Abstract
Purpose
Sunitinib is an oral multi-targeted receptor tyrosine kinase inhibitor. The purpose of this study was to determine the recommended phase 2 dose, pharmacokinetics, pharmacodynamic effects, and preliminary anti-tumor activity of sunitinib in a pediatric population.
Experimental Design
Patients 2-21 years of age with refractory solid tumors were eligible if they had measurable or evaluable disease and met baseline organ function requirements. Patients received sunitinib once daily for 28 days followed by a 14-day break between each cycle. Dose levels of 15 and 20 mg/m2/day were evaluated, with dose escalation based on a 3+3 design. Sunitinib pharmacokinetics and biomarkers of angiogenesis were also evaluated during the first cycle.
Results
Twenty-three patients were treated (median age 13.9 years; range, 3.9 – 20.6 years). The most common toxicities were neutropenia, thrombocytopenia, elevated liver transaminases, gastrointestinal symptoms, and fatigue. Two patients developed dose-limiting reductions in cardiac ejection fraction prompting a protocol amendment to exclude patients with prior exposure to anthracyclines or cardiac radiation. In patients without these cardiac risk factors, the maximum tolerated dose was 15 mg/m2/day. Steady-state plasma concentrations were reached by day 7. No objective responses were observed. Four patients with sarcoma and glioma had stable disease for 2 - 9 cycles.
Conclusions
Cardiac toxicity precluded determination of a recommended dose for pediatric patients with prior anthracycline or cardiac radiation exposure. The maximum tolerated dose of sunitinib for patients without risk factors for cardiac toxicity is 15 mg/m2/day for 28 days followed by a 14-day break.
Keywords: sunitinib, pediatric, pharmacokinetics, angiogenesis, VEGF
Introduction
Sunitinib is an oral small molecule multi-targeted receptor tyrosine kinase inhibitor that inhibits vascular endothelial growth factor receptors (VEGFR), platelet derived growth factor receptors (PDGFR), c-KIT, Flt3, CSF-1 receptor, and RET (1-3). Sunitinib has been studied in adults with a range of malignancies, particularly gastrointestinal stromal tumor (GIST) and renal cell carcinoma (4-6). The recommended adult dose is 50 mg/day for 28 days followed by a 14-day break (5, 7), though some groups have evaluated daily dosing without a 14-day break (8). In adults, common toxicities were fatigue, gastrointestinal symptoms, and myelosuppression (4-7, 9). Pharmacodynamic effects of sunitinib observed in studies in adults include: increased plasma VEGF; increased circulating endothelial cells (CECs); decreased soluble VEGFR2; and decreased monocyte counts (10-13).
In preclinical model systems, sunitinib has activity against a number of pediatric solid tumors, including neuroblastoma and sarcoma models with demonstrated growth inhibition during sunitinib exposure (14, 15). Sunitinib has not previously been evaluated in children with the exception of eleven children with GIST (16, 17) who were treated with doses ranging from 25 to 50 mg/day. The toxicity profile of sunitinib in this small number of children was similar to the adult experience.
The current report describes the results of a pediatric phase I study of sunitinib in children with refractory or recurrent solid tumors. The primary aims of the study were: to define the maximum tolerated dose and toxicities associated with sunitinib; and to characterize the pharmacokinetics of sunitinib in children. Secondary endpoints included an assessment of anti-tumor activity as well as pharmacodynamic biomarker studies.
Materials and Methods
Patients
Patients were eligible for participation if they were 2 – 21 years of age, had histologic diagnosis of solid malignancy with measurable or evaluable disease, and had no known curative options. Patients were required to have a Karnofsky (age >10 years) or Lansky (age ≤ 10 years) performance score ≥ 50 and to have recovered from prior therapy. Patients were required to have adequate baseline bone marrow, renal, hepatic, pancreatic, and cardiac function according to defined protocol criteria. Patients with pre-existing hyper- or hypothyroidism were required to have stable thyroid function. Patients could not be receiving concomitant antihypertensive medications and had to have blood pressure < 95th percentile for age, height, and gender.
Exclusion criteria included: inability to swallow capsules; body surface area < 0.5 m2 (due to available capsule sizes); concurrent use of strong CYP3A4 inducers or inhibitors; treatment with agents that might increase the risk of bleeding complications; presence of pleural based tumors; or uncontrolled infection.
An amendment to the eligibility criteria was made after enrollment of the first twelve patients (Part A) because of cardiac and hematologic toxicity. In the amended protocol (Part B), patients with prior anthracycline treatment or cardiac radiation exposure were excluded. Patients with known bone marrow metastases were also excluded from Part B in order to provide a clear assessment of the hematological toxicity of sunitinib after a patient in Part A developed dose limiting neutropenia. This amendment also allowed enrollment of patients with central nervous system tumors. These patients were excluded from Part A since experience with the use of anti-angiogenic agents in children with central nervous system tumors was limited at the time of study initiation.
The protocol was approved by each institutional review board. Written informed consent (and assent when applicable) was obtained for all patients.
Treatment and Evaluations
Patients received sunitinib orally once daily for 28 days followed by a 14-day break, such that the duration of one cycle of therapy was 42 days. The starting dose level was 20 mg/m2/dose (approximately 80% of the approved adult dose) in Part A and 15 mg/m2/dose in Part B. All doses were given as capsules and were rounded to the nearest 12.5 mg using a dosing nomogram. Patients without disease progression or unacceptable toxicity could receive up to 9 cycles of therapy.
Patients had routine physical examinations and surveillance laboratory testing to evaluate for toxicity. Electrocardiograms and echocardiograms were obtained at the end of cycles 1 and 2 and thyroid stimulating hormone (TSH) levels were monitored at the end of cycle 3 and then every other cycle. Based on the toxicities observed in Part A, patients in Part B were required to have additional electrocardiograms and echocardiograms at the end of cycles 3, 6, and 9 as well as a TSH levels on day 28 of cycle 1, days 1 and 28 of cycle 2, and day 28 of every subsequent odd number cycle. All patients had baseline plain radiographs of the proximal tibial growth plate. Patients with open tibial growth plates had repeat radiographs at the end of cycle 3 and then every other cycle.
Toxicities were graded according to the NCI Common Terminology Criteria, version 3.0. Dose-limiting toxicity (DLT) was defined as any of the following that were attributed as at least possibly related to sunitinib: grade 4 neutropenia; grade 4 thrombocytopenia; any grade 4 non-hematologic toxicity; grade 2 cardiac systolic dysfunction; blood pressure > 25 mmHg above the 95th percentile for age, height, and gender; any grade 2 non-hematologic toxicity for ≥ 7 days that required drug interruption; any non-hematologic toxicity that required drug interruption for > 7 days; or any grade 3 non-hematologic toxicity with the exception of nausea and vomiting of < 3 days duration, ALT elevation that returned to baseline prior to the next cycle, AST elevation, fever of < 5 days duration, electrolyte abnormalities responsive to oral supplementation, asymptomatic elevations of amylase or lipase resolving to < grade 1 within 7 days of drug interruption.
Patients underwent disease re-evaluation at the end of cycle 1 and then every other cycle. For patients with measurable disease, tumor response was evaluated by RECIST (18).
Pharmacokinetic Analysis
All patients were required to have trough plasma samples obtained on days 7, 14, 21, and 28 of cycle 1. Patients were also asked to participate in extended pharmacokinetic sampling after the first dose with samples obtained prior to sunitinib and 1, 2, 4, 6, 8, 24, and 48 hours after the first dose. Plasma concentrations of sunitinib and its main active metabolite, SU12662, were measured using liquid chromatography-tandem mass spectrometry by Bioanalytical Systems, Inc (BASi; West Lafayette, IN) as previously described (19). Sunitinib and SU12662 plasma concentration–time data were analyzed by standard noncompartmental methods using WinNonlin Pro (Pharsight Corp; Mountain View, CA).
Biomarker Studies
Plasma was obtained at baseline and day 28 of cycle 1 in consenting patients to assess the impact of sunitinib on angiogenic biomarkers. VEGF, VEGFR1, VEGFR2, placental growth factor (PlGF), stromal cell -derived factor-1 (SDF-1), and endoglin levels were measured by ELISA using commercially available kits (R&D Systems, Inc; Minneapolis, MN). Circulating endothelial cells (CECs) and endothelial progenitor cells (CEPs) were measured using a multiparameter FACSCalibur flow cytometer (BD Biosciences; San Jose, CA) as previously described (20). CECs were defined as CD31+CD45-CD133- and CPCs were defined as CD31+CD45-CD133+. Additional testing was performed to determine if CECs and CEPs were apoptotic, expressed endoglin, or expressed VEGFR2 (21, 22). Monocyte counts were obtained at each institution using standard methods.
Dose Escalation Strategy and Statistical Methods
The primary endpoint for determining dose escalation was DLT in cycle 1. In Part A, a standard 3+3 phase I dose escalation design was utilized. Briefly, patients were enrolled in cohorts of three patients each and, if no first cycle DLT was observed, the dose was escalated. If one patient had a DLT, then up to three additional patients were treated at that same dose level. If two or more patients in a given cohort of six patients had DLT, then the maximum tolerated dose (MTD) was exceeded and the sunitinib dose was de-escalated. In Part B, a more conservative dose escalation strategy was adopted such that an additional 3 patients were enrolled at a dose level if 0 or 1 of the first three patients had a DLT. The dose was then only escalated if ≤ 1 patient in the cohort of 6 had a DLT. Therefore, a minimum of 6 patients were to be evaluated per dose level in Part B before the dose could be escalated.
Changes in biomarkers of angiogenesis obtained at baseline and at the end of cycle 1 were assessed using the paired t-test or Wilcoxon signed rank test for non-normal data. Differences between dose level or clinical response (progressive disease vs. stable disease) based on changes in laboratory markers from baseline to end of cycle 1 were assessed using the Wilcoxon rank sum test.
Results
Patient Characteristics
Characteristics of the 23 patients, who received a median (range) of 1 (1 to 9) cycle of sunitinib, are shown in Table 1. Eight patients were < 12 years of age at study entry. One patient had received prior therapy with another VEGFR tyrosine kinase inhibitor, sorafenib.
Table 1.
Patient characteristics
| Overall Study (n = 23) | Part A (n = 12) | Part B (n = 11) | |
|---|---|---|---|
| Median age, years (range) | 13.9 (3.9 – 20.6) | 14.5 (10.6 – 20.6) | 11.1 (2.9 – 18.2) |
|
| |||
| Male : Female | 11 : 12 | 8 : 4 | 3 : 8 |
|
| |||
| Diagnosis | |||
| Brain tumor (Part B only) | 8 | 8 | |
| Soft tissue sarcoma | 4 | 3 | 1 |
| Ewing sarcoma | 2 | 2 | |
| Neuroblastoma | 2 | 2 | |
| Osteosarcoma | 2 | 2 | |
| Other | 5* | 3 | 2 |
|
| |||
| Measurable disease by RECIST | 17 | 8 | 9 |
|
| |||
| Bone marrow involved at study entry (Part A only) | 1 | ||
|
| |||
| Prior receptor tyrosine kinase inhibitor | 5** | 3 | 2 |
|
| |||
| Prior anthracycline (Part A only) | 10 | 10 | |
|
| |||
| Prior radiation therapy | 14 | 7 | 7 |
|
| |||
| Open tibial growth plate | 15 | 6 | 9 |
|
| |||
| Median number sunitinib cycles (range) | 1 (1 – 9) | 1 (1 – 4) | 1 (1 – 9) |
Includes one patient each with: desmoplastic small round cell tumor (Part A); renal cell carcinoma (Part A); spindle epithelial tumor with thymus like differentiation (Part A); gastrointestinal stromal tumor (Part B); and malignant meningioma (Part B).
Includes two patients treated with imatinib (one each on Parts A and B) and one patient each treated with dasatinib (Part A), sorafenib (Part A), and gefitinib (Part B).
Dose Escalation and Toxicity
In Part A, 1 of 3 patients treated at the starting dose level of 20 mg/m2 developed grade 2 systolic dysfunction. This dose level was expanded to 6 patients and 2 additional patients had DLTs consisting of grade 3 anorexia and grade 4 neutropenia. The dose was then de-escalated to 15 mg/m2. In the first 3 patients treated at this dose level, one patient had a prolonged grade 3 ALT elevation that was dose-limiting. Three more patients were enrolled, one of whom developed dose-limiting cardiac toxicity with grade 3 diastolic and grade 2 systolic dysfunction (Table 2). Enrollment to Part A was suspended and, following a detailed evaluation of both cases of reversible cardiac dysfunction, the protocol was amended to exclude patients with prior anthracycline or cardiac radiation exposure.
Table 2.
Details of cardiac toxicity observed in two patients treated with sunitinib.
| Patient 1 | Patient 2 | |
|---|---|---|
| Demographics | 10 year old, male | 20 year old, male |
| Disease | Desmoplastic small round cell tumor | Ewing sarcoma |
| Cumulative doxorubicin exposure | 150 mg/m2 | 367 mg/m2 |
| Prior cardiac radiation | No | Yes |
| Sunitinib dose level | 20 mg/m2 | 15 mg/m2 |
| Toxicity first noted | Day 28, Cycle 1 | Day 28, Cycle 1 |
| Toxicity | Asymptomatic systolic dysfunction on planned surveillance echocardiogram | Symptomatic diastolic and systolic dysfunction; transient ischemic changes on EKG |
| Toxicity grade | Grade 2 | Grade 3 and Grade 2 |
| Attribution to sunitinib | Probable | Definite |
| Associated hypertension | No | No |
| Day 21 Css sunitinib | 40 ng/mL | 18 ng/mL |
| Outcome | Resolved 4 weeks off sunitinib | Required digoxin and lisinopril; Resolved 8 weeks off sunitinib |
Eight patients were enrolled on Part B of the study at a starting dose level of 15 mg/m2. This starting dose was chosen for Part B since it was anticipated that the risk of cardiac toxicity would be less in this cohort of patients who had not received prior anthracycline therapy or cardiac radiation. Two patients had early disease progression during cycle 1 and were therefore not fully evaluable for DLT. None of the 6 evaluable patients at this dose level experienced DLT in cycle 1. Three patients were treated at 20 mg/m2, two of whom had DLTs in cycle 1. One patient developed grade 4 hyperuricemia in the setting of grade 2 diarrhea and dehydration. The second patient developed grade 5 aspiration pneumonia in the setting of grade 4 punctate hemorrhage into a progressive diffuse pontine glioma. While this episode may have been related to the natural history of her disease, (23) a possible attribution to study therapy could not be excluded. These results established 15 mg/m2/day for 28 days followed by a 14-day break as the MTD for patients without prior cardiac radiation or anthracycline exposure. No cardiac dysfunction was observed in Part B of the trial.
Due to dose rounding to accommodate capsule size, the 14 patients treated at the 15 mg/m2 dose level received a mean actual dose of 14.8 ± 2.1 mg/m2. The mean dose for the 6 evaluable patients treated at 15 mg/m2 in Part B was 14.5 mg/m2. The 9 patients treated at the 20 mg/m2 dose level received a mean actual dose of 18.8 ± 2.8 mg/m2. Patients with DLT were not more likely to receive an increased dose due to rounding compared to patients without DLT.
Details of toxicity reported in more than 10% of patients in cycle 1 are shown in Table 3. Neutropenia, thrombocytopenia, and transaminase elevation were the most commonly reported toxicities. Gastrointestinal symptoms and fatigue were the most commonly reported symptoms. The incidence of neutropenia and thrombocytopenia did not appear to increase in subsequent cycles. Non-hematologic toxicity was uncommon in subsequent cycles of therapy. The main differences in toxicity profiles between Parts A and B were the absence of reported cardiac toxicity and thrombocytopenia in Part B.
Table 3.
Hematologic and non-hematologic toxicities observed in 21 evaluable patients in cycle 1 and in 23 subsequent cycles of therapy with sunitinib.*
| PART A | Cycle 1 | Subsequent Cycles (2-4) | ||||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | |
| N = 12 cycles | N = 5 cycles | |||||||
| Hematologic Toxicity | ||||||||
| Leukopenia | 33% | 17% | 25% | 40% | ||||
| Thrombocytopenia | 42% | 25% | 40% | |||||
| Neutropenia | 8% | 17% | 33% | 8% | 20% | 20% | ||
| Lymphopenia | 17% | 17% | 8% | 20% | ||||
| Anemia | 25% | 8% | 20% | |||||
| Non-Hematologic Toxicity | ||||||||
| AST elevation | 33% | 8% | 20% | |||||
| ALT elevation | 17% | 8% | 8% | 20% | ||||
| Anorexia | 8% | 17% | 8% | |||||
| Diarrhea | 33% | |||||||
| Fatigue | 8% | 8% | 8% | |||||
| Hypoalbuminemia | 25% | |||||||
| Hypocalcemia | 25% | |||||||
| Vomiting | 25% | |||||||
| Amylase elevation | 17% | |||||||
| Hyponatremia | 8% | 8% | ||||||
| Hypophosphatemia | 17% | |||||||
| Hypothyroidism | 8% | 8% | 20% | |||||
| Left ventricular systolic dysfunction | 17% | 20% | ||||||
| Mucositis | 17% | |||||||
| Nausea | 8% | 8% | ||||||
| Weight loss | 17% | |||||||
| PART B | Cycle 1 | Subsequent Cycles (2-9) | ||||||
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | |
| N = 9 cycles** | N = 18 cycles | |||||||
| Hematologic Toxicity | ||||||||
| Neutropenia | 29% | 14% | 14% | 6% | 6% | 6% | ||
| Leukopenia | 43% | 6% | 6% | |||||
| Lymphopenia | 14% | 14% | ||||||
| Non-Hematologic Toxicity | ||||||||
| AST elevation | 44% | 6% | ||||||
| ALT elevation | 22% | 11% | 11% | |||||
| Diarrhea | 11% | 11% | ||||||
| Fatigue | 22% | |||||||
| Hypermagnesemia | 22% | |||||||
| Hyperuricemia | 11% | 11% | ||||||
| Muscle or joint pain | 11% | 11% | 6% | |||||
| Rash or hypopigmentation | 22% | 12% | ||||||
| Amylase elevation | 11% | |||||||
| Anorexia | 11% | |||||||
| CNS hemorrhage | 11% | |||||||
| Constipation | 11% | |||||||
| Cranial neuropathy | 11% | |||||||
| Decreased serum bicarbonate | 11% | |||||||
| Dehydration | 11% | |||||||
| Epistaxis | 11% | |||||||
| Eye swelling | 11% | |||||||
| Headache | 11% | |||||||
| Hypernatremia | 11% | 6% | ||||||
| Hypertension | 11% | 6% | 6% | |||||
| Hypoalbuminemia | 11% | |||||||
| Hypomagnesemia | 11% | |||||||
| Hypophosphatemia | 11% | 11% | ||||||
| Hypothyroidism | 11% | 6% | ||||||
| Lipase elevation | 11% | |||||||
| Mucositis | 11% | |||||||
| Vomiting | 11% | |||||||
Data are presented for patients in Parts A and B of the study. Only toxicities possibly, probably, or definitely related to sunitinib and which occurred in more than 10% of patients in cycle 1 are displayed. Values represent percent of patient cycles with listed toxicity according to grade.
Of 9 evaluable patients in Part B, only 7 were evaluable for hematologic toxicity.
Three of 23 patients developed hypothyroidism (≤ grade 2) after one cycle of therapy. Two patients developed hypothyroidism (≤ grade 2) during subsequent cycles of therapy. Hypertension was rarely reported. One patient had grade 1 and another patient had grade 2 QTc interval prolongation, with maximal QTc of 490 msec. Of the 15 patients with open tibial growth plates at study entry, one patient received 9 cycles of therapy and did not demonstrate any growth plate thickening. The remaining 14 patients were removed from protocol therapy prior to undergoing a second growth plate evaluation. Changes in hair color and texture observed in adult studies of sunitinib (7) were not reported.
Pharmacokinetics
All 23 patients submitted at least one sample for evaluation of steady-state trough levels (Table 4). Steady state concentrations of sunitinib, the active metabolite SU12662, and total drug (sunitinib + SU12662) were reached by Day 7. Five of 14 patients (36%) at the 15 mg/m2 dose level and five of nine patients (56%) at the 20 mg/m2 dose level had steady-state total drug trough concentrations > 50 ng/mL, the target total drug concentration for kinase inhibition based on preclinical studies (2). Nine patients participated in the extended sampling study (Table 5). A median peak plasma concentration of 16.8 (range 9.5 – 61.4) ng/mL sunitinib was achieved 7 hours after the first dose. Median values of the sunitinib and SU12662 half-life, based on the accumulation ratios calculated from the steady-state data were 38.7 (range 23.9 – 61.7) and 93.4 (range 47.4 – 176) hours, respectively. Steady state trough concentrations did not appear to correlate with occurrence of DLT (data not shown).
Table 4.
Trough plasma concentrations (ng/mL) of sunitinib and total drug (sunitinib plus SU12662) on days 7-28 of sunitinib therapy in 23 children and young adults.
| Dose Level | Day 7 | Day 14 | Day 21 | Day 28 | Css | ||
|---|---|---|---|---|---|---|---|
| 15 mg/m2 | |||||||
| N = 14 | N = 14 | N = 13 | N = 11 | ||||
| Sunitinib | Median | 27.8 | 29.7 | 37.7 | 22.5 | 28.0 | |
| Min | 13.4 | 11.9 | 8.9 | 16.1 | 13.2 | ||
| Max | 50.4 | 61.9 | 64.2 | 69.7 | 57.9 | ||
| Total Drug | Median | 36.9 | 42.5 | 43.7 | 34.9 | 38.8 | |
| Min | 22.0 | 19.5 | 10.1 | 15.6 | 21.0 | ||
| Max | 69.7 | 94.2 | 90.5 | 90.5 | 83.8 | ||
| Number with total drug concentration > 50 ng/mL | 6 | 5 | 6 | 4 | 5 | ||
| 20 mg/m2 | N = 9 | N = 8 | N = 8 | N = 7 | |||
| Sunitinib | Median | 35.9 | 31.0 | 37.4 | 46.7 | 36.5 | |
| Min | 23.1 | 26.9 | 24.2 | 23.4 | 28.2 | ||
| Max | 55.1 | 58.6 | 62.9 | 86.2 | 65.0 | ||
| Total Drug | Median | 48.2 | 45.4 | 49.3 | 66 | 50.6 | |
| Min | 32.8 | 39.2 | 41.1 | 34.5 | 43.2 | ||
| Max | 83.2 | 90.5 | 104.7 | 147.3 | 106.4 | ||
| Number with total drug concentration > 50 ng/mL | 4 | 2 | 4 | 6 | 5 | ||
Absolute doses received per assigned dose level: 15 mg/m2 dose level - 12.5 mg (n = 4), 25 mg (n = 10); 20 mg/m2 dose level - 12.5 mg (n = 1), 25 mg (n = 7), 37.5 mg (n = 1).
Table 5.
Detailed day 1 pharmacokinetic parameters for sunitinib and SU12662 in 9 children and young adults treated with sunitinib.
| Dose Level | N | Sunitinib | SU12662 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmax (hours) | Cmax (ng/mL) | AUC0-48h (h·ng/L) | AUC0-∞ (h·ng/L) | Cl/F (L/hr/m2) | AR | Tmax (h) | Cmax (ng/mL) | AUC0-48h (h·ng/L) | AUC0-∞ (h·ng/L) | AR | |||
| 15 g/m2 | 8 | Median | 7.0 | 16.8 | 492 | 747 | 19.5 | 3.1 | 8.0 | 2.3 | 76 | 384 | 6.4 |
| Min | 2.0 | 9.5 | 247 | 298 | 6.8 | 2.2 | 4.0 | 1.5 | 38 | 202 | 3.4 | ||
| Max | 48.0 | 61.4 | 1111 | 5365 | 43.3 | 4.2 | 48.7 | 8.5 | 285 | 2605 | 11.1 | ||
| 20 mg/m2 | 1 | 8.0 | 26.3 | 698 | 879 | 19.9 | 2.00 | 8.0 | 5.3 | 206 | 522 | 4.2 | |
Calculations for sunitinib Cl/F reflect data from 6 patients. Tmax = time to maximum plasma concentration; Cmax = maximum plasma concentration; AUC0-∞ = AUC0-48h + C48h/kel, where kel (terminal elimination rate constant) was calculated by linear least squares regression of the linear terminal elimination phase of the graph of ln(plasma concentration) versus time. AR = accumulation ratio = Css/C24h.
Efficacy
No objective responses were observed in the 17 patients evaluable for response. Four patients had a best response of stable disease, including patients with diffuse pontine glioma (2 cycles), osteosarcoma (4 cycles), epithelioid sarcoma (9 cycles), and ganglioglioma (9 cycles). The latter two patients did not progress on study therapy, but were removed from study after receiving the maximum of 9 cycles. Thirteen patients had a best response of progressive disease. Of the six patients not evaluable for best response, two patients did not complete cycle 1 due to toxicity. The remaining four patients had stable disease at the completion of cycle 1, but were removed from protocol therapy for toxicity after cycle 1. Of these, one patient with neuroblastoma who received one cycle at the 20 mg/m2 dose level had resolution of the disease noted at baseline on a 123I-meta-iodobenzylguanidine scan but had persistent bone marrow disease. The patient was removed from study due to toxicity and a subsequent 123I-MIBG scan was not obtained.
Correlative Studies
Twelve patients had paired baseline and day 28 plasma samples available for evaluation of angiogenic factor biomarkers by ELISA. Plasma VEGF and placental growth factor concentrations increased significantly over the course of the first cycle of therapy (p = 0.002 and 0.0003, respectively; Figures 1A and 1B). Plasma soluble VEGFR2 and endoglin concentrations decreased significantly over the course of the first cycle of therapy (p = 0.0009 and 0.01, respectively; Figures 1C and 1D). Changes in VEGF, placental growth factor, soluble VEGFR2, and endoglin did not vary by sunitinib dose level or by response to therapy over the course of cycle 1. Plasma soluble VEGFR1 and SDF-1 concentrations did not change in a consistent manner with therapy.
Figure 1.
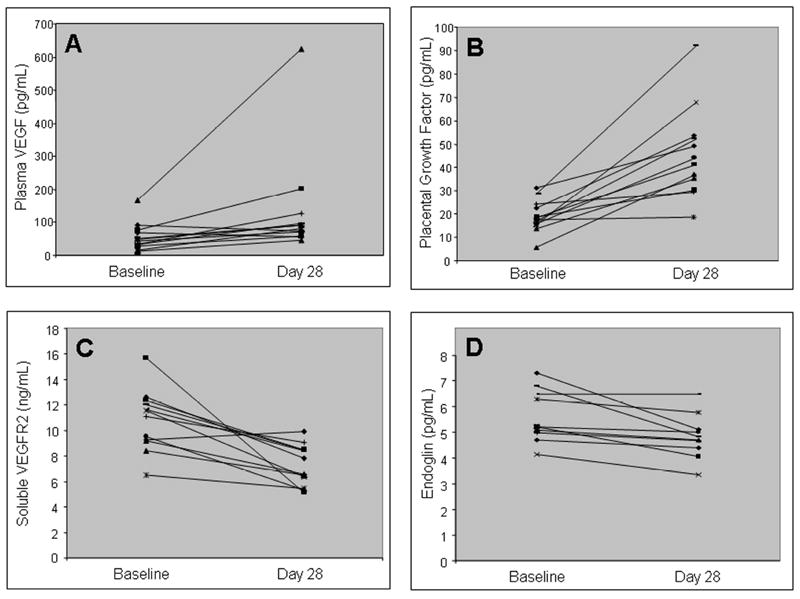
Changes in plasma VEGF (A), placental growth factor (B), soluble VEGFR1 (C), and endoglin (D) concentrations from baseline to Day 28 of cycle 1 of sunitinib in 12 patients.
Totals and subsets of CECs and CEPs in 11 patients with paired baseline and day 28 samples did not reliably change with therapy. Paired day 1 and day 28 absolute monocyte counts (AMC) were available for 18 patients. One patient had an increase in AMC. The remaining 17 patients had decreases in AMC (median decrease 117/μL; range 18-1253/μL).
Discussion
The maximum tolerated dose of sunitinib for pediatric patients without prior anthracycline exposure or prior cardiac radiation was 15 mg/m2/day. This dose is lower than the approved adult dose of 50 mg/day (approximately 28 mg/m2/day based on an average adult body surface area of 1.8 m2). Previous case series have indicated that children with GIST are able to tolerate sunitinib doses of 25 – 50 mg (16, 17). The lower doses tolerated in this formal dose escalation study may reflect more intensive prior therapy in the patient population in the current study. In addition, adults treated with sunitinib require dose reductions in 11-50% of cases (5, 6, 24, 25). The typical reduced adult dose of 37.5 mg/day (approximately 21 mg/m2/day based on an average adult body surface area of 1.8 m2) is still greater than the pediatric dose determined in the current study. In addition, the toxicity profile of sunitinib in children may differ from the toxicity profile in adults. In the current study, myelosuppression and transaminase elevations were the most commonly reported toxicities compared to fatigue and gastrointestinal symptoms in adults (4-6).
The pharmacokinetic data of the current study found that the apparent clearance of sunitinib was similar between children and adults (7, 26-29). These results are consistent with a recent population pharmacokinetic analysis of sunitinib in adults (median age 55 years; range 18 – 87) that showed that clearance did not correlate with age (27). As a consequence of the lower doses tolerated in this study, steady state trough total drug concentrations fell below target concentrations (50 ng/mL) in many of the patients. While peak concentrations would be predicted to exceed target concentrations, these data suggest that kinase inhibition may be intermittent for some patients treated at the maximum tolerated dose in this study. It is possible that plasma drug concentrations may not reflect intratumoral drug concentrations and that there may be different target exposures required for anti-tumor activity in different model tumor types.
Due to cardiac toxicity, a recommended phase 2 dose was not determined in Part A of the study, which included patients with prior anthracycline exposure or prior cardiac radiation. Reports from adult patients treated with sunitinib demonstrate an incidence of grade 3 or symptomatic congestive heart failure of 7-15% (30-32). As in the current study, the onset of cardiac toxicity is often early in the course of therapy with sunitinib (33, 34). While both patients in the current study recovered normal cardiac function after discontinuing sunitinib, not all adults have had reversible cardiac dysfunction (32, 33). The mechanism of cardiac toxicity with sunitinib remains unclear, though some preclinical studies have demonstrated an increase in myocyte apoptosis in response to sunitinib (30, 35). Since cardiac toxicity precluded the determination of a recommended sunitinib dose in patients with prior anthracycline exposure, future evaluation of this agent is likely to be limited to specific pediatric oncology populations who do not routinely receive therapy with anthracyclines, e.g. children with primary central nervous system tumors.
There were no objective anti-tumor responses in this trial. However, several patients with a variety of different tumors, including sarcoma and glioma, may have benefited as evidenced by stable disease for multiple cycles. Of note, the two patients with stable disease for 9 cycles had histologies which may demonstrate indolent growth: epithelioid sarcoma and ganglioglioma. In addition to recognized activity in GIST, sunitinib and other anti-angiogenic tyrosine kinase inhibitors (sorafenib and pazopanib) have shown clinical activity in several other subtypes of sarcoma, including desmoplastic small round cell tumor, alveolar soft part sarcoma, synovial sarcoma, and angiosarcoma (8, 36, 37). Sunitinib has been effective in the management of adult patients with renal cell carcinoma and central nervous system metastases, indicating that this agent is distributed to central nervous system tumors (38-40). Experience with sunitinib for primary central nervous system tumors is limited to studies demonstrating preclinical activity in glioma models (41, 42). Given a reported relationship between drug exposure and clinical benefit in adults (43), it is possible that the lack of objective responses in the current study was a result of inadequate drug exposure given the lower doses tolerated in children versus adults. Intra-patient dose escalation may be possible in a less heavily pre-treated phase 2 population, which would result in increased sunitinib exposure, thereby maximizing the potential for anti-tumor activity.
As with previous studies, sunitinib resulted in consistent increases in plasma VEGF and placental growth factor along with decreases in soluble VEGFR2 and monocyte counts (10, 11, 13). In contrast, changes in plasma SDF-1, total CECs, and CEC subsets noted in other studies could not be confirmed in the present study. Plasma endoglin concentrations decreased significantly with sunitinib treatment. Previous published studies have not described the effects of sunitinib on plasma endoglin levels. Since endoglin is expressed on tumor-associated endothelial cells and increased levels of plasma endoglin have been reported in patients with malignant tumors, (44) plasma endoglin levels may provide another pharmacodynamic tool for evaluating patients treated with sunitinib.
In conclusion, cardiac toxicity precluded a determination of a recommended phase 2 dose of sunitinib in children with prior therapy with a potential for cardiac toxicity. The maximum tolerated dose of sunitinib in children without prior anthracycline exposure or radiation therapy involving a cardiac field is lower than in adults (15 mg/m2 versus 28 mg/m2), despite similar apparent drug clearance. As a result, trough plasma concentrations at the MTD may be below “target” concentrations, as defined in preclinical studies. The implications of these findings are that further development of sunitinib in children with refractory malignancies is currently limited to a patient population without prior cardiotoxic therapy, specifically children with primary central nervous system tumors. In an attempt to maximize the potential for benefit from this agent in a planned phase II study in this population, a single intra-patient dose escalation is planned if the first cycle of therapy at 15 mg/m2 is well-tolerated. This strategy has successfully been employed for other anti-cancer agents (45) and may allow specific patients to safely receive higher doses of sunitinib than those identified in the current study.
Statement of Translational Relevance.
Sunitinib is an oral antiangiogenic tyrosine kinase inhibitor approved for use in adults with advanced renal cell carcinoma and gastrointestinal stromal tumor (GIST). Given the critical role of angiogenesis in the growth of pediatric solid tumors as well as the paucity of prior experience with sunitinib in children, we conducted a formal phase I study to determine the recommended phase II dose of sunitinib in a pediatric population. We found that children tolerated lower doses of sunitinib than adults, despite similar pharmacokinetic parameters. As with adults, pediatric patients are also at risk for cardiac toxicity with sunitinib. The results of this trial have direct clinical relevance for clinicians prescribing sunitinib for pediatric patients with renal cell carcinoma or GIST. These results will also inform future planned phase II studies of sunitinib in children with recurrent or refractory cancer.
Acknowledgments
Support: Supported by the Campini Foundation, Pfizer, NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130, and NCI U01 CA97452.
Footnotes
Prior Presentations: Presented in part at the 2008 American Society of Clinical Oncology Annual Meeting (Chicago, IL).
ClinicalTrials.gov Identifier: NCT00387920
Disclosure: Pfizer provided partial support for the analyses of sunitinib pharmacokinetics.
References
- 1.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–8. [PubMed] [Google Scholar]
- 2.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 3.O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 5.Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–73. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 8.George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27:3154–60. doi: 10.1200/JCO.2008.20.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 10.Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–50. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 11.Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3743–8. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 12.Vroling L, van der Veldt AA, de Haas RR, Haanen JB, Schuurhuis GJ, Kuik DJ, et al. Increased numbers of small circulating endothelial cells in renal cell cancer patients treated with sunitinib. Angiogenesis. 2009;12:69–79. doi: 10.1007/s10456-009-9133-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–35. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maris JM, Courtright J, Houghton PJ, Morton CL, Kolb EA, Lock R, et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51:42–8. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Smith KM, Chong AL, Stempak D, Yeger H, Marrano P, et al. In vivo antitumor and antimetastatic activity of sunitinib in preclinical neuroblastoma mouse model. Neoplasia. 2009;11:426–35. doi: 10.1593/neo.09166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agaram NP, Laquaglia MP, Ustun B, Guo T, Wong GC, Socci ND, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204–15. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janeway KA, Albritton KH, Van Den Abbeele AD, D’Amato GZ, Pedrazzoli P, Siena S, et al. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52:767–71. doi: 10.1002/pbc.21909. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Bello CL, Sherman L, Zhou J, Verkh L, Smeraglia J, Mount J, et al. Effect of food on the pharmacokinetics of sunitinib malate (SU11248), a multi-targeted receptor tyrosine kinase inhibitor: results from a phase I study in healthy subjects. Anticancer Drugs. 2006;17:353–8. doi: 10.1097/00001813-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 21.Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–9. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabascio C, Muratori E, Mancuso P, Calleri A, Raia V, Foutz T, et al. Assessing tumor angiogenesis: increased circulating VE-cadherin RNA in patients with cancer indicates viability of circulating endothelial cells. Cancer Res. 2004;64:4373–7. doi: 10.1158/0008-5472.CAN-04-0265. [DOI] [PubMed] [Google Scholar]
- 23.Pollack IF, Jakacki RI, Blaney SM, Hancock ML, Kieran MW, Phillips P, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007;9:145–60. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–6. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boven E, Massard C, Armand JP, Tillier C, Hartog V, Brega NM, et al. A phase I, dose-finding study of sunitinib in combination with irinotecan in patients with advanced solid tumours. Br J Cancer. 2010 doi: 10.1038/sj.bjc.6605852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houk BE, Bello CL, Kang D, Amantea M. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res. 2009;15:2497–506. doi: 10.1158/1078-0432.CCR-08-1893. [DOI] [PubMed] [Google Scholar]
- 28.Bello CL, Garrett M, Sherman L, Smeraglia J, Ryan B, Toh M. Pharmacokinetics of sunitinib malate in subjects with hepatic impairment. Cancer Chemother Pharmacol. 66:699–707. doi: 10.1007/s00280-009-1213-4. [DOI] [PubMed] [Google Scholar]
- 29.Khosravan R, Toh M, Garrett M, La Fargue J, Ni G, Marbury TC, et al. Pharmacokinetics and safety of sunitinib malate in subjects with impaired renal function. J Clin Pharmacol. 50:472–81. doi: 10.1177/0091270009347868. [DOI] [PubMed] [Google Scholar]
- 30.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Lorenzo G, Autorino R, Bruni G, Carteni G, Ricevuto E, Tudini M, et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol. 2009 doi: 10.1093/annonc/mdp025. [DOI] [PubMed] [Google Scholar]
- 32.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19:1613–8. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 33.Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C, et al. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112:2500–8. doi: 10.1002/cncr.23460. [DOI] [PubMed] [Google Scholar]
- 34.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–12. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 35.Hasinoff BB, Patel D, O’Hara KA. Mechanisms of myocyte cytotoxicity induced by the multiple receptor tyrosine kinase inhibitor sunitinib. Mol Pharmacol. 2008;74:1722–8. doi: 10.1124/mol.108.050104. [DOI] [PubMed] [Google Scholar]
- 36.Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–40. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schoffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–32. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 38.Koutras AK, Krikelis D, Alexandrou N, Starakis I, Kalofonos HP. Brain metastasis in renal cell cancer responding to sunitinib. Anticancer Res. 2007;27:4255–7. [PubMed] [Google Scholar]
- 39.Medioni J, Cojocarasu O, Belcaceres JL, Halimi P, Oudard S. Complete cerebral response with sunitinib for metastatic renal cell carcinoma. Ann Oncol. 2007;18:1282–3. doi: 10.1093/annonc/mdm275. [DOI] [PubMed] [Google Scholar]
- 40.Thibault F, Billemont B, Rixe O. Regression of brain metastases of renal cell carcinoma with antiangiogenic therapy. J Neurooncol. 2008;86:243–4. doi: 10.1007/s11060-007-9449-5. [DOI] [PubMed] [Google Scholar]
- 41.de Bouard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, et al. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9:412–23. doi: 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannopoulou E, Dimitropoulos K, Argyriou AA, Koutras AK, Dimitrakopoulos F, Kalofonos HP. An in vitro study, evaluating the effect of sunitinib and/or lapatinib on two glioma cell lines. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9290-0. [DOI] [PubMed] [Google Scholar]
- 43.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66:357–71. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 44.Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003;22:6557–63. doi: 10.1038/sj.onc.1206813. [DOI] [PubMed] [Google Scholar]
- 45.Blaney SM, Phillips PC, Packer RJ, Heideman RL, Berg SL, Adamson PC, et al. Phase II evaluation of topotecan for pediatric central nervous system tumors. Cancer. 1996;78:527–31. doi: 10.1002/(SICI)1097-0142(19960801)78:3<527::AID-CNCR21>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]


