Abstract
Plants orient their growth depending on directional stimuli such as light and gravity, in a process known as tropic response. Tropisms result from asymmetrical accumulation of auxin across the responding organ relative to the direction of the stimulus, which causes differential growth rates on both sides of the organ. Here, we show that gibberellins (GAs) attenuate the gravitropic reorientation of stimulated hypocotyls of dark-grown Arabidopsis (Arabidopsis thaliana) seedlings. We show that the modulation occurs through induction of the expression of the negative regulator of auxin signaling INDOLE-3-ACETIC ACID INDUCIBLE19/MASSUGU2. The biological significance of this regulatory mechanism involving GAs and auxin seems to be the maintenance of a high degree of flexibility in tropic responses. This notion is further supported by observations that GA-deficient seedlings showed a much lower variance in the response to gravity compared to wild-type seedlings and that the attenuation of gravitropism by GAs resulted in an increased phototropic response. This suggests that the interplay between auxin and GAs may be particularly important for plant orientation under competing tropic stimuli.
One hundred thirty years ago, Darwin described that plants can sense their environment and orient themselves for optimal growth and development (Darwin, 1880). Among the signals that promote a tropic response in plants, gravity is unique in that it is constant and unidirectional. In addition, it generally induces the underground tissues to bend toward the signal, and the aerial parts against the stimulating vector. Like in other tropisms, when plants perceive a change in their position relative to the gravity vector, they respond by differential growth on either side of the affected organ (Esmon et al., 2005), and several hormones have been involved in the control of these responses. Among them, auxin is instrumental because it forms a lateral gradient in response to the stimulus and thus establishes the framework for differential growth (Rashotte et al., 2000; Esmon et al., 2006). The differential response to auxin on either side of an organ has been shown to depend on the correct functioning of polar auxin transport and activity of auxin efflux carriers (Friml et al., 2002), and also on the activity of specific Aux/IAA and AUXIN RESPONSE FACTOR (ARF) transcriptional regulators (Harper et al., 2000; Tatematsu et al., 2004). Moreover, brassinosteroids have been proposed to enhance tropic reorientation by facilitating polar auxin transport (Meudt, 1987; Li et al., 2005; Kim et al., 2007).
GAs are also known to promote cell expansion (Cowling and Harberd, 1999). The molecular mechanism of GA signaling proceeds through GA-induced degradation of repressor proteins of the DELLA family by the proteasome, thereby activating transcription of growth-promoting genes (Schwechheimer, 2008). Given that GAs regulate growth, sometimes as a subsidiary signal of auxin action (Frigerio et al., 2006), the obvious hypothesis is that GAs would mediate the promotion of differential growth during gravitropic reorientation. However, here we show molecular evidence for a different role of GAs on gravitropism through the attenuation of auxin responsiveness, that results in an increased ability to modulate growth under competing tropic signals.
RESULTS
GA Deficiency Enhances Gravitropic Reorientation
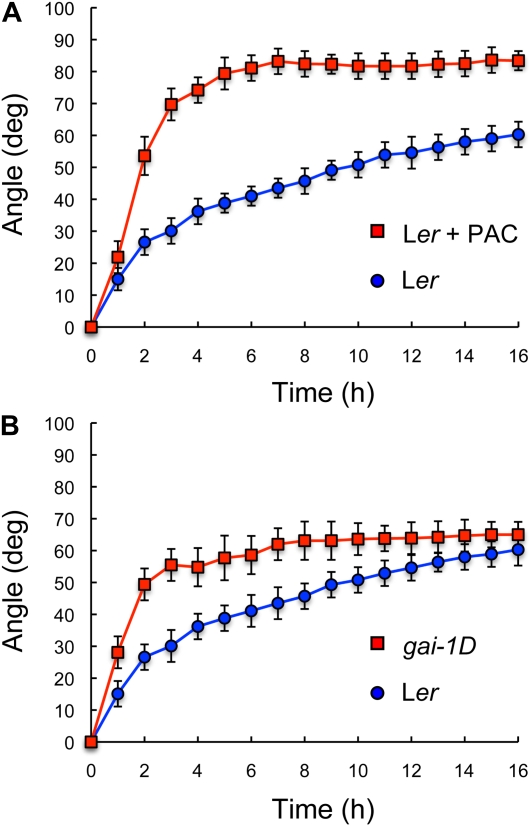
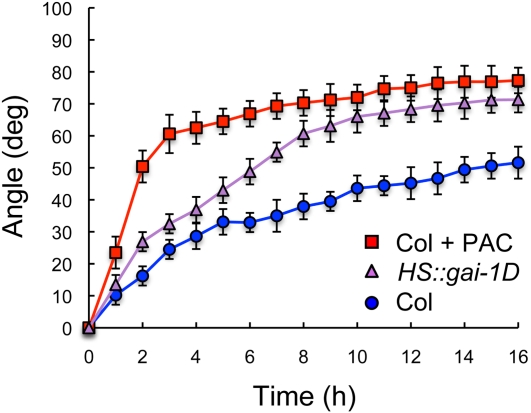
To test if GAs are necessary for the promotion of the differential cell expansion that underlies a tropic response, we examined the response of Arabidopsis (Arabidopsis thaliana) etiolated hypocotyls to a gravitropic stimulus, under GA-limiting conditions. Surprisingly, paclobutrazol (PAC)-induced deficiency in GA biosynthesis not only did not impair gravitropic reorientation but, on the contrary, the hypocotyls of GA-deficient seedlings displayed an enhanced response to the gravitropic stimulus and a faster reorientation (Fig. 1A). This effect was observed at a low PAC concentration that did not inhibit seed germination, and was fully reverted by GA3 application, demonstrating the specificity of the inhibitor. Moreover, an enhanced rate of response was also evident in the gai-1D and rga-Δ17 mutants, which express dominant versions of the DELLA proteins GIBBERELLIN INSENSITIVE (GAI) and REPRESSOR OF GA1 (RGA), respectively, that constitutively block GA-induced growth (Peng et al., 1997; Dill et al., 2001; Fig. 1B; Supplemental Fig. S1). Since GA deficiency also causes dwarfism, it is possible that the enhanced gravitropic response was due to an intrinsic capacity of smaller seedlings to display differential growth and bending. However, this is not the case, because transient induction of the dominant allele gai-1D increased the response to a gravitropic stimulus without affecting the size of the seedlings (Alabadí et al., 2008; Fig. 2). Therefore, we conclude that GAs attenuate the gravitropic response in aerial tissues, and that this regulation is likely a direct consequence of DELLA activity.
Figure 1.
Gravitropic reorientation of hypocotyls of GA-deficient seedlings. Seedlings derived from Ler and Col accessions were grown for 3 d in darkness, and the plates were turned 90° at time 0. Reorientation of the hypocotyls was monitored in darkness as explained in “Materials and Methods.” When used, PAC concentration was 0.4 μm. Error bars represent sd (n > 16 individual seedlings). The effect of PAC and gai-1D with respect to the wild type was statistically significant (P < 0.05) as indicated by a two-way ANOVA test. [See online article for color version of this figure.]
Figure 2.
Gravitropic reorientation after transient induction of gai-1D. Col and HS::gai-1D seedlings were grown for 3 d in darkness and subject to a 30-min heat shock at 37°C before turning the plates 90°. Reorientation of the hypocotyls was monitored in darkness as explained in “Materials and Methods.” When used, PAC concentration was 0.4 μm. Error bars represent sd (n > 16 individual seedlings). The effect of PAC and HS::gai-1D with respect to the wild type was statistically significant (P < 0.05) as indicated by a two-way ANOVA test. [See online article for color version of this figure.]
Expression of IAA19/MSG2 Is Repressed by DELLA Proteins
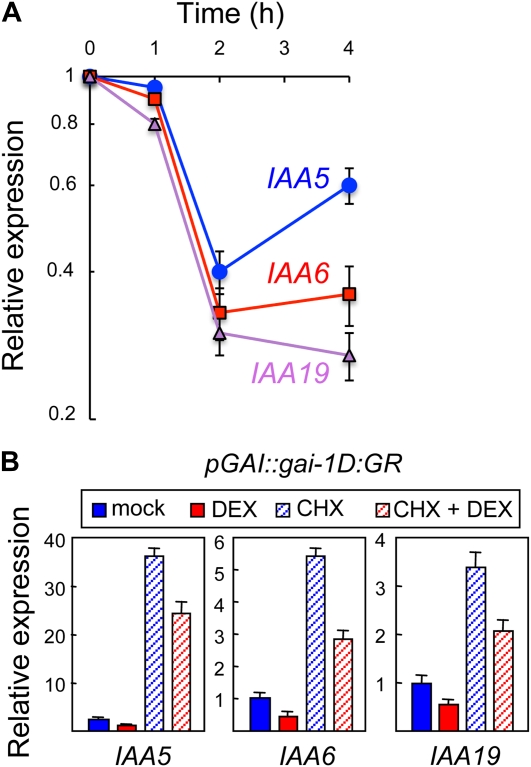
DELLA proteins regulate gene expression in response to GAs (Zentella et al., 2007; de Lucas et al., 2008; Feng et al., 2008). Thus, to elucidate the molecular mechanism that underlies the regulation of gravitropism by GAs, we investigated by microarray analysis the transcriptional changes associated with transient expression of gai-1D in 2-d-old dark-grown seedlings, i.e. under conditions where it promotes gravitropism (Fig. 2). To achieve transient expression of gai-1D, we used a transgenic line harboring the gai-1D gene under the control of a heat-shock inducible promoter (Alabadí et al., 2008). Incubation of 3-d-old etiolated seedlings at 37°C for 30 min led to altered expression of around 150 genes 4 h after the inductive stimulus when compared with wild-type seedlings subject to the same treatment (J. Gallego-Bartolomé, D. Alabadí, and M.A. Blázquez, unpublished data). Remarkably, expression of INDOLE-3-ACETIC ACID INDUCIBLE19/MASSUGU2 (IAA19/MSG2), which encodes a member of the Aux/IAA family of proteins that negatively regulate auxin signaling (Tatematsu et al., 2004; Overvoorde et al., 2005), was steadily down-regulated (Fig. 3A). The two closest paralogs of IAA19/MSG2, IAA5, and IAA6/SHORT HYPOCOTYL1 (IAA6/SHY1), were also repressed following gai-1D induction (Fig. 3A), and the expression of all these genes was consistently lower in dark-grown seedlings of the gai-1D and rga-Δ17 mutants, or in the presence of 1 μm PAC (Supplemental Fig. S2).
Figure 3.
Regulation of Aux/IAA gene expression by DELLA proteins. A, Expression of IAA5, IAA6, and IAA19 determined by reverse transcription quantitative PCR in 3-d-old dark-grown HS::gai-1D seedlings after a 30-min heat shock at 37°C. Values at each time point are relative to the expression of each gene in seedlings not subject to heat shock. Error bars represent sem (n = 3 biological replicates). B, Expression of IAA5, IAA6, and IAA19 determined by reverse transcription quantitative PCR in 3-d-old dark-grown seedlings expressing a fusion between gai-1D and the glucocorticoid receptor (GR) under the control of the GAI promoter. Three-day-old dark-grown seedlings were transferred into flasks and incubated with soft shaking for 6 h with a mock solution or with 10 μm dexamethasone (DEX) to allow gai-1D:GR moving to the nucleus and regulate target genes. Alternatively, seedlings were also incubated with 10 μm cycloheximide (CHX) to prevent de novo protein synthesis during mock or DEX treatments. Values are relative to the expression of each gene in mock-treated seedlings. Error bars represent sem (n = 3 biological replicates).
To determine if the regulation of these genes by GAI was direct, we constructed a glucocorticoid-inducible version of gai-1D by fusing it to the rat glucocorticoid receptor domain (GR; Lloyd et al., 1994; Aoyama and Chua, 1997), under the control of the GAI promoter. As expected, induction of gai-1D translocation into the nucleus by dexamethasone application caused repression of IAA19/MSG2, IAA5, and IAA6/SHY1, and this repression was not blocked by cycloheximide (Fig. 3B).
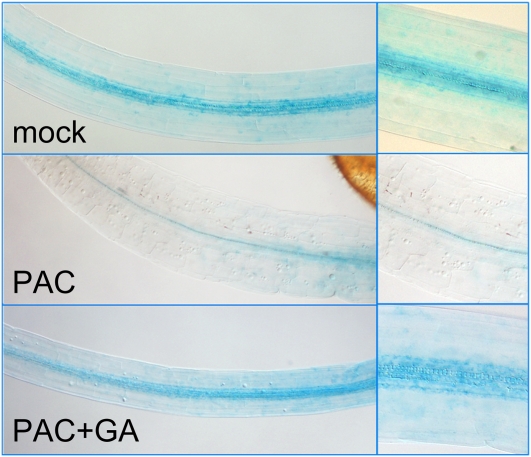
The repression of IAA19/MSG2 expression upon GA deficiency was also evident in etiolated seedlings harboring a transcriptional fusion between the IAA19/MSG2 promoter and the GUS reporter gene (Fig. 4). Higher expression levels were detected, as previously reported (Tatematsu et al., 2004), in the apical part of dark-grown seedlings, and the level of expression decreased upon PAC application in a dose-dependent manner (Supplemental Fig. S3).
Figure 4.
Transcriptional regulation of MSG2/IAA19 by GA in etiolated seedlings during gravitropic reorientation. GUS staining in 3-d-old dark-grown seedlings was done as indicated in “Materials and Methods.” PAC concentration was 1 μm. GA3 concentration was 10 μm. Scale bars represent 0.5 mm.
IAA19/MSG2 Mediates the Regulation of Gravitropism by GAs
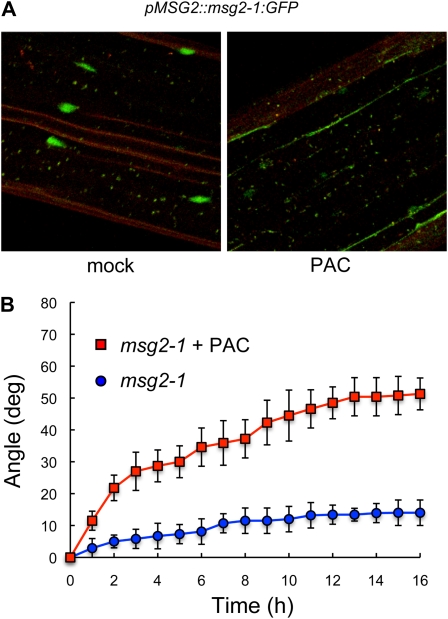
The observation that IAA19/MSG2 is a direct target for GAI transcriptional regulation provides a likely mechanism for the attenuation of gravitropism by GAs. The dominant msg2-1 mutation that prevents IAA19/MSG2 destabilization by auxin has been shown to impair gravitropic responses presumably by blocking ARF activity (Tatematsu et al., 2004). Thus, to investigate the degree of involvement of IAA19/MSG2 in the repression of the gravitropic response by GAs, we asked whether DELLA accumulation would alleviate the agravitropic phenotype of the seedlings that carry the hyperstable allele msg2-1. In agreement with our observation that PAC decreases the activity of the IAA19/MSG2 promoter (Fig. 4; Supplemental Fig. S3), growth of etiolated seedlings in the presence of 0.4 μm PAC prevented the accumulation in the nuclei of msg2-1:GFP protein expressed from the IAA19/MSG2 promoter (Muto et al., 2007; Fig. 5A). And, concurrently, this treatment restored an almost normal reorientation capacity to msg2-1 mutant seedlings (Fig. 5B). We thus conclude that GAs modulate gravitropism at least partly through the transcriptional regulation of IAA19/MSG2 expression.
Figure 5.
Involvement of IAA19/MSG2 in the regulation of gravitropism by GA. A, Reduction of IAA19/Msg2-1 protein levels caused by impairment of GA biosynthesis. A pMSG2::msg2-1:GFP transgenic line was grown for 3 d in darkness with and without 0.4 μm PAC, and GFP fluorescence in hypocotyls was visualized under a confocal microscope. B, Gravitropic reorientation of hypocotyls of msg2-1 seedlings. Seedlings were grown for 3 d in darkness on control media or in media supplemented with 0.4 μm PAC, and the plates were turned 90° at time 0. Reorientation of the hypocotyls was monitored in darkness as explained in “Materials and Methods.” Error bars represent sd (n > 16 individual seedlings).
Physiological Relevance of the Regulation of Gravitropism by GAs
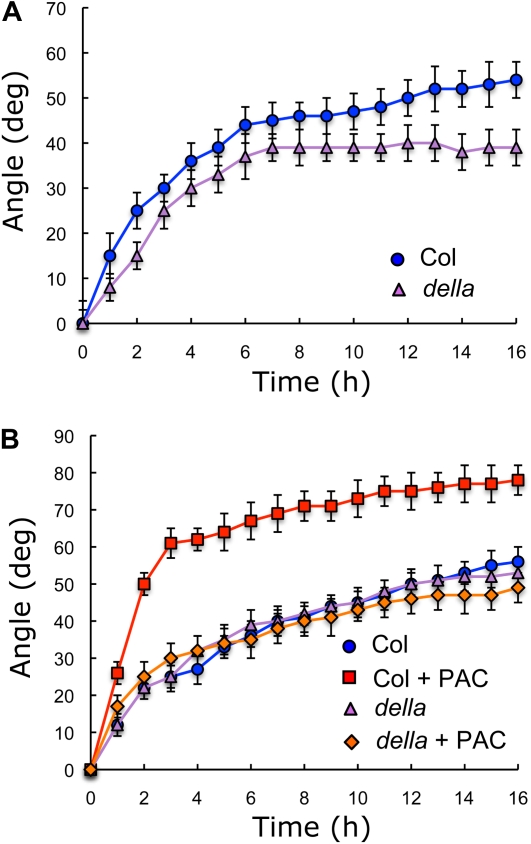
The results shown here indicate that GAs—and hence the level of DELLA proteins—influence the gravitropic response of aerial tissues, but what is the physiological relevance of this regulation? Can changes in DELLA abundance within the physiological range still affect gravitropism? Indeed, seedlings of the quadruple DELLA KO mutant did suffer a delay in the gravitropic response when grown in the light (Fig. 6A), a situation that causes DELLA accumulation in the wild type (Achard et al., 2007), confirming that the ability to respond to gravistimulation directly depends on the relative concentration of DELLA proteins. In agreement with this, a quadruple DELLA KO mutant did not show any difference, compared to the wild type, in the speed or extent of reorientation after gravistimulation in darkness, when GAs are not in limiting concentrations (Fig. 6B).
Figure 6.
Gravitropic reorientation of hypocotyls of quadruple della (gai rga rgl1 rgl2) knockout mutants. Seedlings were grown for 3 d in darkness on Murashige and Skoog, and the plates were turned 90° at time 0. In A, seedlings were exposed to 150 μmol m−2 s−1 of white light during 8 h prior to reorientation, while in B, dark-grown seedlings were used. All values between 2 and 16 h in A are statistically different (P < 0.01) between the della mutant and the parental control. PAC concentration was 0.4 μm. Error bars represent sd (n > 46 individual seedlings). The behavior of the della mutant was statistically significant (P < 0.05) with respect to the wild type as indicated by a two-way ANOVA test. [See online article for color version of this figure.]
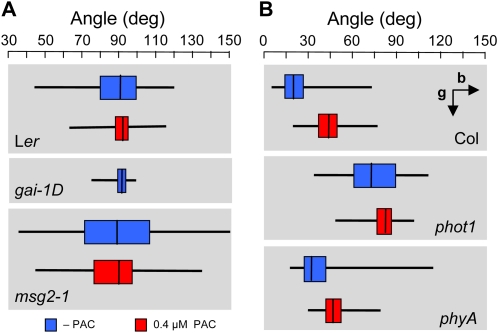
However, a more critical consequence of the attenuation of auxin responsiveness by GAs is the increase in variance of the gravitropic response when large populations of Arabidopsis seedlings were examined right after germination. As shown in Figure 7A, individual seedlings grown for 3 d in darkness displayed certain degree of inclination (between 5° and 10°) with respect to the gravity vector under normal GA concentrations, but this variation practically disappeared when GA biosynthesis or GA signaling were compromised (with PAC, or in the gai-1D mutant). The mechanism by which GAs cause this variance is very likely equivalent to the one through which GAs control gravitropic reorientation (i.e. through IAA19/MSG2), given that msg2-1 mutants showed a much larger variance compared to the wild type, which was consequently reduced by PAC (Fig. 7A).
Figure 7.
Physiological relevance of the regulation of gravitropism by GAs. A, Distribution of hypocotyl orientation of 3-d-old seedlings in darkness in the presence and absence of PAC. Seedlings were grown on vertical Murashige and Skoog plates and the angle with respect to a horizontal line was recorded, with 90° being perfectly vertical. B, Distribution of hypocotyl orientation of 3-d-old seedlings under continuous unilateral blue light (10 nmol m−2 s−1), coming from the left as indicated by the arrow. The angle with respect to a horizontal line was recorded, with 90° being perfectly vertical. Boxes represent the middle quartiles around the median (bisecting line), while the whiskers represent the upper and lower quartiles. The data represent the values of 65 to 130 seedlings.
To study whether the increase in variance of the gravitropic response caused by GAs could confer any adaptive advantage, we examined the behavior of seedling populations grown under two competing signals: a light source perpendicular to the gravity vector (i.e. photo- versus gravitropism). As shown in Figure 7B, the increased gravitropic response caused by the presence of PAC, led to a reduction in phototropic orientation in wild-type seedlings. This effect was also observed when phototropic reorientation was monitored after illumination of dark-grown seedlings with lateral blue light (Supplemental Fig. S4). On the contrary, the aphototropic phototropin1 (phot1) mutant, impaired in the main light receptor that regulates phototropism (Esmon et al., 2005), still responded to PAC with a severe increase in gravitropic response, arguing against the direct regulation of phototropism by GAs (Tsuchida-Mayama et al., 2010). Competition between gravi- and phototropism has been proposed to be mediated by the phytochrome A (phyA) photoreceptor (Lariguet and Fankhauser, 2004; Whippo and Hangarter, 2004; Iino, 2006). Interestingly, the phyA mutant shows reduced response to PAC (Fig. 7B), suggesting a connection between GA action and the regulation of gravitropism by phyA. These observations highlight a specific role of GA-induced regulation of gravitropic response in a situation of competing environmental signals.
DISCUSSION
The work presented here reveals an unexpected role for GAs in the control of the response of plants to gravity, and highlights the physiological relevance of a novel interaction between DELLA proteins and the expression of Aux/IAA genes.
The main line of evidence that supports the relevance of IAA19/MSG2 in the control of gravitropism by GAs is the observation that the agravitropic phenotype caused by the dominant msg2-1 allele was alleviated by inhibiting GA biosynthesis (Fig. 5B). This result at least indicates that transcriptional regulation of IAA19/MSG2 by GAs has a significant impact in the gravitropic response of etiolated seedlings, and rules out the hypothesis that the cells on the lower side of a reorienting hypocotyl simply respond proportionally better to auxin if cells on both sides are smaller due to GA deficiency. Our work also shows that the regulation of IAA19/MSG2 expression by DELLA proteins does not require protein synthesis (Fig. 3B). Since DELLA proteins do not bind DNA directly, it is highly likely that they regulate transcription of IAA9/MSG2 through the interaction with other transcription factors. Two such mechanisms have been proposed so far: the inhibition of DNA binding of members of the PHYTOCHROME-INTERACTING FACTOR (PIF) family of bHLH transcription factors (de Lucas et al., 2008; Feng et al., 2008), and the inhibitory interaction with JASMONATE-ZIM-DOMAIN proteins (Hou et al., 2010), which in turn regulate MYC2 activity in jasmonic acid signaling. There are no indications that jasmonic acid signaling is involved in gravitropic responses, but it is reasonable to think that DELLA proteins interact with PIFs already present at high levels in dark-grown seedlings to directly regulate the expression of the target Aux/IAA genes. In agreement with this model, the expression of IAA19 is strongly reduced in etiolated pifq mutants (Leivar et al., 2009), although there is no experimental evidence to date showing direct interaction between any PIF transcription factor and the IAA19 promoter.
A critical issue in the control of the gravitropic response is the spatial localization of the machinery that perceives gravity and directs reorientation. Starch-loaded amyloplasts have been shown to be an integral part of the mechanism that allows gravity perception (Boonsirichai et al., 2003), and cells accumulating amyloplasts are located in the tip of the roots and in the endodermis of aerial tissues, such as the hypocotyl. Given that GAs affect gravitropic responses in hypocotyls, it is tempting to suggest that GA signaling interferes with the early events after gravity perception in the endodermis, but this hypothesis requires additional experimental evidence.
In any case, our observation that GAs regulate the gravitropic response at an early stage does not diminish the role of the auxin gradient as the driving force in the orientation of the plant with respect to the gravity vector. Rather, GAs would act by fine tuning the formation of this gradient and modulating the responsiveness to this gradient in auxin-responding cells. However, we cannot rule out that GAs also affect auxin relocalization, therefore establishing a reinforcing mechanism.
Interestingly, our results do not support an important role of GAs in the execution of cell expansion during gravitropic reorientation, but highlight a function of GAs in the generation of variance in the response. A higher degree of variance or noise in biological responses is a trait often selected by nature, and proposed to be at the core of the mechanisms that drive robust morphogenesis (Houchmandzadeh et al., 2002; Yucel and Small, 2006) and speciation (Braendle and Félix, 2008). In microorganisms, even adaptation of a population to the environment seems to be based, to some extent, on cell-to-cell variance within genetic circuits (Balaban et al., 2004; Sánchez and Kondev, 2008). Not many molecular mechanisms have been proposed that explain the generation of noise in cellular systems (Casal et al., 2004), and the attenuation of auxin response by GAs might represent one of such mechanisms to provide flexibility in situations under which plants face competing tropic signals. Such could be the case of plants that must optimize their access to light because of neighbors’ proximity. The observation that DELLA protein concentration decreases in seedlings during the shade avoidance response (Djakovic-Petrovic et al., 2007) supports this scenario. In that case, part of the shade avoidance mechanism may involve the attenuation of gravitropism to allow bending against the gravity vector.
MATERIALS AND METHODS
Plants and Growth Conditions
Arabidopsis (Arabidopsis thaliana) GA signaling dominant mutants gai-1D and rga-Δ17, and the quadruple loss-of-function rga-24 gai-t6 rgl2-1 rgl1-1 (Cheng et al., 2004) are in the Landsberg erecta (Ler) background, while the other lines used in this work, such as msg2-1 (Tatematsu et al., 2004), phot1 (Huala et al., 1997), phyA (Nagatani et al., 1993), HS::gai-1D (Alabadí et al., 2008), pMSG2::GUS (Tatematsu et al., 2004), and pMSG2::msg2-1:GFP (Muto et al., 2007), are derived from Columbia-0 (Col-0) accession. For all experiments, seeds were surface sterilized and stratified for 4 to 7 d at 4°C in darkness. Germination was induced under continuous white fluorescent light (90–100 μmol m−2 s−1) for 8 h, and then the plates were kept in darkness at 22°C.
Construction of Transgenic Lines
To obtain the transgenic line pGAI::gai-1D:GR, the gai-1D coding sequence was amplified from genomic DNA of the gai-1D mutant with primers: GAId-BamHI-F (GGA TCC ATG AAG AGA GAT CAT CAT CAT CA) and GAId-SacI-R (GAG CTC ATT GGT GGA GAG TTT CCA AGC CGA) and cloned into the pCR2.1 vector (Invitrogen), and the BamHI-SacI fragment was subcloned into pGreen0029-35S::GR (Hellens et al., 2000) to give rise to pG35::gai-1D:GR. The GAI promoter was PCR amplified from genomic DNA using oligos pGAI-KpnI-F (GGT ACC TGG GAC CAC AGT CTA AAT GGC GT) and pGAI-XbaI-R (TCT AGA GGT TGG TTT TTT TTC AGA GAT GGA), cloned into the pCR2.1 vector, and the KpnI-XbaI fragment transferred into pG35::gai-1D:GR to construct pGAI::gai-1D:GR. Agrobacterium C58 pSOUP cells were transformed with pGAI::gai1-GR and Arabidopsis Ler plants were transformed using the floral-dip method (Clough and Bent, 1998). Transgenic seedlings with a 3:1 segregation ratio for kanamycin resistance conferred by the transgene marker were selected for further work.
Gene Expression Analysis
Total RNA extraction, cDNA synthesis, and quantitative PCR were carried out as described previously (Frigerio et al., 2006) using EF1-α expression for normalization. The primers used were: IAA19 (CTC GGG CTT GAG ATA ACG GA and CCA CAT CTC TCC CCG GAA), IAA5 (AAC TAC GGC TAG GTC TTC CCG and AGA TGG ACT CAC CGG AGA CG), and IAA6 (TGG CAA AGG AAG GTC TAG CAC and TGG AAG ACC CAA TCG AAG CT).
GUS staining was carried out as described previously (Frigerio et al., 2006).
Detailed description of the microarray experiment will be published somewhere else. Briefly, HS::gai-1D and Col-0 seedlings were grown for 3 d at 22°C in darkness on half-strength Murashige and Skoog medium (Duchefa) with 0.8% (w/v) agar and 1% (w/v) Suc, and then the plates were transferred to 37°C for 30 min. Samples were collected before, and 1, 2, and 4 h after the beginning of the heat treatment. Three independent biological replicates were used for the analysis. RNA amplification, labeling, and hybridization of microarray slides (70-mer oligonucleotide arrays that represent the majority of the Arabidopsis genes: http://www.ag.arizona.edu/microarray) were carried out as previously described (Bueso et al., 2007). Hybridization was set up to isolate genes differentially expressed in HS::gai-1D compared to Col-0 at each time point.
Tropism Tests
To determine the angle of seedling emergence, seedlings were grown for 3 d in darkness at 22°C in a vertical orientation on plates containing half-strength Murashige and Skoog medium (Duchefa) with 0.8% (w/v) phytoagar and without Suc, and supplemented with mock or 0.4 μm PAC. After 3 d the plates were photographed.
For reorientation experiments, the conditions were the same as above but after 3 d of growth the plates were reoriented 90° relative to the initial growth angle. Reorientation was recorded every hour under infrared light using CCD cameras coupled to Metamorph software as described by Schepens et al. (2008).
To test blue-light-induced phototropism, seedlings were grown for 3 d under 10 nmol m−2 s−1 of continuous unilateral blue light at 22°C in a vertical orientation on plates containing half-strength Murashige and Skoog medium (Duchefa) with 0.8% (w/v) phytoagar and without Suc and supplemented with mock or 0.4 μm PAC. After 3 d, the plates were photographed.
In all cases, angles were measured using Image J software.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Gravitropic reorientation of rga-Δ17 seedlings.
Supplemental Figure S2. Repression of IAA genes in DELLA mutants.
Supplemental Figure S3. MSG2/IAA19 expression in etiolated seedlings.
Supplemental Figure S4. Effect of GA deficiency on phototropic reorientation.
Acknowledgments
We thank Kiyosi Tatematsu (National Institute of Agrobiological Sciences, Ibaraki, Japan) and the Arabidopsis Biological Resource Center for providing us with mutant and transgenic lines. We also thank François Parcy (Laboratoire de Physiologie Cellulaire Végétale, CNRS, Grenoble, France) for fruitful discussions and hosting M.A.B., and Cristina Ferrándiz (Instituto de Biología Molecular y Celular de Plantas, Valencia, Spain) and Detlef Weigel (Max Planck Institute for Developmental Biology, Tübingen, Germany) for very useful comments on the manuscript.
References
- Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, Harberd NP. (2007) DELLAs contribute to plant photomorphogenesis. Plant Physiol 143: 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabadí D, Gallego-Bartolomé J, Orlando L, García-Cárcel L, Rubio V, Martínez C, Frigerio M, Iglesias-Pedraz JM, Espinosa A, Deng XW, et al. (2008) Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J 53: 324–335 [DOI] [PubMed] [Google Scholar]
- Aoyama T, Chua NH. (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11: 605–612 [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. (2004) Bacterial persistence as a phenotypic switch. Science 305: 1622–1625 [DOI] [PubMed] [Google Scholar]
- Boonsirichai K, Sedbrook JC, Chen R, Gilroy S, Masson PH. (2003) ALTERED RESPONSE TO GRAVITY is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes. Plant Cell 15: 2612–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braendle C, Félix MA. (2008) Plasticity and errors of a robust developmental system in different environments. Dev Cell 15: 714–724 [DOI] [PubMed] [Google Scholar]
- Bueso E, Alejandro S, Carbonell P, Perez-Amador MA, Fayos J, Bellés JM, Rodriguez PL, Serrano R. (2007) The lithium tolerance of the Arabidopsis cat2 mutant reveals a cross-talk between oxidative stress and ethylene. Plant J 52: 1052–1065 [DOI] [PubMed] [Google Scholar]
- Casal JJ, Fankhauser C, Coupland G, Blázquez MA. (2004) Signalling for developmental plasticity. Trends Plant Sci 9: 309–314 [DOI] [PubMed] [Google Scholar]
- Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J. (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cowling RJ, Harberd NP. (1999) Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J Exp Bot 50: 1351–1357 [Google Scholar]
- Darwin C. (1880) The Power of Movement in Plants. John Murray, London [Google Scholar]
- de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S. (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484 [DOI] [PubMed] [Google Scholar]
- Dill A, Jung HS, Sun TP. (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakovic-Petrovic T, de Wit M, Voesenek LA, Pierik R. (2007) DELLA protein function in growth responses to canopy signals. Plant J 51: 117–126 [DOI] [PubMed] [Google Scholar]
- Esmon CA, Pedmale UV, Liscum E. (2005) Plant tropisms: providing the power of movement to a sessile organism. Int J Dev Biol 49: 665–674 [DOI] [PubMed] [Google Scholar]
- Esmon CA, Tinsley AG, Ljung K, Sandberg G, Hearne LB, Liscum E. (2006) A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc Natl Acad Sci USA 103: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, et al. (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio M, Alabadí D, Pérez-Gómez J, García-Cárcel L, Phillips AL, Hedden P, Blázquez MA. (2006) Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol 142: 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E. (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Hou X, Lee LY, Xia K, Yan Y, Yu H. (2010) DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell 19: 884–894 [DOI] [PubMed] [Google Scholar]
- Houchmandzadeh B, Wieschaus E, Leibler S. (2002) Establishment of developmental precision and proportions in the early Drosophila embryo. Nature 415: 798–802 [DOI] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR. (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Iino M. (2006) Toward understanding the ecological functions of tropisms: interactions among and effects of light on tropisms. Curr Opin Plant Biol 9: 89–93 [DOI] [PubMed] [Google Scholar]
- Kim TW, Lee SM, Joo SH, Yun HS, Lee Y, Kaufman PB, Kirakosyan A, Kim SH, Nam KH, Lee JS, et al. (2007) Elongation and gravitropic responses of Arabidopsis roots are regulated by brassinolide and IAA. Plant Cell Environ 30: 679–689 [DOI] [PubMed] [Google Scholar]
- Lariguet P, Fankhauser C. (2004) Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism. Plant J 40: 826–834 [DOI] [PubMed] [Google Scholar]
- Leivar P, Tepperman JM, Monte E, Calderon RH, Liu TL, Quail PH. (2009) Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21: 3535–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xu J, Xu ZH, Xue HW. (2005) Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell 17: 2738–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AM, Schena M, Walbot V, Davis RW. (1994) Epidermal cell fate determination in Arabidopsis: patterns defined by a steroid-inducible regulator. Science 266: 436–439 [DOI] [PubMed] [Google Scholar]
- Meudt WJ. (1987) Investigations on the mechanism of the brassinosteroid response: VI. Effect of brassinolide on gravitropism of bean hypocotyls. Plant Physiol 83: 195–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto H, Watahiki MK, Nakamoto D, Kinjo M, Yamamoto KT. (2007) Specificity and similarity of functions of the Aux/IAA genes in auxin signaling of Arabidopsis revealed by promoter-exchange experiments among MSG2/IAA19, AXR2/IAA7, and SLR/IAA14. Plant Physiol 144: 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. (1993) Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol 102: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde PJ, Okushima Y, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Liu A, Onodera C, Quach H, et al. (2005) Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 17: 3282–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK. (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A, Kondev J. (2008) Transcriptional control of noise in gene expression. Proc Natl Acad Sci USA 105: 5081–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens I, Boccalandro HE, Kami C, Casal JJ, Fankhauser C. (2008) PHYTOCHROME KINASE SUBSTRATE4 modulates phytochrome-mediated control of hypocotyl growth orientation. Plant Physiol 147: 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C. (2008) Understanding gibberellic acid signaling—are we there yet? Curr Opin Plant Biol 11: 9–15 [DOI] [PubMed] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT. (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida-Mayama T, Sakai T, Hanada A, Uehara Y, Asami T, Yamaguchi S. (2010) Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J 62: 653–662 [DOI] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP. (2004) Phytochrome modulation of blue-light phototropism. Plant Cell Environ 27: 1223–1228 [Google Scholar]
- Yucel G, Small S. (2006) Morphogens: precise outputs from a variable gradient. Curr Biol 16: R29–R31 [DOI] [PubMed] [Google Scholar]
- Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, et al. (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19: 3037–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]