Abstract
Lung cancer is the leading cause of cancer deaths worldwide. Standard chemotherapy has been shown to improve quality of life and has a modest influence on overall survival. This modest improvement in survival is partly due to the choice of chemotherapy regimens that have been based on prognostic factors such as age, performance status and comorbidities of the patient. This underlines the importance of developing a more personalized therapy for patients with non-small cell lung cancer. Such an approach may reduce the variation in how individual patients respond to medications by tailoring therapies to their genetic profile. In this review we focus on several aspects of customized therapy, looking not only at patient characteristics but also to tumor histology and specific tumor biomarkers.
Keywords: bevacizumab, histology, non-small cell lung cancer, pemetrexed, personalized therapy, tyrosine kinase inhibitors
Introduction
Lung cancer is the leading cause of cancer-related deaths in Western countries, with non-small-cell lung cancer (NSCLC) accounting for more than 85% of primary lung cancers. Importantly, the majority of lung cancers are metastasized at the time of diagnosis, making them illegible for curative treatment by either surgery or radiotherapy.
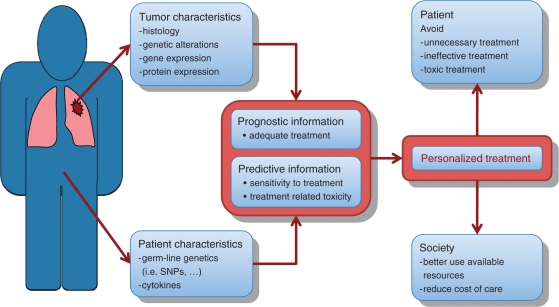
Systemic treatment with either chemotherapy or biologic therapy is the key component for the treatment of patients with disseminated NSCLC. Indeed, chemotherapy in addition to supportive care has been shown to improve quality of life, control symptoms and overall survival (i.e. ∼10% increase in the 1-year survival rate) in patients with advanced NSCLC [Non-small Cell Lung Cancer Collaborative Group, 1995]. However these improvements are modest (response rates ∼30% for first-line treatment and ∼10% for second-line treatment, with ∼2 month increase in median overall survival), indicating that many NSCLC patients fail to respond to ‘standard’ chemotherapy. In part, this is due to the fact that until recently the choice of chemotherapy regimens was based mainly on either the age, performance status (PS) and comorbidities of the patient or on the physician’s experience and patient’s preferences. These facts underline the importance of developing a more personalized therapy for patients with NSCLC. Ideally, such a personalized treatment should be based on patient and/or tumor characteristics, which together provide prognostic and/or predictive information. This information should then facilitate the selection of appropriate systemic treatment for an individual patient by avoiding unnecessary, less effective or more toxic treatments (Figure 1).
Figure 1.
Development of personalized therapy for non-small cell lung cancer.
There are three considerations to produce a successful molecularly targeted biological treatment. First, the oncogenic target controlling cancer growth and survival must be identified; second, an effective therapy targeting this oncogenic target needs to be made available; and, third, a biomarker that predicts a favorable outcome of the targeted therapy needs to be identified.
Recently, advances have been made to elucidate molecular pathways that play a role in NSCLC carcinogenesis and that may also correlate with clinical response. Many biomarkers are suggested to have both prognostic and predictive properties; however, randomized clinical trials and meta-analyses are necessary to determine the usefulness of a given marker in clinical setting (Figure 2). To date, the only biomarkers, which have been tested or validated in large phase III trials, are tumor histology and the presence of an activating epidermal growth factor receptor (EGFR) mutation.
Figure 2.
Many biomarkers have both prognostic and predictive properties. Randomized controlled trials and/or meta-analyses are required to determine their clinical usefulness.
Clinical patient-based treatment decisions
Age, performance and comorbidities
The choice of chemotherapy regimens until recently was based on prognostic factors such as age, PS and comorbidities of the patient.
The retrospective analysis of the large International Staging Committee database confirmed that PS is a very important prognostic factor, while age and gender were other independent significant variables [Sculier et al. 2008]. In a number of trials, the presence of comorbidities was also found to be associated with poorer survival [Lee et al. 2011; Irisa et al. 2010]. Nevertheless, NSCLC patients with a poorer PS, older age, or the presence of comorbidities derive a similar survival benefit from chemotherapy as patients without these poor prognostic factors, but they experience significantly more toxicities [Davidoff et al. 2010; Gronberg et al. 2010; Asmis et al. 2008; Weiss et al. 2006; Lilenbaum et al. 2005; Langer et al. 2002]. In other words, age, PS and the presence of comorbidities may predict for tolerability and toxicity, but not for survival benefits associated with single-agent or platinum-based chemotherapy.
Pemetrexed
Pemetrexed is a multitargeted antifolate inhibitor of thymidilate syntethase (TS) dihydrofolate reductase and glycinamide ribonucleotide formyl transferase [Hong et al. 2010; Shih et al. 1998].
Several trials studied the role pemetrexed as a first- or second-line treatment in patients with advanced NSCLC. The results of these phase III trials are summarized in Table 1. In summary, there was no difference between first-line pemetrexed plus cisplatin and cisplatin plus gemcitabine with regard to progression-free survival and median overall survival [Scagliotti et al. 2008]. Similarly, pemetrexed was not inferior to docetaxel when given as second-line treatment [Hanna et al. 2004]. Recently, pemetrexed was also compared with placebo in the maintenance or ‘early second line’ following four cycles of first-line platinum-based chemotherapy. The use of pemetrexed in this maintenance setting resulted in a significantly longer progression-free survival and overall survival [Ciuleanu et al. 2009].
Table 1.
Pemetrexed in the treatment of non-small cell lung cancer. Data from Scagliotti et al., [2008], Hanna et al., [2004], and Ciuleanu et al., [2009].
| 1st line: |
2nd line: |
Maintenance |
||||
|---|---|---|---|---|---|---|
| Cis-Gem | Cis-Pem | Doc | Pern | Plac | Pern | |
| Median PFS | 5.1 m | 4.8 m | 2.9 m | 2.9 m | 2.0 m | 4.0 m |
| HR 1.04 | HR 0.97 | HR 0.60* | ||||
| Median OS | 10.3 m | 10.3 m | 7.9 m | 8.3 m | 10.6 m | 13.4 m |
| HR 0.99 | HR 0.99 | HR 0.79* | ||||
HR, hazard ratio; PFS, progression-free survival; OS, overall survival; Cis-Gem, cisplatin plus gemcitabine; Doc, docetaxel; Pem, pemetrexed; Plac, placebo; m, months; *p < 0.05.
A retrospective analysis of the trial comparing pemetrexed with docetaxel in previously treated NSCLC patients found a significant treatment-by-histology interaction. Docetaxel resulted in a better survival in the squamous cell subgroup, while pemetrexed had a superior survival in the nonsquamous subgroup [Hanna et al. 2004].
A preplanned subgroup analysis in the first-line study confirmed that the effect on survival of pemetrexed-containing chemotherapy is significantly different in patients with nonsquamous NSCLC and patients with squamous cell carcinoma. In patients with adenocarcinoma and large-cell carcinoma, the overall survival was superior in the cisplatin plus pemetrexed arm, while in patients with squamous cell carcinoma there was a significant improvement in survival with cisplatin plus gemcitabine. The overall survival for a fourth group, consisting of all of those patients in whom a generic cytological diagnosis of NSCLC without further subtype classification was made (NOS or ‘not otherwise specified’), did not show a significant difference between the two arms. [Scagliotti et al. 2008]
Similar results were found in the pemetrexed maintenance trial: the improvements in progression-free survival and overall survival were confined to patients with nonsquamous histology. In patients with squamous cell cancer, the progression-free survival and overall survival was similar in the pemetrexed and the placebo arms [Ciuleanu et al. 2009]. The hazard ratios (HRs) for overall survival according to tumor histology are summarized in Table 2.
Table 2.
Pemetrexed and non-small cell lung cancer histology: squamous versus non-squamous. Data shown as hazard ratio. Data from Ciuleanu et al., [2009].
| Histology | 1st line: Cis-Pem vs. Cis-Gem | 2nd line: Pern vs. Doc | Maintenance: Pern vs. Plac |
|---|---|---|---|
| Non-squamous | 0.81* | 0.78* | 0.70* |
| Squamous | 1.23 | 1.56* | 1.07 |
Cis-Gem, cisplatin plus gemcitabine; Pem, pemetrexed; Plac, placebo; *p < 0.05.
Resistance to pemetrexed in cancer cell lines has been shown to be due to upregulation of TS [Sigmond et al. 2003]. Pemetrexed in combination with cisplatin was first licensed for the treatment of malignant pleural mesothelioma [Vogelzang et al. 2003]. Recently, low TS protein levels were found to be predictive of improved time-to-progression and overall survival in patients with mesothelioma treated with pemetrexed-based chemotherapy [Righi et al. 2010]. Similarly, the observed treatment-by-histology interaction for pemetrexed in NSCLC has been related to differences in TS expression in the different histologic subtypes. In chemotherapy-naive patients, the baseline expression of TS is significantly higher in squamous cell carcinoma compared to adenocarcinoma [Ceppi et al. 2006]. Large-cell carcinomas, when considered as a single group of tumors, have a median TS expression comparable with squamous cell cancer, and higher than in adenocarcinoma. However, the TS expression in large-cell carcinomas differs according to their immunohistochemical profile: in large-cell carcinomas expressing markers of squamous cell differentiation (i.e. p63) the TS levels were significantly higher than in those expressing markers of adenocarcinoma (i.e. TTF-1). Thus, the expression of TS in large-cell carcinoma may resemble that of adenocarcinomas or squamous cell carcinomas [Monica et al. 2009]. These findings may explain why the HRs for overall survival are consistently in favor of the pemetrexed-treated adenocarcinoma patients, but vary for the large-cell and ‘NOS’ subgroups (Table 3).
Table 3.
Pemetrexed and non-small cell lung cancer histology: squamous versus adenocarcinoma, large-cell carcinoma and not other specified types. Data shown as hazard ratio. Data from Scagliotti et al., [2008] and Ciuleanu et al., [2009].
| Histology | 1st line: Cis-Pem vs. Cis-Gem | 2nd line: Pern vs. Doc | Maintenance: Pern vs. Plac |
|---|---|---|---|
| Non-squamous | 0.81* | 0.78* | 0.70* |
| Adenocarcinoma | 0.84* | 0.92 | 0.73* |
| Large cell | 0.67 | 0.27* | 0.98 |
| NOS | 1.08 | 0.57 | 0.61* |
| Squamous | 1.23 | 1.56* | 1.07 |
Cis-Gem, cisplatin plus gemcitabine; Doc, docetaxel; Pem, pemetrexed; Plac, placebo; NOS, not otherwise specified; *p < 0.05
In summary, it may be concluded that in patients with nonsquamous NSCLC, pemetrexed-containing treatments are superior to gemcitabine- or docetaxel-containing treatments. Furthermore, these results should warrant a prospective study that is specifically designed to evaluate TS-expression as a more reliable marker to select those patients most likely to benefit from cisplatin plus pemetrexed therapy.
Bevacizumab
Bevacizumab is a monoclonal vascular endothelial growth factor (VEGF) antibody binding directly to VEGF and removing it from the environment [Presta et al. 1997]. It is not considered cytotoxic bearing in mind it has mostly anti-angiogenic effects [Hanahan and Weinberg, 2000], but it normalizes the tumor vasculature thereby improving the drug delivery to the tumor [Jain, 2001]. Indeed, several trials have shown the beneficial effects of adding bevacizumab to standard chemotherapy in a variety of tumors [Willett et al. 2007, 2004; Sandler et al. 2006].
The initial randomized phase II trial evaluating the role of bevacizumab in patients with advanced or recurrent NSCLC demonstrated that addition of bevacizumab (high dose) to carboplatin and paclitaxel resulted in higher response rates (31.5% versus 18.8%, respectively) and longer time-to-progression (7.4 months with bevacizumab versus 4.2 months for chemotherapy alone). However, an alarming rate (9%) of life-threatening pulmonary bleeding was observed in the bevacizumab-treated patients. This life-threatening pulmonary hemorrhage was found to be associated with squamous cell histology (four of the six cases occurred in patients with squamous cell histology), tumor necrosis and cavitation, and disease location close to major blood vessels [Johnson et al. 2004].
As a result of these safety concerns observed in the phase II trials, the subsequent phase III trials excluded patients with tumors with a predominant squamous component. The rates of grade ≥3 pulmonary hemorrhage in the bevacizumab plus chemotherapy arms of these phase III trials in patients with nonsquamous cell NSCLC were <2% [Reck et al. 2009; Sandler et al. 2006].
The ECOG-trial showed that the addition of bevacizumab to paclitaxel plus carboplatin resulted in a significant increase in progression-free survival (6.2 versus 4.5 months; HR 0.66) and overall survival (12.3 months versus 10.3 months; HR 0.79) [Sandler et al. 2006]. The AVAiL trial confirmed progression-free survival of bevacizumab when combined with cisplatin plus gemcitabine (HR 0.75). However, in the AVAiL trial the progression-free survival benefit did not translate into a significant overall survival benefit. This lack of overall survival benefit has been attributed to the use of efficacious second-line therapies [Reck et al. 2010].
Thus, by using nonsquamous histology as a biomarker for selecting patients eligible for bevacizumab treatment, undue toxicity could be avoided without compromising the antitumoral effects.
Tumor biomarker-based treatment decisions
EGFR tyrosine kinase inhibitors
EGFR belongs to a family of four related transmembrane receptors, namely EGFR (HER1), HER2, HER3 and HER4. It regulates important processes including proliferation, apoptosis, angiogenesis and invasion. As EGFR is frequently expressed in NSCLC [Rusch et al. 1997], it has been an important target for the development of new agents. The EGFR tyrosine kinase inhibitors (TKIs), erlotinib and gefitinib, have been extensively studied in NSCLC.
Even with the first clinical trials of EGFR-TKIs, it was observed that female patients, nonsmokers, patients of Asian ethnicity and patients with adenocarcinomas had a greater response rate following treatment with EGFR-TKIs. However, it has now been demonstrated that the EGFR-TKI sensitivity is associated with the presence of activating mutations in the tyrosine kinase domain of EGFR in the NSCLC tumor and not with these clinical characteristics per se [Rosell et al. 2009; Lynch et al. 2004; Paez et al. 2004]. However, these mutations occur with higher frequency in women, nonsmokers, adenocarcinoma, and in patients of Asian ethnicity, thus explaining the higher response rates to treatment with EGFR-TKIs in these patient groups [Pao et al. 2004]. Importantly, the constitutive EGFR activation caused by these mutations causes oncogene addiction to the EGFR pathway. This means that de-induction of mutant EGFR expression leads to dramatic regression of tumors suggesting the need for persistent mutant EGFR activity for continued tumor survival [Herbst et al. 2008].
These EGFR mutations were also found to be a favorable prognostic factor for survival in advanced NSCLC patients treated with chemotherapy with or without concomitant EGFR-TKI treatment [Eberhard et al. 2005].
The presence of the activating mutation in exons 19 and 21 of the EGFR results in an activation of the Akt and STAT pathways without having an effect on ERK/MAP signaling. It has been suggested that EGFR-TKIs affect wild-type and mutant cells differently. Namely, gefinitib triggers apoptosis in cell-lined harboring EGFR exon 19 or 21 mutations that results in complete or partial response. On the other hand, it induces G1 arrest in EGFR wild-type cells that leads to stable disease [Pao and Miller, 2005].
Recently, several trials have examined the potential role of EGFR-TKIs in the first-line treatment of patients with tumors with activating mutations on the EGFR [Mitsudomi et al. 2010; Maemondo et al. 2010]. These trials all showed that the presence of EGFR mutation is a strong predictive marker for improved response rate and improved progression free survival with EGFR-TKI versus chemotherapy (Table 4). The IPASS trial, which also included patients without activating EGFR mutations, demonstrated that the presence of an EGFR mutation is associated with a higher response rate to chemotherapy and is a favorable prognostic factor regardless of treatment (Table 5).
Table 4.
Progression-free survival and response rate in epidermal growth factor receptor (EGFR) mutation positive patients following first-line treatment. Data from Mitsudomi et al. [2009] and Makato et al. [2010].
| Progression-free survival (months) |
Response rate |
|||
|---|---|---|---|---|
| Chemotherapy | EGFR-TKI | Chemotherapy | EGFR-TKI | |
| Mitsudomi et al. [2009] Cisplatin-docetaxel versus gefinitib | 6.3 | 9.3 | 32.2% | 62.1% |
| Makato etal. [2010] Carboplatin-paclitaxel versus gefinitib | 5.5 | 10.4 | 30.7% | 73.7% |
TKI, tyrosine kinase inhibitor.
Table 5.
First-line epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) versus chemotherapy: IPASS trial. The presence of EGFR mutation is a strong predictive marker for response rate and progression-free survival (PFS). Data from Mok et al. [2009].
| EGFR wild type |
EGFR-mutation+ |
|||
|---|---|---|---|---|
| Gefitinib | Carbo/Pacli | Gefitinib | Carbo/Pacli | |
| Response rate | 1% | 23%* | 71%* | 47% |
| PFS | 1.5 m | 5.5 m* | 9.6 m* | 6.3 m |
| HR2.85* | HR 0.48* | |||
| OS | MST ∼12 m | MST ∼20 m | ||
| HR1.38 | HR0.78 | |||
Carbo, carboplatin; HR, hazard ratio; MST, musculoskeletal toxicity; OS: Overall Survival; Pacli, paclitaxel; *p < 0.05.
Similar findings were reported in the SATURN trial, which explored the role of erlotinib in maintenance or early second-line settings immediately after completion of the initial course of platinum-based chemotherapy. In unselected patients, median progression-free survival was significantly longer with erlotinib than with placebo (12.3 versus 11.1 weeks respectively; HR 0.71; p < 0.0001). Moreover, in patients with tumors harboring an EGFR mutation, erlotinib as maintenance treatment resulted in a dramatic increase in progression-free survival (HR 0.10; p < 0.0001). However, patients with tumors with wild-type EGFR also seemed to derive a benefit from erlotinib maintenance as the overall survival was significantly improved in these patients (HR 0.77; p < 0.05) [Cappuzzo et al. 2010].
Erlotinib has also been shown to prolong survival in patients with NSCLC after failure of first-line or second-line chemotherapy [Shepherd et al. 2005]. In this trial, the presence of an EGFR mutation was shown to increase the responsiveness to erlotinib, but it is not indicative of a survival benefit as the overall survival benefit was also observed in the EGRF wild-type patients [Tsao et al. 2005].
Inhibition of other oncogenic drivers such as anaplastic lymphoma kinase
A number of specific genetic lesions (i.e. KRAS, ALK, BRAF, MET, PGDFR, PIK3CA, HER2) driving tumor proliferation have been identified in primary lung adenocarcinomas. Inhibition of these oncogenic drivers in NSCLC patients harboring these mutated pathways is a logical candidate for future targeted treatments. So far, the most promising results have been observed with the inhibition of anaplastic lymphoma kinase (ALK) in patients with NSCLC with EML4-ALK translocation.
ALK is an oncogene that induces cell transformation in vitro and in vivo. Translocation of ALK results in an abnormal fusion gene, usually with its counterpart in lung cancer, echinoderm microtubule-associated protein-like 4 (ELM4). The translocation produces an ongoing activation of an intercellular tyrosine kinase, which then leads to proliferation and cancer propagation [Horn and Pao, 2009]. In some lung cancers, this will be the sole driver of their existence because the ALK translocation is so dominant [Soda et al. 2008, 2007]. Treatment of patients with ALK rearrangements with crizotinib, an inhibitor of the ALK tyrosine kinase, results in response rates in excess of 50% and estimated probability of 6-month progression-free survival of more than 70% [Kwak et al. 2010].
ALK rearrangements and EGFR mutations seem to be usually mutually exclusive while mainly occurring in patients with the same clinical characteristics, namely younger patients, never or light smokers and adenocarcinomas. Interestingly, the group of patients with ALK rearrangements tends to have a slightly higher proportion of males compared with those who have EGFR mutations. Importantly, patients with ALK rearrangements do not respond to EGFR-TKI treatment [Shaw et al. 2009].
Cisplatin-based therapy and DNA repair systems
Cisplatin and carboplatin are used as standard chemotherapy for solid tumors. The mechanism of action is based on the formation of cisplatin-DNA adducts leading to apoptosis. Removal of these adducts, leading to chemoresistance, is mainly carried out by the nucleotide excision repair system.
Excision repair cross-complementation group 1 (ERCC 1) is a rate-limiting protein in the NER and ICL-R pathways, which works by recognizing and removing platinum adducts and by repairing interstrand DNA crosslinks. Its prognostic effect was assessed in a study by Simon and colleagues in chemotherapy-naïve patients with resected NSCLC [Simon et al. 2005]. Namely, there was a statistically significant difference in median survival for patients with ERCC1 expression >50 (94.6 months) compared with <50 (35.5 months) (p = 0.01). Multivariate analysis revealed that high ERCC1 expression independently predicted longer survival. They also suggested that an intact DNA repair mechanism reduces the accumulation of genetic aberrations that are thought to contribute to a tumors malignant potential and therefore the risk of relapse after definitive treatment. This study is in accordance with the results of the International Adjuvant Lung Cancer Biology Trial (IALT-Bio) [Olaussen et al. 2006] that showed better survival in patients with ERCC1 positive tumors (p = 0.009). In contrast to these studies, Rosell and colleagues showed that ERCC1 overexpression was related to worse prognosis. Their study, however, did not reach a statistical significance of p > 0.05 [Rosell et al. 2007]. Nevertheless, the analysis of patients with advanced disease treated with cisplatin and gemcitabine showed that low expression of ERRC1 was associated with longer survival [Lord et al. 2002].
Ribonucleotide reductase messenger 1 (RRM1) is the regulatory component of ribonucleotide reductase that assists with DNA synthesis and repair. Moreover, it is the predominant target of nucleoside analogue, gemcitabine. Analysis of patients with advanced NSCLC treated with cisplatin-containing combinations or nonplatinum doublets showed a longer median survival in a group with low RRM1 expression (13.7 months) versus a group with high RRM1 expression (3.6 months). Moreover, median survival was significantly longer in the group treated with cisplatin plus gemcitabine who had lower levels of both RMM1 and ERCC1 expression than other subgroups. Therefore, it has been suggested that RRM1 expression is a predictive marker of survival in gemcitabine plus cisplatin-treated patients [Rosell et al. 2004].
Breast cancer type 1 susceptibility protein (BRCA1) plays a central role in DNA repair as a component of multiple repair pathways. Furthermore, BRCA1 is a potential regulator of mitotic spindle assembly as it is found to be colocalized with beta-tubulin to the microtubules of the mitotic spindle [Coate et al. 2009; Lafarge et al. 2001]. The increased expression of BRCA1 appears to be an unfavorable prognostic factor as it is correlated with decreased overall survival in chemotherapy-naïve patients with NSCLC after surgery [Rosell et al. 2007].
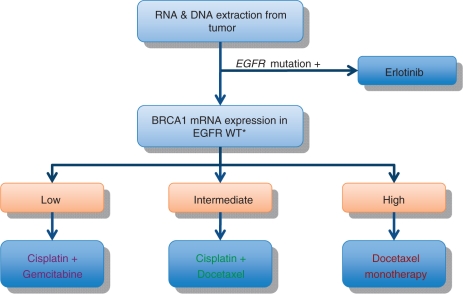
In preclinical models, upregulation of BRCA1 has been linked to platinum resistance and sensitivity to antimicrotubule drugs such as taxanes and vinca alkaloids [Rosell et al. 2004]. This differential sensitivity to different chemotherapeutic agents according to the BRCA1 expression levels makes BRCA1 an interesting candidate marker to customize chemotherapy treatment in NSCLC. This concept has been tested in a phase II trial where nonsquamous cell carcinoma patients were allocated to different treatments based on the EGFR mutation status and BRCA1 mRNA expression levels. Treatment algorithm is shown in Figure 3. In this trial, the influence of receptor- associated protein 80 (RAP 80) expression was also examined as RAP 80 has been shown to act upstream of BRCA1 and be required for the accumulation of BRCA1 to sites of DNA breaks [Rosell et al. 2007; Wang et al. 2007].
Figure 3.
Customized treatment of non-small cell lung cancer based on epidermal growth factor receptor (EGFR) mutation and BRCA1 mRNA expression. WT, wild type.
Median overall survival was 11 months in the lowest BRCA1 expression treated with gemcitabine plus cisplatin and 2-year survival was 41.2%, comparable with the median survival and 2-year survival obtained with cisplatin and gemcitabine in a recent large phase III trial [Scagliotti et al. 2008]. Meanwhile, in patients with the highest BRCA expression treated with docetaxel alone, median survival was 11 months (similar to the survival obtained in a large phase III trial in patients treated with cisplatin plus either docetaxel or vinorelbine) [Fossella et al. 2003]. The survival of patients with low BRCA1 expression decreased as RAP 80 expression increased, confirming that RAP80 can replace the BRCA1 DNA repair function in cells lacking BRCA (Table 6).
Table 6.
Customized treatment of non-small cell lung cancer based on epidermal growth factor receptor mutations and BRCA1 mRNA expression. Correlation of BRCA1 and RAP80 expression with median survival. Purple circle, cisplatin–gemcitabine; green circle, cisplatin–docetaxel; red, docetaxel monotherapy. Data from Rosell et al. [2007].
| RAP80 mRNA |
||||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| BRCA1 mRN | Low | NR | 8 m | 7 m |
| Intermediate | 5 m | 13 m | 16 m | |
| High | 6 m | 12 m | 11 m | |
m, months.
Thus, this trial showed that chemotherapy customized according to BRCA1 expression levels is associated with encouraging overall survival, and that RAP80 could play a crucial modulating effect in this model of customized chemotherapy. The concept of customizing chemotherapy treatment based on BRCA1 and RAP80 expression levels is now being tested in a prospective randomized phase III trial.
Conclusions
The newer systemic therapies for NSCLC are directed towards specific targets but their efficacy will be strongly influenced by inherent tumor heterogeneity and the multiple robust and compensatory signaling pathways involved in tumor homeostasis [Thomson et al. 2008; Amit et al. 2007]. Therefore, identification of genotypes that respond better to specific therapies, thus actually adjusting the therapies to the genetic profile of the patients, remains an important and interesting issue.
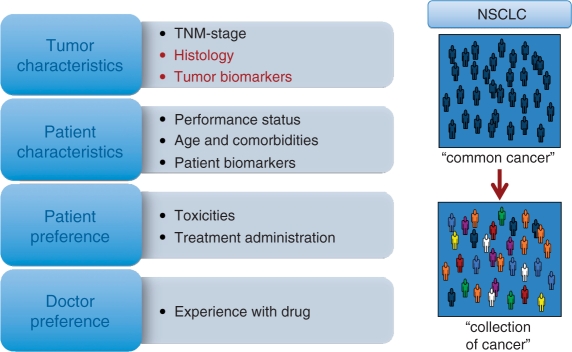
The recent advances in our understanding of NSCLC tumor biology and the mechanisms of action of various systemic treatments have led to the first steps towards personalized treatment of patients with NSCLC. A number of potential selection factors for customizing systemic treatment in advanced NSCLC are summarized in Figure 4. At present, only tumor histology and EGFR-mutation status have been validated in phase III trials as predictive markers, which can help us guide treatment choices. In patients with squamous cell cancers, treatment with bevacizumab leads to life-threatening toxicity. Furthermore, squamous cell tumors appear to be more or less resistant to pemetrexed-containing chemotherapy. Thus, by restricting treatment with bevacizumab and pemetrexed to patients with nonsquamous NSCLC it should be possible to obtain better clinical outcomes (i.e. better tolerability and/or survival). The presence of an activating EGFR mutation predicts for a better response rate and progression-free survival with EGFR-TKI treatment compared with first-line chemotherapy regimens. In the maintenance and second-line treatment trials with erlotinib, an overall survival benefit was also observed in patients with wild-type EGFR [Cappuzzo et al. 2010]. Thus, EGFR-TKI treatment is a valid choice for first-line treatment in patients with such an EGFR mutation. However, this does not mean that treatment with EGFR-TKIs should be restricted to patients with EGFR mutations: in patients with wild-type EGFR, erlotinib can be considered for second- or third-line treatment (Table 7).
Figure 4.
Potential selection factors for customizing systemic treatment in advanced non-small cell lung cancer (NSCLC). Moving from thinking of NSCLC as a single entity towards customized treatment of a collection of tumors grouped under the term NSCLC.
Table 7.
Selection factors for customizing systemic treatment in advanced non-small cell lung cancer.
| Category | Drug | Selection factor | Effect |
|---|---|---|---|
| Tumor histology | EGFR-TKI | Adeno | Improved response rate |
| Pemetrexed | Nonsquamous | Exclusion non-benefiting pts | |
| Bevacizumab | Non-Squamous | Safety concerns in squamous | |
| Molecular tumor biomarkers | EGFR-TKI | EGFR-mutation | Improved PFS |
| Pemetrexed | Low TS expression | Selection of benefiting pts | |
| Gemcitabine | Low RRM1 | Selection of benefiting pts | |
| Platinum | ERCC1.BRCA1, RAP80 | Improved RR, PFS and/or OS |
EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; PFS, progression-free survival; OS, overall survival; pts, patients; RR, response rate; TS, thymidilate syntethase; RRM1; ribonucleotide reductase messenger 1.
These first steps towards personalized medicine represent a shift in the management of NSCLC. Indeed, NSCLC should no longer be viewed as one common generic tumor but rather as a collection of more rare tumors with differing biological behaviors and different sensitivities to various systemic treatments.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors declare no conflict of interest in preparing this manuscript.
References
- Amit I., Wides R., Yarden Y. (2007) Evolvable signaling networks of receptor tyrosine kinases: relevance of robustness to malignancy and to cancer therapy. Mol Syst Biol 3: 151–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmis T.R., Ding K., Seymour L., Shepherd F.A., Leighl N.B., Winton T.L., et al. (2008) Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol 26(1): 54–59 [DOI] [PubMed] [Google Scholar]
- Cappuzzo F., Ciuleanu T., Stelmakh L., Cicenas S., Szczesna A., Juhasz E., et al. (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11: 521–529 [DOI] [PubMed] [Google Scholar]
- Ceppi P., Volante M., Saviozzi S., Rapa I., Novello S., Cambieri A., et al. (2006) Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 107: 1589–1596 [DOI] [PubMed] [Google Scholar]
- Ciuleanu T., Brodowicz T., Zielinski C., Kim J.H., Krzakowski M., Laack E., et al. (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374: 1432–1440 [DOI] [PubMed] [Google Scholar]
- Coate L.E., John T., Tsao M.S., Shepherd F.A. (2009) Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol 10: 1001–1010 [DOI] [PubMed] [Google Scholar]
- Davidoff A.J., Tang M., Seal B., Edelman M.J. (2010) Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 28: 2191–2197 [DOI] [PubMed] [Google Scholar]
- Eberhard D.A., Johnson B.E., Amler L.C., Goddard A.D., Heldens S.L., Herbst R.S., et al. (2005) Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 23: 5900–5909 [DOI] [PubMed] [Google Scholar]
- Fossella F., Pereira J.R., von Pawel J., Pluzanska A., Gorbounova V., Kaukel E., et al. (2003) Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 21: 3016–3024 [DOI] [PubMed] [Google Scholar]
- Gronberg B.H., Sundstrom S., Kaasa S., Bremnes R.M., Flotten O., Amundsen T., et al. (2010) Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer 46: 2225–2234 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Hanna N., Shepherd F.A., Fossella F.V., Pereira J.R., De Marinis F., von Pawel J., et al. (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22: 1589–1597 [DOI] [PubMed] [Google Scholar]
- Herbst R.S., Heymach J.V., Lippman S.M. (2008) Lung cancer. N Engl J Med 359: 1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J., Kyung S.Y., Lee S.P., Park J.W., Jung S.H., Lee J.I., et al. (2010) Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small cell lung cancer. Korean J Intern Med 25: 294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn L., Pao W. (2009) EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol 27: 4232–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisa K., Masago K., Togashi Y., Fujita S., Hatachi Y., Fukuhara A., et al. (2010) Significance of pretreatment comorbidities in elderly patients with advanced non-small-cell lung cancer treated with chemotherapy or epidermal growth factor receptor-tyrosine kinase inhibitor. Med Oncol in press [DOI] [PubMed] [Google Scholar]
- Jain R.K. (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7: 987–989 [DOI] [PubMed] [Google Scholar]
- Johnson D.H., Fehrenbacher L., Novotny W.F., Herbst R.S., Nemunaitis J.J., Jablons D.M., et al. (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22: 2184–2191 [DOI] [PubMed] [Google Scholar]
- Kwak E.L., Bang Y.J., Camidge D.R., Shaw A.T., Solomon B., Maki R.G., et al. (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363: 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafarge S., Sylvain V., Ferrara M., Bignon Y.J. (2001) Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene 20: 6597–6606 [DOI] [PubMed] [Google Scholar]
- Langer C.J., Manola J., Bernardo P., Kugler J.W., Bonomi P., Cella D., et al. (2002) Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst 94: 173–181 [DOI] [PubMed] [Google Scholar]
- Lee L., Cheung W.Y., Atkinson E., Krzyzanowska M.K. (2011) Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol 29: 106–117 [DOI] [PubMed] [Google Scholar]
- Lilenbaum R.C., Herndon J.E., II, List M.A., Desch C., Watson D.M., Miller A.A., et al. (2005) Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 23: 190–196 [DOI] [PubMed] [Google Scholar]
- Lord R.V., Brabender J., Gandara D., Alberola V., Camps C., Domine M., et al. (2002) Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res 8: 2286–2291 [PubMed] [Google Scholar]
- Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139 [DOI] [PubMed] [Google Scholar]
- Makato et al. (2010)
- Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388 [DOI] [PubMed] [Google Scholar]
- Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121–128 [DOI] [PubMed] [Google Scholar]
- Mitsudomi et al. (2009)
- Mok et al. (2009)
- Monica V., Scagliotti G.V., Ceppi P., Righi L., Cambieri A., Lo Iacono M., et al. (2009) Differential Thymidylate Synthase Expression in Different Variants of Large-Cell Carcinoma of the Lung. Clin Cancer Res 15: 7547–7552 [DOI] [PubMed] [Google Scholar]
- Non-small Cell Lung Cancer Collaborative Group (1995) Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Br Med J 311: 899–909 [PMC free article] [PubMed] [Google Scholar]
- Olaussen K.A., Dunant A., Fouret P., Brambilla E., Andre F., Haddad V., et al. (2006) DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 355: 983–991 [DOI] [PubMed] [Google Scholar]
- Paez J.G., Janne P.A., Lee J.C., Tracy S., Greulich H., Gabriel S., et al. (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500 [DOI] [PubMed] [Google Scholar]
- Pao W., Miller V., Zakowski M., Doherty J., Politi K., Sarkaria I., et al. (2004) EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101: 13306–13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W., Miller V.A. (2005) Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol 23: 2556–2568 [DOI] [PubMed] [Google Scholar]
- Presta L.G., Chen H., O'Connor S.J., Chisholm V., Meng Y.G., Krummen L., et al. (1997) Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 57: 4593–4599 [PubMed] [Google Scholar]
- Reck M., von Pawel J., Zatloukal P., Ramlau R., Gorbounova V., Hirsh V., et al. (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27: 1227–1234 [DOI] [PubMed] [Google Scholar]
- Reck M., von Pawel J., Zatloukal P., Ramlau R., Gorbounova V., Hirsh V., et al. (2010) Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 21: 1804–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi L., Papotti M.G., Ceppi P., Bille A., Bacillo E., Molinaro L., et al. (2010) Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol 28: 1534–1539 [DOI] [PubMed] [Google Scholar]
- Rosell R., Danenberg K.D., Alberola V., Bepler G., Sanchez J.J., Camps C., et al. (2004) Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res 10: 1318–1325 [DOI] [PubMed] [Google Scholar]
- Rosell R., Moran T., Queralt C., Porta R., Cardenal F., Camps C., et al. (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361: 958–967 [DOI] [PubMed] [Google Scholar]
- Rosell R., Skrzypski M., Jassem E., Taron M., Bartolucci R., Sanchez J.J., et al. (2007) BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One 2: e1129–e1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch V., Klimstra D., Venkatraman E., Pisters P.W., Langenfeld J., Dmitrovsky E. (1997) Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res 3: 515–522 [PubMed] [Google Scholar]
- Sandler A., Gray R., Perry M.C., Brahmer J., Schiller J.H., Dowlati A., et al. (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550 [DOI] [PubMed] [Google Scholar]
- Scagliotti G.V., Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C., et al. (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26: 3543–3551 [DOI] [PubMed] [Google Scholar]
- Sculier J.P., Chansky K., Crowley J.J., Van Meerbeeck J., Goldstraw P. (2008) The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol 3: 457–466 [DOI] [PubMed] [Google Scholar]
- Shaw A.T., Yeap B.Y., Mino-Kenudson M., Digumarthy S.R., Costa D.B., Heist R.S., et al. (2009) Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27: 4247–4253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd F.A., Rodrigues Pereira J., Ciuleanu T., Tan E.H., Hirsh V., Thongprasert S., et al. (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353: 123–132 [DOI] [PubMed] [Google Scholar]
- Shih C., Habeck L.L., Mendelsohn L.G., Chen V.J., Schultz R.M. (1998) Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine-based antifolate LY231514 (MTA). Adv Enzyme Regul 38: 135–152 [DOI] [PubMed] [Google Scholar]
- Sigmond J., Backus H.H., Wouters D., Temmink O.H., Jansen G., Peters G.J. (2003) Induction of resistance to the multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem Pharmacol 66: 431–438 [DOI] [PubMed] [Google Scholar]
- Simon G.R., Sharma S., Cantor A., Smith P., Bepler G. (2005) ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest 127: 978–983 [DOI] [PubMed] [Google Scholar]
- Soda M., Choi Y.L., Enomoto M., Takada S., Yamashita Y., Ishikawa S., et al. (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448: 561–566 [DOI] [PubMed] [Google Scholar]
- Soda M., Takada S., Takeuchi K., Choi Y.L., Enomoto M., Ueno T., et al. (2008) A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A 105: 19893–19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S., Petti F., Sujka-Kwok I., Epstein D., Haley J.D. (2008) Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis 25: 843–854 [DOI] [PubMed] [Google Scholar]
- Tsao M.S., Sakurada A., Cutz J.C., Zhu C.Q., Kamel-Reid S., Squire J., et al. (2005) Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med 353: 133–144 [DOI] [PubMed] [Google Scholar]
- Vogelzang N.J., Rusthoven J.J., Symanowski J., Denham C., Kaukel E., Ruffie P., et al. (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21: 2636–2644 [DOI] [PubMed] [Google Scholar]
- Wang B., Matsuoka S., Ballif B.A., Zhang D., Smogorzewska A., Gygi S.P., et al. (2007) Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 316: 1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G.J., Langer C., Rosell R., Hanna N., Shepherd F., Einhorn L.H., et al. (2006) Elderly patients benefit from second-line cytotoxic chemotherapy: a subset analysis of a randomized phase III trial of pemetrexed compared with docetaxel in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 24: 4405–4411 [DOI] [PubMed] [Google Scholar]
- Willett C.G., Boucher Y., di Tomaso E., Duda D.G., Munn L.L., Tong R.T., et al. (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett C.G., Duda D.G., di Tomaso E., Boucher Y., Czito B.G., Vujaskovic Z., et al. (2007) Complete pathological response to bevacizumab and chemoradiation in advanced rectal cancer. Nat Clin Pract Oncol 4: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]