ABSTRACT
Doctors accuse individual variability or lack of quality of generic drugs for adverse reactions or lack of efficacy. The variability of effect of generic substitution, although accepted by clinicians as possible, is little discussed or even understood by them. The situation is really serious in the case of generic substitution of drugs with narrow therapeutic index (NTI) or critical dose. In this paper we review the basic notions of variability and effectiveness of generic medication and change of attitude that would improve the use of these drugs.
Keywords: Generic Drugs, Generic-generic substitution, brand-generic substitution, narrow terapeutic index
INTRODUCTION
Managers are trying to save available health care resources, using generic medicines, as they are cheaper. For a long period of time generic drugs were of no interest to drug manufacturers. All the steps required for registration of a new drug requested for innovative drug producers, had to be followed for a generic drug. Although research was limited to the substance already used in practice, skipping the necessary tests with thousands of other candidate substances, the costs of Phase I, II, III studies diminished the interest of various generic drugs manufacturers. Everything have transformed after a U.S. legislative changes in 1984, remained in history as "the Drug Price Competition and Patent Term Restoration Act" (also known as the Hatch-Waxman Act). This law allowed the producers to skip the trials, demonstration of bioequivalence with the original product being considered enough to prove equivalence. Shortly after, the American model has been followed by other countries; the first country to license its generic substitution was Australia in 1994. Today, most of the countries accept the replacement of an original medicine with a copy product (1).
Specialists estimate that, for each drug, on average 30% of the patients experience benefits, 30% do not experience any major benefit, 10% only experience side effects, and 30% discontinue treatment because they have either no benefits or side effects (2). Often, the blame for the lack of effective treatment is attributed to the use of generic drugs.
Concerns for the replacement of those original with copy medicines are linked to a certainty: large studies certify their efficacy and safety for brand drugs, while for generics efficacy and safety is only presumed. All drugs paid a price for their beneficial effects - being accompanied by side effects or adverse effects - some serious. In 2001, in the Netherlands 1.83% of hospital admissions had the main cause an adverse drug reaction. 6% of these patients died (3).
Other authors estimate the frequency rate of adverse reactions as 20% of cases and increase the likelihood of an adverse reaction to 40% in patients who need to receive more than 15 drugs per period of hospitalization! (4) In these circumstances the doctor's clinical reasoning should be based on as many certainties. Doubts about the effect of insufficient efficacy or toxicity of a generic drug make things more confusing.
In Romania both generic prescribing (when doctor is writing the non-proprietary – generic- name on a prescription) and generic substitution (the pharmacist may dispense any brand of the drug) are allowed. Even endorsed by law this practice is sometime unsuitable and should take in consideration some clinical situations and particular drugs.
Certainties and conventions on generic drugs
Innovative drugs have a patent that extends protection, usually for a period of 20 years. This rule was originally established in the U.S. through the 1984 Hatch-Waxman Act. In this way have been covered two aspects: on one hand to legislate that drugs are protected by patent, on the other hand has also established a sufficient time limit after which a drug can be copied. They sought to protect the interests of companies that invest astronomical sums in research, but also to remove an unlimited monopoly on a special character commercial product which contributes to improving the health of the population (5).
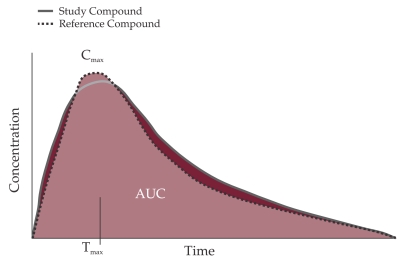
The fundamental convention upon which is based the generic drugs concept is of acceptance of their therapeutically equivalent if the original product is pharmaceutically equivalent (have the same active ingredients, the same dose and route of administration, the same concentration) and similar pharmacokinetics (PK) (bioequivalence). Bioequivalence is demonstrated through studies conducted on 12-24-36 young volunteers, men, healthy, fasting, who are receiving a dose of each product. Assessment is made by comparing the rates of drug absorption - peak concentration (Cmax) and the extent to which it occurs in the area under the curve (AUC) (6). AUC is determined by collecting serial blood samples at designated times after subjects have received the drug formulation (innovator or generic) (7).
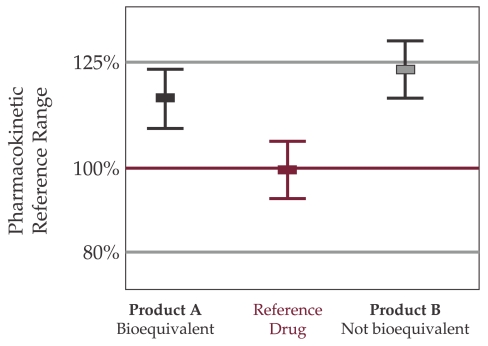
Two drugs are considered bioequivalent when demonstrates that their bioavailability (the percentage of drug that reaches the blood unchanged) is similar, within certain limits. Acceptable limits are – 20% to + 25%! (the 90% confidence interval (CI) of the Test-to-Reference ratio (T/R) of AUC0-t, and Cmax fall completely within the 80-125% boundary, which correspond to ln-transformations of the AUC to -/+ 22,314%) (9).
These limits are considered by some to be relatively large, but they determined this way to respect other conventions. Variations are taking into account the 90% CI - the average measurement being within more limited limits.
In 1999, the Food and Drug Administration (FDA) made an analysis of standard deviations of the parameters occurring in bioequivalence study for the proposed drugs in 1997. Three measures were reviewed: the area under the plasma drug concentration–time curve from time 0 to time t (AUC [0-t]), the AUC from time 0 to infinity (AUC [0-Inf]), and the highest drug concentration (Cmax). The observed mean difference between the innovator's product and the generic product for AUC (0-t) was ± 3.47% (SD, 2.84), for AUC (0-Inf) it was ±3.25% (SD, 2.97), and for Cmax it was ± 4.29% (SD, 3.72). (10) So the concerns seem to be unjustified.
A much smaller range could lead to an unacceptable paradox: a reference medicinal product could not be "bioequivalent" with itself. Various pharmacopoeia admit variations of + / -5% margin in active ingredient between the products form the same batch (9).
Another convention accepted for generic drugs is that different immediate release oral formulations are considered to be the same pharmaceutical form.
Moreover, "different salts, esters, ethers, isomers, mixtures of isomers, complexes or derivatives of active substances are considered the same active substance, if not show significantly different properties in terms of safety and/or efficacy" (11). Table 1 1 2
Table 1.
FDA Therapeutic Equivalence Codes (7)
| Rating | Comments |
|---|---|
| A | Drug products that FDA considers to be therapeutically equivalent to other pharmaceutically equivalent products because either there are no known or suspected bioequivalence problems, or bioequivalence problems have been resolved with in vivo or in vitro data confirming bioequivalence |
| AA | Products in conventional dosage forms not presenting bioequivalence problems |
| AB | Products meeting necessary bioequivalence requirements |
| AN | Solutions and powders for aerosolization |
| AO | Injectable oil solutions |
| AP | Injectable aqueous solutions and, in certain instances, intravenous nonaqueous solutions |
| B | Drug products that FDA, at present, considers not to be therapeutically equivalent to other pharmaceutically equivalent drug products |
| BC | Extended release dosage forms (capsules, injectables, and tablets |
| BD | Active ingredients and dosage forms with documented bioequivalence problems |
| BE | Delayed-release oral dosage forms |
| BN | Products in aerosol-nebulizer drug delivery systems |
| BP | Active ingredients and dosage forms with potential bioequivalence problems |
| BR | Suppositories or enemas that deliver drugs for systemic absorption |
| BS | Products having drug standard deficiencies |
| BT | Topical products with bioequivalence issues |
| BX | Drug products for which data are insufficient to determine therapeutic equivalence |
| B* | Drug products requiring further FDA investigation and review to determine therapeutic equivalence |
Figure 1. Comparison of pharmacokinetic (PK) Profiles to Determine Bioequivalence (8).
AUC = aria under curve.
Figure 2. FDA Requirements for Bioequivalence (8).
Many of the doubts concerning the effectiveness of different generic drugs compared with the original are assigned to the excipients. These suspicions are not justified. By definition excipients are inert substances that have no biological activity. Generic excipients have to be previously used for approved drugs for which there is evidence that they have not affected the safety or effectiveness (6)! Conventions on therapeutic equivalence were complemented with more rigorous FDA information for doctors, pharmacists and patients on the bioequivalence available data on the use of generic by coding them (the code is printed on the label).
Sources of preconceived ideas and misperceptions about generic drugs
There is the lingering suspicion among physicians and patients that generic versions may differ in quality and therapeutic efficacy from the brand name drug. This suspicion is based mainly on preconceived ideas and incorrect use of generic drugs.
1. Convention regarding compliance with Good Manufacturing Practice (GMP) as a guarantee of maintaining bioequivalence
The law does not impose a retest of a generic product at a random time, considering that once the quality criteria are met, they will be respected because producers are respecting the GMP. Such retesting is required in extremely rare situations, where a significant number of complaints arise regarding the quality of a product. For example The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended this year the precautionary recall of all batches of eight centrally-authorized generic clopidogrel-containing medicines, for which the active substance was manufactured by an Indian manufacturer. The Committee's recommendation followed an inspection of the manufacturing site, which identified failings in Good Manufacturing Practices (GMP) (12). Such inspections, however, are extremely difficult to make in distant countries like India, China, etc.
2. The way drugs are chosen for bioequivalence test
Some other conventions open the way to the assumptions of equivalence. Thus, the manufacturer has the right to choose their own production lot for testing and even the lot from a local pharmacy where from to be taken the original comparative drug. Thus it is able, at least in theory, to make its own checks on pharmaceutical and bioequivalence before actual testing, if desired.
3. Bioequivalence testing only on healthy young subjects
For ethical reasons, bioequivalence studies are performed on healthy volunteers. It is conventionally accepted that the similar bioavailability found in these people is a solid proof of a similar bioavailability in sick people! There are opinions that require that in certain cases, bioavailability tests to be carried out on patients (13)!
4. The existence of variability inter and intra-patients even when use brand drugs
Someone can test and compare the inter-patient variability, by observing the concentration versus time profile of a drug following administration of a fixed dose to several patients. Bioequivalence testing is not giving any information regarding the intra-patient variability, the differences in pharmacokinetics that happen within the same patient from dose to dose during the course of drug therapy (14).
There are people who respond unusual strongly to low doses or do not respond even to high doses due to a genetic determinism. Besides these, there are factors that influence absorption: age, sex, weight, condition of organs providing metabolism and excretion. In case of oral medicines many gastrointestinal factors may contribute to the variability in absorption: gastric emptying, intestinal transit speed, luminal pH, luminal surfactant concentrations, and/or presence or absence of food, a decrease of enterocites absorption capacity. Other factors that determine differences in drugs effect can vary in the binding capacity of the receptor, influenced by various pathological conditions, prior administration of drugs that act on the receptor itself, a variability in number of receptors, changes in the integrity of body functions according with age, the existence of some of preexisting conditions (15).
5. Widespread use of generic-generic substitution instead of brand-generic substitution
Physicians should bear in mind that brand to generic substitution is acceptable for drugs with high-medium therapeutic index and unacceptable for the generic-generic substitution! Therefore, after a clinical result was obtained using a specific generic drug, patient should be instructed about the need not to change it by his will or by the pharmacist will. This rule is often violated.
At least theoretically, in the case of two generic drugs administration may exist individuals who can show a difference in Cmax and AUC up to 45% between the two drugs! We can imagine that a patient receives a drug with drug bioavailability parameters located at one end of target range -20%, compared with the brand drug while there is and another patient who received another generic drug with parameters of bioavailability as upper limit of + 25% compared with the original product. If we add to this range and tolerance between quantities of active ingredient from the same batch - of +/- 5%, resulting that between two generic drugs may exist theoretical differences that might go to extremes up to 55%! This is why the authorities in the field of medicine do not put equal sign between two generic (9).
6. Use of generic substitution for drugs with narrow therapeutic index and critical-Dose Drugs
Therapeutic index provides information about the existing links between the desired and undesired effect. It is an important parameter in preclinical research phase of a substance with the potential to become a drug because it offers information about risks. A substance with a narrow therapeutic index is usually stopped from further research if the benefits are not large enough or if there are alternatives to treat the target clinical situation. Therapeutic index is calculated using two other indicators – ED 50 - the effective dose to 50% of laboratory animals (dose that causes the clinical effect to half of the laboratory animals) and LD50% - the dose that kills half the laboratory animals. Therapeutic index is the ratio between LD50 and DE50. This index extrapolates the data by comparing the dose per unit weight, but then they are correct by some data obtained during clinical trials or communications in the field of toxicology (15).
It is generally considered that a drug has a good safety spectrum in terms of therapeutic index, if its value exceeds value of 10. The therapeutic index is considered small when the ratio between LD50 and DE50 is 2 or below 2. NTI is often defined as a maximally safe serum levels no more than twice the minimally effective one. Drugs with narrow therapeutic index require careful titration and individualized (16).
By definition, generic substitution is not applicable for drugs with narrow therapeutic index! Drugs with narrow therapeutic index present small differences between the effective and toxic doses. Small variations in concentration of these drugs can result in an insufficient therapeutic response or toxic appearance.
FDA indicates over 25 NTI drugs used in various therapeutic indications: heart failure, thromboembolism, seizures, asthma, depression, thyroid dysfunction. Examples of drugs with narrow therapeutic index or critical dose drugs are: carbamazepine, digoxin, levothyroxine, phenytoin, theophylline, cyclosporine, warfarin, fentanyl, and immunosuppressants (17).
Legislative and administrative factors influencing improper substitution of generic substitution in Romania
Product substitution decisions are influenced by therapeutic issues, legal matters and pharmacy practice factors, including work flow, supply issues, access to current resources (18).
1. Legislative factors
In Romania, generic substitution is encouraged, National Insurance House only cover the cost of the cheaper generic (for drugs without generics compensation is set at a therapeutic class level).
The law impose to the pharmacy to have available at least one commercial product for every International Nonproprietary Names (INN) included on the reimbursement list. As the Romanian legislation did not allow the doctors to stipulate on their prescription in certain justified cases - "dispense as written" and compensate the patient for the prescribed product instead of compensation to the minimum reference price, treatment was left to the pharmacist or the patient choice for any generic alternative. In these circumstances, indirectly, the patient is advised to use the cheapest generic to qualify for a maximum compensation level, the generic-generic substitution being encouraged. Generic-generic substitution is a common practice in Romania, which should be revised.
2. Factors related to hospital drug delivery
In hospital, in the absence of specific rules of supply, from a day to another the patient may receive generics from different manufacturers, depending on the pharmacist's option or the financial arrangements established by the chief pharmacist or manager. During hospital stay the patient may also receive a brand name drug. After discharge is very likely that the patient will benefit from another drug trade than the one received during hospitalization.
3. Generic-generic substitution and brand-generic substitution for NTI drugs
Under financial and legislative pressure it has been overlooked the possibility that in certain clinical situations and for certain medications with narrow therapeutic index the pharmacist should be able to release exactly the commercial product that has been shown a benefit for the patient. Generic substitution for drugs with narrow therapeutic index should be avoided and made exclusively to strictly medical indication. ❑
CONCLUSIONS
In administrative whirlpool or due to a deficient instruction regarding the proper use of generic medication, doctors may be exposed to violate one of the most important driving forces in the medical art: "Primum Non Nocere." Improper use of generics can result in harm to patient health or inability to obtain the best result for them.
To warn against the appearance of clinical situations dangerous to patient safety we need to change attitude towards pharmacovigilance reports and a more effective post marketing surveillance system that will help to identify therapeutic inequivalence after formulation substitutions, and separate these incidents from those caused by drug failure and/or disease progression (9). It is not enough to rely on data from abroad. This way, we can gather reliable information about generic drugs and increase our trust in them.
The patient has the right to be informed about his treatment and particularly about the consequences of improper generic-generic substitution, substitution for NTI or critical dose drugs. In special cases a written consent in generic to brand substitution may be advisable.
The pharmacy must be able to provide any NTI brand name or critical dose drug, not only any generic of this drugs, as in present.
The National Agency for Drugs and Medical Devices should take in consideration adopting the FDA labeling for generics in order to provide the Romanian doctors with supplementary information's towards these drugs.
Legislation should take in consideration that a distinction is made between three forms of rationality: that of the physician, of the pharmacologist, and of the patient.
Health authorities should have a constant concern for education of the public, pharmacists, physicians on the details and complexities involved with the approval and use of formulation alternatives (9).
A decision whether to substitute an alternative product for a prescribed medication is a clinically based process that must be grounded in appropriate medical evidence, therapeutic equivalence information, financial factors, and consideration of how the substitution will impact the patient (18). ❑

Easter Light
Mircea Cinteza, MD, PhD
ARFOMED Art Photography Club of Romanian Physicians
References
- 1.Andersson K, Sonesson C, Petzold M, et al. What are the obstacles to generic substitution? An assessment of the behaviour of prescribers, patients and pharmacies during the first year of generic substitution. Sweden Pharmacoepidemiology and drug safety. 2005;14:341–348. doi: 10.1002/pds.1055. [DOI] [PubMed] [Google Scholar]
- 2.Rumel D, Nishioka Sde A, Santos AA. Drug interchangeability: clinical approach and consumer's point of view. Rev Saúde Pública. 2006:40–40. doi: 10.1590/s0034-89102006000600024. [DOI] [PubMed] [Google Scholar]
- 3.van der Hooft CS, Sturkenboom MC, van Grootheest K, et al. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf. 2006;29:161–8. doi: 10.2165/00002018-200629020-00006. [DOI] [PubMed] [Google Scholar]
- 4.Harrison's Principles of Internal Medicine. McGraw-Hill Professional; 17 editions. 2008 [Google Scholar]
- 5.Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514–26. doi: 10.1001/jama.2008.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters JR, Hixon DR, Conner DP, et al. Generic drugs-safe, effective, and affordable. Dermatol Ther. 2009 May-Jun;22:229–40. doi: 10.1111/j.1529-8019.2009.01236.x. Review. [DOI] [PubMed] [Google Scholar]
- 7.Henderson JD, Esham RH. Generic substitution: issues for problematic drugs. South Med J. 2001;94:16–21. [PubMed] [Google Scholar]
- 8.Approved Drug Products With Therapeutic Equivalence Evaluations. 23rd ed. 2003. FDA/CDER Web site. Available at: http://www.fda.gov/cder/ob/docs/preface/ecpreface.htm#Therapeutic Equivalence-Related Terms. [Accessed Septembrie 2003].
- 9.Reiffel JA. Formulation Substitution and Other Pharmacokinetic Variability: Underappreciated Variables Affecting Antiarrhythmic Efficacy and Safety in. Clinical Practice Am J Cardiol. 2000;85(25):46D–52D. doi: 10.1016/s0002-9149(00)00906-1. [DOI] [PubMed] [Google Scholar]
- 10.Henney J. Review of generic bioequivalence studies. JAMA. 1999;282:1995–1995. [Google Scholar]
- 11.Law no. 95/2006 on health reform
- 12.European Medicines Agency Press release 25 March 2010. EMA/179606/2010 http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/03/WC500076546.pdf. [Accessed Sept 2010].
- 13.Crawford P, Feely M, Guberman A, et al. Are there potential problems with generic substitution of antiepileptic drugs? A review of issues. Seizure. 2006;15:165–76. doi: 10.1016/j.seizure.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Balthasar J. Bioequivalence and bioequivalency testing. Amer J Pharm Ed. 1999;63:194–198. [Google Scholar]
- 15.Katzung Bertram G. Basic & Clinical Pharmacology 10th Ed. McGraw-Hill Professional. 2006 [Google Scholar]
- 16.Zheng JK. John Wiley and Sons; 2009. Formulation and Analytical Development for Low-Dose Oral Drug Products. pp. 432–432. [Google Scholar]
- 17.Burns M, et al. Management of narrow therapeutic index drugs. J Thromb Thrombolysis. 1999;7:137–43. doi: 10.1023/a:1008829403320. [DOI] [PubMed] [Google Scholar]
- 18.Manolakis PG. Prescription drug product substitution decision support. J Am Pharm Assoc. 2007;47:328–38. doi: 10.1331/JAPhA.2007.07502. [DOI] [PubMed] [Google Scholar]