Abstract
The effect of proline (Pro) accumulation on heat sensitivity was investigated using transgenic Arabidopsis (Arabidopsis thaliana) plants ectopically expressing the Δ(1)-pyrroline-5-carboxylate synthetase 1 gene (AtP5CS1) under the control of a heat shock protein 17.6II gene promoter. During heat stress, the heat-inducible expression of the AtP5CS1 transgene was capable of enhancing Pro biosynthesis. Twelve-day-old seedlings were first treated with heat at 37°C for 24 h to induce Pro and then were stressed at 50°C for 4 h. After recovery at 22°C for 96 h, the growth of Pro-overproducing plants was significantly more inhibited than that of control plants that do not accumulate Pro, manifested by lower survival rate, higher ion leakage, higher reactive oxygen species (ROS) and malondialdehyde levels, and increased activity of the Pro/P5C cycle. The activities of antioxidant enzymes superoxide dismutase, guaiacol peroxidase, and catalase, but not those of glutathione reductase and ascorbate peroxidase, increased in all lines after heat treatment, but the increase was more significant in Pro-overproducing seedlings. Staining with MitoSox-Red, reported for being able to specifically detect superoxide formed in mitochondria, showed that Pro accumulation during heat stress resulted in elevated levels of ROS in mitochondria. Interestingly, exogenous abscisic acid (ABA) and ethylene were found to partially rescue the heat-sensitive phenotype of Pro-overproducing seedlings. Measurement of ethylene and ABA levels further confirmed that these two hormones are negatively affected in Pro-overproducing seedlings during heat stress. Our results indicated that Pro accumulation under heat stress decreases the thermotolerance, probably by increased ROS production via the Pro/P5C cycle and inhibition of ABA and ethylene biosynthesis.
Pro accumulation is a widespread phenomenon in higher plants in response to various environmental stresses and was demonstrated to be protective for plants under adverse conditions. Pro accumulation was proposed to act as a compatible osmolyte, free radical scavenger, cell redox balancer, cytosolic pH buffer, and stabilizer for subcellular structures during various stresses, especially osmotic and salt stresses (for review, see Hare and Cress, 1997; Kishor et al., 2005; Trovato et al., 2008; Verbruggen and Hermans, 2008; Szabados and Savouré, 2010). Recently, increasing evidence suggested that Pro accumulation may also play regulatory roles during plant growth and flowering (Nanjo et al., 1999b; Samach et al., 2000; Maggio et al., 2002; Mattioli et al., 2008). However, the exact physiological function of Pro is still controversial, and several researchers have attributed its beneficial function to the process of Pro metabolism rather than to the Pro molecule itself. The interconversion of Pro and pyrroline-5-carboxylate (P5C) in different cellular compartments might be involved in metabolic signaling and the regulation of intracellular redox potential in higher plants (Hare and Cress, 1997; Miller et al., 2009).
In plants, the biochemical routes of Pro biosynthesis and degradation have been well studied. Pro biosynthesis is derived from two different precursors, Glu and Orn. Glu is converted into Pro by two successive reductions, catalyzed by pyrroline-5-carboxylate synthetase (P5CS) and pyrroline-5-carboxylate reductase (P5CR), respectively, with P5C as an intermediate (Hu et al., 1992; Verbruggen et al., 1993; Yoshiba et al., 1995). Alternatively, Orn is converted to P5C by Orn-δ-aminotransferase and then reduced further to Pro by P5CR (Roosens et al., 1998). The up-regulation of the Glu pathway is thought to be responsible for Pro accumulation under various stresses. For degradation, Pro is oxidized to Glu via P5C, catalyzed by proline dehydrogenase (PDH) and P5C dehydrogenase (P5CDH) in mitochondria. P5CS and PDH appeared to catalyze the rate-limiting step in Pro biosynthesis and degradation, respectively (Hu et al., 1992; Verbruggen et al., 1996; Székely et al., 2008).
Numerous reports have been published that transgenic plants overproducing Pro exhibited increased tolerance to various environmental stresses, such as freezing, high salinity, and drought (Kishor et al., 1995; Nanjo et al., 1999a; Hong et al., 2000; Kumar et al., 2010). On the contrary, transgenic Arabidopsis (Arabidopsis thaliana) plants with an antisense AtP5CS cDNA displayed lower Pro content and decreased tolerance to osmotic stress (Nanjo et al., 1999b). Similarly, lower salt tolerance was observed in the P5CS1 insertional mutant of Arabidopsis with impaired Pro accumulation (Székely et al., 2008).
Compared with other stresses, however, only a few reports demonstrated that Pro accumulated during heat stress (HS). In the first leaf of barley (Hordeum vulgare) and radish (Raphanus sativus), Pro content showed a slight increase under 41°C HS (Chu et al., 1974). In chickpea (Cicer arietinum), heat treatment resulted in a marginal increase in Pro content compared with the control, and the increase was more significant after salicylic acid (SA) pretreatment (Chakraborty and Tongden, 2005). In suspensions cells of cowpea (Vigna unguiculata), the Pro synthesis rate was increased 2.7-fold when cells had been transferred from 26°C to 42°C (Mayer et al., 1990). In a salt-tolerant strain of tobacco (Nicotiana tabacum) suspension cells, NrEs-1, a transient initial rise in Pro content was reported at high temperature or at high temperature plus salinity, but high Pro content could last a longer time in response to salinity solely (Kuznetsov and Shevyakova, 1997). In Arabidopsis, Pro accumulation was not observed during HS (Yoshiba et al., 1995; Hua et al., 2001; Rizhsky et al., 2004). This has posed a question whether Pro accumulation is beneficial in all kinds of stresses, or is it only so in a specific set of stresses where Pro accumulates naturally? It has been reported that high temperature exaggerated the toxic effect of exogenous Pro (Rizhsky et al., 2004), raising the possibility that the presence of high concentrations of Pro during high temperature is inhibitory. In supporting this hypothesis, PDH insertional mutants, with higher Pro content, exhibited lower thermotolerance compared with the wild type (Larkindale and Vierling, 2008); the p5cdh mutant, unable to completely oxidize Pro, was also demonstrated to be more sensitive to heat shock (Miller et al., 2009). However, direct evidence for the effect of Pro accumulation on thermotolerance is still lacking.
In this study, we have ectopically expressed P5CS1 under the control of the heat shock protein AtHSP17.6II promoter in Arabidopsis. These transgenic plants, where Pro accumulated in a heat-inducible manner, displayed reduced heat tolerance compared with the wild type. Our data indicated that, unlike during osmotic stresses, Pro accumulation during HS induced the expression of the Pro oxidase gene, resulting in enhanced activity of the Pro/P5C cycle and production of reactive oxygen species (ROS). In addition, the function of hormones such as abscisic acid (ABA) and ethylene also seemed to be affected.
RESULTS
Identification of Transgenic Arabidopsis
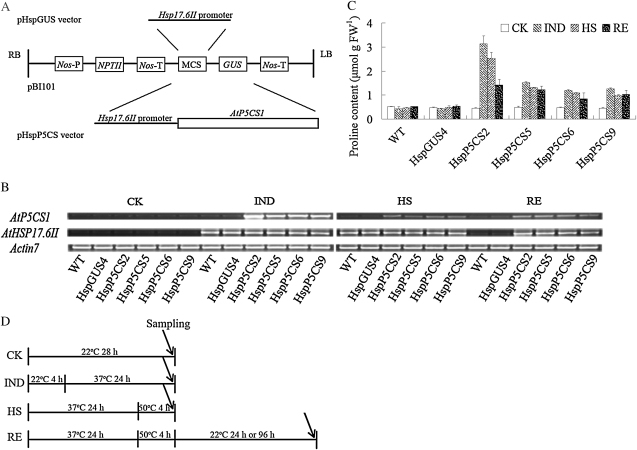
In order to investigate the effect of Pro accumulation on the heat response in Arabidopsis, transgenic plants containing the AtP5CS1 gene under the control of the promoter of HSP17.6II were generated and designated as HspP5CS. Transgenic plants containing the GUS gene under the control of the same promoter were used as a control and designated as HspGUS (Fig. 1A). Thirty-two and 21 independent transgenic lines were obtained for HspP5CS and HspGUS, respectively. The homozygous T3 generation of four independent HspP5CS lines, namely HspP5CS2, HspP5CS5, HspP5CS6, and HspP5CS9, and one HspGUS line, namely HspGUS4, were used for further analysis. Reverse transcription (RT)-PCR analysis showed that AtP5CS1 was expressed in a heat-inducible manner in HspP5CS lines, among which HspP5CS2 showed the highest induction, while the expression of AtP5CS1 was not detectable in the wild type and HspGUS4 under either control or heat conditions. As expected, the expression of HSP17.6II was similarly induced in all lines tested (Fig. 1B), indicating that endogenous HSP17.6II expression was not affected in HspP5CS lines.
Figure 1.
Construction of plasmid vectors, the mRNA expression of AtP5CS1, and determination of free Pro content. A, Schematic presentation of vectors pHspGUS and pHspP5CS. LB, Left border; RB, right border. B, Expression level of AtP5CS1 in wild-type (WT), HspGUS4, and transgenic lines, as determined by semiquantitative RT-PCR. C, Measurement of free Pro content in all stages. FW, Fresh weight. D, Schematic drawing of the experimental design for HS. CK, Control; IND, Pro induction at 37°C for 24 h; HS, heat stress at 50°C for 4 h after Pro induction; RE, recovery from HS. For semiquantitative RT-PCR analysis and Pro accumulation measurements, seedlings were recovered for 24 h. The IND and HS treatments started after 8 h of light, with the same photoperiods and light conditions as for the control. Results were calculated from measurements of at least three repeats.
Free Pro content in different lines was also determined. Under unstressed conditions, the Pro content was nearly the same in all lines. In comparison with the control condition, heat shock (Pro induction at 37°C for 24 h [IND]) caused a significant increase in Pro content in HspP5CS lines but not in the wild type and the vector control (HspGUS4; Fig. 1C). These results indicate that heat-inducible expression of AtP5CS is functional in promoting Pro biosynthesis.
The Effect of Pro Accumulation on the Heat Response
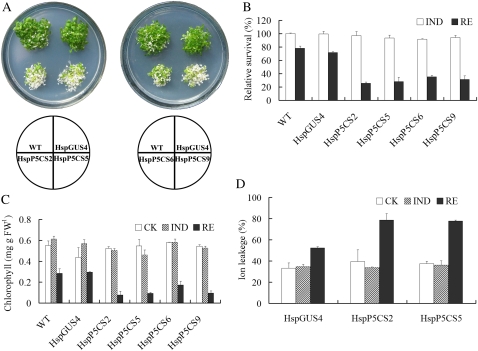
To test whether Pro accumulation has any effects on heat tolerance, we followed the experimental procedures as depicted in Figure 1D. Twelve-day-old seedlings that have been grown at 22°C (control) were first treated with heat at 37°C for 24 h to induce Pro (IND) and were then transferred to 50°C for 4 h (HS). After recovery at 22°C for 96 h (RE), the growth of the seedlings was evaluated. As shown in Figure 2A, HspP5CS lines were more severely bleached than the wild type and HspGUS4. The relative survival rate was only about 32% for HspP5CS seedlings, while that for the wild type and the vector control was about 75% (Fig. 2B). Total chlorophyll content was also examined. During RE, a significant decrease in chlorophyll content was observed in all lines, but the decrease was more dramatic in HspP5CS lines than in the wild type and HspGUS4 (Fig. 2C). No significant difference in chlorophyll content was found in different lines under control conditions and during IND. Unsurprisingly, ion leakage measured in lines HspP5CS2 and HspP5CS5 was 79% and 77%, respectively, during the recovery period, much higher than that in line HspGUS4, which was 52% (Fig. 2D). These data clearly demonstrate that high levels of free Pro resulted in decreased tolerance to high temperature.
Figure 2.
Heat response in transgenic lines and the control line. After HS, the seedlings were allowed to recover for 96 h and photographed (A), relative survival rate was calculated according to the equation relative survival = survival rate (IND or RE)/survival rate (control [CK]) × 100% (B), chlorophyll content was determined (C), and ion leakage was measured (D). Data are means ± se of at least three independent experiments. FW, Fresh weight; WT, wild type.
To test whether Pro accumulation upon induction is causing growth inhibition, we transferred 37°C-induced wild-type, HspGUS4, and HspP5CS lines directly to 22°C. The result showed that without heat treatment at 50°C, the growth of HspP5CS lines was not significantly different from that of the wild type and HspGUS (Supplemental Fig. S1).
The P5CS knockout mutant has been shown to be deficient in salt-induced Pro accumulation and in salt tolerance (Székely et al., 2008). Therefore, we tested whether this mutant is also affected in sensitivity to HS. As shown in Supplemental Figure S2, following the same procedure of heat treatment, p5cs1-1 did not significantly differ from the wild type in heat sensitivity.
Oxidative Damage and Antioxidant Enzymes
In plants, ROS has been demonstrated to cause oxidative damage to the cell membrane during various environmental stresses, including extreme temperature (Larkindale and Knight, 2002; Apel and Hirt, 2004). Even very short HS is able to bring about the increase of ROS, among which hydrogen peroxide (H2O2) and superoxide (O2−) are believed to be the most important components (Apel and Hirt, 2004).
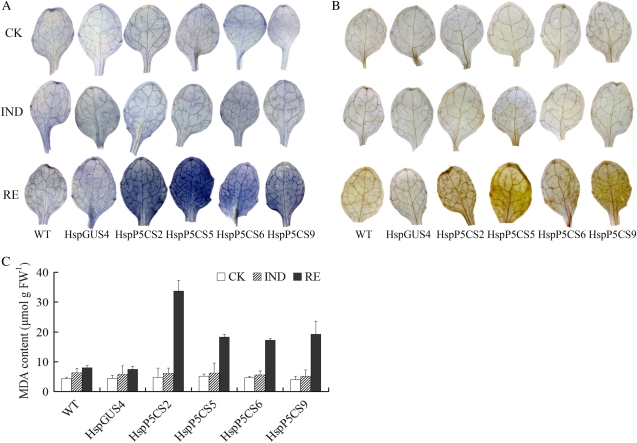
To explore whether decreased heat tolerance in Pro-accumulating lines is related to ROS generation during HS, seedlings were analyzed for H2O2 and O2− levels by 3,3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining, respectively, during different stages of heat treatment. Low levels of H2O2 and O2− were detected in both HspP5CS lines and the control line during the control and IND periods. However, H2O2 and O2− levels increased dramatically in HspP5CS lines, in comparison with only a minor increase in the control line and the wild type after 24 h of recovery from HS (Fig. 3, A and B).
Figure 3.
ROS level in HspP5CS and HspGUS4 lines during different stages of heat treatment. To detect O2−H2O2, 12-d-old seedlings were stained with NBT (A) and DAB (B), respectively. Levels of lipid peroxidation (MDA) were also measured (C). Results were obtained from measurements in at least three independent experiments. CK, Control; FW, fresh weight; WT, wild type.
Malondialdehyde (MDA) level, often used as an index for lipid peroxidation, was also measured. During the recovery stage after heat treatment, MDA content in HspP5CS lines was two to four times as much as that in the wild type and HspGUS4 (Fig. 3C). However, there was no visible difference found during the control and IND periods. These results suggested that the heat sensitivity of Pro-accumulating lines was probably caused, at least in part, by oxidative damage.
Plants have multiple strategies to prevent oxidative damage to cells, accomplished by enzymatic and nonenzymatic antioxidants. Superoxide dismutase (SOD), peroxidase, catalase (CAT), glutathione reductase (GR), and ascorbate peroxidase (APX) are among the enzymatic antioxidants. It is well known that dismutation of O2− catalyzed by SOD produces H2O2 and oxygen. CAT exterminates H2O2 and is thought to be one of the most important antioxidant enzymes. GR and APX act in the stress-regulated glutathione-ascorbate cycle. The activities of these enzymes have been proved to be inducible by the rise of intracellular ROS levels (Apel and Hirt, 2004).
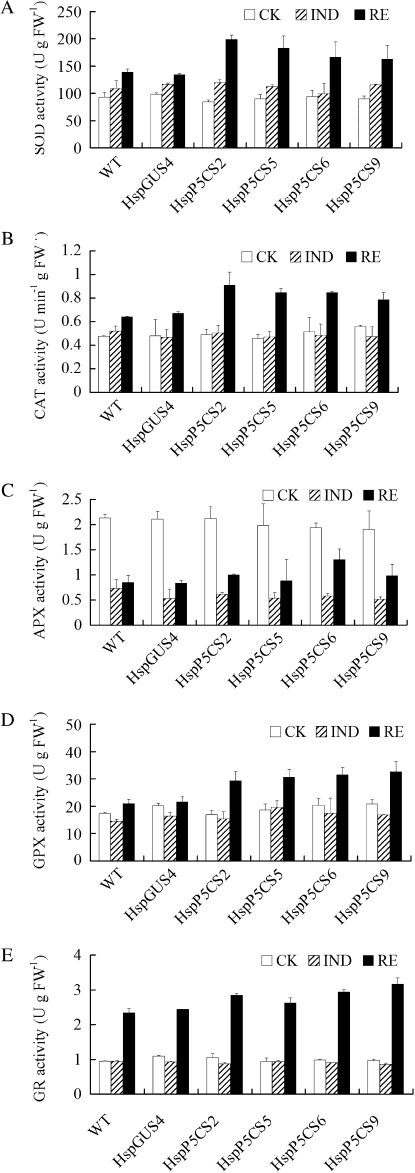
To gain more insights into the differences in the enzymatic antioxidative system in response to heat treatment between HspP5CS and HspGUS lines, the activities of SOD, guaiacol peroxidase (GPX), CAT, GR, and APX were measured (Fig. 4). SOD, GPX, and CAT displayed increased activities in all the lines after heat treatment, but the increase was more significant in HspP5CS lines than in the wild type and HspGUS4. GR and APX activities showed only a marginal difference between HspP5CS lines and the wild type in both normal growth and HS conditions.
Figure 4.
Activities of ROS-scavenging enzymes in different transgenic lines during heat treatment. Activities of SOD (A), CAT (B), APX (C), GPX (D), and GR (E) in 12-d-old seedlings were determined. Data are means ± se of at least three independent experiments. CK, Control; FW, fresh weight; WT, wild type.
Characterization of Gene Expression
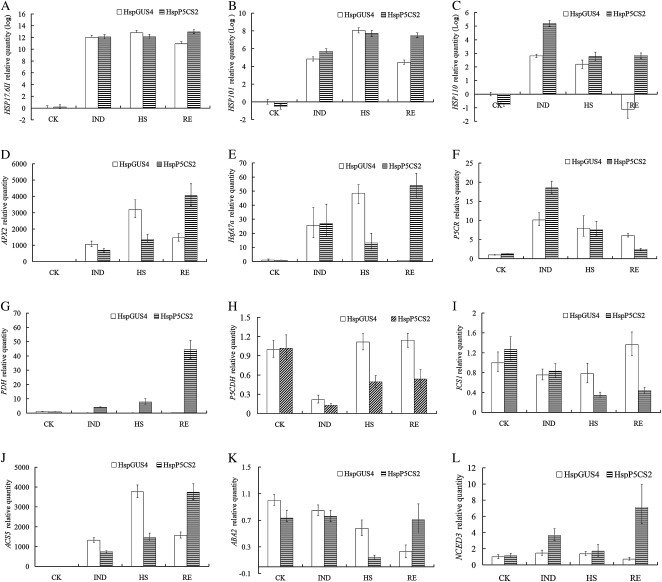
To elucidate the mechanisms underlying Pro-induced heat sensitivity, we have characterized the expression of several genes in HspGUS4 and HspP5CS2 through quantitative real-time PCR. The genes known to be involved in plant heat response, such as heat shock transcription factor A7a (HsfA7a), HSP17.6II, HSP101, HSP110, and APX2, were selected (Hong and Vierling, 2000; Sun et al., 2001; Larkindale and Vierling, 2008). It has been reported that some plant hormones, such as ABA, ethylene, and SA, are involved in heat response signaling (Larkindale and Knight, 2002; Larkindale et al., 2005). Therefore, genes related to the synthesis of these hormones, such as ABA2, nine-cis-epoxycarotenoid dioxygenase 3 (NCED3), 1-aminocyclopropane-1-carboxylate synthase 5 (ACS5), and isochorismate synthase 1 (ICS1), were also chosen. The expression of the genes in the Pro metabolic pathway, such as P5CR, PDH, and P5CDH, was also analyzed to understand the modification of Pro metabolism itself during HS. The expression level of the genes was measured at four stages, namely control, IND, HS, and RE, in HspP5CS2 and HspGUS. As shown in Figure 5, the transcript levels of HSP17.6II and HSP101 were higher in HspP5CS2 than that in HspGUS4 only during the RE stage (Fig. 5, A and B). The expression of HSP110 was higher during the IND and RE stages, correlated with the fact that HspP5CS2 was more severely injured than the control (Fig. 5C). T-DNA insertional mutants of APX2 and HsfA7a have been reported to be sensitive to HS (Larkindale and Vierling, 2008), suggesting that APX2 and HsfA7a are important for heat resistance. Our data demonstrated that compared with HspGUS4, the expression of both APX2 and HsfA7a in HspP5CS2 was lower under the HS condition but was higher during RE (Fig. 5, D and E), correlated with the heat-hypersensitive phenotype of HspP5CS. In agreement with a previous study (Hua et al., 1997), AtP5CR showed significant up-regulation in both lines during induction and the HS stage. Interestingly, AtPDH expression increased gradually during different stages only in line HspP5CS2, probably due to the induction by accumulated Pro. On the contrary, AtP5CDH expression was repressed during the IND stage in both lines and restored to normal levels during HS and RE in control line HspGUS4 but not in HspP5CS2 (Fig. 5, F–H).
Figure 5.
Analysis of gene expression by real-time PCR. Fold change of expression levels of genes (HSP17.6II, HSP101, HSP110, APX2, HsfA7a, P5CR, PDH, P5CDH, ICS1, ACS5, ABA2, and NCED3) was detected by quantitative real-time PCR. The raw data (cycle threshold values) were normalized using ACTIN2 as an internal reference, of which the results of HSPs were log2 transformed. Data are means ± se of at least three independent experiments. This is a representation of at least two replicates. CK, Control.
The expression of the SA biosynthetic gene ICS1 was found to be constant in HspGUS4 during all stages but repressed in HspP5CS2 during the HS and RE stages (Fig. 5I). The expression of AtNCED3, an important gene in ABA biosynthesis, was induced only in HspP5CS2 during the IND and RE stages (Fig. 5L). Another ABA biosynthetic gene, ABA2, was suppressed during the processes of the experiment and was more suppressed in HspP5CS2 during the HS stage, which was restored to control level during RE, whereas in HspGUS4, the expression of ABA2 declined continuously (Fig. 5K). AtACS5 is a member of ACS family, which has been implicated in the plant response to high temperature (Wang et al., 2005). The expression of AtACS5 was clearly induced in both HspP5CS2 and HspGUS4. In HspP5CS2, the induction of AtACS5 was to a lesser extent during the IND and HS stages but was higher during RE compared with that in HspGUS4 (Fig. 5J). This implied that insufficient ethylene production during HS may be partly responsible for the heat hypersensitivity of HspP5CS2.
Pro/P5C Cycle
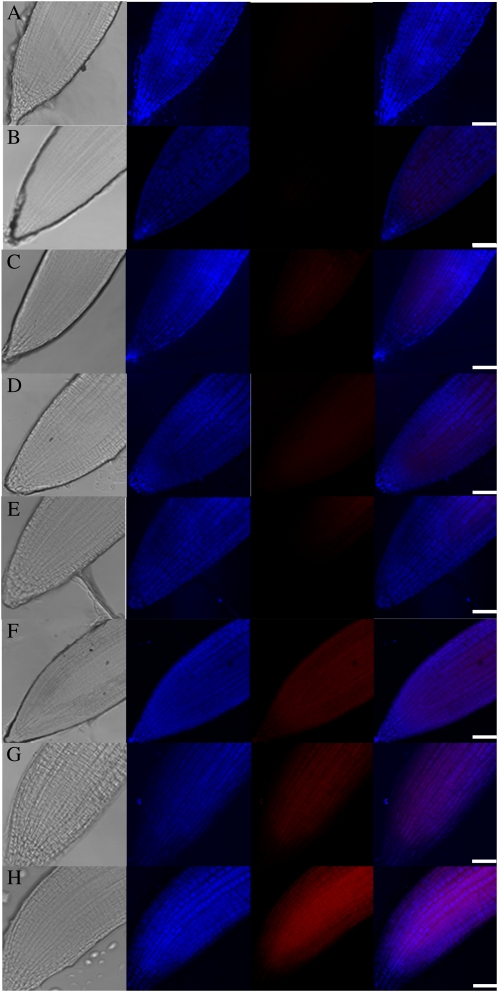
The Pro/P5C cycle, which was well characterized in mammalian cells, was recently reported to be also operative in plant cells. That is, the P5C generated from Pro oxidation in mitochondria can be transported to cytosol, where it is converted back to Pro by P5CR. This cycle helps to maintain the intracellular Pro-to-P5C ratio and may play roles in many physiological processes (Miller et al., 2009). When Pro was overproduced, such as in HspP5CS lines during HS, the activity of the Pro/P5C cycle would increase, compared with that in HspGUS4, as AtPDH expression was induced in HspP5CS lines (Fig. 5G), coinciding with the reduced expression of AtP5CDH. This might increase the chance that the electron generated from Pro oxidation would overflow from the mitochondria electron transport chain into the generation of ROS. To analyze whether there is a difference in mitochondrial ROS levels between HspP5CS2 and HspGUS4, the roots of HspGUS4 and HspP5CS2 were stained with MitoSox-Red and MitoTracker-Deep Red for O2− detection in mitochondria and mitochondrial localization, respectively. As shown in Figure 6, similarly weak MitoSox-Red fluorescent signal was detected under control and IND conditions in both HspGUS4 and HspP5CS2 (Fig. 6, A–D). The fluorescent signal increased in both lines during RE after HS, but more significantly in HspP5CS2 than in HspGUS4 (Fig. 6, E and F). Interestingly, addition of Pro (50 mm) to HspGUS4 before HS to mimic the situation in HspP5CS2 enhanced the fluorescent signal (Fig. 6G), suggesting that Pro overaccumulation enhances ROS generation in mitochondria during HS.
Figure 6.
Mitochondrial O2− generation in roots of HspGUS4 and HspP5CS2 seedlings during HS. Columns from left to right are as follows: light image, mitochondria localization in blue pseudocolor stained with MitoTracker-Deep Red, O2− generation in red pseudocolor stained with MitoSox-Red, and the merged fluorescence. Staining was performed with lines HspGUS (A, C, E, and G) and HspP5CS (B, D, F, and H) during control conditions (A and B), the Pro-inducing period (C and D), and the recovery stage after heat treatment at 50°C without (E and F) or with (G and H) exogenous Pro pretreatment. Bars = 50 μm.
Ethylene Signaling May Be Affected
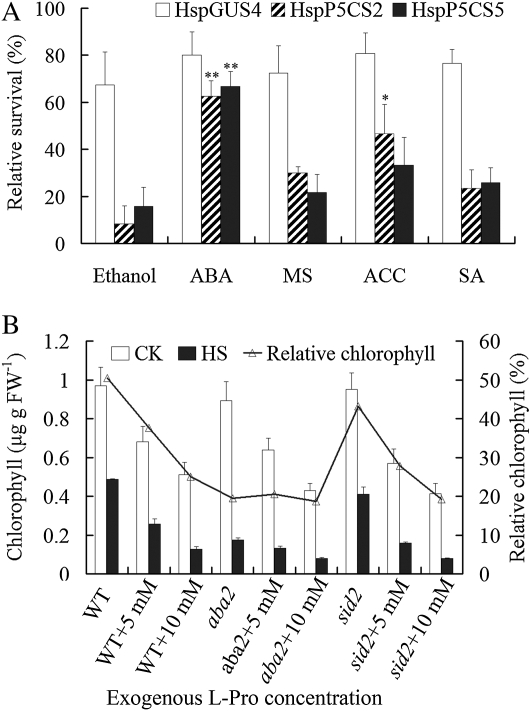
Ethylene has been demonstrated to be involved in the heat response by enhancing the activities of ROS-scavenging enzymes and thus protecting plants against heat-induced oxidative damage in Arabidopsis (Larkindale and Knight, 2002; Larkindale and Huang, 2004). Our results with AtACS5 expression (Fig. 5J) suggested that attenuated ethylene signaling is probably affected by Pro accumulation. To confirm this possibility, 50 μm 1-aminocyclopropane-l-carboxylic acid (ACC), the product of ACS in the ethylene biosynthetic pathway, was added into the medium just before HS. Exogenous ACC could significantly enhance the survival rate of HspP5CS2 (P < 0.05), although that of HspP5CS5 was not as significant. However, exogenous ACC has failed to improve the survival rate HspGUS4 (Fig. 7A). This result suggested that Pro accumulation during HS impaired ethylene biosynthesis, probably contributing to the heat hypersensitivity.
Figure 7.
Effects of ABA, SA, and ethylene on Pro-induced heat sensitivity. A, After inducing Pro, seedlings were pretreated with 100 μm ABA, 50 μm ACC, or 10 μm SA, and the respective solvent, as indicated, for 2 h before HS. Relative survival rate was calculated after 96 h of recovery. Relative survival rate was expressed according to the following equation: relative survival = survival rate (RE)/survival rate (control [CK]) × 100%. ABA was dissolved in ethanol, and all other compounds were dissolved in liquid MS medium. B, The effect of Pro on chlorophyll content of aba2 and sid2 during HS. Four-day-old seedlings grown vertically on MS medium containing exogenous l-Pro were transferred to 50°C for 2 h. After recovery for 96 h, chlorophyll content was measured, and relative chlorophyll was calculated as follows: chlorophyll (treated seedlings)/chlorophyll (control seedlings) × 100%. Data are means ± se of at least three independent experiments. FW, Fresh weight; WT, wild type.
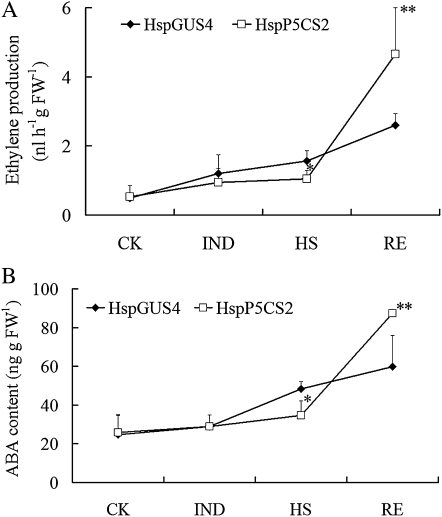
To investigate whether ethylene biosynthesis is actually affected by Pro accumulation during HS, ethylene production during all stages were measured. As shown in Figure 8A, during the HS stage, ethylene production was gradually induced in control line HspGUS4 and reached a significantly higher level than in HspP5CS2 during the HS stage (P < 0.05). During the RE stage, ethylene content in HspP5CS2 was significantly higher than that in HspGUS4, probably due to the fact that HspP5CS2 was more severely injured than the control.
Figure 8.
Measurement of ethylene (A) and ABA (B) levels in lines HspP5CS and HspGUS during different stages of heat treatment. CK, Control; FW, fresh weight.
ABA Signaling May Be Affected
ABA-insensitive mutants (abi1 and abi2) and ABA-deficient mutants (aba1 and aba2) have previously been shown to have much higher MDA contents and much lower survival rates than did the wild type under high-temperature conditions (Larkindale et al., 2005). To evaluate whether ABA signaling is also affected by Pro accumulation during HS, 12-d-old seedlings of HspP5CS2 and HspP5CS5 were treated with exogenous ABA before heat treatment at 50°C. After recovery, the relative survival rates in ABA-pretreated HspP5CS2 and HspP5CS5 seedlings were 62.5% and 66.7%, respectively, significantly higher than those in corresponding controls treated with ethanol (Fig. 7A), the solvent we used to dissolve ABA. On the contrary, ABA treatment did not significantly enhance the heat tolerance of HspGUS4.
To test whether endogenous ABA is involved in Pro-mediated heat sensitivity, we developed an assay using exogenous Pro to mimic endogenously produced Pro in mediating heat sensitivity. As heat treatment generally causes the seedlings to bleach, we measured chlorophyll content as an indication of heat injury. As shown in Figure 7B, after heat treatment, the wild-type seedlings grown on the medium containing l-Pro showed decreased chlorophyll content compared with wild-type seedlings without Pro treatment, and the relative chlorophyll decreased in a dose-dependent manner. This suggested that, similar to endogenous Pro, exogenous Pro can also increase heat sensitivity. However, the relative chlorophyll content of the ABA-deficient mutant aba2 is already low without Pro treatment, similar to that of the wild type with Pro treatment, supporting that aba2 is more heat sensitive. Interestingly, unlike in the wild type, the addition of exogenous Pro had almost no effect on the relative chlorophyll content in aba2, suggesting that ABA biosynthesis may be inhibited by Pro accumulation during HS.
Therefore, we further measured ABA content in HspP5CS2 and HspGUS4 during different stages (Fig. 8B). Under IND conditions, ABA content did not increase in both HspP5CS2 and HspGUS4. However, during the HS stage, there was a rise in ABA production in HspGUS4 but not significantly in HspP5CS2. After recovery for 24 h, ABA content increased in both lines, with the increase in HspP5CS2 being more dramatic, which was consistent with the phenotype that HspP5CS2 was more severely damaged than HspGUS4. These findings suggested that Pro accumulation during HS may disturb ABA biosynthesis, resulting in heat sensitivity.
On the other hand, SA pretreatment did not have any effects on the heat tolerance in HspGUS and HspP5CS lines (Fig. 7A). Pro had a similar effect on the heat sensitivity in sid2 (mutated in ICS1) and the wild type (Fig. 7B), implying that SA signaling seems not to be involved in Pro-induced heat sensitivity.
DISCUSSION
In this report, we have studied the effect of Pro accumulation on heat tolerance using transgenic Arabidopsis plants ectopically expressing AtP5CS1 in a heat-inducible manner. Since in wild-type Arabidopsis plants AtP5CS1 is not induced by heat treatment and neither is Pro accumulation (Yoshiba et al., 1995; Hua et al., 2001), these transgenic lines provided ideal materials for specifically evaluating the effect of Pro accumulation during HS without disturbing normal growth and development. Pro measurement indicated that Pro level is not altered under normal growth conditions but increases up to 6-fold under HS in line HspP5CS2, comparable to the Pro level under salt stress; in other lines, however, such as HspP5CS5, HspP5CS6, and HspP5CS9, the Pro accumulation is relatively lower. These result suggested, on the one hand, that the unaltered expression of P5CS1 is probably one of the major limiting factors for Pro not accumulating in Arabidopsis during HS, and on the other hand, that the lower accumulation of Pro in the majority of the lines is probably due to the induced expression of PDH. In our experiment, care has been taken to avoid any possible changes in water status during all the stages of heat treatment (data not shown). Therefore, Pro accumulation in transgenic lines was not owing to the desiccation resulting from HS.
Pro accumulation has been well documented to play positive roles in enhancing plant tolerance to many environmental stresses, such as osmotic, salt, heavy metal, cold, UV radiation, and oxidative stress (Saradhi et al., 1995; Nanjo et al., 1999a; Hong et al., 2000; Molinari et al., 2007; Wang et al., 2009). However, its role during HS has seldom been reported. Our results indicated that Pro accumulation is harmful to plants under HS, manifested by lower survival rate, higher ion leakage, higher ROS and MDA levels, and increased activity of the Pro/P5C cycle in HspP5CS lines, in agreement with previous data that exogenous Pro can exaggerate the inhibitory effects of HS on seedling growth in Arabidopsis (Rizhsky et al., 2004). In our experiments, all HspP5CS lines and control line HspGUS4 showed similar basal thermotolerance without Pro induction (data not shown), suggesting that Pro accumulation is responsible for the reduced thermotolerance.
Therefore, it seems that Pro is not always helpful for plants subjected to environmental stresses. This poses interesting questions. Why is Pro accumulation beneficial during some stresses but not others? Are there any common features in stresses where Pro accumulation is protective? One interesting finding is that the expression of the PDH gene is induced in HspP5CS lines during HS by accumulated Pro. Pro, the substrate of PDH, is an inducer for PDH expression under normal growth conditions. However, during salt and drought stress, the expression of PDH is normally suppressed, in spite of the Pro accumulation. It has been proposed that there might be a mechanism that can override the Pro inducibility toward PDH expression, functioning to suppress PDH expression (Verbruggen et al., 1996). Therefore, the suppression of PDH expression may have served dual roles during salt and drought stresses. The first is to facilitate the accumulation of Pro by reducing its degradation. The second is to avoid the consequences of Pro degradation, which are reported to be coupled with the mitochondrial electron transport chain capable of producing ROS (Miller et al., 2009). Our results of mitochondrial ROS measurement strongly supported this notion. Contradictory to the above proposition, Larkindale and Vierling (2008) reported that two insertional mutants of AtPDH, with higher Pro content and presumably reduced PDH activity, showed reduced tolerance to heat treatment. The reason behind this discrepancy is not clear. One possibility is that Pro itself might also be toxic during HS.
Another unique feature of HS, differing from other stresses, is that the expression of AtP5CR, another enzyme involved in the Pro/P5C cycle, is strongly up-regulated under heat conditions. This has been illustrated by Hua et al. (1997) and is further confirmed by the quantitative real-time PCR results in our study. Therefore, the Pro/P5C cycle appeared ready to go once PDH was induced.
To further confirm the involvement of the Pro/P5C cycle in Pro-induced heat sensitivity, it would be helpful to cross our HspP5CS lines to other lines with potentially reduced Pro/P5C cycle activity and to analyze the heat sensitivity of the hybrids. Although pdh mutants are blocked in the Pro/P5C cycle, their heat hypersensitivity might render them unsuitable for this purpose. Transgenic lines overexpressing P5CDH would be a better candidate, since they have been reported to be more tolerant to Pro toxicity (Deuschle et al., 2004), possibly by facilitating the oxidation of P5C into Glu. Alternatively, introducing a mitochondria-localized SOD, such as MnSOD (Liu et al., 2005), would also help to illustrate the role of mitochondrial ROS in Pro-induced heat sensitivity.
It has been previously reported that during a combination of drought and HS, sugar instead of Pro accumulated (Rizhsky et al., 2004). Thus, sugar appeared to play protective roles during HS. In agreement with this, we have observed that the increase in soluble sugar level is significantly lower in HspP5CS lines than in HspGUS4 and the wild type (data not shown), suggesting that Pro accumulation during HS may have negative effects on sugar biosynthesis.
Multiple signaling pathways, such as ABA, H2O2, ethylene, SA, and calcium signaling pathways, are reported to be involved in defense responses to HS (Larkindale and Knight, 2002; Liu et al., 2006; Clarke et al., 2009). Several mutants defective in ABA biosynthesis and signaling are more sensitive to HS (Larkindale and Knight, 2002; Larkindale et al., 2005; Zimmerli et al., 2008; Dobra et al., 2010). Acquired thermotolerance induced by β-aminobutyric acid in Arabidopsis is thought to be correlated with the up-regulation of ABA signaling components (Zimmerli et al., 2008). Ethylene has also been demonstrated to protect Arabidopsis against heat-induced oxidative damage by enhancing the activities of ROS-scavenging enzymes (Larkindale and Knight, 2002; Larkindale et al., 2005). In Arabidopsis, pretreatment with exogenous ACC could decrease the MDA level and enhance the survival rate during subsequent HS (Larkindale and Knight, 2002). The ethylene signaling mutants ethylene resistant1 and ethylene insensitive2 show decreased tolerance to lethal HS under high-light conditions (Larkindale et al., 2005).
In our study, ABA and ethylene biosynthesis were partially impaired by Pro accumulation during HS. The expression of the ABA biosynthetic gene ABA2 and the ethylene biosynthetic gene ACS5 during HS was significantly lower in HspP5CS lines than in HspGUS4, correlated with reduced levels of the respective hormones. In addition, pretreatment of exogenous ABA and ACC improved the thermotolerance of the Pro-accumulating HspP5CS lines but not in HspGUS4. Moreover, in aba2, Pro could not further reduce its thermotolerance, probably because Pro cannot inhibit ABA biosynthesis further. Interestingly, a transient decrease in ABA content after 2 h of 40°C HS has been recorded in tobacco plants constitutively overproducing Pro (Dobra et al., 2010). Therefore, it is possible that the lower levels of ABA and ethylene in HspP5CS2 during HS may contribute to Pro-induced heat sensitivity. However, how Pro accumulation affects ABA and ethylene levels remains to be investigated.
In summary, our data demonstrate that Pro accumulation during HS is detrimental for plant growth. Pro is not always a compatible solute during environmental stresses. It may well be a toxic compound under certain stress conditions where it does not naturally accumulate.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) wild-type and transgenic plants used in this study were in the Columbia background. Wild-type and transgenic seeds were surface sterilized with 10% sodium hypochlorite for 15 min and rinsed seven times in sterile, distilled water. Seeds were cultured in petri dishes (9.0 cm in diameter) on Murashige and Skoog (MS) medium (MS salts, MS vitamins, 1% Suc, and 0.8% agar, pH 5.8). After being stored at 4°C for 72 h, seeds were germinated in a growth chamber at 22°C under a 16-h-light (120 μmol m−2 s−1)/8-h-dark regime.
Vector Construction and Transformation of Arabidopsis
A 1,085-bp promoter fragment of HSP17.6II was amplified from Arabidopsis genomic DNA with two primers (forward primer, 5′-underline>CCCGGG/underline>TTGCAGAAATTATTACTGTTC-3′; reverse primer, 5′-underline>GAATTC/underline>TCCTAAATCCATTGTTAGTTG-3′; the SmaI and EcoRI sites, respectively, are underlined). The HSP17.6II promoter fragment was digested with SmaI and EcoRI and cloned into the same restriction sites in pBI101 in front of a GUS reporter gene, yielding pHspGUS containing the HSP17.6II promoter:GUS construct. The 2,154-bp coding region of AtP5CS1 cDNA was amplified by PCR from poly(A)-primed reverse-transcribed total RNA with two primers (forward primer, 5′-underline>GAATTC/underline>ATGGAGGAGCTAGATCGTTC-3′; reverse primer, 5′-underline>GAGCTC/underline>TTAAGCTTGGATGGGAATGT-3′; the EcoRI and SacI sites, respectively, are underlined). The cDNA fragment was digested with EcoRI and SacI and used to replace the GUS gene in pHspGUS, yielding pHspP5CS with AtP5CS1 driven by the HSP17.6II promoter. Transgenic lines were generated by Agrobacterium tumefaciens-mediated transformation as described previously (Clough and Bent, 1998) and selected on 50 mg L−1 kanamycin. The kanamycin-resistant plants were used to breed the next generation. Homozygous T3 generation plants with a single copy of the transgene were used in this study.
Semiquantitative RT-PCR and Quantitative PCR Analysis
Semiquantitative RT-PCR
Total RNA extracted from 12-d-old seedlings was reverse transcribed by SuperScript II (Invitrogen) according to the manufacturer’s instructions. The gene-specific primers were as follows: AtP5CS1 (At2g39800), 5′-AGTCTATGCTTGATTTGAGGGT-3′ (forward) and 5′-AAGCTCATTAAGCACAGCATTC-3′ (reverse); AtHSP17.6II (At5g12020), 5′-TCACGAGTTTACATGCGAGA-3′ (forward) and 5′-ACCATATCCCTCACGCATTC-3′ (reverse); ACTIN7 (At5g09810; internal reference), 5′-TGGACAAGTCATAACCATCGG-3′ (forward) and 5′-TGTGAACAATCGATGGACCTGAC-3′ (reverse). PCR was then performed using first-strand cDNA as a template under conditions of an initial denaturation at 94°C for 5 min followed by 25 cycles of denaturing at 94°C for 30 s, annealing at 58°C for 30 s, extending at 72°C for 60 s, and a final extension at 72°C for 10 min. One percent agarose gel electrophoresis was performed to measure the expression of AtP5CS.
Quantitative PCR
RNA from HspGUS4 and HspP5CS2 samples was treated with DNase I to remove remnant genomic DNA. The total RNA was converted into cDNA by SuperScript II according to the manufacturer’s instructions. Real-time PCR was optimized and performed in triplicate using a SYBR Green Real-Time PCR Master Mix (QPK-201; Toyobo) on a Mx3000P thermocycler under conditions of an initial denaturation at 95°C for 2 min followed by 40 cycles of denaturing at 95°C for 15 s, annealing at 59°C for 20 s, and extending at 72°C for 15 s. Quantification was performed using the 2−ΔΔCT method, and the data were normalized through the quantity of the reference gene ACTIN2 (At3g18780). The dissociation curves were analyzed in all amplifications. The sequences of all gene-specific primers are listed in Supplemental Table S1.
Pro Induction and HS Tolerance Analysis
To induce Pro, 12-d-old seedlings were placed in a chamber at 37°C for 24 h. Samples were harvested, frozen at −80°C, and ground in liquid nitrogen. Free Pro content was measured by colorimetric assay (Bates et al., 1973).
For heat tolerance evaluation, after inducing Pro, 13-d-old seedlings were transferred to 50°C for 4 h and then returned to 22°C. Plants were photographed, and survival rate was assessed after recovery at 22°C for 96 h.
MDA Assay
After 24 h of recovery, samples were harvested and MDA level was assayed as described (Zhang et al., 2009a). Seedlings frozen in liquid nitrogen were ground and homogenized with 1.5 mL of 20% (w/v) TCA and centrifuged at 10,000g for 5 min. A 1-mL aliquot of the supernatant was added with 2 mL of thiobarbituric acid solution (0.5% [w/v] in 20% TCA). The mixture was heated at 95°C for 15 min, quickly cooled in an ice bath, and then centrifuged at 12,000g for 10 min. The absorbance of the supernatant was monitored at 450, 532, and 600 nm. The concentration of MDA was measured according to the following equation: concentration (μmol L−1) = 6.45 × (OD532 − OD600) − 0.56 × OD450, where OD stands for optical density.
Determination of Chlorophyll, and Electrolyte Leakage Measurement
Tissues were homogenized in 8 mL of 80% (v/v) acetone, and chlorophyll was extracted for 72 h at 4°C in the dark. The supernatant was quantified through monitoring the absorbance at 663 and 645 nm using a spectrophotometer (Zhang et al., 2008). Absolute chlorophyll content was calculated according to the following equation: total chlorophyll (mg L−1) = 8.02 × OD663 + 20.21 × OD645, where OD stands for optical density.
For electrolyte leakage measurement, seeds were sown on soil and grown in a chamber at 22°C under a 16-h photoperiod. Four-week-old plantlets were measured according to described methods (Clarke et al., 2004) with some modifications. Samples were weighed and washed three times in deionized water and then immersed into 25 mL of deionized water at 25°C. After 24 h, the conductivity of the solution was measured using a conductivity meter (initial conductivity). The tube was heated at 95°C for 15 min and cooled in an ice bath, and conductivity was remeasured (final conductivity). The relative ion leakage was calculated according to the following equation: ion leakage = initial conductivity/final conductivity × 100%.
H2O2 and O2− Radical Detection
H2O2 level was measured by DAB staining. Seedlings were immersed in freshly prepared 0.1% (w/v) DAB-HCl (pH 3.8) solution, vacuum infiltrated, and then incubated in darkness at 22°C for 10 h. After incubation, the seedlings were placed in acetic acid:glycerol:ethanol (1:1:3, v/v/v) solution at 95°C for 10 min and then stored in 95% ethanol until photographed (Orozco-Cardenas and Ryan, 1999). The detection of O2− was done by NBT staining (Kawai-Yamada et al., 2004). Seedlings were placed in 0.1% (w/v) NBT in 10 mm potassium phosphate buffer (pH 7.8) containing 10 mm NaN3, vacuum infiltrated, and then incubated in darkness at 22°C for 1 h. Stained plants were bleached in a solution containing acetic acid:glycerol:ethanol (1:1:3, v/v/v). After boiling for 5 min, samples were stored in 95% ethanol until photographs were taken.
Measurement of Enzyme Activities
Seedlings (1 g) were homogenized at 4°C with 100 mm potassium phosphate (pH 7.0) containing 0.1 mm EDTA and 1% polyvinylpyrrolidone (for APX extraction, 0.5 mm ascorbate was supplemented). The homogenate was centrifuged at 15,000g at 4°C for 20 min. The supernatant was used as the crude extract for the assay of enzyme activities.
SOD activity was assayed by monitoring the inhibition of the photochemical reduction of NBT (Beauchamp and Fridovich, 1971). The 3-mL reaction mixture contained 50 mm phosphate buffer (pH 7.8), 13 mm Met, 2 μm riboflavin, 100 mm EDTA, 75 μm NBT, and 30 μL of enzyme extract. Riboflavin was added last, and the reaction was started by switching on the light and allowed to run for 20 min. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition in the reduction of NBT.
CAT activity was measured by monitoring the disappearance of H2O2 (extinction coefficient of 39.4 mm−1 cm−1) at 240 nm for 180 s (Lu et al., 2007). The reaction mixture contained 100 mm potassium phosphate buffer (pH 7.0) and plant extract. The reaction was initiated by the addition of 10 mm H2O2, and 1 unit of CAT activity was defined as μm H2O2 degraded per min.
GPX activity was assayed on the basis of guaiacol oxidation using H2O2 (extinction coefficient of 26.6 mm−1 cm−1) at 470 nm for 180 s (Wu et al., 2009). The reaction mixture containing 100 mm potassium phosphate buffer (pH 7.0), 1 mm guaiacol, and 0.3% H2O2, and enzyme extract. One unit of GPX activity was defined as μm guaiacol oxidation per min.
GR activity was determined following the disappearance of NADPH (extinction coefficient of 6.2 mm−1 cm−1) at 340 nm (Rao, 1992). The reaction mixture contained 100 mm potassium phosphate buffer (pH 7.8), 2 mm EDTA, 0.2 mm NADPH, 0.5 mm oxidized glutathione, and enzyme extract. One unit of GR activity was defined as μm NADPH oxidation per min.
APX activity was measured by monitoring the rate of H2O2-dependent oxidation of ascorbate (extinction coefficient of 2.8 mm−1 cm−1) at 290 nm for 3 min (Chen and Asada, 1989). The reaction mixture consisted of 50 mm potassium phosphate buffer (pH 7.5), 0.5 mm ascorbate, and 0.2 mm H2O2. One unit of APX activity was defined as μm ascorbate oxidized per min.
Measurement of Ethylene Production
Ethylene level was measured with a gas chromatograph as described (Zhang et al., 2009b). Seedlings were placed in a 20-mL vial. After fumigation for 2 h, 1-mL samples of the air inside the vials were used for the determination of ethylene content with a gas chromatograph (Hitachi).
ABA Extraction and Determination
ABA concentration in seedlings was determined by HPLC as described (Mwange et al., 2003). Seedlings (0.5 g) were finely ground in liquid N2, and 2.5 mL of cold acetonitrile containing 1 mm 2,6-di-tert-butyl-methyl phenol was added. After homogenization in an ultrasonic processor for 30 min, ABA was extracted on ice for 12 h and then centrifuged at 4°C for 10 min. A total of 1.5 mL of the supernatant was evaporated in a Speedvac concentrator. The samples were resuspended in 1 mL of 0.4 m phosphate buffer (pH 8.0), and 80 mg of polyvinylpolypyrrolidone was added in the solution. After vortexing for 20 s, the extracts were centrifuged at 4°C for 10 min, and a 0.5-mL aliquot of the supernatant was adjusted to pH 3.0 using pure methanoic acid. The solution was then extracted by 0.5 mL of ethyl acetate three times. A total of 1.5 mL of the extract was evaporated in a Speedvac concentrator and then dissolved in 0.5 mL of mobile phase (methanol:0.5% acetic acid, 45:55). Samples were injected on a reverse-phase Luna C18 column (5-μm particle size, 4.6 × 250 mm; Waters 510) with the flow rate of 0.8 mL min−1 after filtration through a 0.45-μm membrane filter. Elution from the column was monitored with a Waters 2487 Dual λ absorbance detector at a wavelength of 254 nm. Quantification was obtained by comparing the peak areas with those of known amounts of ABA (Sigma).
Detection of Mitochondrial O2−
Roots of the seedlings were immersed in 5 μm MitoSox-Red work solution (Molecular Probes, Invitrogen) and then incubated for 25 min at 37°C in darkness. Subsequently, seedlings were washed gently three times with liquid MS medium and stained with 250 nm MitoTracker-Deep Red (Molecular Probes, Invitrogen). After incubation for 15 to 45 min at 37°C in darkness, the seedlings were washed gently with liquid MS medium (Miller et al., 2009). Fluorescence was imaged using a confocal microscope (Leica; TCS, SP5) with excitation/emission of 514/580 nm and 633/680 nm for MitoSox-Red and MitoTracker-Deep Red, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of Pro accumulation on Arabidopsis seedlings in the absence of HS.
Supplemental Figure S2. Heat response of the p5cs1-1 mutant.
Supplemental Table S1. Primers used in quantitative real-time PCR.
Acknowledgments
We thank Dr. Jing-Bo Jin (Institute of Botany, Chinese Academy of Science) for kingly providing seeds of Arabidopsis mutant sid2.
References
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207 [Google Scholar]
- Beauchamp C, Fridovich I. (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44: 276–287 [DOI] [PubMed] [Google Scholar]
- Chakraborty U, Tongden C. (2005) Evaluation of heat acclimation and salicylic acid treatments as potent inducers of thermotolerance in Cicer arietinum L. Curr Sci 89: 384–389 [Google Scholar]
- Chen GX, Asada K. (1989) Ascorbate peroxidase in tea leaves: occurrence of 2 isozymes and the differences in their enzymatic and molecular-properties. Plant Cell Physiol 30: 987–998 [Google Scholar]
- Chu TM, Aspinall D, Paleg LG. (1974) Stress metabolism. VI. Temperature stress and the accumulation of proline in barley and radish. Aust J Plant Physiol 1: 87–97 [Google Scholar]
- Clarke SM, Cristescu SM, Miersch O, Harren FJM, Wasternack C, Mur LAJ. (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182: 175–187 [DOI] [PubMed] [Google Scholar]
- Clarke SM, Mur LAJ, Wood JE, Scott IM. (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38: 432–447 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Deuschle K, Funck D, Forlani G, Stransky H, Biehl A, Leister D, van der Graaff E, Kunze R, Frommer WB. (2004) The role of Δ1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell 16: 3413–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobra J, Motyka V, Dobrev P, Malbeck J, Prasil IT, Haisel D, Gaudinova A, Havlova M, Gubis J, Vankova R. (2010) Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J Plant Physiol 167: 1360–1370 [DOI] [PubMed] [Google Scholar]
- Hare PD, Cress WA. (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21: 79–102 [Google Scholar]
- Hong SW, Vierling E. (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97: 4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong ZL, Lakkineni K, Zhang ZM, Verma DPS. (2000) Removal of feedback inhibition of Δ(1)-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122: 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CA, Delauney AJ, Verma DP. (1992) A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci USA 89: 9354–9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XJ, van de Cotte B, Van Montagu M, Verbruggen N. (1997) Developmental regulation of pyrroline-5-carboxylate reductase gene expression in Arabidopsis. Plant Physiol 114: 1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XJ, Van de Cotte B, Van Montagu M, Verbruggen N. (2001) The 5′ untranslated region of the At-P5R gene is involved in both transcriptional and post-transcriptional regulation. Plant J 26: 157–169 [DOI] [PubMed] [Google Scholar]
- Kawai-Yamada M, Ohori Y, Uchimiya H. (2004) Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell 16: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishor PBK, Hong ZL, Miao GH, Hu CAA, Verma DPS. (1995) Overexpression of delta-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108: 1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishor PBK, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N. (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88: 424–438 [Google Scholar]
- Kumar V, Shriram V, Kishor PBK, Jawali N, Shitole MG. (2010) Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol Rep 4: 37–48 [Google Scholar]
- Kuznetsov VV, Shevyakova NI. (1997) Stress responses of tobacco cells to high temperature and salinity: proline accumulation and phosphorylation of polypeptides. Physiol Plant 100: 320–326 [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E. (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138: 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Huang B. (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161: 405–413 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR. (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146: 748–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Liu YY, Pan QH, Yang HR, Zhan JC, Huang WD. (2006) Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J Exp Bot 57: 3337–3347 [DOI] [PubMed] [Google Scholar]
- Liu YM, Borchert GL, Donald SP, Surazynski A, Hu CA, Weydert CJ, Oberley LW, Phang JM. (2005) MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis 26: 1335–1342 [DOI] [PubMed] [Google Scholar]
- Lu ZQ, Liu DL, Liu SK. (2007) Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep 26: 1909–1917 [DOI] [PubMed] [Google Scholar]
- Maggio A, Miyazaki S, Veronese P, Fujita T, Ibeas JI, Damsz B, Narasimhan ML, Hasegawa PM, Joly RJ, Bressan RA. (2002) Does proline accumulation play an active role in stress-induced growth reduction? Plant J 31: 699–712 [DOI] [PubMed] [Google Scholar]
- Mattioli R, Marchese D, D’Angeli S, Altamura MM, Costantino P, Trovato M. (2008) Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol Biol 66: 277–288 [DOI] [PubMed] [Google Scholar]
- Mayer RR, Cherry JH, Rhodes D. (1990) Effects of heat shock on amino acid metabolism of cowpea cells. Plant Physiol 94: 796–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Honig A, Stein H, Suzuki N, Mittler R, Zilberstein A. (2009) Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J Biol Chem 284: 26482–26492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari HBC, Marur CJ, Daros E, de Campos MKF, de Carvalho JFRP, Bespalhok JC, Pereira LFP, Vieira LGE. (2007) Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant 130: 218–229 [Google Scholar]
- Mwange KN, Hou HW, Cui KM. (2003) Relationship between endogenous indole-3-acetic acid and abscisic acid changes and bark recovery in Eucommia ulmoides Oliv. after girdling. J Exp Bot 54: 1899–1907 [DOI] [PubMed] [Google Scholar]
- Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. (1999a) Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett 461: 205–210 [DOI] [PubMed] [Google Scholar]
- Nanjo T, Kobayashi M, Yoshiba Y, Sanada Y, Wada K, Tsukaya H, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. (1999b) Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J 18: 185–193 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA. (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96: 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV. (1992) Cellular detoxifying mechanisms determine the age-dependent injury in tropical trees exposed to SO2. J Plant Physiol 140: 733–740 [Google Scholar]
- Rizhsky L, Liang HJ, Shuman J, Shulaev V, Davletova S, Mittler R. (2004) When defense pathways collide: the response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134: 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosens NH, Thu TT, Iskandar HM, Jacobs M. (1998) Isolation of the ornithine-Δ-aminotransferase cDNA and effect of salt stress on its expression in Arabidopsis thaliana. Plant Physiol 117: 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Saradhi PP, Alia, Arora S, Prasad KV. (1995) Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem Biophys Res Commun 209: 1–5 [DOI] [PubMed] [Google Scholar]
- Sun W, Bernard C, van de Cotte B, Van Montagu M, Verbruggen N. (2001) At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J 27: 407–415 [DOI] [PubMed] [Google Scholar]
- Szabados L, Savouré A. (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15: 89–97 [DOI] [PubMed] [Google Scholar]
- Székely G, Abrahám E, Cséplo A, Rigó G, Zsigmond L, Csiszár J, Ayaydin F, Strizhov N, Jásik J, Schmelzer E, et al. (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53: 11–28 [DOI] [PubMed] [Google Scholar]
- Trovato M, Mattioli R, Costantino P. (2008) Multiple roles of proline in plant stress tolerance and development. Rend Lincei-Sci Fis 19: 325–346 [Google Scholar]
- Verbruggen N, Hermans C. (2008) Proline accumulation in plants: a review. Amino Acids 35: 753–759 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hua XJ, May M, Van Montagu M. (1996) Environmental and developmental signals modulate proline homeostasis: evidence for a negative transcriptional regulator. Proc Natl Acad Sci USA 93: 8787–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Villarroel R, Van Montagu M. (1993) Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiol 103: 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FJ, Zeng B, Sun ZX, Zhu C. (2009) Relationship between proline and Hg2+-induced oxidative stress in a tolerant rice mutant. Arch Environ Contam Toxicol 56: 723–731 [DOI] [PubMed] [Google Scholar]
- Wang NN, Shih MC, Li N. (2005) The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J Exp Bot 56: 909–920 [DOI] [PubMed] [Google Scholar]
- Wu GL, Cui J, Tao L, Yang H. (2009) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 19: 124–132 [DOI] [PubMed] [Google Scholar]
- Yoshiba Y, Kiyosue T, Katagiri T, Ueda H, Mizoguchi T, Yamaguchi-Shinozaki K, Wada K, Harada Y, Shinozaki K. (1995) Correlation between the induction of a gene for delta 1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J 7: 751–760 [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhou RG, Gao YJ, Zheng SZ, Xu P, Zhang SQ, Sun DY. (2009a) Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol 149: 1773–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wollenweber B, Jiang D, Liu FL, Zhao J. (2008) Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J Exp Bot 59: 839–848 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Quan R, Wang XC, Huang R. (2009b) Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150: 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli L, Hou BH, Tsai CH, Jakab G, Mauch-Mani B, Somerville S. (2008) The xenobiotic beta-aminobutyric acid enhances Arabidopsis thermotolerance. Plant J 53: 144–156 [DOI] [PubMed] [Google Scholar]