Abstract
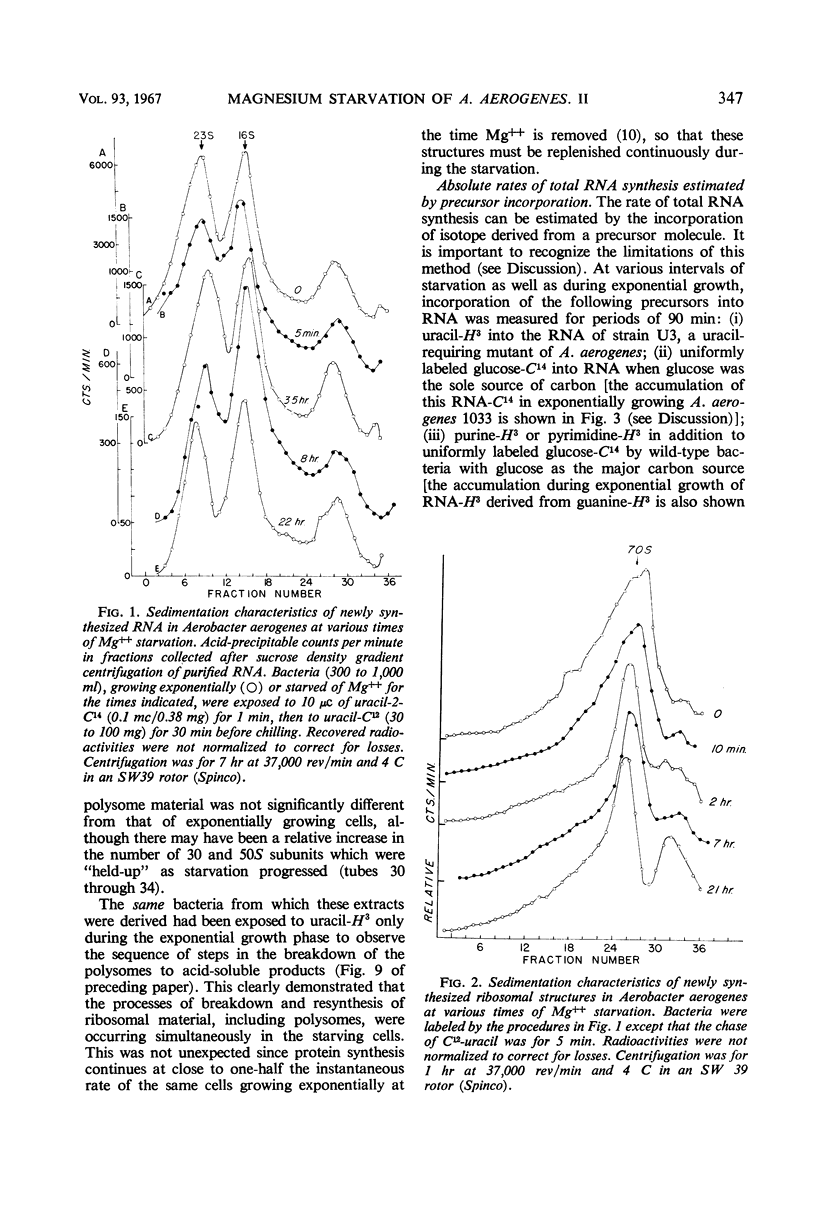
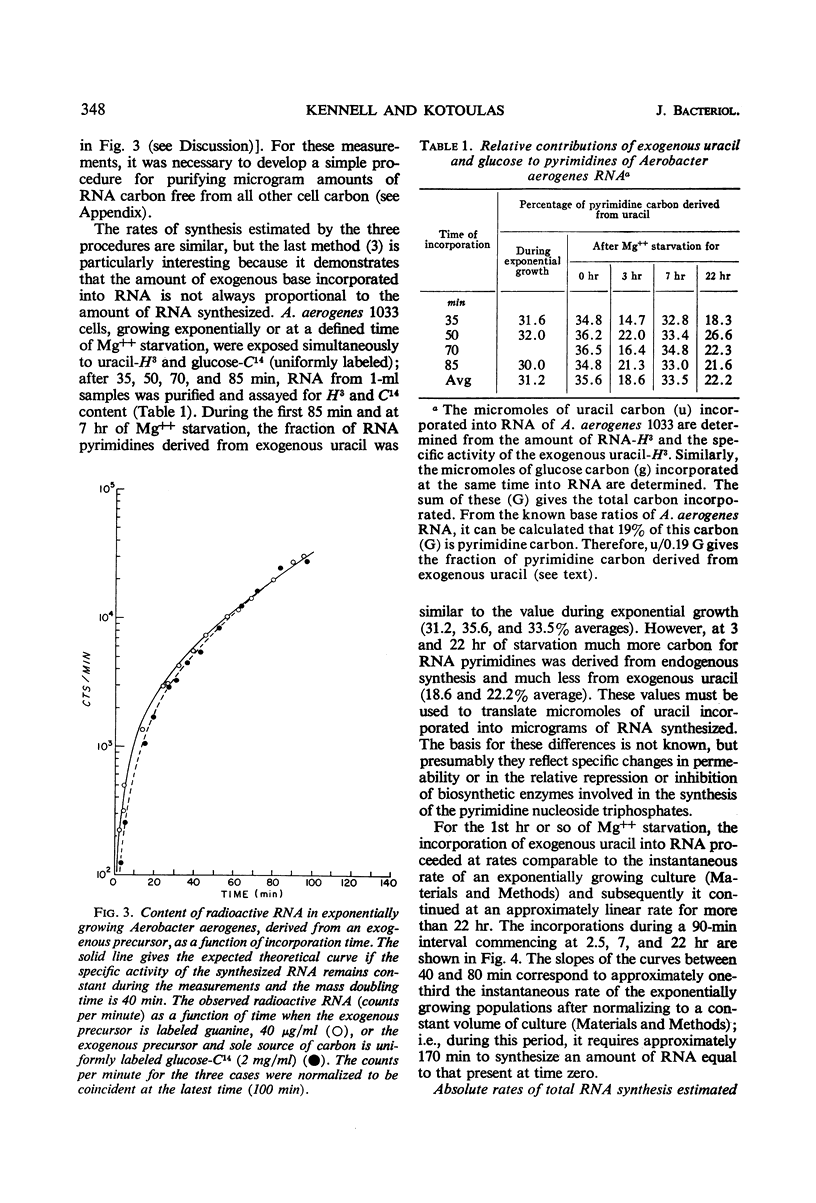
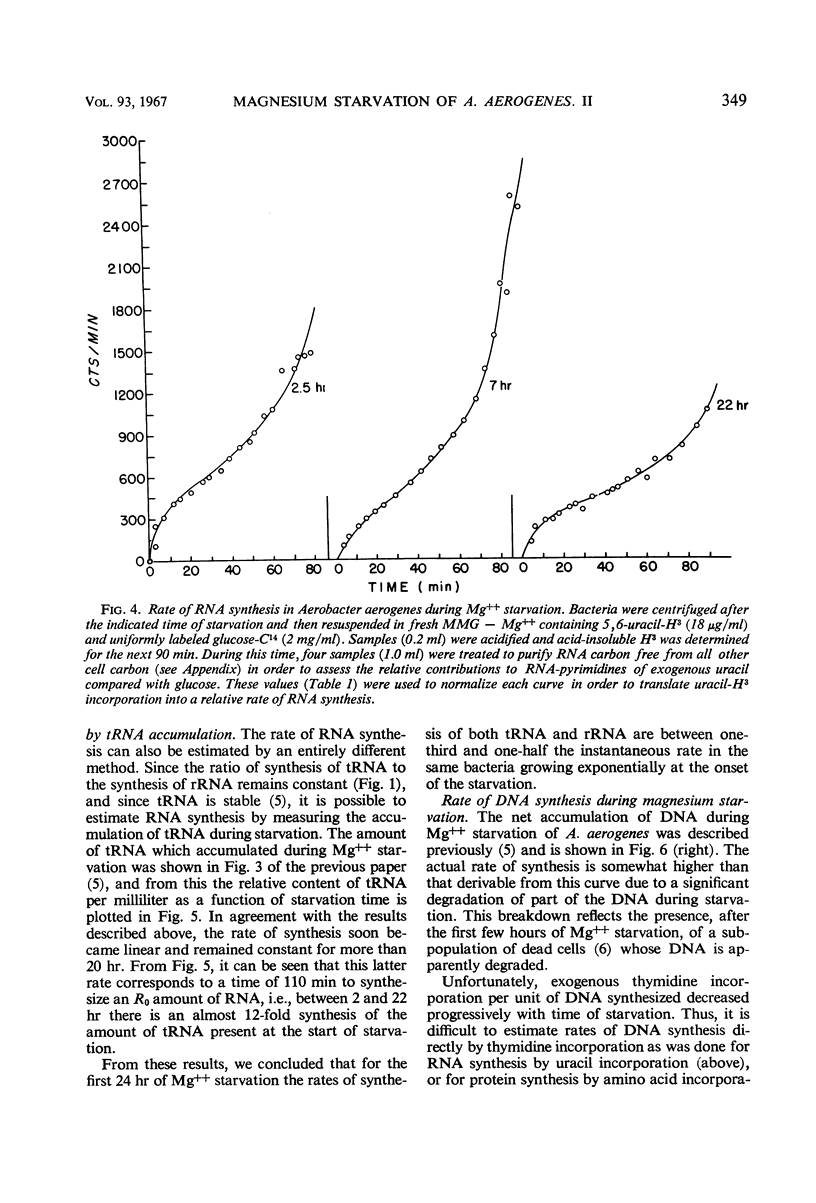
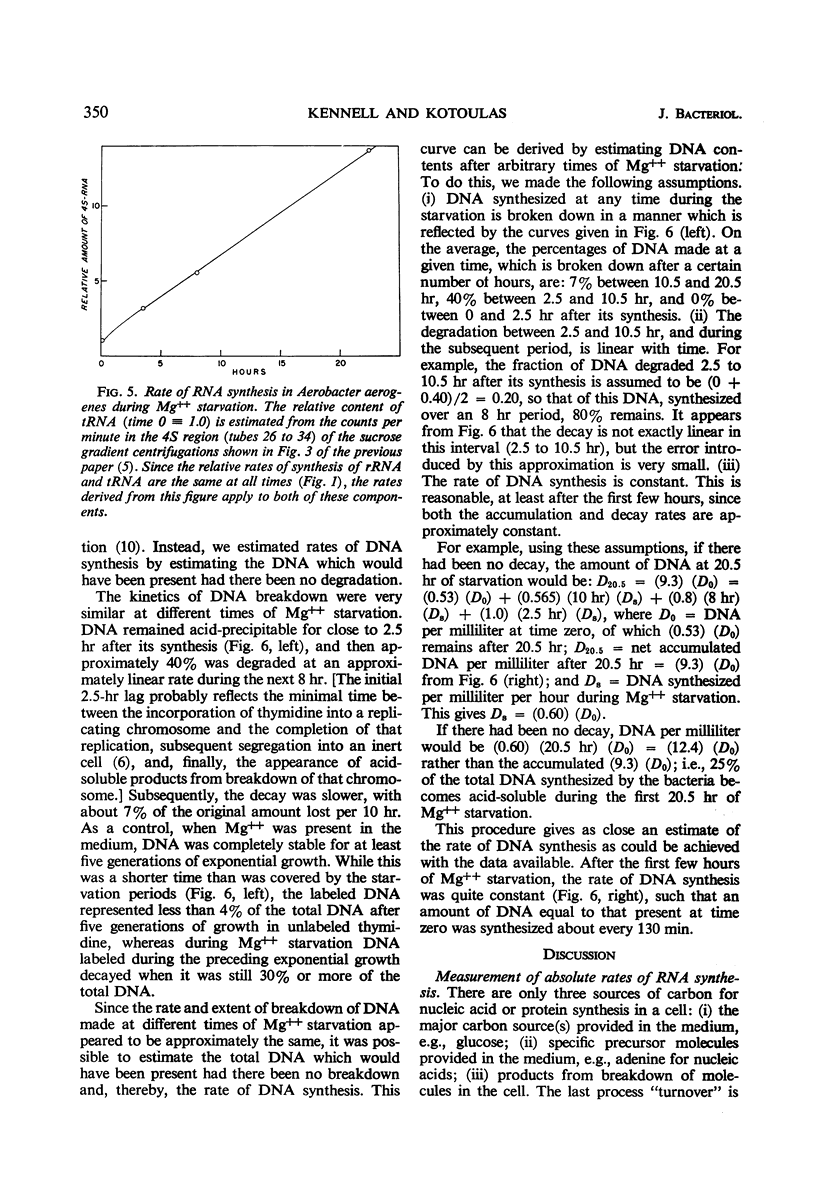
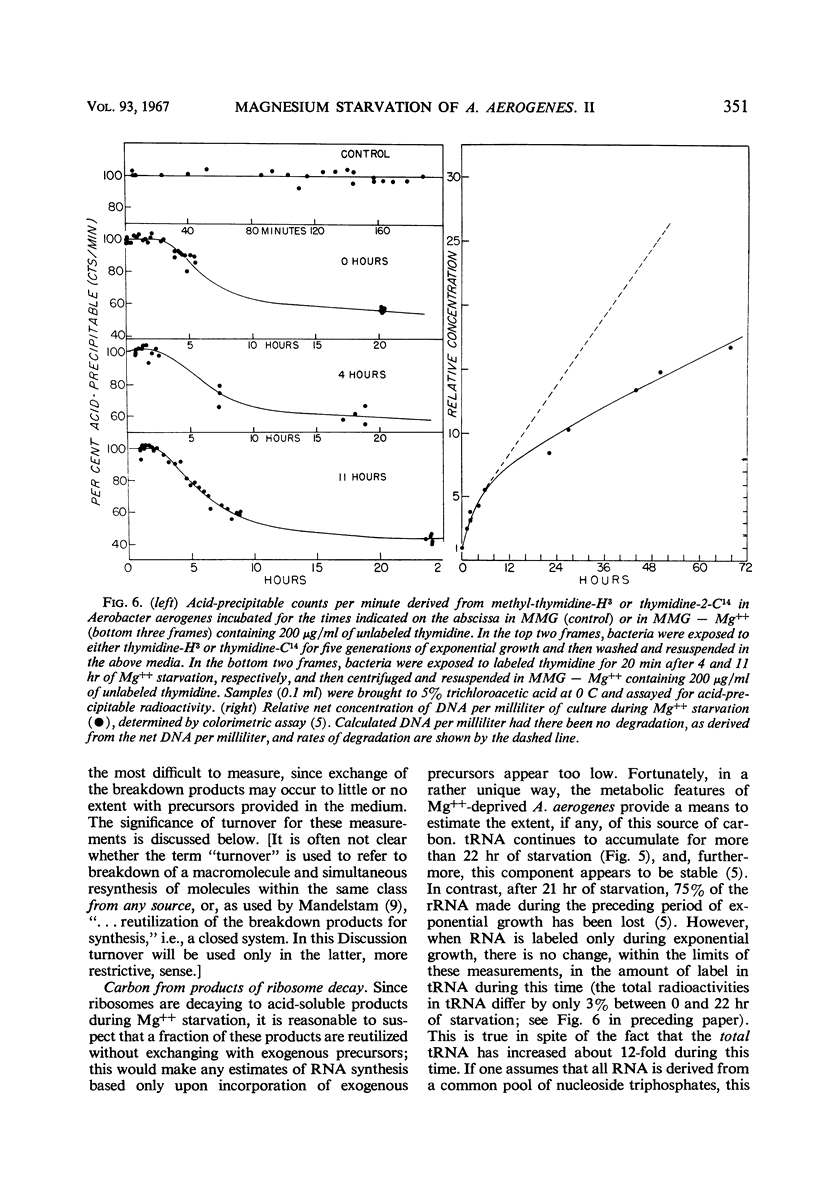
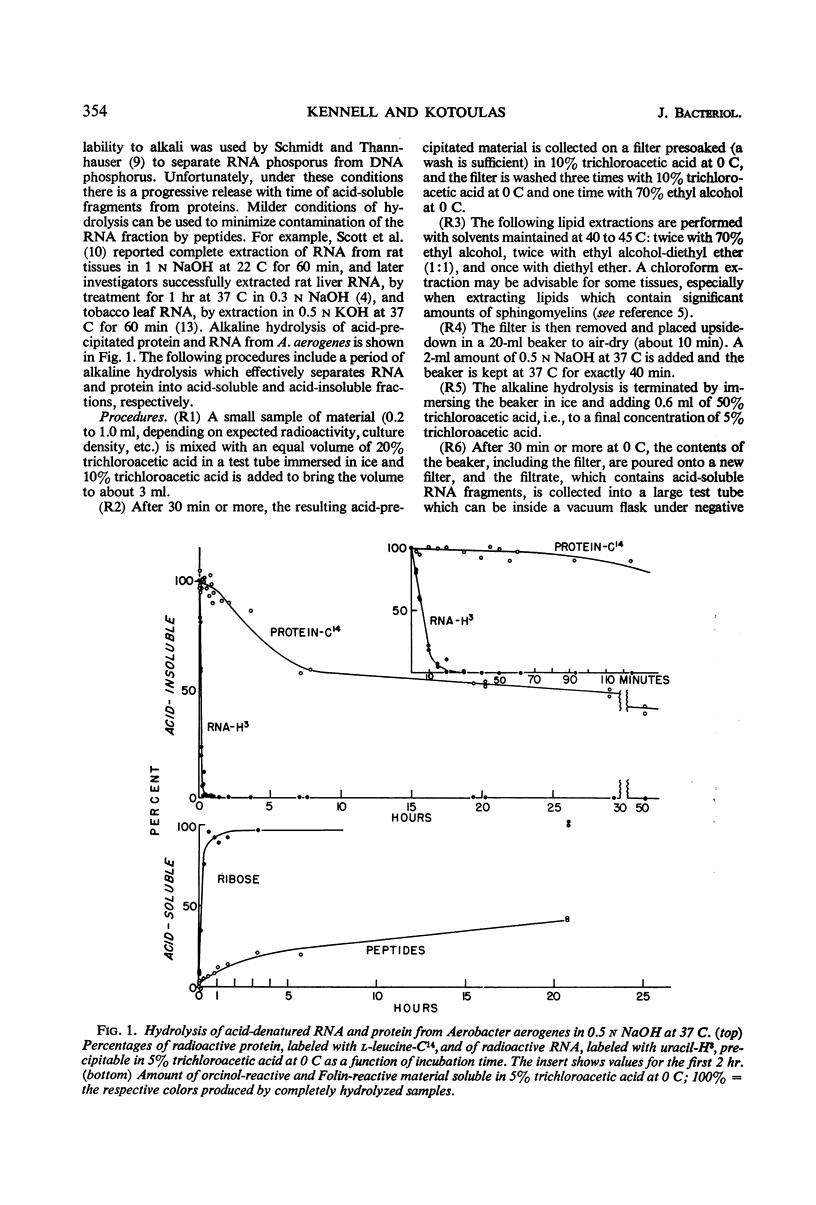
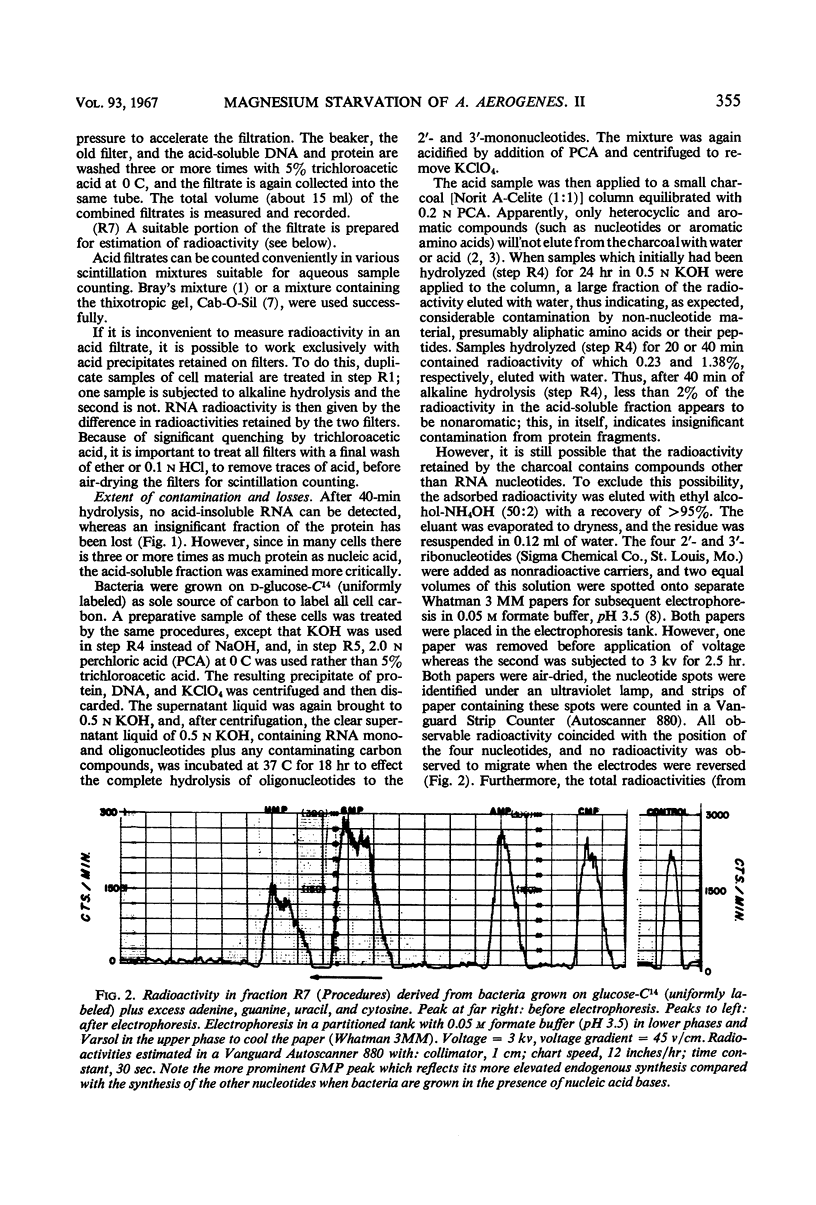
The rates of synthesis of Aerobacter aerogenes nucleic acids were estimated during incubation of the bacteria in a Mg++-free medium. Deoxyribonucleic acid (DNA) synthesized during Mg++ starvation, or in the preceding exponential growth, remained acid-precipitable for 2.5 hr before breaking down to acid-soluble products during a period of many hours. Rates of DNA synthesis were calculated by correcting the net amounts of DNA per milliliter to values that would have appeared had there been no decay. After the first few hours, this rate was constant, the amount of DNA present at the start of Mg++ starvation being synthesized every 130 min. Rates of synthesis of total ribonucleic acid (RNA) were established in two ways: (i) by measurements of the incorporation of exogeneous uracil and glucose carbon into RNA, and (ii) by the accumulation of transfer RNA (tRNA), since this component is stable during Mg++ starvation. After the first few hours, this rate was constant, the amount of RNA present at the start of Mg++ starvation being synthesized about every 120 min. Fractionation by gradient centrifugation revealed that at all times of starvation the ratio of newly synthesized tRNA-rRNA was the same as it was during exponential growth. Furthermore, newly synthesized ribosomal RNA (rRNA) became a part of polysomal structures. Thus, in the absence of Mg++, DNA, tRNA, and rRNA were synthesized in the same relative proportions as during exponential growth, at rates close to one-half the instantaneous rates of synthesis in the bacteria growing exponentially at the start of starvation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLTON E. T., McCARTHY B. J. A general method for the isolation of RNA complementary to DNA. Proc Natl Acad Sci U S A. 1962 Aug;48:1390–1397. doi: 10.1073/pnas.48.8.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. PRE-RIBOSOMAL PARTICLES FORMED IN POTASSIUM-DEPLETED CELLS. STUDIES ON DEGRADATION AND STABILIZATION. Biochim Biophys Acta. 1965 Apr 19;95:605–623. doi: 10.1016/0005-2787(65)90515-0. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fiske C. H. The Nature of the Depressor Substance of the Blood. Proc Natl Acad Sci U S A. 1934 Jan;20(1):25–27. doi: 10.1073/pnas.20.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- HUTCHISON W. C., MUNRO H. N. The determination of nucleic acids in biological materials. A review. Analyst. 1961 Dec;86:768–813. doi: 10.1039/an9618600768. [DOI] [PubMed] [Google Scholar]
- Johnson M. J. Optimum operation of recording count rate meters for radioactive paper chromatograms. J Chromatogr. 1965 Oct;20(1):100–106. doi: 10.1016/s0021-9673(01)97369-8. [DOI] [PubMed] [Google Scholar]
- KENNELL D., MAGASANIK B. The relation of ribosome content to the rate of enzyme synthesis in Aerobacter aerogenes. Biochim Biophys Acta. 1962 Jan 22;55:139–151. doi: 10.1016/0006-3002(62)90940-x. [DOI] [PubMed] [Google Scholar]
- KREISBERG R. A., WILLIAMSON J. R. METABOLIC EFFECTS OF GLUCAGON IN THE PERFUSED RAT HEART. Am J Physiol. 1964 Sep;207:721–727. doi: 10.1152/ajplegacy.1964.207.3.721. [DOI] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Magnesium starvation of Aerobacter aerogenes. I. Changes in nucleic acid composition. J Bacteriol. 1967 Jan;93(1):334–344. doi: 10.1128/jb.93.1.334-344.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Magnesium starvation of Aerobacter aerogenes. IV. Cytochemical changes. J Bacteriol. 1967 Jan;93(1):367–378. doi: 10.1128/jb.93.1.367-378.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acid. I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J. 1952 Dec;52(4):552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDGLEY J. E., McCARTHY B. J. The synthesis and kinetic behavior of deoxyribonucleic acid-like ribonucleic acid in bacteria. Biochim Biophys Acta. 1962 Nov 26;61:696–717. doi: 10.1016/0926-6550(62)90053-1. [DOI] [PubMed] [Google Scholar]
- Marchesi S. L., Kennell D. Magnesium starvation of Aerobacter aerogenes. 3. Protein metabolism. J Bacteriol. 1967 Jan;93(1):357–366. doi: 10.1128/jb.93.1.357-366.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J. The Synthesis of Ribosomes in E. coli: I. The Incorporation of C-Uracil into the Metabolic Pool and RNA. Biophys J. 1962 Jan;2(1):35–47. doi: 10.1016/s0006-3495(62)86839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT J. F., FRACCASTORO A. P., TAFT E. B. Studies in histochemistry. I. Determination of nucleic acids in microgram amounts of tissue. J Histochem Cytochem. 1956 Jan;4(1):1–10. doi: 10.1177/4.1.1. [DOI] [PubMed] [Google Scholar]
- VENKATARAMAN P. R., LOWE C. U. Effect of ethanol on ratliver ribonucleoprotein previously exposed to cold trichloroacetic acid. Biochem J. 1959 Jul;72:430–435. doi: 10.1042/bj0720430. [DOI] [PMC free article] [PubMed] [Google Scholar]



