Abstract
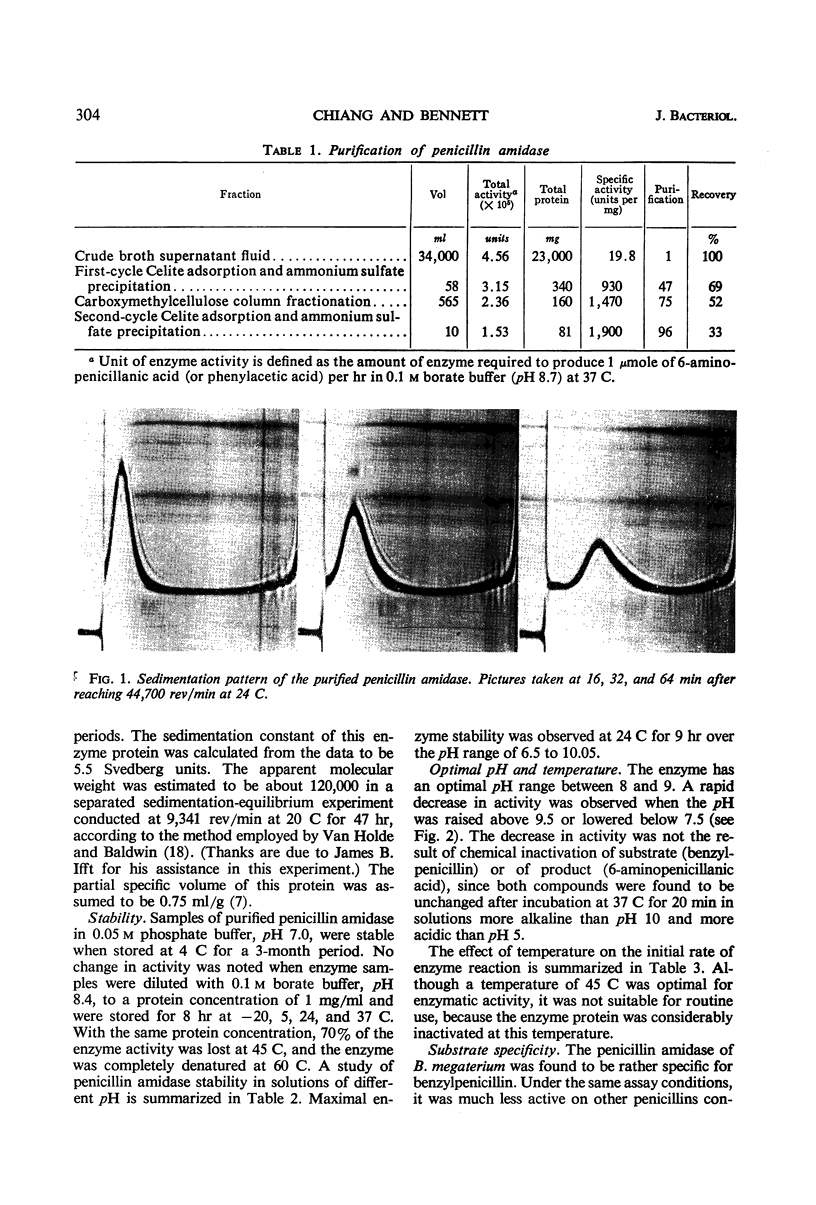
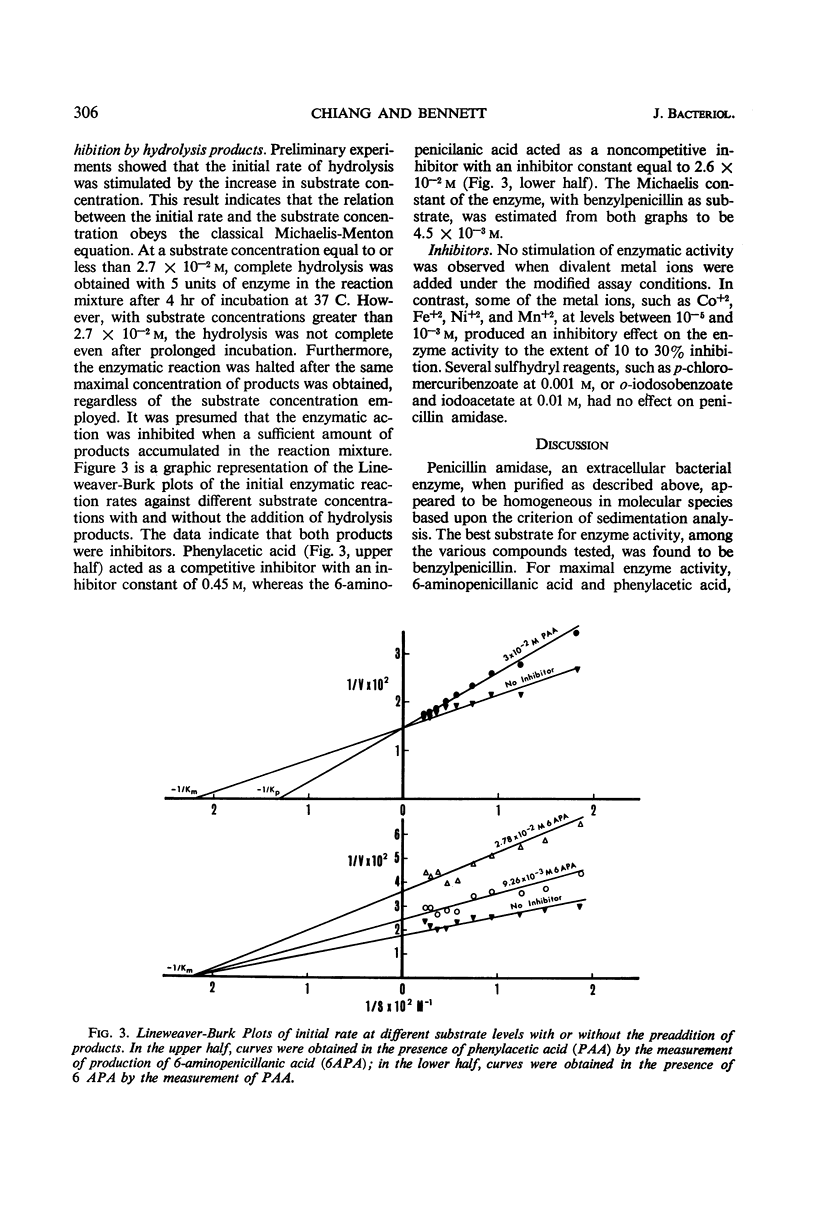
A penicillin amidase, obtained from the exogenous medium of a Bacillus megaterium culture, was purified approximately 96-fold by means of two cycles of adsorption on, and elution from, Celite, followed by a further fractionation on carboxymethylcellulose. On the basis of sedimentation centrifugation analysis, the final preparation was deemed to be homogeneous with an apparent molecular weight of approximately 120,000. The enzyme is specific for benzylpenicillin and has a pH optimum between 8 and 9. Complete hydrolysis of benzylpenicillin was obtained at low substrate concentrations. At higher substrate concentrations, the hydrolysis of benzylpenicillin was incomplete, apparently due to enzyme inhibition by phenylacetic acid and 6-aminopenicillanic acid, which were formed during the hydrolysis. Under the assay conditions, phenylacetic acid was a competitive inhibitor of penicillin amidase with an inhibitor constant (Ki) of 0.45 m, whereas 6-aminopenicillanic acid was noncompetitive in nature with a Ki of 2.6 × 10−2m. The Michaelis constant of this enzyme was found to be 4.5 × 10−3m when benzylpenicillin was used as substrate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATCHELOR F. R., CHAIN E. B., HARDY T. L., MANSFORD K. R., ROLINSON G. N. 6-Aminopenicillanic acid. III. Isolation and purification. Proc R Soc Lond B Biol Sci. 1961 Aug 15;154:498–508. doi: 10.1098/rspb.1961.0047. [DOI] [PubMed] [Google Scholar]
- BATCHELOR F. R., CHAIN E. B., RICHARDS M., ROLINSON G. N. 6-Aminopenicillanic acid. VI. Formation of 6-aminopenicillanic acid from penicillin by enzymic hydrolysis. Proc R Soc Lond B Biol Sci. 1961 Aug 15;154:522–531. doi: 10.1098/rspb.1961.0050. [DOI] [PubMed] [Google Scholar]
- CLARIDGE C. A., GOUREVITCH A., LEIN J. Bacterial penicillin amidase. Nature. 1960 Jul 16;187:237–238. doi: 10.1038/187237a0. [DOI] [PubMed] [Google Scholar]
- CLARIDGE C. A., LUTTINGER J. R., LEIN J. SPECIFICITY OF PENICILLIN AMIDASES. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:1008–1012. doi: 10.3181/00379727-113-28559. [DOI] [PubMed] [Google Scholar]
- COLE M. PROPERTIES OF THE PENICILLIN DEACYLASE ENZYME OF ESCHERICHIA COLI. Nature. 1964 Aug 1;203:519–520. doi: 10.1038/203519a0. [DOI] [PubMed] [Google Scholar]
- HUANG H. T., SETO T. A., SHULL G. M. Distribution and substrate specificity of benzylpenicillin acylase. Appl Microbiol. 1963 Jan;11:1–6. doi: 10.1128/am.11.1.1-6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMEDA Y., KIMURA Y., TOYOURA E., OMORI T. A method for isolating bacteria capable of producing 6-aminopenicillanic acid from benzylpenillin. Nature. 1961 Sep 9;191:1122–1123. doi: 10.1038/1911122a0. [DOI] [PubMed] [Google Scholar]
- KAUFMANN W., BAUER K. VARIETY OF SUBSTRATES FOR A BACTERIAL BENZYL PENICILLIN-SPLITTING ENZYME. Nature. 1964 Aug 1;203:520–520. doi: 10.1038/203520a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ROLINSON G. N., BATCHELOR F. R., BUTTERWORTH D., CAMERON-WOOD J., COLE M., EUSTACE G. C., HART M. V., RICHARDS M., CHAIN E. B. Formation of 6-aminopenicillanic acid from penicillin by enzymatic hydrolysis. Nature. 1960 Jul 16;187:236–237. doi: 10.1038/187236a0. [DOI] [PubMed] [Google Scholar]



