Abstract
Introduction: The treatment of advanced non-small cell cancer (NSCLC) has changed with multiple new treatment algorithms proposed based on histological and molecular subtyping but low mutation rates will ensure the dominance of cytotoxic chemotherapy. Accordingly, we undertook a detailed review of our practice delivering multiple lines of systemic therapy.
Method: We undertook a retrospective review of consecutive patients presenting with advanced (stage IIIb/IV) NSCLC treated with systemic therapy at two UK hospitals during a 2-year period, January 2007 to December 2008.
Results: A total of 130 patients were identified, treated with predominantly carboplatin/gemcitabine (20 initially radically). Fifty of 110 patients (45%) treated with first-line systemic therapy subsequently received second-line therapy, of which 10 patients received third-line and two patients fourth-line therapy. Sixty three of 110 first-line patients (58%) achieved clinical benefit, 19 out of 50 (38%) in the second-line, 6 out of 10 (60%) in third-line but both patients progressed at fourth-line. Median overall survival for 110 patients was 10 months (95% confidence interval [CI] 8.6–11.4); but 16 months (95% CI 14-17.9) in those receiving multiple lines. Median survival from the first cycle of last-line treatment to death in the multiple therapy lines was 5 months (95% CI 2.6-7.3) and the majority of patients spent more time off treatment.
Conclusion: Overall our outcomes are consistent with published data and show good survival times can be achieved. The future of advanced NSCLC is in selecting the best treatment approach on a histological and genotypic basis.
Keywords: non-small cell lung cancer, retrospective analysis, systemic therapy
Introduction
Non-small cell lung cancer (NSCLC) is a major cause of death from cancer worldwide. In the UK, despite overall falls in tobacco consumption, lung cancer remains the second most commonly diagnosed cancer and is the leading cause of cancer mortality [Cancer Research UK, 2010].
Combination cytotoxic chemotherapy is the mainstay of management in advanced NSCLC with response rates of 20–40% and a median survival time of 7–10 months [Schiller et al. 2002]. Historically, standard first-line chemotherapy regimens have varied and previous clinical trials failed to reveal significant differences between a variety of platinum-based regimens [Schiller et al. 2002]. The most recent American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small Cell Lung Cancer [Azzoli et al. 2009] (using research published from January 2002 to July 2008) endorses the use of a doublet platinum-based regimen for patients of good WHO performance status (PS) of 0 or 1.
Recent data suggest a differential response to pemetrexed or gemcitabine-containing chemotherapy for squamous and nonsquamous histologies [Scagliotti et al. 2008]. The importance of histological subtyping of NSCLC has become increasingly recognized and systemic treatment is now routinely adapted to histological subtype. In addition, the advent of molecular testing of epidermal growth factor receptor (EGFR) mutations in NSCLC has now come of age, but with low mutation rates in patients of non-Asian ethnicity, cytotoxic chemotherapy remains the mainstay of management. From the nihilistic era of the past, the last decade has seen significant changes in treatment algorithms with the emergence of multiple lines of therapy.
The goal of this retrospective study was to review our clinical practice in delivering multiple lines of therapy in comparison to published data worldwide and highlight areas of practice to change in light of the new developments in molecular testing.
Methods
We undertook a retrospective review of consecutive patients presenting with advanced NSCLC treated with systemic therapy at two UK Hospitals within the same cancer network according to national and local Cancer Network clinical guidelines during a 2-year period, January 2007 to December 2008. Patient records and prescribing databases were scrutinized to generate data for all eligible patients who had received any form of systemic therapy in the study period. All patients were treated by site-specialized lung oncologists and all management plans were discussed within local lung multidisciplinary team meetings.
Eligibility included any patient diagnosed with inoperable (stage IIIb or IV) NSCLC whose primary treatment was palliative systemic therapy. Patients previously treated radically with surgery, radiotherapy or chemoradiotherapy were analysed separately to avoid bias in analysis of outcome.
Statistical analysis was performed using the SPSS statistical package version 17. The Kaplan–Meier method was used to calculate median overall survival (OS).
Results
A total of 130 patients were identified. Data were collected and censored as of March 2010. Median duration of follow up was 10 months. Individual patients receiving more than one line of therapy during the study period were captured more than once, with partial overlap of data for patients from one line of therapy to the subsequent line of treatment. Full demographics are shown in Table 1 and are typical for UK lung cancer chemotherapy practice. The majority of patients were male, exsmokers of PS 1 with stage IV disease.
Table 1.
Demographics and baseline characteristics of patient and tumour.
| All patients |
Second- and subsequent-line of therapy only |
Previous radically treated patients |
||||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| Age – year at diagnosis | ||||||
| Median | 66 | 65 | 65 | |||
| Range | 46-84 | 52-79 | 57-75 | |||
| Sex – n (%) | ||||||
| Male | 64 | 58 | 31 | 62 | 13 | 65 |
| Female | 46 | 42 | 19 | 38 | 7 | 35 |
| Ethnic group – n (%) | ||||||
| White | 109 | 99 | 49 | 98 | 19 | 95 |
| Asian | 1 | 1 | 1 | 2 | 1 | 5 |
| Smoking history – n (%) | ||||||
| Never smoked | 3 | 3 | 2 | 4 | 0 | 0 |
| Exsmoker | 62 | 56 | 23 | 46 | 8 | 40 |
| Current smoker | 35 | 32 | 19 | 38 | 9 | 45 |
| Unknown | 10 | 9 | 6 | 12 | 3 | 15 |
| WHO performance status at diagnosis – n (%) | ||||||
| 0 | 14 | 13 | 11 | 22 | 3 | 15 |
| 1 | 71 | 64 | 34 | 68 | 12 | 60 |
| 2 | 25 | 23 | 5 | 10 | 5 | 25 |
| Disease stage – n (%) | ||||||
| IIIB | 27 | 25 | 12 | 24 | ||
| IV | 83 | 75 | 38 | 76 | ||
| Histological feature of tumour – n (%) | ||||||
| NSCLC not otherwise specified | 47 | 43 | 20 | 40 | 11 | 55 |
| Adenocarcinoma | 22 | 20 | 13 | 26 | 4 | 20 |
| Squamous | 31 | 28 | 13 | 26 | 5 | 25 |
| Large cell | 4 | 4 | 1 | 2 | 0 | 0 |
| Bronchioalveolar | 3 | 3 | 2 | 4 | 0 | 0 |
| Mixed | 1 | 1 | 1 | 2 | 0 | 0 |
| No pathology | 2 | 1 | 0 | 0 | 0 | 0 |
| Method of histological diagnosis – n (%) | ||||||
| Biopsy | 65 | 59 | ||||
| Brushings/washings | 35 | 32 | ||||
| Cytology (e.g. FNA, pleural fluid) | 9 | 8 | ||||
| No histology | 1 | 1 | ||||
| Total | 110 | 50 | 20 | |||
WHO, World Health Organization; NSCLC, non-small cell lung cancer; FNA, fine needle aspiration.
Twenty patients were initially treated with radical intent, either by surgery and adjuvant chemotherapy (n = 9), chemoradiotherapy (n = 9) or radical radiotherapy (n = 2). All four patients who received adjuvant chemotherapy received a cisplatin and vinorelbine doublet for up to four cycles. The demographics of this group were comparable to the whole population.
Twenty six of the 110 patients with advanced disease at first presentation were treated with palliative radiotherapy in addition to chemotherapy.
First-line therapy: numbers of patients treated by regimen
One hundred and ten patients received palliative chemotherapy as their first oncological treatment. Seventy eight (71%) were treated with first-line carboplatin and gemcitabine. This regimen was the most commonly used platinum doublet regimen locally and within the UK prior to published data favouring pemetrexed for nonsquamous histology [Scagliotti et al. 2008; Rudd et al 2005]. For reasons of PS, clinician preference or comorbidity, a different regimen was substituted in 32 (29%) cases. Rationale behind choice of chemotherapy was not collected. In decreasing order of frequency, the other regimens used were carboplatin/paclitaxel (n = 5), cisplatin/vinorelbine (n = 4), single-agent vinorelbine (n = 3), erlotinib (n = 3), carboplatin/vinorelbine (n = 2), single-agent gemcitabine (n = 2), gemcitabine/carboplatin/thalidomide (n = 1 on trial) and other (n = 12). EGFR mutation status was unknown for the patients receiving erlotinib. No patients received maintenance treatment.
For 20 patients previously treated with radical intent, the most frequently used chemotherapy regimen in the first-line palliative setting was again carboplatin and gemcitabine (75%) with other regimens used in small numbers.
Second- and subsequent-line therapy by regimen
Fifty out of 110 patients (45%) treated with initial first-line systemic therapy subsequently received second-line therapy. A minority went on to receive third- or fourth-line systemic therapy. Twenty six out of 50 patients (52%) were treated with erlotinib in line with previously published National Institute of Clinical Excellence (NICE) guidance. At the time of treatment, EGFR mutation status was not routinely assessed. Remaining patients were treated with docetaxel (n = 9, 18%) or pemetrexed (n = 8, 16%). Seven patients (14%) received various other regimens including gemcitabine/carboplatin, single-agent gemcitabine and cyclophosphamide, adriamycin and vincristine (CAV).
Ten out of 110 patients (9%) went on to receive third-line therapy. Treatment was with docetaxel (n = 3), gemcitabine/carboplatin (n = 3), gemcitabine (n = 2), pemetrexed (n = 1) or paclitaxel (n = 1). One patient remains progression-free without further therapy at 19 months.
Two out of 110 patients (2%) went on to receive fourth-line therapy, one receiving erlotinib and one cisplatin/vinorelbine.
In the group of patients previously treated initially with radical intent, 20 patients were treated with first-line palliative systemic therapy, 11 received second-line therapy, 6 third-line therapy and 1 fourth-line therapy. The choice of regimen was broadly similar to the 110 patients treated with initial first-line systemic therapy (docetaxel 36%, erlotinib 36% and pemetrexed 27%).
Response rates
Response rates were assessed radiologically by contrast enhanced computerized tomography (CT) reported by an onsite consultant radiologists. Formal RECIST criteria were not used in this nontrial setting. Sixty three of out of 110 patients (58%) receiving first-line chemotherapy achieved clinical benefit (any radiological response or stable disease). Forty eight (44%) patients achieved a radiological response, 15 (14%) stable disease, 41 (37%) progressive disease and 6 patients had mixed or unassessable response.
In the second-line setting, clinical benefit was seen in 19 of 50 patients (38%): 6 (12%) radiological responses, 13 (26%) stable disease; the remaining 28 (56%) had progressive disease and 3 (6%) a mixed response.
In the third-line setting, 6 out of 10 patients achieved clinical benefit (60%): two (20%) radiological response, four (40%) stable disease; the remaining four (40%) had progressive disease. Neither patient treated with fourth-line therapy responded. Table 2 gives full details.
Table 2.
Response rate by line of treatment.
| Line of treatment | Number treated | Radiological response | Stable disease | Progressive disease | Mixed/ unassessable response | TTP |
|
|---|---|---|---|---|---|---|---|
| Line of therapy | Median TTP in months (range) | ||||||
| 1 | 110 | 48 (44%) | 15 (14%) | 41 (37%) | 6 (4.5%) | 1 | 5 (0–25) |
| 2 | 50 | 6 (12%) | 13 (26%) | 28 (56%) | 3 (6%) | 2 | 3 (0–21) |
| 3 | 10 | 2 (20%) | 4 (40%) | 4 (40%) | 0 | 3 | 3.75 (2–8) |
| 4 | 2 | 0 | 0 | 2 (100%) | 0 | 4 | 1.75 (1–2.5) |
TTP, time to progression.
Response rates to patients previously treated with radical intent in the first-line palliative setting were 35% radiological response, 15% stable disease, 50% progressive disease.
Number of cycles of chemotherapy
The median number of chemotherapy cycles delivered in the first- to third-line settings was 4 (range 1–6). Median time on erlotinib in the second-line setting was 2 months with a wide range (0–20 months). The two patients receiving fourth-line treatment received two cycles only.
Time to progression
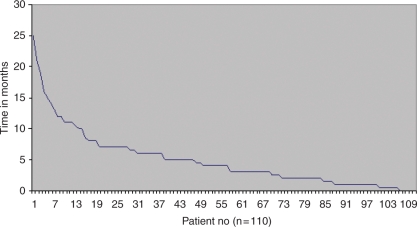
Time to progression (TTP) for each line of therapy was calculated from the start of that line of therapy to documented progression by radiological or clinical criteria. The median time to progression was 5 months (range 0–25 months) following first-line palliative therapy (n = 110); see Figure 1. Median TTP fell after each line of therapy (Table 2).
Figure 1.
Time to progression (months) from first-line chemotherapy by imaging or clinically.
Overall survival
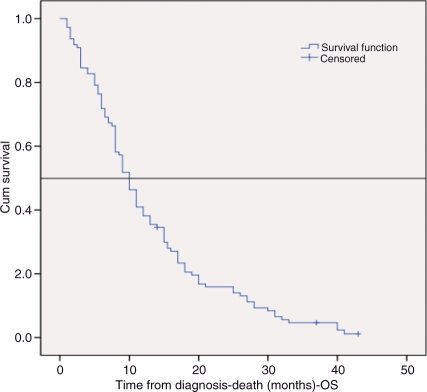
OS from the initial date of histological diagnosis until death was calculated using the Kaplan–Meier method. Median survival for the 110 patients receiving palliative chemotherapy as their first oncological treatment was 10 months (95% confidence interval [CI] 8.6–11.4 months); see Figure 2. Patients still alive (n = 3) were censored at the most recent follow-up appointment. Of note, for patients who received more than one line of therapy, median OS was 16 months (95% CI 14–17.9). Median survival from the first cycle of last-line treatment to death in the 50 patients who had received two to four lines of therapy was 5 months (95% CI 2.6–7.3), which is in line with other published data [Massarelli et al. 2003]. The range was <1 to 23 months. One-year and two-year survival rates were 41% and 15%, respectively.
Figure 2.
Median overall survival.
For the 20 patients who had previously been treated with radical intent, survival was significantly longer at 28 months from initial date of diagnosis until death (range 14–41 months).
Prognostic factors and demographics
Survival did not differ significantly depending on histology; however, we recognize the high proportion of NSCLC NOS (‘not otherwise specified’) may skew this finding. The median OS was 10 months for NSCLC NOS and squamous histology, 11 months for adenocarcinoma, 8 months for large cell (n = 4) and 5 months for bronchioalveolar carcinoma (n = 3). The latter two histology types had small patient numbers with wide ranges skewing the survival figures. OS for the 110 patients receiving palliative chemotherapy as their first oncological treatment was strongly related to PS. Median survival for patients with PS 0 was 15 months, PS 1 was 11 months and PS 2 was 5.5 months. Of note, 45 of 85 patients of PS 0–1 (53%) went on to receive two or more lines of chemotherapy. For patients of PS 2, only 5 of 25 (20%) patients received two or more lines of therapy.
Time on/off chemotherapy
Despite the use of multiple lines of systemic therapy, the majority of patients spent more time off treatment than time spent receiving active treatment. A total of 90% of patients spent over 50% of their time from histological diagnosis to death off therapy and 58% of patients spent over 75% time off therapy.
Discussion
We describe detailed information regarding the clinical course of 130 patients with advanced NSCLC treated with multiple lines of systemic therapy in a 2-year period from January 2007 to December 2008. The goal of this retrospective study was to review our clinical practice in comparison with published data worldwide and highlight areas of practice to change in light of the new developments in molecular testing. Overall, our outcomes are broadly consistent with published phase III data from the last 10 years [Rudd et al. 2005; Schiller et al. 2002]. Median survival for this unselected cohort of patients was 10 months with a 1-year survival of 41% and 2-year survival of 15%. Although we did not evaluate RECIST criteria response rates, chemotherapy resulted in radiological response or disease stabilisation in the majority of patients. For first-line therapy, 58% of patients achieved radiological response or disease stabilization.
Although the majority of patients received only one line of chemotherapy, a significant proportion (45%) of patients went on to receive second-line therapy. This figure compares favourably with clinical trials of immediate versus delayed second-line (docetaxel) chemotherapy where, even in the setting of a closely monitored trial setting, only 63% of patients in the delayed arm went on to receive second-line therapy [Fidias et al. 2009]. The likelihood of patients going on to receive second-line therapy appeared closely related to response to first-line treatment. The majority of patients who responded or achieved stable disease (54% and 53%, respectively) to first-line chemotherapy went on to receive second-line therapy. In contrast, only 27% of patients who progressed on first-line chemotherapy received any further treatment. Better PS at baseline was also strongly associated with a higher chance of going on to receive multiple lines of treatment (53% PS 0/1 versus 20% PS 2 patients). For those selected patients who did receive second- or third-line systemic therapy, survival was in keeping with published data [de Marinis and Grossi, 2008; Tambo et al. 2007; Shepherd et al. 2005]. These figures emphasize that where possible we should use the most effective therapy as first line.
A striking feature from this review is the degree to which treatment algorithms have changed in a relatively short period of time. For patients treated in 2007–2008, carboplatin/gemcitabine represented one UK standard for the first-line treatment of advanced NSCLC [Rudd et al. 2005]. Elsewhere in Europe and North America, cisplatin/gemcitabine or carboplatin/paclitaxel were more commonly used. In 2008, published data suggested differential outcomes to pemetrexed- or gemcitabine-containing, platinum-based chemotherapy for squamous and nonsquamous histologies [Azzoli et al. 2009]. Prior to the publication of this study, histological subtyping was not uniformly undertaken by all UK pathologists. Our data reveal a relative a paucity of a definite histological subtype with a significant proportion of patients (43%) reported as NSCLC NOS. Reporting of histological subtype is now considered of diagnostic importance and we have moved more into an era of histology-adapted chemotherapy.
In 2009, publication of the IPASS data [Mok et al. 2009] comparing gefitinib with chemotherapy strikingly demonstrated the importance of epidermal growth factor mutation status in predicting response and progression-free survival to treatment with the EGFR tyrosine kinase inhibitor (TKI), gefitinib, with profound ramifications on the treatment algorithm of advanced NSCLC. In 2007–2008, EGFR mutation status was not routinely undertaken for patients with advanced NSCLC. Treatment with the EGFR TKI, erlotinib, was given in an unselected manner and largely confined to the second-line setting in line with NICE guidance and the results from BR21 trial [Shepherd et al. 2005]. Although long-term control was achieved for a few patients, the majority of patients failed to respond to treatment. The advent of molecular testing in NSCLC has now come of age. Activating mutations in the EGFR are considered both prognostically favourable and strongly predict response to treatment. All patients should now be routinely tested for mutation status and, for activating mutation-positive patients, treatment with an EGFR TKI is considered standard first-line therapy. Mutation status appears to ‘trump’ all other variables and even for patients with poor PS, treatment can prove both effective and tolerable. A trial [Inoue et al. 2009] looking at poor performance patients (including PS 4) treated with first-line gefitinib illustrated the ‘Lazarus’ effect these drugs can have on some patients, indicating we should test all patients, including those who in the past would be treated with supportive/palliative care only. It is worth noting however, that within the UK population, the EGFR mutation rate is low (around 10%) and cytotoxic chemotherapy will remain the dominant treatment modality for the majority of patients both in the primary treatment of advanced disease, and at relapse or progression. The use of first-line EGFR TKIs brings with it new uncertainties. For mutation positive patients, the optimal approach to the management of progressive disease following first-line treatment with an EGFR TKI is uncertain. Along with this tide of change in molecular testing (not only EGFR but KRAS, ALK and Her2) come new challenges. What happens to mutation status with advancing disease, the development of metastases and the effect of chemotherapy is unclear. There is some evidence of discordance between primary and metastatic mutation status [Gow et al. 2009] and that sequencing of targeted treatment and chemotherapy may affect response rate perhaps due to acquisition of new mutations/resistance mechanisms [Maemondo et al. 2010]. Molecular testing was not carried out in our cohort but we theorize that those patients with more extreme OS and progression-free survival may have an inherently less aggressive biology with good prognosis mutations.
Since 2007–2008, several studies have suggested a benefit from the use of maintenance therapy, an approach not in routine use at that time [Cappuzzo et al. 2010; Ciuleanu et al. 2009]. The rapid pace of change in NSCLC, however, means that the applicability of these studies to a broad population of patients with advanced NSCLC is uncertain, particularly in group of patients who will receive pemetrexed or gefitinib as a first-line treatment approach.
The future of advanced NSCLC is in selecting the best treatment approach on a histological and genotypic basis. Adequate tissue sampling with detailed histopathological assessment and molecular profiling has become a new standard of care perhaps with repeat biopsy a new consideration. For patients with advanced NSCLC, we have moved from a situation of one size fits all to the dawn of individualized cancer therapy.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors declare no conflicts of interest in preparing this article.
References
- Azzoli, C.G., Baker, S., Jr, Temin, S., Pao, W., Aliff, T., Brahmer, J., et al. (2009) American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small Cell Lung Cancer. J Clin Oncol 27: 6251-6266. Available at: http://www.asco.org/guidelines/nsclc. [DOI] [PMC free article] [PubMed]
- Cancer Research UK (2010) Cancer research UK mortality statistics, July 2010, http://info.cancerresearchuk.org/cancerstats/mortality/
- Cappuzzo F., Ciuleanu T., Stelmakh L., Cicenas S., Szczésna A., Juhász E., et al. (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11: 521–529 [DOI] [PubMed] [Google Scholar]
- Ciuleanu T., Brodowicz T., Zielinski C., Kim J.H., Krzakowski M., Laack E., et al. (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374: 1432–1440 [DOI] [PubMed] [Google Scholar]
- de Marinis F., Grossi F. (2008) Clinical evidence for second- and third-line treatment options in advanced non-small cell lung cancer. The Oncologist 13(Suppl. 1): 14–20 [DOI] [PubMed] [Google Scholar]
- Fidias P.M., Dakhil S.R., Lyss A.P., Loesch D.M., Waterhouse D.M., Bromund J.L., et al. (2009) Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small cell lung cancer. J Clin Oncol 27: 591–598 [DOI] [PubMed] [Google Scholar]
- Gow C.-H., Chang Y.-L., Hsu Y.-C., Tsai M.-F., Wu C.-T., Yu C.-J., et al. (2009) Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naïve non-small-cell lung cancer. Ann Oncol 20: 696–702 [DOI] [PubMed] [Google Scholar]
- Inoue A., Kobayashi K., Usui K., Maemondo M., Okinaga S., Mikami I., et al. (2009) First-line gefitinib for patients with advanced non–small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 27: 1394–1400 [DOI] [PubMed] [Google Scholar]
- Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., et al. (2010) Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med 362: 25–25 [DOI] [PubMed] [Google Scholar]
- Massarelli E., Andre F., Liu D.D., Lee J.J., Wolf M., Fandi A., et al. (2003) A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer 39: 55–61 [DOI] [PubMed] [Google Scholar]
- Mok T.S., Wu Y.-L., Thongprasert S., Yang C.-H., Chu D.T., Saijo N., et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947–957 [DOI] [PubMed] [Google Scholar]
- Rudd R., Gower N., Spiro S., Eisen T.G., Harper P.G., Littler J.A.H., et al. (2005) Gemcitabine plus carboplatin versus mitomycin, ifosfamide, and cisplatin in patients with stage IIIB or IV non-small-cell lung cancer: a phase III randomized study of the London Lung Cancer Group. J Clin Oncol 23: 142–153 [DOI] [PubMed] [Google Scholar]
- Scagliotti G.V., Parikh P., Von Pawel J., Biesma B., Vansteenkiste J., Manegold C., et al. (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemextrexed in chemotherapy-naïve patients with advanced–stage non-small cell lung cancer. J Clin Oncol 26: 3543–3551 [DOI] [PubMed] [Google Scholar]
- Schiller J.H., Harrington D., Belani C.P., Langer C., Sandler A., Krook J., et al. (2002) Comparison of four chemotherapy regimens for advanced non-small cell lung cancer. N Engl J Med 346: 92–98 [DOI] [PubMed] [Google Scholar]
- Shepherd F.A., Rodrigues Pereira J., Ciuleanu T., Tan E.H., Hirsh V., Thongprasert S., et al. (2005) Erlotinib in previously treated non-small-cell-lung cancer. N Engl J Med 353: 123–132 [DOI] [PubMed] [Google Scholar]
- Tambo Y., Kasahara K., Kimura H., Sone T., Araya T., Tamori S., et al. (2007) A retrospective study of salvage chemotherapy for advanced non-small cell lung cancer; the efficacy of third and fourth chemotherapy: P2-312. J Thoracic Oncol 2: S697–S697 [Google Scholar]