. Plant Cell Environ 27: 413–421 [Google Scholar]
- Heredia A. (2003) Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochim Biophys Acta 1620: 1–7 [DOI] [PubMed] [Google Scholar]
- Holloway PJ. (1969) The effects of superficial wax on leaf wettability. Ann Appl Biol 63: 145–153 [Google Scholar]
- Järvinen R, Kaimainen M, Kallio H. (2010) Cutin composition of selected northern berries and seeds. Food Chem 122: 137–144 [Google Scholar]
- Jeffree CH. (2006) The fine structure of the plant cuticle. Riederer M, Müller C, , Annual Plant Reviews, Vol 23: Biology of the Plant Cuticle. Blackwell, Oxford, pp 11–125 [Google Scholar]
- Jetter R, Kunst L, Samuels AL. (2006) Composition of plant cuticular waxes. Riederer M, Müller C, , Annual Plant Reviews, Vol 23: Biology of the Plant Cuticle. Blackwell, Oxford, pp 145–181 [Google Scholar]
- Jetter R, Schäffer S. (2001) Chemical composition of the Prunus laurocerasus leaf surface: dynamic changes of the epicuticular wax film during leaf development. Plant Physiol 126: 1725–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EJ, Chefetz B, Xing B. (2007) Spectroscopic characterization of aliphatic moieties in four plant cuticles. Commun Soil Sci Plant Anal 38: 2461–2478 [Google Scholar]
- Johnson HB. (1975) Plant pubescence: an ecological perspective. Bot Rev 41: 233–258 [Google Scholar]
- Johnstone JA, Dawson TE. (2010) Climatic context and ecological implications of summer fog decline in the coast redwood region. Proc Natl Acad Sci USA 107: 4533–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabourniotis G, Bornman JF. (1999) Penetration of UV-A, UV-B and blue light through the leaf trichome of two xeromorphic plants, olive and oak, measured by optical fibre microprobes. Physiol Plant 105: 655–661 [Google Scholar]
- Karabourniotis G, Kofidis G, Fasseas C, Liakoura V, Drossopoulos I. (1998) Polyphenol deposition in leaf hairs of Olea europaea (Oleaceae) and Quercus ilex (Fagaceae). Am J Bot 85: 1007–1012 [PubMed] [Google Scholar]
- Karabourniotis G, Kotsabassidis D, Manetas Y. (1995) Trichome density and its protective potential against ultraviolet-B radiation damage during leaf development. Can J Bot 73: 376–383 [Google Scholar]
- Karabourniotis G, Liakopoulos G. (2005) Phenolic compounds in plant cuticles: physiological and ecophysiological aspects. Hemantaranjan A, , Advances in Plant Physiology, Vol 8 Scientific Publishers, Varanasi, India, pp 33–47 [Google Scholar]
- Karabourniotis G, Tzobanoglou D, Nikolopoulos D, Liakopoulos G. (2001) Epicuticular phenolics over guard cells: exploitation for in situ stomatal counting by fluorescence microscopy and combined image analysis. Ann Bot (Lond) 87: 631–639 [Google Scholar]
- Kenzo T, Yoneda R, Azani MA, Majid NM. (2008) Changes in leaf water use after removal of leaf lower surface hairs on Mallotus macrostachyus (Euphorbiaceae) in a tropical secondary forest in Malaysia. J For Res 13: 137–142 [Google Scholar]
- Khayet M, Feng CY, Matsuura T. (2003) Morphological study of fluorinated asymmetric polyetherimide ultrafiltration membranes by surface modifying macromolecules. J Membr Sci 213: 159–180 [Google Scholar]
- Khayet M, Vazquez Alvarez M, Khulbe KC, Matsuura T. (2007) Preferential surface segregation of homopolymer and copolymer blend films. Surf Sci 601: 885–895 [Google Scholar]
- Koch K, Ensikat HJ. (2008) The hydrophobic coatings of plant surfaces: epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 39: 759–772 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE. (1980) Biopolyester membranes of plants: cutin and suberin. Science 208: 990–1000 [DOI] [PubMed] [Google Scholar]
- Kosma DK, Bourdenx B, Bernard A, Parsons EP, Lü S, Joubès J, Jenks MA. (2009) The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol 151: 1918–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon PA, Williamson G. (1999) Hydroxycinnamates in plants and food: current and future perspectives. J Sci Food Agric 79: 355–361 [Google Scholar]
- Kwok DY, Neumann AW. (1999) Contact angle measurement and contact angle interpretation. Adv Colloid Interface Sci 81: 167–249 [Google Scholar]
- Lee MH, Bostock RM. (2007) Fruit exocarp phenols in relation to quiescence and development of Monilinia fructicola infections in Prunus spp.: a role for cellular redox? Phytopathology 97: 269–277 [DOI] [PubMed] [Google Scholar]
- Leide J, Hildebrandt U, Reussing K, Riederer M, Vogg G. (2007) The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: effects of a deficiency in a β-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol 144: 1667–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL. (1970) The origin of cultivated plants in Southeast Asia. Econ Bot 24: 3–19 [Google Scholar]
- Liakopoulos G, Stavrianakou S, Karabourniotis G. (2001) Analysis of epicuticular phenolics of Prunus persica and Olea europaea leaves: evidence on the chemical origin of the UV-induced blue fluorescence of stomata. Ann Bot (Lond) 87: 641–648 [Google Scholar]
- Liakopoulos G, Stavrianakou S, Karabourniotis G. (2006) Trichome layers versus dehaired lamina of Olea europaea leaves: differences in flavonoid distribution, UV-absorbing capacity, and wax yield. Environ Exp Bot 55: 294–304 [Google Scholar]
- Lichtenthaler HK, Schweiger J. (1998) Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J Plant Physiol 152: 272–282 [Google Scholar]
- Limm EB, Dawson TE. (2010) Polystichum munitum (Dryopteridaceae) varies geographically in its capacity to absorb fog water by foliar uptake within the redwood forest ecosystem. Am J Bot 97: 1121–1128 [DOI] [PubMed] [Google Scholar]
- Limm EB, Simonin KA, Bothman AG, Dawson TE. (2009) Foliar water uptake: a common water acquisition strategy for plants of the redwood forest. Oecologia 161: 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirong W. (2005) Status Report on Genetic Resources of Peach in East Asia. Surveying Report. International Plant Genetic Resources Institute, Rome [Google Scholar]
- Luque P, Bruque S, Heredia A. (1995) Water permeability of isolated cuticular membranes: a structural analysis. Arch Biochem Biophys 317: 417–422 [DOI] [PubMed] [Google Scholar]
- Miller RH. (1984) The multiple epidermis-cuticle complex of medlar fruit Mespilus germanica L. (Rosaceae). Ann Bot (Lond) 53: 779–792 [Google Scholar]
- Mittal KL. (1993) Contact Angle, Wettability and Adhesion. VSP International Science Publishers, Utrecht, The Netherlands [Google Scholar]
- Nosonovsky M, Bhushan B. (2009) Superhydrophobic surfaces and emerging applications: non-adhesion, energy, green engineering. Curr Opin Colloid Interface Sci 14: 270–280 [Google Scholar]
- Owens DK, Wendt RC. (1969) Estimation of the surface free energy of polymers. J Appl Polym Sci 13: 1741–1747 [Google Scholar]
- Pandey S, Nagar PK. (2003) Pattern of leaf surface wetness in some important medicinal and aromatic plants of western Himalaya. Flora 198: 349–357 [Google Scholar]
- Pfündel EE, Agati G, Cerovic ZG. (2006) Optical properties of plant surfaces. Riederer M, Müller C, , Annual Plant Reviews, Vol 23: Biology of the Plant Cuticle. Blackwell, Oxford, pp 216–249 [Google Scholar]
- Ramírez FJ, Luque P, Heredia A, Bukovac MJ. (1992) Fourier transform IR study of enzymatically isolated tomato fruit cuticular membrane. Biopolymers 32: 1425–1429 [Google Scholar]
- Reicosky DA, Hanover JW. (1978) Physiological effects of surface waxes. I. Light reflectance for glaucous and nonglaucous Picea pungens. Plant Physiol 62: 101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RG, Kolattukudy PE. (1975) Evidence for covalently attached p-coumaric acid and ferulic acid in cutins and suberins. Plant Physiol 56: 650–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Schönherr J. (1993) Contact areas between leaf surfaces and aqueous solutions: quantitative determination of specific leaf surface contact areas. J Exp Bot 44: 1653–1661 [Google Scholar]
- Skaltsa H, Verykokidou E, Harvala C, Karabourniotis G, Manetas Y. (1994) UV-B protective potential and flavonoid content of leaf hairs of Quercus ilex. Phytochemistry 37: 987–990 [Google Scholar]
- Stavrianakou S, Liakopoulos G, Miltiadou D, Markoglou AN, Ziogas BN, Karabourniotis G. (2010) Antifungal and antibacterial capacity of extracted material from non-glandular and glandular leaf hairs applied at physiological concentrations. Plant Stress 4: 25–30 [Google Scholar]
- Stone EC. (1963) Ecological importance of dew. Q Rev Biol 38: 328–341 [Google Scholar]
- Suh MC, Samuels AL, Jetter R, Kunst L, Pollard M, Ohlrogge J, Beisson F. (2005) Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol 139: 1649–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás-Barberán FA, Gil MI, Cremin P, Waterhouse AL, Hess-Pierce B, Kader AA. (2001) HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J Agric Food Chem 49: 4748–4760 [DOI] [PubMed] [Google Scholar]
- van Oss CJ, Chaudhury MK, Good RJ. (1987) Monopolar surfaces. Adv Colloid Interface Sci 28: 35–64 [DOI] [PubMed] [Google Scholar]
- van Oss CJ, Chaudhury MK, Good RJ. (1988) Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem Rev 88: 927–941 [Google Scholar]
- Villena JF, Domínguez E, Heredia A. (2000) Monitoring biopolymers present in plant cuticles by FT-IR spectroscopy. J Plant Physiol 156: 419–422 [Google Scholar]
- Villena JF, Domínguez E, Stewart D, Heredia A. (1999) Characterization and biosynthesis of non-degradable polymers in plant cuticles. Planta 208: 181–187 [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Wang E, Shepherd RW. (2004) New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot (Lond) 93: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P, Fürstner R, Barthlott W, Neinhuis C. (2003) Quantitative assessment to the structural basis of water repellency in natural and technical surfaces. J Exp Bot 54: 1295–1303 [DOI] [PubMed] [Google Scholar]
- Werker E. (2000) Trichome diversity and development. Adv Bot Res 31: 1–35 [Google Scholar]
- Xia Y, Yu K, Navarre D, Seebold K, Kachroo A, Kachroo P. (2010) The glabra1 mutation affects cuticle formation and plant responses to microbes. Plant Physiol 154: 833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue CH, Jia ST, Ma JZ. (2010) Large-area fabrication of superhydrophobic surfaces for practical applications: an overview. Sci Technol Adv Mater 11: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]