Abstract
Hepatocyte growth factor receptor (HGFR), the product of the MET gene, plays an important role in normal cellular function and oncogenesis. In cancer, HGFR has been implicated in cellular proliferation, cell survival, invasion, cell motility, metastasis and angiogenesis. Activation of HGFR can occur through binding to its ligand, hepatocyte growth factor (HGF), overexpression/amplification, mutation, and/or decreased degradation. Amplification of HGFR can occur de novo or in resistance to therapy. Mutations of HGFR have been described in the tyrosine kinase domain, juxtamembrane domain, or semaphorin domain in a number of tumors. These mutations appear to have gain of function, and also reflect differential sensitivity to therapeutic inhibition. There have been various drugs developed to target HGFR, including antibodies to HGFR/HGF, small-molecule inhibitors against the tyrosine kinase domain of HGFR and downstream targets. Different HGFR inhibitors are currently in clinical trials in lung cancer and a number of solid tumors. Several phase I trials have already been completed, and two specific trials have been reported combining HGFR with epidermal growth factor receptor (EGFR) inhibition in non-small cell lung cancer. In particular, trials involving MetMAb and ARQ197 (tivantinib) have gained interest. Ultimately, as individualized therapies become a reality for cancers, HGFR will be an important molecular target.
Keywords: hepatocyte growth factor, kinase inhibitors, MET, targeted therapy
Biology of lung cancer
The most common cancer to be diagnosed in the United States is lung cancer. There are estimated an 222,520 cases diagnosed in 2010. The mortality burden from this disease is quite high, with an estimated 157,300 deaths expected in that year [Jemal et al. 2010]. According to Surveillance, Epidemiology, and End Results (SEER) 17 data, the age-adjusted incidence of cancer of the lung and bronchus was 62.5 per 100,000 persons per year from 2003 to 2007. There are a large number of molecular and cellular abnormalities that can occur in lung cancer [Salgia and Skarin, 1998]. As an example, there can be oncogenic activation of receptor tyrosine kinases, activation of signal transduction pathways, and deletion of tumor suppressors such as p53 and Rb. One of the emerging themes in lung cancer is the alteration of receptor tyrosine kinases (RTKs). Over the past several years, the epidermal growth factor receptor (EGFR) has been shown to be mutated (in particular the tyrosine kinase domain), with mutation being predictive of therapeutic response to small-molecule inhibition with agents such as erlotinib and gefitinib. Another RTK that can be genetically altered is ALK on chromosome 2. A translocation on chromosome 2p results in the generation of the potent oncogene EML4-ALK. This fusion protein has recently been shown to be a target of the small-molecule inhibitor, crizotinib (formerly PF02341066). In this review, we focus on the RTK MET. MET RTK has been shown to be important in normal homeostasis, and signal transduction, and is abnormal in various cancers.
The hepatocyte growth factor receptor is expressed on functionally different cells
There is a considerable amount of systems biology established as related to hepatocyte growth factor (HGF) and its receptor (HGFR). The biological activities triggered by binding of HGF to HGFR, the product of the MET proto-oncogene, initiates a complex variety of responses. This is in part due to the expression of HGFR on functionally different cells (Table 1); its functions may include growth, transformation, cell motility, invasion, metastasis, epithelial to mesenchymal transition (EMT), angiogenesis, wound healing, or tissue regeneration depending on the cellular context [Weidner et al. 1996; Jeffers et al. 1996; Rong et al. 1994; Grant et al. 1993; Montesano et al. 1991; Stoker et al. 1987]. These broad biological functions are reflected in mice with MET gene disruption, which results in embryonic lethality with severe defects in liver and placenta development [Uehara et al. 1995]; the phenotype is similar in mice with a disruption of the HGF gene [Schmidt et al. 1995]. Further, both genetic models implicate HGF and HGFR in a broader role in morphogenesis and growth of multiple embryonic tissues, including the nervous system [Maina and Klein, 1999; Bladt et al. 1995; Schmidt et al. 1995; Uehara et al. 1995].
Table 1.
Frequency of abnormalities in the hepatocyte growth factor receptor (HGFR)/MET pathway. The frequency of increased HGFR protein expression or mutation and amplification of MET is thought to be dependent on environmental factors and genetic predisposition. Alteration in the HGFR/MET pathway can ultimately lead to transformation of normal cells. All values are indicated as percentage of total (M = marginal).
| Tumor type | HGFR expression | MET mutation | MET amplification |
|---|---|---|---|
| Brain | 54–88 | 0–9 | 9–20 |
| Breast | 25–60 | M | − |
| Cervical | 30–72 | 0 | − |
| Colon | 55–78 | 0 | 4–89 |
| Gastric | 75–90 | M | 5–10 |
| Head and neck | 52–68 | 11–27 | − |
| Hepatoma | 68–69 | 0–30 | − |
| Lung (NSCLC) | 41–72 | 8–13 | 5–10 |
| Melanoma | 17–39 | M | − |
| Mesothelioma | 74–100 | 0 | − |
| Multiple myeloma | 48–80 | − | − |
| Ovarian | 64 | 0–4 | 0 |
| Renal cell | 54–87 | 13–100 | (Trisomy 7) |
| Sarcoma | 20–87 | 0–3 | − |
| Thyroid | 40–91 | 6–10 | − |
NSCLC, non-small cell lung cancer.
In addition to the induction of cell growth and reduced apoptosis, there are profound biological effects of activated HGFR on cytoskeletal function. The ability of activated HGFR to induce growth and scattering of epithelial cells has been well documented [Stoker et al. 1987]. Cell scattering is a process regulated through the cytoskeleton that involves cell migration, cell–cell dissociation, and cell spreading. For example, in a small-cell lung cancer (SCLC) model, stimulation of HGFR with HGF enhances cell motility, a process that involves the formation and retraction of filopodia/lamellipodia, changes in actin filament formation, and cell migration [Maulik et al. 2002]. However, altered motility can also be induced by mutationally activated HGFR signaling in epithelial cells [Jeffers et al. 1998]. Progress has been made in understanding the signaling mechanisms by which HGF stimulation of HGFR leads to increased motility, migration, and invasion. A major downstream molecule is the phosphatidylinositol-3′-kinase, which is required for HGF-induced mitogenesis, motogenesis, or morphogenesis in renal epithelial cells. Inhibition of its activity decreased branching formation on collagen and chemotaxis [Derman et al. 1996]. Also of interest is the focal adhesion protein paxillin, which is phosphorylated (pY31) in response to HGFR stimulation [Maulik et al. 2002]. Paxillin is highly overexpressed in lung cancers, in particular in metastatic tumors and frequently along with HGFR and EGFR. It is also mutated in almost 10% of all lung cancer cases [Jagadeeswaran et al. 2008]. The exact role of paxillin in HGFR transformation is not known, but activating mutations of HGFR (R988C and T1010I) in SCLC that modulate cellular transformation, anchorage-dependent proliferation, cytoskeletal motility and migration, also increase tyrosine phosphorylation of paxillin (pY31) [Ma et al. 2003].
Owing to the pleiotropic effects regulated through activated HGFR and the variety of cells that express the receptor, it is not surprising that this pathway has gained interest as a driving force in tumor initiation, maintenance, and progression of not only lung cancer but also other malignancies. Additional efforts are being made to further understand the effectors of HGFR signaling and to evaluate its potential for targeted therapies. Initial clinical trials with various inhibitors of HGFR activation hold promise but the value of the activation and mutational status of MET as prognostic factors needs further assessment. It is not known whether HGFR cooperates with additional oncoproteins, or if the oncogenic potential of HGFR is increased by the deregulation of other signaling pathways.
Structure and function of HGFR and HGF
The human MET gene is located on chromosome 7 (7q31) and encodes a transmembrane protein. The first observation of MET as a potential proto-oncogene stems from its discovery as the fusion partner with Tpr in the transforming fusion oncogene TPR-MET in an immortalized osteosarcoma cell line that had been chemically mutagenized with N-methyl-N′-nitro-N-nitrosoguanidine [Cooper et al. 1984]. In the Tpr-MET translocation the TPR gene (chromosome 1) is fused with the MET kinase gene (chromosome 7). Similar to other tyrosine kinase oncogenes, such as BCR-ABL and TEL-ABL, where the fusion partners BCR and TEL provide an oligomerization domain for the activation of ABL [Banerji and Sattler, 2004], the TPR sequence provides two leucine zipper domains, which facilitate oligomerization and substitute for ligand-stimulated activation. The structural changes as a result of the fusion with Tpr are thought to lead to the release of structural constraints that allow for constitutive activation of the HGFR kinase activity, thus turning it into a transforming protein [Zhen et al. 1994; Rodrigues and Park, 1993]. Other than in this example of chemically induced oncogenesis, there is little evidence that the TPR-MET translocation has clinical relevance or occurs in vivo.
HGFR expression has been described in a majority of normal cells and tissues. The receptor is synthesized as a single precursor that is posttranslationally digested and glycosylated, forming a 50 kDa extracellular α-chain and a transmembrane 140 kDa ß-chain, which are then linked by disulfide bonds. HGFR is related to RON and the avian SEA receptor tyrosine kinases, which have extracellular structures related to the semaphorin receptor (or plexin) family [Iwama et al. 1994; Huff et al. 1993; Ronsin et al. 1993]. The HGFR ß-chain contains homologous structural domains shared with other proteins, including a semaphorin (sema) domain, a PSI domain (found within plexins, semaphorins, and integrins), four IPT repeats (found within immunoglobulins, plexins, and transcription factors), a transmembrane domain, a juxtamembrane (JM) domain, a tyrosine kinase domain, and a carboxy-terminal tail region.
HGFR is normally activated by ligation through its natural ligand HGF (or scatter factor). HGF is a member of the plasminogen-related growth factor family and was originally identified as a growth factor for hepatocytes and as a fibroblast-derived cell motility or scatter factor [Nakamura et al. 1987; Stoker et al. 1987; Gohda et al. 1986]. The HGF precursor is predominantly produced by mesenchymal cells, acting on epithelial cells [Sonnenberg et al. 1993; Stoker et al. 1987]. The secreted product is proteolytically cleaved into a disulfide-linked heterodimer. HGF consists of six domains (N-terminal domain, four kringle domains, and a C-terminal domain, which is structurally similar to the catalytic domain of serine proteinases). The stoichiometry of HGF binding to HGFR is 2:2. HGF has been shown to bind to the sema domain, suggesting an important role for activation and receptor dimerization [Gherardi et al. 2006; Stamos et al. 2004]. The HGFR sema domain contains seven beta sheets that fold into the shape of a seven-bladed propeller structure, where the second and third sheet bind to the HGF ß-chain active site region [Stamos et al. 2004].
Aberrant HGFR activation can occur through HGF ligation or ligand-independent mechanisms. In transformed cells, the most likely mechanism of HGFR activation is overexpression and MET gene amplifications and mutations (germline and somatic) in a variety of malignancies. Thus, for the development of successfully targeted therapies, it will be necessary to understand the structural requirements involved in ligand-induced HGFR activation as well as the biochemical consequences that elicit a specific biological response. Advances in the development of targeted therapies for tyrosine kinase oncogenes suggest that HGFR may provide several domains that are rational targets for clinical therapeutics.
Dysregulation of HGFR expression
HGF-dependent autocrine HGFR activation has been found in human primary and metastatic tumors, including breast cancer, glioblastoma, osteosarcoma, melanoma, and others [Li et al. 2001; Koochekpour et al. 1997; Tuck et al. 1996; Ferracini et al. 1995]. Nonautocrine mechanisms can also activate the metastatic growth and potential of cancer cells. HGFR expressing tumor cells transplanted in HGF expressing transgenic mice resulted in pulmonary metastases, which could be altered by a HGF antagonist [Yu and Merlino, 2002]. The regulation of HGFR activity in oncogenic transformation is likely to be different compared with normal HGFR signaling. This is exemplified by the fact that overexpression of HGFR can have oncogenic potential by itself. Elevated HGFR levels and functional activation of the HGFR pathway have been shown to be sufficient for transformation of normal osteoblasts. In vitro, overexpression of HGFR resulted in the conversion of primary human osteoblasts into transformed osteosarcoma cells, also causing an osteosarcoma-like disease in vivo [Patane et al. 2006]. Overexpression of HGFR in hepatocytes is sufficient to induce hepatocellular carcinoma in transgenic mice [Wang et al. 2001]. Another important example is lung cancer, where HGFR overexpression is associated with higher pathological tumor stage and worse outcome [Rossi et al. 2005; Ichimura et al. 1996; Olivero et al. 1996]. HGFR was also found to be overexpressed in 20 of 52 squamous cell carcinomas, in 34 of 47 adenocarcinomas, and in all 11 non-small cell lung cancer (NSCLC) cell lines studied [Ichimura et al. 1996]. In addition to HGFR, higher levels of HGF can also be associated with a poorer prognosis in NSCLC [Siegfried et al. 1998]. In NSCLC, HGFR levels can be 2–10 times increased and HGF levels can be 10- to 100-fold higher compared with adjacent normal lung tissue [Siegfried et al. 1997]. Interestingly, susceptibility to carcinogen-induced lung cancer is increased in transgenic mice with HGF overexpression in the airways [Stabile et al. 2006]. A number of other proteins have been correlated with Met expression, and the downstream target paxillin and the transcription factor pax5 can be co-expressed with Met and/or phosphorylated Met in neuroendocrine tumors [Song et al. 2010]. Thus, in contrast to most cancers, at least in lung cancer, there does not seem to be a requirement for abnormal expression of both HGF and its receptor for cancer susceptibility or development.
The transcriptional regulation of HGF is poorly understood, but several key factors that increase HGFR expression have been identified. For example, HGFR expression can be driven by hypoxia and hypoxia can increase HGF-dependent invasion [Pennacchietti et al. 2003]. In colorectal cancer, upregulation of HGFR may be an early event that is controlled by Wnt signaling [Boon et al. 2002]. Also, members of the Pax family of transcriptional activators involved in development are important regulators of HGFR expression. Both Pax3 and Pax7 modulate the expression of HGFR during limb muscle development, partially through unique mechanisms [Relaix et al. 2004; Epstein et al. 1996]. In lung cancer, PAX5 is expressed in SCLC and not NSCLC, whereas PAX8 is expressed in NSCLC and not SCLC [Kanteti et al. 2009]. PAX5 is shown to be a transcription factor for HGFR in SCLC and activated HGFR can be found within the nucleus. There are likely tissue- and lineage-specific additional factors that may act in concert with factors that function in a more general fashion to regulate HGFR expression. The participation of specific activators or co-activators of MET transcription in transformation of HGFR-dependent tumors is an intriguing possibility of additional therapeutic value that requires further attention.
Mutations of MET in cancer
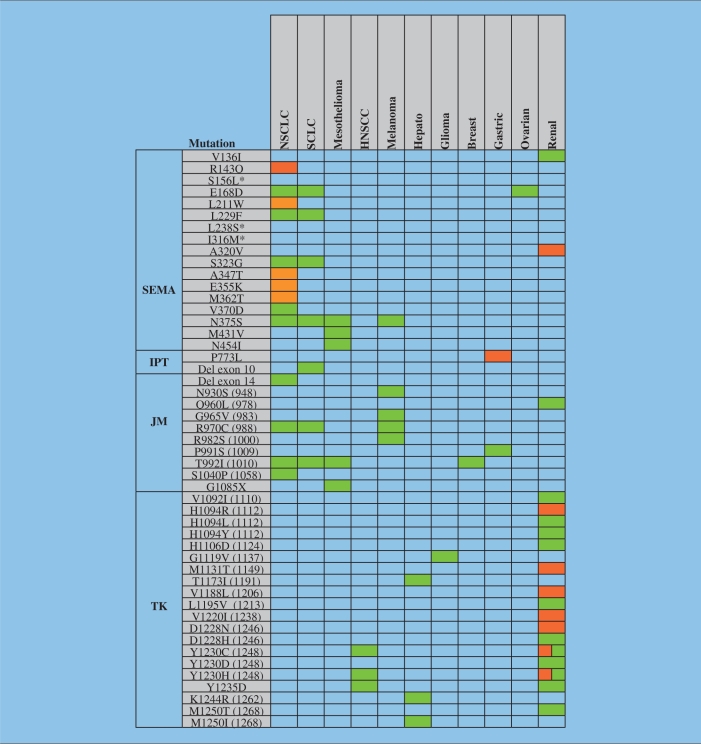
There are two major mechanisms involving HGFR that can lead to activation or hyperresponsiveness of the HGFR/HGF pathway. MET can be either mutated in the extracellular or in the cytoplasmic domain (Table 2). Mutations can alter inhibitory constraints that allow the kinase to be either active or hyperresponsive to stimuli. Mutations can also alter the degree of activation or prevent efficient degradation of the protein, therefore prolonging the duration of the biochemical signals. Another mechanism of ligand-independent activation is the overexpression of the functional wild-type protein. Both mechanisms of HGFR activation have been observed, either individually or concomitantly. Tyrosine kinase mutations in MET were first identified in hereditary papillary renal cell carcinoma [Schmidt et al. 1997]. A systematic analysis of the complete exons, initially for lung cancer and thereafter for a number of other solid tumors, revealed ‘hot-spots’ of mutations, especially in the sema and JM domains.
Table 2.
Hepatocyte growth factor receptor (HGFR) is a target of mutations in various malignancies. Known mutations within the semaphorin (SEMA), immunoglobulin plexin transcription (IPT), juxtamembrane (JM), and tyrosine kinase (TK) domain of MET are summarized. Mutations were found in non-small cell lung cancer (NSCLC), small-cell lung cancer (SCLC), mesothelioma, head and neck squamous cell carcinoma (HNSCC), melanoma, glioma, breast cancer, gastric cancer, and renal cell carcinoma in somatic (green) or germ line (orange) cells. There are two isoforms of HGFR and the amino acids are numbered according to the canonical isoform 1. The sequence of isoform 2 was used in some publications (differences indicated in brackets) and contains the following sequence after S755: TWWKEPLNIVSFLFCFAS (see also http://www.uniprot.org and [Krishnaswamy et al. 2009]). Hepato, hepatocellular carcinoma. *Tissue not indicated in publication.
MET mutations in the semaphorin domain
The extracellular sema domain within MET is encoded by exon 2, and provides a specific binding site for HGF. Mutational analysis suggests that this domain is required for receptor activation and dimerization [Kong-Beltran et al. 2004]. The crystal structure of the HGFR sema domain revealed that it adopts a seven-blade ß-propeller fold [Gherardi et al. 2006]. This important structural information will be helpful for the development of targeted therapies. Interference with the regulatory function of this domain would be expected to alter HGFR function [Kong-Beltran et al. 2004]. Interestingly, a portion of mesothelioma patients (7%) were found to have mutations (N375S, M431V, and N454I, the sequence of the canonical isoform 1 of HGFR was used for the numbering presented here) within this domain; however, the functional role of these MET mutations in transformation is not known [Jagadeeswaran et al. 2006]. Most recently, there have been germline MET mutations described in the sema domain in lung cancer [Krishnaswamy et al. 2009]. There is also a cluster of mutations that are different in various ethnic groups. As an example, N375S is identified in lung cancer patients from Asian and Caucasian origin; however, this mutation is not identified in African-American patients with lung cancer. In lung cancer, N375S is more common in squamous cell carcinomas and not adenocarcinomas or large-cell carcinomas. The squamous cell carcinomas harboring the N375S mutation were also smokers, whereas EGFR mutations are somatic, associated with adenocarcinomas, and more common in nonsmokers. These MET sema mutations result in differential binding to HGF, and the N375S mutation results in increased resistance to small-molecule inhibition.
MET mutations in the JM domain
Gain-of-function mutations in the JM domain have been well characterized for the receptor tyrosine kinase FLT3. They are present in about 20% of adult acute myeloid leukemia (AML) patients and thought to regulate the catalytic activity of the FLT3 kinase [Yokota et al. 1997]. Recently, a T992I JM domain MET mutation was identified in AML, although AML is usually not associated with JM domain mutations of MET [Loriaux et al. 2008]. The molecular mechanisms affected by JM mutations in MET are not well characterized. Interestingly, MET mutations of the JM domain in Rottweiler dogs appear to increase cancer risk. The G966S gain-of-function germline mutation of MET occurs at a frequency of 74% in these dogs. Specifically, the carriers of this mutation appear to be predisposed to cancers, including lymphoma, osteosarcoma, and histiocytic sarcoma [Liao et al. 2006].
Some of the JM mutations found in human cancers may accelerate tumor formation rather than being fully transformed by themselves. A good example is the HGFR-T992I mutation, which is not associated with full activation of the HGFR kinase activity. However, tumors form faster in athymic nude mice injected with HGFR-T992I expressed in NIH3T3 cells compared with wild-type HGFR expressing cells [Lee et al. 2000].
In addition to the T992I mutation, R970C and T992A germline mutations were identified from 126 patients with adenocarcinomas. A screen of 79 mouse inbred strains also identified the R968C variation in SWR/J mice; genetic evidence suggests the importance of this variation in lung tumorigenesis [Zaffaroni et al. 2005]. The T992I mutation is present in some cases of hereditary papillary renal cell carcinoma and has been seen in a patient with breast cancer [Lee et al. 2000; Schmidt et al. 1999]. The same mutation is also found in the human H513 and H2596 mesothelioma cell lines [Jagadeeswaran et al. 2006]. Mutational analysis of SCLC tissue samples and cell lines revealed R970C, T992I, and S1040P mutations in the JM domain. The R970C mutation has been described in lung cancer from African-Americans and Caucasians but not in Asians. In vitro data suggest that these alterations may contribute to enhanced tumorigenicity, cell migration, and phosphorylation of HGFR protein in this disease [Ma et al. 2003]. Increased levels of reactive oxygen species (ROSs) have also been found to be associated with the R970C and T992I variants [Jagadeeswaran et al. 2007]. ROS in most cancer cells are of mitochondrial origin and do not only play a role in cancer cell signaling, but may also contribute to genomic instability [Rodrigues et al. 2008].
In 21% of human melanomas, the HGFR receptor was found to be phosphorylated at the Y1003 activation site. Further, a N930S HGFR missense mutation was also identified in tumor tissue in addition to the HGFR-R970C mutation in melanoma cell lines [Puri et al. 2007]. The Y1003 residue is a binding site for the ubiquitin E3 ligase CBL and is crucial for the regulation of HGFR activity. When replaced by phenylalanine (Y1003F), a loss of ubiquitination of the HGFR receptor occurs and HGFR gains transforming activity in fibroblast and epithelial cells [Peschard et al. 2001]. The P991S germline mutation in the HGFR JM domain was found in a patient with gastric carcinoma. This mutation does not appear to induce ligand-independent activation of HGFR but it showed increased persistent response to HGF stimulation when expressed in fibroblasts [Lee et al. 2000].
MET mutations in the kinase domain
The kinase activity of HGFR is essential for the activation of signaling pathways and biological responses. Owing to its essential role, efforts had initially been directed on identifying mutations in the HGFR kinase domain of HGFR-expressing tumors. However, this mechanism of deregulating HGFR does not appear common and the majority of tyrosine kinase domain activating mutations in MET have been described in sporadic papillary renal carcinomas (somatic) and hereditary papillary renal cell carcinomas (germline) [Schmidt et al. 1997]. These mutations are sufficient to result in an increase in HGFR kinase activity and increased motility and metastasis [Jeffers et al. 1998; Schmidt et al. 1997]. Even though recent analyses aimed at identifying additional mutations have included all exons coding for the MET gene, it is conceivable that in vivo, the use of small-molecule tyrosine kinase inhibitors may lead to a reemergence of HGFR kinase domain mutations, as it has been shown for BCR-ABL and other kinases during targeted therapy [Walz and Sattler, 2006].
Amplification of MET
In addition to missense mutations, HGFR can also be activated by amplification. During development, the HGFR receptor is mainly expressed by epithelial cells but can also be found in a variety of human cancer cell lines or tumor tissue. Overexpression of MET occurs in many tumors to various degrees. For example, 10–20% of human gastric carcinomas have MET amplification [Sakakura et al. 1999], and gastric cancer cell lines show increased susceptibility to HGFR kinase inhibition [Smolen et al. 2006]. The breakage–fusion–bridge (BFB) mechanism is thought to be a major cause for MET amplifications in these cancers [Hellman et al. 2002]. Interestingly, in NSCLC, amplification of MET strongly correlates with paxillin expression, a focal adhesion protein involved in the regulation of cytoskeletal functions. The potential prognostic value of paxillin expression in HGFR-dependent cancers is not known but it should be noted that paxillin is also a target of somatic mutations in approximately 9% of NSCLC [Jagadeeswaran et al. 2008]. Since amplification of MET is sufficient for transformation, one could envision MET amplification may also be a mechanism of a drug-resistant phenotype in cancers transformed by other oncogenes. In vitro, this mechanism has already been demonstrated in EGFR-dependent lung tumors that are resistant to the EGFR small-molecule inhibitor gefitinib [Engelman et al. 2007]. Although these data were not confirmed in the human disease [Bean et al. 2007], additional analysis of the mutational status of MET might provide a better understanding about its role in drug-resistant tumors.
HGF-dependent signaling through its receptor
Physiological activation of the HGFR signaling pathway is initiated by binding of soluble HGF to monomeric cell surface expressed mature HGFR receptors. HGF stimulation is followed by receptor dimerization, activation of its tyrosine kinase and rapid initiation of signaling cascades. One of the initial events of HGFR activation is thought to be phosphorylation at the three conserved tyrosine residues Y1230, Y1234, and Y1235 in the activation loop of the kinase domain. Phosphorylation at Y1234 and Y1235 correlates with increased tyrosine kinase activity [Rodrigues and Park, 1994] and is required for HGFR kinase activity [Longati et al. 1994]. Additional phosphorylation sites in HGFR lead to the recruitment of signaling proteins, which mediate downstream signaling events. These sites are either directly phosphorylated by HGFR or by other protein kinases. The unique multisubstrate docking sites Y1349 and Y1356 lead to the recruitment of a signaling complex when phosphorylated [Ponzetto et al. 1994]. Y1356 of HGFR is required for the binding of the adapter protein GRB2 through its SH2 (Src homology 2) domain [Nguyen et al. 1997]. Phosphorylated Y1349 interacts with the MBD (MET binding domain) containing adapter GAB1 (GRB2 associated binder 1), which may be sufficient for partial receptor interaction. The related GAB2 requires additional SH3 domain binding from receptor bound GRB2 [Lock et al. 2002; Nguyen et al. 1997]. GAB1 is the major substrate for HGFR in epithelial cells and is also required for the morphogenic response [Nguyen et al. 1997; Weidner et al. 1996]. Cell morphogenesis is also mediated in part through Y1365 [Weidner et al. 1995]. An important regulator of this response with pleiotropic effects is phosphatidylinositol-3′kinase (PI3K), which is regulated indirectly through recruitment of Gab1 and binding of the p85 regulatory subunit of PI3K [Bardelli et al. 1997; Ponzetto et al. 1994]. Additional posttranslational modifications and domain structures are likely to contribute to the biological functions induced by the activated HGFR receptor and may also include nontyrosine residues that can alter HGFR function. As an example, S985 negatively regulates HGFR, likely involving phosphorylation by protein kinase C [Gandino et al. 1994].
A major difference between normal signaling through receptor activation and oncogenic, ligand-independent signaling is the transient nature of the stimulus. In transformed cells, regulatory mechanisms can be disrupted, whereas normal signaling is terminated by activation of specific phosphatases and internalization of HGFR into clathrin-coated vesicles. As part of the endosomal complex, HGFR is then finally degraded via the lysosomal pathway [Hammond et al. 2003, 2001]. Y1003 within the juxtamembrane domain of HGFR is an important regulatory site, which participates in this degradation process through recruitment of CBL, an E3-ubiquitin ligase. CBL binds to Y1003 when phosphorylated and facilitates ubiquitination of the HGFR receptor. HGFR containing the Y1003F mutation is not ubiquitinated and does not show altered HGFR internalization but increased stability of HGFR due to decreased lysosomal receptor degradation and thus further recycling to the membrane and signaling as well as oncogenic activation [Abella et al. 2005]. It would be predicted that the function of CBL is disrupted or altered at some level to enable prolonged or chronic signaling through HGFR in cancer cells. Activating mutations of CBL have been identified in AML and myeloproliferative disorders [Fernandes et al. 2010; Grand et al. 2009; Dunbar et al. 2008; Caligiuri et al. 2007; Sargin et al. 2007]. In lung cancer, novel CBL mutations may also indicate an essential role for tumorigenesis and metastasis [Tan et al. 2010]. Somatic CBL mutations were not found to be mutually exclusive of MET or EGFR mutations, but they appear independent of p53 and KRAS mutations. In addition, pairwise analysis (normal/tumor) indicates a significant loss of heterozygosity (LOH) for the CBL locus (22%, n = 8/37), in the absence of CBL mutations. CBL LOH was positively correlated with EGFR and MET mutations, suggesting an important role in EGFR and HGFR receptor regulation.
HGFR inhibition
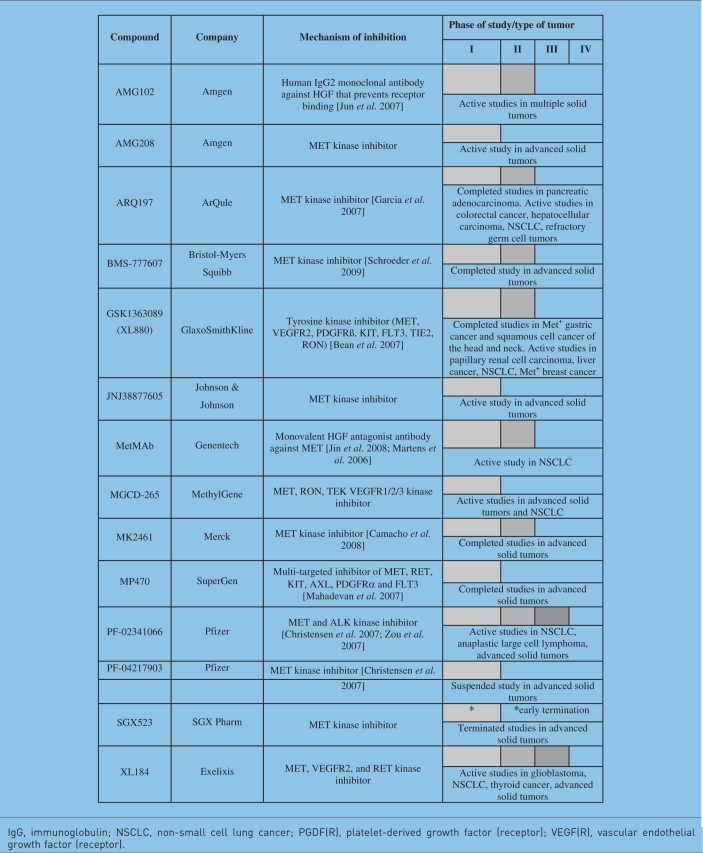
Several novel agents targeting the HGFR pathway are currently under clinical investigation. (Table 3). Some of these molecules target other molecules along with HGFR. Ongoing clinical trials will potentially validate HGFR as a therapeutic target, improving the outcome of patients with HGFR-dependent cancers. HGFR small-molecule inhibitors have been utilized as single agents as well as in combination regimens. As we showed early on [Puri and Salgia, 2008], EGFR and HGFR synergize in biological functions such as cancer cell growth and cell motility/migration. There is also increased phosphorylation of various signal transduction molecules such as AKT and mammalian target of rapamycin (mTOR), thereby leading to activation. When combined with small-molecule inhibitors such as gefitinib, there was increased and synergistic apoptosis with a small-molecule MET inhibitor in a NSCLC cell line. Most recently, the small-molecule MET inhibitor SGX523 has been shown to be synergistic with erlotinib in preclinical models [Zhang et al. 2010]. Synergism has also been reported for MET inhibition with mTOR inhibition [Ma et al. 2005], PI3K inhibition [Ma et al. 2005], and cisplatin [Seiwert et al. 2009]. In addition, MET inhibition may have an additive effect when combined with topoisomerase-I inhibition in SCLC cells [Kanteti et al. 2009]. MET inhibition can also overcome sunitinib resistance, as well synergize with sunitinib [Shojaei et al. 2010].
Table 3.
Hepatocyte growth factor (HGF) and HGF receptor inhibitors under clinical evaluation (see also http://clinicaltrials.gov).
IgG, immunoglobulin; NSCLC, non-small cell lung cancer; PGDF(R), platelet-derived growth factor (receptor); VEGF(R), vascular endothelial growth factor (receptor).
In NSCLC, recent trials of two HGFR-targeted agents have suggested the efficacy of HGFR inhibition in combination with EGFR inhibition (utilizing erlotinib). A randomized controlled phase II trial of erlotinib combined with the HGFR selective TKI, ARQ 197 (also known as tivantinib), or placebo in patients with advanced NSCLC who had not been previously treated with EGFR inhibition did not demonstrate a significant improvement in progression-free survival (PFS) or overall survival (OS) in the intent-to-treat (ITT) population. However, there was a significant improvement in PFS in the ITT population when a Cox regression model was used to include key prognostic factors (hazard ratio [HR] 0.68, p < 0.05). In planned subset analyses, there was a significant improvement in both PFS and OS in patients with nonsquamous histology using the Cox regression model. In addition, patients with activating KRAS mutations gained significant improvement in PFS from treatment on the ARQ197-containing arm (HR 0.18) with a trend toward improvement in PFS in patients with wild-type EGFR as well (HR 0.7). The ARQ197-containing arm achieved an overall response rate of 10%, with additional responses after crossover from the placebo to ARQ197 containing arm [Schiller et al. 2010].
Currently, phase I and II studies are ongoing to evaluate the monovalent antagonist antibody to MET (MetMAb; also known as onartuzumab). Preliminary phase I data demonstrated MetMAb to be well tolerated in patients with advanced solid tumors, with a report of a complete response in a gastric cancer patient for a duration of approximately 2 years [Moss et al. 2010]. A randomized controlled phase II trial of MetMAb in combination with erlotinib in patients with advanced NSCLC did not demonstrate an improvement in PFS or OS in the overall ITT population. However, the primary objective of this trial included the evaluation of PFS in patients with HGFR overexpression. Tumor tissue was obtained from 100% of the patients. A total of 54% patients had ‘Met High’ NSCLC by immunohistochemistry, as defined by 2 + or 3 + staining on a 0–3 + scale. In this predefined subset of patients, a trend towards improvement in both PFS (HR 0.56, p = 0.0547) and OS (HR 0.55, p = 0.1113) was seen. Interestingly, patients with low HGFR expression had a significantly worse PFS and OS with the addition of MetMAb, compared with erlotinib alone. As a biomarker, high HGFR expression in the placebo arm was prognostic of a worse OS in patients treated with erlotinib alone (HR 2.52, p = 0.0350) [Spigel et al. 2010].
Conclusions
MET is involved in many mechanisms of cancer proliferation and metastasis. MET overexpression and genetic alterations play a role in the pathogenesis of several tumors, including lung cancer. The recent development of MET-targeted agents offers the potential for improving patient outcomes in malignant diseases. The incorporation of further biomarker development studies of MET and other potential oncogenes into the design of clinical trials is essential to further individualize cancer care by carefully defining true prognostic and predictive markers in oncology.
Funding
This work is supported in part by the National Institutes of Health (grant numbers R01CA134660-03 [to MS] and R01CA100750-07, R01CA125541-04, R01CA129501-03A1, P01HL058064-140009, and R21CA140003-02 to [RS]), and grants by the Mesothelioma Applied Research Foundation (Jeffrey P. Hayes Memorial Grant), V-Foundation (Guy Geleerd Memorial Foundation), Respiratory Health Association of Metropolitan Chicago, and Cancer Research Foundation (Goldblatt Award to RS).
Conflict of interest statement
The authors declare no conflict of interest in preparing this document.
References
- Abella J.V., Peschard P., Naujokas M.A., Lin T., Saucier C., Urbe S., et al. (2005) Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol Cell Biol 25: 9632–9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji L., Sattler M. (2004) Targeting mutated tyrosine kinases in the therapy of myeloid leukaemias. Expert Opin Ther Targets 8: 221–239 [DOI] [PubMed] [Google Scholar]
- Bardelli A., Longati P., Gramaglia D., Stella M.C., Comoglio P.M. (1997) Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene 15: 3103–3111 [DOI] [PubMed] [Google Scholar]
- Bean J., Brennan C., Shih J.Y., Riely G., Viale A., Wang L., et al. (2007) MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 104: 20932–20937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. (1995) Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376: 768–771 [DOI] [PubMed] [Google Scholar]
- Boon E.M., van der Neut R., van de Wetering M., Clevers H., Pals S.T. (2002) Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 62: 5126–5128 [PubMed] [Google Scholar]
- Caligiuri M.A., Briesewitz R., Yu J., Wang L., Wei M., Arnoczky K.J., et al. (2007) Novel c-CBL and CBL-b ubiquitin ligase mutations in human acute myeloid leukemia. Blood 110: 1022–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L.H., Moulder S.L., LoRusso P.M., Blumenschein G.R., Bristow P.J., Kurzrock R., et al. (2008) First in human phase I study of MK-2461, a small molecule inhibitor of c-Met, for patients with advanced solid tumors. J Clin Oncol 26abstract, 14657 [Google Scholar]
- Christensen J.G., Zou H.Y., Arango M.E., Li Q., Lee J.H., McDonnell S.R., et al. (2007) Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther 6: 3314–3322 [DOI] [PubMed] [Google Scholar]
- Cooper C.S., Park M., Blair D.G., Tainsky M.A., Huebner K., Croce C.M., et al. (1984) Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311: 29–33 [DOI] [PubMed] [Google Scholar]
- Derman M.P., Chen J.Y., Spokes K.C., Songyang Z., Cantley L.G. (1996) An 11-amino acid sequence from c-met initiates epithelial chemotaxis via phosphatidylinositol 3-kinase and phospholipase C. J Biol Chem 271: 4251–4255 [DOI] [PubMed] [Google Scholar]
- Dunbar A.J., Gondek L.P., O'Keefe C.L., Makishima H., Rataul M.S., Szpurka H., et al. (2008) 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res 68: 10349–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O., et al. (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316: 1039–1043 [DOI] [PubMed] [Google Scholar]
- Epstein J.A., Shapiro D.N., Cheng J., Lam P.Y., Maas R.L. (1996) Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci U S A 93: 4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M.S., Reddy M.M., Croteau N.J., Walz C., Weisbach H., Podar K., et al. (2010) Novel oncogenic mutations of CBL in human acute myeloid leukemia that activate growth and survival pathways depend on increased metabolism. J Biol Chem 285: 32596–32605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracini R., Di Renzo M.F., Scotlandi K., Baldini N., Olivero M., Lollini P., et al. (1995) The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene 10: 739–749 [PubMed] [Google Scholar]
- Gandino L., Longati P., Medico E., Prat M., Comoglio P.M. (1994) Phosphorylation of serine 985 negatively regulates the hepatocyte growth factor receptor kinase. J Biol Chem 269: 1815–1820 [PubMed] [Google Scholar]
- Garcia A., Rosen L., Cunningham C.C., Nemunaitis J., Li C., Rulewski N., et al. (2007) Phase 1 study of ARQ 197, a selective inhibitor of the c-Met RTK in patients with metastatic solid tumors reaches recommended phase 2 dose. J Clin Oncol 25abstract, 3525 [Google Scholar]
- Gherardi E., Sandin S., Petoukhov M.V., Finch J., Youles M.E., Ofverstedt L.G., et al. (2006) Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc Natl Acad Sci U S A 103: 4046–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohda E., Tsubouchi H., Nakayama H., Hirono S., Takahashi K., Koura M., et al. (1986) Human hepatocyte growth factor in plasma from patients with fulminant hepatic failure. Exp Cell Res 166: 139–150 [DOI] [PubMed] [Google Scholar]
- Grand F.H., Hidalgo-Curtis C.E., Ernst T., Zoi K., Zoi C., McGuire C., et al. (2009) Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood 113: 6182–6192 [DOI] [PubMed] [Google Scholar]
- Grant D.S., Kleinman H.K., Goldberg I.D., Bhargava M.M., Nickoloff B.J., Kinsella J.L., et al. (1993) Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A 90: 1937–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D.E., Carter S., McCullough J., Urbe S., Vande Woude G., Clague M.J. (2003) Endosomal dynamics of Met determine signaling output. Mol Biol Cell 14: 1346–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D.E., Urbe S., Vande Woude G.F., Clague M.J. (2001) Down-regulation of MET, the receptor for hepatocyte growth factor. Oncogene 20: 2761–2770 [DOI] [PubMed] [Google Scholar]
- Hellman A., Zlotorynski E., Scherer S.W., Cheung J., Vincent J.B., Smith D.I., et al. (2002) A role for common fragile site induction in amplification of human oncogenes. Cancer Cell 1: 89–97 [DOI] [PubMed] [Google Scholar]
- Huff J.L., Jelinek M.A., Borgman C.A., Lansing T.J., Parsons J.T. (1993) The protooncogene c-sea encodes a transmembrane protein-tyrosine kinase related to the Met/hepatocyte growth factor/scatter factor receptor. Proc Natl Acad Sci U S A 90: 6140–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura E., Maeshima A., Nakajima T., Nakamura T. (1996) Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn J Cancer Res 87: 1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama A., Okano K., Sudo T., Matsuda Y., Suda T. (1994) Molecular cloning of a novel receptor tyrosine kinase gene, STK, derived from enriched hematopoietic stem cells. Blood 83: 3160–3169 [PubMed] [Google Scholar]
- Jagadeeswaran R., Jagadeeswaran S., Bindokas V.P., Salgia R. (2007) Activation of HGF/c-Met pathway contributes to the reactive oxygen species generation and motility of small cell lung cancer cells. Am J Physiol Lung Cell Mol Physiol 292: L1488–L1494 [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran R., Ma P.C., Seiwert T.Y., Jagadeeswaran S., Zumba O., Nallasura V., et al. (2006) Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res 66: 352–361 [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran R., Surawska H., Krishnaswamy S., Janamanchi V., Mackinnon A.C., Seiwert T.Y., et al. (2008) Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res 68: 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M., Fiscella M., Webb C.P., Anver M., Koochekpour S., Vande Woude G.F. (1998) The mutationally activated Met receptor mediates motility and metastasis. Proc Natl Acad Sci U S A 95: 14417–14422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M., Rao M.S., Rulong S., Reddy J.K., Subbarao V., Hudson E., et al. (1996) Hepatocyte growth factor/scatter factor-Met signaling induces proliferation, migration, and morphogenesis of pancreatic oval cells. Cell Growth Differ 7: 1805–1813 [PubMed] [Google Scholar]
- Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300 [DOI] [PubMed] [Google Scholar]
- Jin H., Yang R., Zheng Z., Romero M., Ross J., Bou-Reslan H., et al. (2008) MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res 68: 4360–4368 [DOI] [PubMed] [Google Scholar]
- Jun H.T., Sun J., Rex K., Radinsky R., Kendall R., Coxon A., et al. (2007) AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res 13: 6735–6742 [DOI] [PubMed] [Google Scholar]
- Kanteti R., Nallasura V., Loganathan S., Tretiakova M., Kroll T., Krishnaswamy S., et al. (2009) PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription. Lab Invest 89: 301–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong-Beltran M., Stamos J., Wickramasinghe D. (2004) The Sema domain of Met is necessary for receptor dimerization and activation. Cancer Cell 6: 75–84 [DOI] [PubMed] [Google Scholar]
- Koochekpour S., Jeffers M., Rulong S., Taylor G., Klineberg E., Hudson E.A., et al. (1997) Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res 57: 5391–5398 [PubMed] [Google Scholar]
- Krishnaswamy S., Kanteti R., Duke-Cohan J., Loganathan S., Ma P.C., Sattler M., et al. (2009) Ethnic differences and functional analysis of MET mutations in lung cancer. Clin Cancer Res in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Han S.U., Cho H., Jennings B., Gerrard B., Dean M., et al. (2000) A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene 19: 4947–4953 [DOI] [PubMed] [Google Scholar]
- Li G., Schaider H., Satyamoorthy K., Hanakawa Y., Hashimoto K., Herlyn M. (2001) Downregulation of E-cadherin and Desmoglein 1 by autocrine hepatocyte growth factor during melanoma development. Oncogene 20: 8125–8135 [DOI] [PubMed] [Google Scholar]
- Liao A.T., McMahon M., London C.A. (2006) Identification of a novel germline MET mutation in dogs. Anim Genet 37: 248–252 [DOI] [PubMed] [Google Scholar]
- Lock L.S., Maroun C.R., Naujokas M.A., Park M. (2002) Distinct recruitment and function of Gab1 and Gab2 in Met receptor-mediated epithelial morphogenesis. Mol Biol Cell 13: 2132–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longati P., Bardelli A., Ponzetto C., Naldini L., Comoglio P.M. (1994) Tyrosines1234-1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor). Oncogene 9: 49–57 [PubMed] [Google Scholar]
- Loriaux M.M., Levine R.L., Tyner J.W., Frohling S., Scholl C., Stoffregen E.P., et al. (2008) High-throughput sequence analysis of the tyrosine kinome in acute myeloid leukemia. Blood 111: 4788–4796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P.C., Kijima T., Maulik G., Fox E.A., Sattler M., Griffin J.D., et al. (2003) c-MET mutaional anlysis in small cell lung cancer: Novel juxtamembrance domain mutations regulating cytoskeletal functions. Cancer Res 63: 6272–6281 [PubMed] [Google Scholar]
- Ma P.C., Schaefer E., Christensen J.G., Salgia R. (2005) A selective small molecule c-MET Inhibitor, PHA665752, cooperates with rapamycin. Clin Cancer Res 11: 2312–2319 [DOI] [PubMed] [Google Scholar]
- Mahadevan D., Cooke L., Riley C., Swart R., Simons B., Della Croce K., et al. (2007) A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene 26: 3909–3919 [DOI] [PubMed] [Google Scholar]
- Maina F., Klein R. (1999) Hepatocyte growth factor, a versatile signal for developing neurons. Nat Neurosci 2: 213–217 [DOI] [PubMed] [Google Scholar]
- Martens T., Schmidt N.O., Eckerich C., Fillbrandt R., Merchant M., Schwall R., et al. (2006) A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res 12: 6144–6152 [DOI] [PubMed] [Google Scholar]
- Maulik G., Kijima T., Ma P.C., Ghosh S.K., Lin J., Shapiro G.I., et al. (2002) Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res 8: 620–627 [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. (1991) Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 67: 901–908 [DOI] [PubMed] [Google Scholar]
- Moss R.A., Patel P., Bothos J., Peterson A.C., Eppler S., Yu W., et al. (2010) Complete results from phase I dose escalation study of MetMAb, a monovalent antagonist antibody to the receptor Met, dosed as single agent and in combination with bevacizumab in patients with advanced solid malignancies. Ann Oncol 21: viii165(Suppl. 168): abstract 504P [Google Scholar]
- Nakamura T., Nawa K., Ichihara A., Kaise N., Nishino T. (1987) Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett 224: 311–316 [DOI] [PubMed] [Google Scholar]
- Nguyen L., Holgado-Madruga M., Maroun C., Fixman E.D., Kamikura D., Fournier T., et al. (1997) Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem 272: 20811–20819 [DOI] [PubMed] [Google Scholar]
- Olivero M., Rizzo M., Madeddu R., Casadio C., Pennacchietti S., Nicotra M.R., et al. (1996) Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br J Cancer 74: 1862–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patane S., Avnet S., Coltella N., Costa B., Sponza S., Olivero M., et al. (2006) MET overexpression turns human primary osteoblasts into osteosarcomas. Cancer Res 66: 4750–4757 [DOI] [PubMed] [Google Scholar]
- Pennacchietti S., Michieli P., Galluzzo M., Mazzone M., Giordano S., Comoglio P.M. (2003) Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3: 347–361 [DOI] [PubMed] [Google Scholar]
- Peschard P., Fournier T.M., Lamorte L., Naujokas M.A., Band H., Langdon W.Y., et al. (2001) Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol Cell 8: 995–1004 [DOI] [PubMed] [Google Scholar]
- Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., et al. (1994) A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77: 261–271 [DOI] [PubMed] [Google Scholar]
- Puri N., Ahmed S., Janamanchi V., Tretiakova M., Zumba O., Krausz T., et al. (2007) c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res 13: 2246–2253 [DOI] [PubMed] [Google Scholar]
- Puri N., Salgia R. (2008) Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog 7: 9–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F., Rocancourt D., Mansouri A., Buckingham M. (2004) Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev 18: 1088–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G.A., Park M. (1993) Dimerization mediated through a leucine zipper activates the oncogenic potential of the met receptor tyrosine kinase. Mol Cell Biol 13: 6711–6722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G.A., Park M. (1994) Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene 9: 2019–2027 [PubMed] [Google Scholar]
- Rodrigues M.S., Reddy M.M., Sattler M. (2008) Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: from molecular redox mechanisms to health implications. Antioxid Redox Signal 10: 1813–1848 [DOI] [PubMed] [Google Scholar]
- Rong S., Segal S., Anver M., Resau J.H., Vande Woude G.F. (1994) Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci U S A 91: 4731–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsin C., Muscatelli F., Mattei M.G., Breathnach R. (1993) A novel putative receptor protein tyrosine kinase of the met family. Oncogene 8: 1195–1202 [PubMed] [Google Scholar]
- Rossi G., Cavazza A., Marchioni A., Longo L., Migaldi M., Sartori G., et al. (2005) Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 23: 8774–8785 [DOI] [PubMed] [Google Scholar]
- Sakakura C., Mori T., Sakabe T., Ariyama Y., Shinomiya T., Date K., et al. (1999) Gains, losses, and amplifications of genomic materials in primary gastric cancers analyzed by comparative genomic hybridization. Genes Chromosomes Cancer 24: 299–305 [DOI] [PubMed] [Google Scholar]
- Salgia R., Skarin A.T. (1998) Molecular abnormalities in lung cancer. J Clin Oncol 16: 1207–1217 [DOI] [PubMed] [Google Scholar]
- Sargin B., Choudhary C., Crosetto N., Schmidt M.H., Grundler R., Rensinghoff M., et al. (2007) Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood 110: 1004–1012 [DOI] [PubMed] [Google Scholar]
- Schiller J.H., Akerley W.L., Brugger W., Ferrari D., Garmey E.G., Gerber D.E., et al. (2010) Results from ARQ 197-209: A global randomized placebo-controlled phase II clinical trial of erlotinib plus ARQ 197 versus erlotinib plus placebo in previously treated EGFR inhibitor-naive patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 28(18 Suppl): abstract, LBA7502 [Google Scholar]
- Schmidt C., Bladt F., Goedecke S., Brinkmann V., Zschiesche W., Sharpe M., et al. (1995) Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373: 699–702 [DOI] [PubMed] [Google Scholar]
- Schmidt L., Duh F.M., Chen F., Kishida T., Glenn G., Choyke P., et al. (1997) Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 16: 68–73 [DOI] [PubMed] [Google Scholar]
- Schmidt L., Junker K., Nakaigawa N., Kinjerski T., Weirich G., Miller M., et al. (1999) Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 18(14): 2343–2350 [DOI] [PubMed] [Google Scholar]
- Schroeder G.M., An Y., Cai Z.W., Chen X.T., Clark C., Cornelius L.A., et al. (2009) Discovery of N-(4-(2-Amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluor ophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J Med Chem 52: 1251–1254 [DOI] [PubMed] [Google Scholar]
- Seiwert T.Y., Jagadeeswaran R., Faoro L., Janamanchi V., Nallasura V., El Dinali M., et al. (2009) The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res 69: 3021–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F., Lee J.H., Simmons B.H., Wong A., Esparza C.O., Plumlee P.A., et al. (2010) HGF/c-Met acts as an alternative angiogenic pathway in sunitinib- resistant tumors. Cancer Res 70: 10090–10100 [DOI] [PubMed] [Google Scholar]
- Siegfried J.M., Weissfeld L.A., Luketich J.D., Weyant R.J., Gubish C.T., Landreneau R.J. (1998) The clinical significance of hepatocyte growth factor for non-small cell lung cancer. Ann Thorac Surg 66: 1915–1918 [DOI] [PubMed] [Google Scholar]
- Siegfried J.M., Weissfeld L.A., Singh-Kaw P., Weyant R.J., Testa J.R., Landreneau R.J. (1997) Association of immunoreactive hepatocyte growth factor with poor survival in resectable non-small cell lung cancer. Cancer Res 57: 433–439 [PubMed] [Google Scholar]
- Smolen G.A., Sordella R., Muir B., Mohapatra G., Barmettler A., Archibald H., et al. (2006) Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A 103: 2316–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Li M., Tretiakova M., Salgia R., Cagle P.T., Husain A.N. (2010) Expression Patterns of PAX5, c-Met, and paxillin in neuroendocrine tumors of the lung. Arch Pathol Lab Med 134: 1702–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg E., Meyer D., Weidner K.M., Birchmeier C. (1993) Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 123: 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigel D., Ervin T., Ramlau R., Daniel D., Goldschmidt J., Krzakowski M., et al. (2010) Randomized multicenter double-blind placebo-controlled phase II study evaluating MetMAb, an antibody to Met receptor, in combination with erlotinib, in patients with advanced non-small-cell lung cancer. Ann Oncol 21(viii7 Suppl. 8): abstract, LBA15 [Google Scholar]
- Stabile L.P., Lyker J.S., Land S.R., Dacic S., Zamboni B.A., Siegfried J.M. (2006) Transgenic mice overexpressing hepatocyte growth factor in the airways show increased susceptibility to lung cancer. Carcinogenesis 27: 1547–1555 [DOI] [PubMed] [Google Scholar]
- Stamos J., Lazarus R.A., Yao X., Kirchhofer D., Wiesmann C. (2004) Crystal structure of the HGF beta-chain in complex with the Sema domain of the Met receptor. Embo J 23: 2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. (1987) Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327: 239–242 [DOI] [PubMed] [Google Scholar]
- Tan Y.H., Krishnaswamy S., Nandi S., Kanteti R., Vora S., Onel K., et al. (2010) CBL is frequently altered in lung cancers: its relationship to mutations in MET and EGFR tyrosine kinases. PLoS One 5: e8972–e8972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck A.B., Park M., Sterns E.E., Boag A., Elliott B.E. (1996) Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol 148: 225–232 [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Minowa O., Mori C., Shiota K., Kuno J., Noda T., et al. (1995) Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373: 702–705 [DOI] [PubMed] [Google Scholar]
- Walz C., Sattler M. (2006) Novel targeted therapies to overcome imatinib mesylate resistance in chronic myeloid leukemia (CML). Crit Rev Oncol Hematol 57: 145–164 [DOI] [PubMed] [Google Scholar]
- Wang R., Ferrell L.D., Faouzi S., Maher J.J., Bishop J.M. (2001) Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 153: 1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K.M., Di Cesare S., Sachs M., Brinkmann V., Behrens J., Birchmeier W. (1996) Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384: 173–176 [DOI] [PubMed] [Google Scholar]
- Weidner K.M., Sachs M., Riethmacher D., Birchmeier W. (1995) Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the met receptor in epithelial cells. Proc Natl Acad Sci U S A 92: 2597–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Kiyoi H., Nakao M., Iwai T., Misawa S., Okuda T., et al. (1997) Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia 11: 1605–1609 [DOI] [PubMed] [Google Scholar]
- Yu Y., Merlino G. (2002) Constitutive c-Met signaling through a nonautocrine mechanism promotes metastasis in a transgenic transplantation model. Cancer Res 62: 2951–2956 [PubMed] [Google Scholar]
- Zaffaroni D., Spinola M., Galvan A., Falvella F.S., Pazzaglia S., Saran A., et al. (2005) Met proto-oncogene juxtamembrane rare variations in mouse and humans: differential effects of Arg and Cys alleles on mouse lung tumorigenesis. Oncogene 24: 1084–1090 [DOI] [PubMed] [Google Scholar]
- Zhang Y.W., Staal B., Essenburg C., Su Y., Kang L., West R., et al. (2010) MET kinase inhibitor SGX523 synergizes with epidermal growth factor receptor inhibitor erlotinib in a hepatocyte growth factor-dependent fashion to suppress carcinoma growth. Cancer Res 70: 6880–6890 [DOI] [PubMed] [Google Scholar]
- Zhen Z., Giordano S., Longati P., Medico E., Campiglio M., Comoglio P.M. (1994) Structural and functional domains critical for constitutive activation of the HGF-receptor (Met). Oncogene 9: 1691–1697 [PubMed] [Google Scholar]
- Zou H.Y., Li Q., Lee J.H., Arango M.E., McDonnell S.R., Yamazaki S., et al. (2007) An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 67: 4408–4417 [DOI] [PubMed] [Google Scholar]