Abstract
The world’s first laser was developed by Theodore Maiman in 1960. Over the course of the past five decades, this technology has evolved into a highly specialized entity, also finding a niche market in the field of urology. Lasers obtained from various lasing mediums producing amplified light of different wavelengths have been tested for urological applications. Today, these lasers are most commonly used in the surgical management of benign prostatic hyperplasia and as intracorporeal lithotripters. Other uses include ablation of various urologic tumors and incising strictures of the upper- and lower urinary tract. A continuous process of evolution of this technology is taking place, resulting in surgical lasers becoming ever safer, more effective, and more affordable.
Keywords: ablation, laser, lithotripsy, penetration depth, thermal effects, vaporization, wavelength
Introduction
In broad terms ‘evolution’ can be seen as changes in characteristics through successive generations, brought on by interactions with the environment. Variants of traits of the original become more common if they aid in survival of the entity: this is referred to as ‘natural selection’.
The use of lasers in medicine is not new, and in urology they have been around for more than 40 years. Here too, a form of evolution is taking place. New, streamlined, safe and effective technology that can answer to growing demands is continuously being selected out, and the outdated and hazardous are condemned to history. However, every passing phase is an essential step towards the pinnacle of laser applications in urology.
Lasers: a short history and simplified physics
It was Albert Einstein who in 1917 first proposed the theory of ‘stimulated emission’: the process by which photons (a ‘packet of light energy’) with the correct amount of energy could disturb an excited atom and cause it to drop to a lower energy level, in turn leading to the creation of another identical photon. The original photon interacting with the atom, as well as the photon subsequently released will be discharged simultaneously and will therefore have an identical wavelength and direction of propagation [Einstein, 1917].
The concept of stimulated emission was the foundation on which subsequent laser development would be undertaken.
Development of the MASER (‘microwave amplification by stimulated emission of radiation’) was the first giant leap. Microwaves are electromagnetic waves with fairly long wavelengths (1 mm to 1 m). In 1954, Gordon and colleagues tested the first MASER where stimulated emission at microwave wavelengths (in this case 12.5 mm) was demonstrated in an oscillator [Gordon et al. 1954].
The step from MASER to LASER (light amplification by stimulated emission of radiation) took 3 years. The idea was to extend the principle of stimulated emission from the microwave wavelengths to much shorter wavelengths, also including the optical range or visible spectrum of around 390–750 nm. For this, one would need to build an optical oscillator that could generate coherent light by amplifying stimulated emission [Hecht, 2010]. Theodore Maiman was the first to succeed and in 1960 he built the first LASER using ruby crystals as an active medium [Maiman, 1960].
It is the active medium (also referred to as the lasing medium) in a laser that determines the wavelength (and therefore color) and frequency of the light that it emits. The wavelength and frequency are inversely proportional to one another.
In simple terms the design of a laser is basically that of a laser medium placed within an optical resonator, which is defined by two mirrors. Light at the characteristic laser wavelength receives amplification whenever it passes through the excited laser medium. The reflective surfaces of the optical resonator ensure many passes of the light beam through the medium, leading to repetitive amplification. Excitation energy is required for this amplification process and can be derived from an electrical current. A fraction of the amplified light inside the optical resonator escapes as a beam of light out of one or both mirrors.
Early lasers used gas as active medium: nitrogen (N), carbon dioxide (CO2), helium (He) and neon (Ne). Liquids as medium soon followed: the so-called ‘dye lasers’, because the lasing agent is an organic dye [Gross and Herrmann, 2007]. Dye lasers have the advantage of being able to generate amplified light with a wider range of wavelengths. Some are even tunable. One of the earliest (1964) solid-state lasers utilized Nd:YAG (neodymium-doped yttrium aluminium garnet) as a medium; this is still popular today [Geusic et al. 1964].
A classification of laser output of particular practical importance in urology is that of pulsed wave (PW) versus continuous wave (CW). During CW operation the output of the laser is continuous and of constant amplitude. The clinical effect is a more controlled interaction with the tissue. PW operation on the other hand, delivers forceful bursts of laser energy, which is useful for stone fragmentation [Teichmann and Herrmann, 1994].
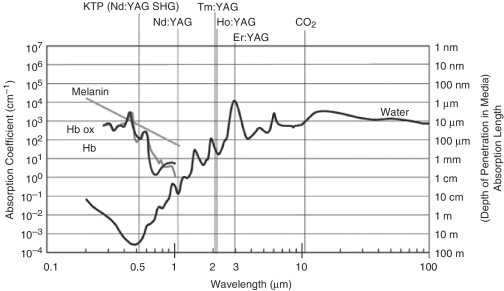
A basic understanding of the light-tissue interaction of lasers is required in order to fully appreciate important aspects such as penetration depth, thermal effects and reflection. These technical terms have major clinical significance. When laser light meets tissue, a percentage of the laser beam will be reflected. The reflected radiation is lost for the surgical purpose and may also cause unintended thermal damage to surrounding areas. Absorption is the most important interaction of laser light with tissue. A chromophore is required in order to achieve absorption: body chromophores accessible for laser light include blood, water and melanin. Absorbed laser light is converted to heat and depending on the amount of heat, the clinical effect will be tissue coagulation or vaporization. Absorption depth is dependent on the wavelength of the laser. Figure 1 illustrates the absorption spectrum of melanin, hemoglobin and water for specific laser wavelengths [Teichmann and Herrmann, 1994].
Figure 1.
Absorption spectrum of melanin, hemoglobin and water for specific laser wavelengths [Teichmann and Herrmann, 2007].
Over the course of the last four decades, many possible applications of lasers in urology have been investigated. This ‘trial and error’ era of the 1980s was a crucial step in the process of evolution of this technology. Every imaginable use was explored, with varying degrees of success and applicability. In the end, the safe and effective have remained, and are constantly being refined.
Today, the types of lasers most commonly used in urology include:
Nd:YAG;
Ho:YAG (holmium:YAG);
Thu:YAG (thulium:YAG);
CO2 (carbon dioxide);
KTP (potassium titanyl phosphate);
LBO (lithium triborate);
diode laser.
Laser applications in urology
Benign prostatic hyperplasia
The ability of the laser to ablate prostatic tissue with minimal hemorrhage has concentrated most of the interest in urologically applied lasers to benign prostatic hyperplasia (BPH) [Anson et al. 1994]. Despite tremendous advances in the surgical and minimally invasive treatment of BPH, transurethral resection of the prostate (TURP) is still considered the ‘gold standard’. The risks of TURP are always mentioned when discussing the reasons for seeking alternative treatment modalities for BPH. Bleeding certainly remains a concern, especially in patients on some form of anticoagulation (heparin, coumarin related compounds, antiplatelet agents) or those with prostates in excess of 60–80 g. On the other hand, with the availability of transurethral resection in saline (TURiS), the TURP syndrome is nowadays considered by many to be a relatively rare complication [Sarf et al. 2010].
Although removal of benign prostatic tissue using a laser was first described in 1986, it was only in 1990 that introduction of the ‘side-firing’ (deflecting device at the tip: 60–90°) laser prompted more widespread use of this modality. The Nd:YAG laser was initially the laser most commonly used and is also the one most extensively studied. One of the earliest techniques used for Nd:YAG laser treatment of BPH was called ‘visual laser ablation of the prostate’ (VLAP) [Norris et al. 1993]. This involves lasing prostatic tissue in a noncontact fashion to create an area of heat-induced coagulative necrosis that extends about 10 mm into the tissue. The method is reasonably simple to learn and perform, is safe in anticoagulated patients and carries no risk of the TURP syndrome. However, edema and prolonged sloughing of the coagulated tissue leads to irritative lower urinary tract syndrome (LUTS) and urinary retention requiring catheterization, often for long periods (3 months), in up to 30% of cases [Cowles et al. 1995].
Another approach in which the Nd:YAG laser can be used is the contact mode, which leads to real-time destruction of prostatic adenoma. A ‘contact tip’ converts laser light to heat, which induces vaporization and immediate creation of a cavity. As with VLAP, no tissue is available for histology, but this approach has the advantages of immediate relief of obstruction, early catheter removal and decreased postoperative LUTS and urinary retention. Tissue ablation advances slowly (so-called repetitive ‘painting’ of the surface that needs to be ablated) and it is therefore not suitable for prostates larger than 40 g. In addition the hemostatic effect is not as good as with VLAP [Floratos and de la Rosette, 1999].
Apart from VLAP and contact ablation, the Nd:YAG laser can also be used for performing interstitial laser coagulation (ILC) of the prostate. First described in 1993 by Hofstetter, the main feature of this method was preservation of the prostatic urethra and its urothelium [Hofstetter and Alvarez, 1993]. The procedure is performed by placing laser-diffusing fibers directly into the prostatic adenoma, either via the transurethral cystoscopic approach, or the perineal approach. Laser energy then produces coagulation necrosis within the adenoma, which subsequently undergoes atrophy [Perlmutter and Muschter, 1998]. As is the case with VLAP, this method is safe in anticoagulated patients, but substantial tissue edema also usually necessitates prolonged (7–21 days) postoperative catheterization. Retreatment rates are problematic: as high as 20% at 2 years, 41% at 3 years and 50% at 54 months. Several authors have concluded that this modality should probably be restricted to selected, high-risk patients. It can be safely performed with a combination of local anesthesia and intravenous sedation [Daehlin and Frugârd, 2007].
KTP laser, green light laser, frequency-doubled Nd:YAG laser and photoselective vaporization of the prostate (PVP) all refer to the same modality. Passing the Nd:YAG laser beam (wavelength 1064 nm, invisible) through a KTP crystal doubles the frequency and halves the wavelength (532 nm, visible green light). This wavelength is strongly absorbed by hemoglobin and therefore has a very short absorption depth in well-vascularized tissue such as the prostate [McAllister and Gilling, 2004]. It is used in a noncontact fashion, causes immediate vaporization of prostatic tissue and is a virtually bloodless procedure. Due to the limited absorption depth, necrosis of the tissue underlying the vaporized area, with subsequent edema, is not a problem. Some authors have reported discharging patients on the day of surgery without a catheter, even those with prostates sizes in excess of 100 g [Barber and Muir, 2004].
Application of the KTP laser has changed tremendously over the years. It started out as part of the ‘hybrid technique’ that involved VLAP with the Nd:YAG laser followed by bladder neck incision using the KTP laser at a low-power setting (34 W). The theory of these ‘hybrid techniques’ was that the additional KTP laser incisions would reduce the Nd:YAG laser’s troublesome postoperative irritative symptoms and need for prolonged catheterization [Barber and Muir, 2004]. Further research was aimed at increasing the power output of the KTP laser, initially to 60 W. Although originally a pulsed wave laser, modifications to the system furthermore facilitated the delivery of pulses so rapidly that the effect of continuous wave delivery was created. All of these improvements heralded the ‘high-power’ era of KTP lasers: they were now being used for prostate vaporization independently from the Nd:YAG laser. Today, ‘high-power’ KTP laser prostatectomy refers to a power output of 80 W.
The 120 W lithium triborate (LBO) laser was the next step in the evolution of the 532 nm lasers. The aim with this system was to overcome the still relatively slow tissue ablation ability of the ‘high-power’ 80 W KTP, which leads to time-consuming procedures in patients with large prostate glands [Wosnitzer and Rutman, 2009]. Not only is energy transfer and tissue ablation faster and more efficient, but the working distance is also increased (working distance for KTP is 0.5 mm, for LBO it is up to 3 mm), making the LBO laser technically simpler to use. Unfortunately, a drawback of the higher power setting is a reduction in the hemostatic ability, as demonstrated in a recent study in an ex vivo model [Heinrich et al. 2010].
The Ho:YAG laser is a pulsed laser with a wavelength of 2140 nm. Some consider it to be the pinnacle of evolution of urological lasers: not only is it ideally suited for procedures on the prostate, but it is also extremely effective as an intracorporeal lithotriptor for most stones. In addition it can be used in the setting of ablation of urothelial tumors and incision of strictures of the upper and lower urinary tract [Kuntz, 2006]. The following features of the holmium laser make it such a useful instrument in prostate surgery:
Absorption depth in the prostate is only 0.4 mm, creating a high energy density sufficient for vaporization.
Dissipating heat causes simultaneous coagulation of small blood vessels to a depth of about 2 mm.
This enables precise, char-free and virtually bloodless incision in prostatic tissue.
The holmium laser proceeded through many of the same ‘evolutionary processes’ as the some of its predecessors. Initially, it was also used in ‘hybrid techniques’ (VLAP with the Nd:YAG laser followed by creation of a tunnel and bladder neck incision with the Ho:YAG laser, to attempt shortening of the postoperative catheterization time) just like the early KTP lasers [Gilling and Frauendorfer, 1998]. The next step was prostate vaporization in the same ‘painting’ fashion as the Nd:YAG and KTP lasers. Although the procedure (called HoLAP, holmium laser ablation of the prostate) was easy to learn and effective, it was once again too time consuming when dealing with larger prostates. This led to the development of HoLRP (holmium laser resection of the prostate) which basically simulates traditional TURP [Gilling et al. 1996]. Chips of prostatic tissue are resected with an end-firing laser fiber and the chips are then removed via the urethra. This method is not only technically quite difficult to master, but operative times are still too long in patients with large adenomas. Refinement of the holmium laser technique and development of an efficient tissue morcellator led to HoLEP (holmium laser enucleation of the prostate)-finally size was no longer an issues. This procedure simulates open prostatectomy where the entire adenoma is removed at the level of the surgical capsule. The Ho:YAG laser ensures bloodless incision followed by blunt dissection using the cystoscope and laser fiber as an ‘index finger’ [Elzayat and Elhilali, 2006].
The Thu:YAG laser is the newest addition to the urologists laser armamentarium and use of this laser for BPH surgery was first published in 2005 [Xia et al. 2005]. This report described the so-called ‘thulium laser resection of the prostate tangerine technique’. The next step was vaporesection (simultaneous resection of TURP-like chips and vaporization of tissue), which was proven to be safe and effective [Bach et al. 2009]. The final leaps came with ‘Thu:YAG vapo-enucleation’, followed by ThuLEP (thulium laser enucleation of the prostate) [Bach et al. 2010]. When compared with the holmium laser, thulium seems to deliver improved vaporization ability, ensuring smooth tissue incisions. This allows the surgeon to accurately remove the adenoma at the level of the surgical capsule, as this plane is easier distinguishable. Virtually any sized prostate can be removed transurethrally using this technique.
Diode lasers have been around for a long time, but their clinical application has thus far been limited when compared to the other lasers. There now seems to be some renewed interest in these lasers as an alternative to KTP or LBO for vaporization techniques. As mentioned previously, for KTP and LBO lasers power versus hemostasis is somewhat of a ‘catch 22’ situation: the increased power of the 120 W LBO certainly cuts down on operative times, but this is at the cost of hemostatic ability, which is much better when using the lower powered 80 W KTP system. However, lower power means longer duration of procedures. A recent report on a 980 nm diode laser device demonstrated better hemostasis during prostate vaporization when compared with a 120 W LBO laser. Unfortunately the diode laser was also associated with a higher incidence of complications such as postoperative irritative symptoms and epididymitis [Chiang et al. 2010].
Stones
Initial reports on the use of the pulsed dye laser for stone fragmentation appeared in 1987 [Dretler et al. 1987]. This was a very promising new technology enabling endoscopic fragmentation of more than 80–95% of stones and urothelial injury being rare. Drawbacks were very high initial costs and expensive disposables (coumarin dye), as well as trouble with fragmentation of notoriously ‘hard’ stones composed of calcium oxalate monohydrate (COM) and cysteine [Floratos and de la Rosette, 1999].
The FREDDY (frequency doubled double-pulse Nd:YAG) laser was the next step in laser lithotripsy, and consists of a KTP crystal incorporated into a Nd:YAG laser [Marks and Teichman, 2007]. This enables the laser to produce two pulses: a 20% green light component at 532 nm and an 80% infrared component at a wavelength of 1,064 nm. This combination works in synergy to enable highly effective stone fragmentation, mainly via a mechanical shockwave with very little thermal effects. Another major advantage is the extremely low risk of damage to the ureteral wall when using this laser [Yates et al. 2007]. Unfortunately the ‘hard’ types of calculi also present a challenge to this laser, as is the case with the pulsed dye lasers [Dubosq et al. 2006]. Another problem is that the FREDDY laser is only able to effectively fragment dark or colored stones that absorb the green wavelength. Some urologists later referred to it as the 50% laser because only about 50% of stones could be treated with it.
The alexandrite laser was introduced in 1991 and even though initial results were promising, there was never widespread acceptance of this laser for use as a lithotriptor [Pearle et al. 1998].
Owing to the high costs when investing in a urological laser, the ideal would be to have a system with applications in various pathological conditions. The drawback of the FREDDY lasers negligible effect on soft tissue is that it can be exclusively used for stone procedures. The use of the Ho:YAG laser in BPH surgery was discussed earlier and this laser has also become the one most commonly used for lithotripsy [Lee and Gianduzzo, 2009]. Fragmentation occurs through a photothermal effect and requires direct contact of the laser tip with the stone [Pierre and Preminger, 2007]. A major advantage is minimal retropulsion effects during stone fragmentation [Cinman et al. 2010]. What puts this laser ‘at the top of the food chain’ is its ability to fragment all types of stones, including cysteine, brushite and COM [Leveillee and Lobik, 2003]. The holmium laser can either reduce stones to tiny fragments that are easily cleared from the collecting system with outflow or irrigant, or larger stones can be broken up and fragments removed using baskets or grasping forceps [Bagley, 2002].
Other
As far as other applications of lasers in urology are concerned, none have evolved as far as in the case for the management of BPH and urolithiasis discussed above. The process of investigating the appropriateness of the use of a specific laser in a specific urological condition is ongoing, and in many of the instances that will be discussed, it is an exercise of re-evaluation and refinement. In most cases it is still ‘a work in progress’.
Transitional cell carcinoma
Parsons was the first to experimentally use a pulsed ruby laser on an opened canine bladder [Anson et al. 1994]. In the setting of transitional cell carcinoma (TCC), the Ho:YAG and Nd:YAG lasers are the ones most frequently used for treating lesions located in the renal pelvis and ureter. The development of thin, flexible ureterorenoscopes as well as improvement of previously inadequate biopsy devices have helped establish lasers as a safe and effective option for managing these relatively uncommon but troublesome tumors [Bagley and Grasso, 2010]. The Nd:YAG laser coagulates tissue to a depth of several millimeters (5–10 mm) and is appropriate when dealing with large tumors. Direct contact of the laser fiber with the tumor is unnecessary and should be prevented, as this causes charring at the tip of the fiber with a resulting decrease in the effectiveness of the laser. The depth of penetration of the Ho:YAG laser is significantly less (0.4 mm), which makes this a much safer choice when dealing with small tumors in the ureter [Phillips and Landman, 2007].
Photodynamic therapy (PDT) for superficial TCC of the bladder is a diagnostic and treatment modality where laser light can be used. The PDT mechanism relies on in situ generation of toxic agents by the activation of a light sensitive drug (also called the ‘photosensitizer’) [Pinthus et al. 2006]. The drug is instilled into the bladder and thereafter activated cystoscopically by light of an appropriate wavelength. Diode lasers are popular here as they can be produced to match the desired wavelengths of most photosensitizer drugs (630–760 nm), but other light sources can also be used. The use of PDT in prostate cancer, renal cell carcinoma, and malignant lesions of the penis has been investigated [Stables et al. 1999; Pomer et al. 1995; Windahl et al. 1990].
Strictures of the upper and lower urinary tract
Reports on the use of an argon laser for the treatment of urethral strictures date back to 1978. Using the laser instead of a cold knife, in the same way as was initially described by Sachse [1978], seemed to be effective, but further follow up revealed a high recurrence rate of up to 70.1% at a mean of 15.2 months [Becker et al. 1995]. Other lasers that have also been investigated include the Nd:YAG, KTP and Ho:YAG lasers. There is still a lack of sufficient long-term data and therefore the role of lasers in the treatment of urethral strictures and also vesico-urethral strictures after radical prostatectomy has yet to be defined [Bader et al. 2010]. More recently, the Thu:YAG laser was investigated in this setting. Its penetration depth of only 0.3 mm should cause very little injury to surrounding tissue, making it an ideal instrument for optical urethrotomy [Wang et al. 2010].
Ureteropelvic junction (UPJ) obstruction can also safely be managed with endopyelotomy using the Ho:YAG laser. Results seem to be more or less similar to those achieved with the ‘hot-wire balloon’ [Lee and Gianduzzo, 2009]. The holmium laser is also effective in the minimally invasive treatment of ureterovesical anastomotic strictures after renal transplant as well as ureterointestinal anastomotic strictures after urinary diversion [Kristo et al. 2003].
Laser tissue soldering
Laser tissue soldering (LTS) has been used to obtain a watertight anastomosis in different tissues for more than 20 years. A diode, CO2 or Nd:YAG laser can be used with albumin as a solder [Kirsch et al. 1995]. LTS relies on the photothermal properties of laser light. When performing LTS, laser energy is applied to a solder in conjunction with laser wavelength-specific chromophores to facilitate laser light absorption. The resultant temperature increase denatures the solder, which then forms a coagulum that increases the strength at the repair site [Lee and Gianduzzo, 2009]. In urology it has been successfully utilized in anastomosis of skin (hypospadias repair), bladder, urethra, renal pelvis (UPJ repair), as well as for vasovasostomy. In order to safely perform LTS, the laser power needs to be regulated: although higher power densities provide a stronger initial weld, there will be increased thermal damage to surrounding tissue which compromises tensile strength of the repair after a few days. Average soldering speed is around 1 minute per centimeter of incision length [Cooper et al. 2001].
Penis carcinoma
It is generally accepted that the risk of metastases is low for patients with penile squamous cell carcinoma exhibiting favorable histologic features (stages Tis Ta, T1, grades 1–2). Laser ablation is one of the organ-preserving therapeutic strategies that may be considered in these cases [Busby and Pettaway, 2005]. The CO2 laser is often used in this setting. Some centers have combined it with the Nd:YAG laser to increase the depth of tissue necrosis in the tumor base [Windahl and Andersson, 2003].
Prostate carcinoma
The possibility of focal laser ablation (another term for interstitial laser coagulation) of localized prostate cancer has recently been investigated. Focal laser ablation of unresectable liver metastases and inoperable hepatocellular carcinoma has been studied more extensively. The Nd:YAG laser or diode laser can be used. An exciting recent innovation is the ability to monitor in real-time the lesion created by the laser using magnetic resonance imaging [Lindner et al. 2010].
Genital skin lesions
Condylomata accuminata can occur on the prepuce, glans, penile shaft and intra-urethrally. The CO2 laser has been used since the late 1970s to vaporize multifocal, small lesions. For larger condylomata, coagulation with the Nd:YAG laser was later introduced. Intra-urethral lesions can be treated using the Nd:YAG or KTP laser [Stein, 1986]. Owing to the risk of dispersion of the oncogenic virus in the aerosol, laser vaporization of condylomata is not recommended in patients with HIV [Lee et al. 2001].
Future prospects
Current shortcomings of urological lasers include the high cost of the technology, relatively complex hardware and collateral injury to surrounding normal tissue [Pierre and Albala, 2007]. New lasers are continuously being developed: one of the more recent ones introduced for possible use in urology is the erbium:YAG (Er:YAG) laser. It operates at a wavelength of 2940 nm and can be used in all of the applications where the Ho:YAG laser is used. In an aqueous solution, this laser has a penetration depth of only 3 µm. Early studies seem to indicate that the Er:YAG laser may be slightly more effective than the holmium laser at breaking up stones [Fried, 2001]. Unfortunately there are still some unresolved issues: normal silica fibers cannot be used for this laser and the alternative materials are extremely expensive and potentially toxic. The hemostatic ability at this wavelength is also insufficient.
The year 2010 marks the 50th anniversary of the laser. This technology has changed and evolved tremendously over the course of five decades, but there is still ample room for improvement. During the next 50 years we can expect for even more applications of this versatile technology to be imagined, tested and eventually accepted.
Funding
This research was made possible by an Academic Fellowship Award from The Discovery Foundation, South Africa.
Conflict of interest statement
None declared.
References
- Anson K., Seenivasagam K., Miller R., Watson G. (1994) The role of lasers in urology. BJU Int 73: 225–230 [DOI] [PubMed] [Google Scholar]
- Bach T., Wendt-Nordahl G., Michel M.S., Herrmann T.R.W., Gross A.J. (2009) Feasibility and efficacy of Thulium:YAG laser enucleation (VapoEnucleation) of the prostate. World J Urol 27: 541–545 [DOI] [PubMed] [Google Scholar]
- Bach T., Xia S.J., Yang Y., Mattioli S., Watson G.M., Gross A.J., et al. (2010) Thulium:YAG 2µm cw laser prostatectomy: where do we stand? World J Urol 28: 163–168 [DOI] [PubMed] [Google Scholar]
- Bader M., Tilki D., Gratzke C., Sroka R., Stief C.G., Reich O. (2010) Ho:YAG-laser: treatment of vesicourethral strictures after radical prostatectomy. World J Urol 28: 169–172 [DOI] [PubMed] [Google Scholar]
- Bagley D. (2002) Expanding role of ureteroscopy and laser lithotripsy for treatment of proximal ureteral and intrarenal calculi. Curr Opin Urol 12: 277–280 [DOI] [PubMed] [Google Scholar]
- Bagley D.H., Grasso M., III (2010) Ureteroscopic laser treatment of upper urinary tract neoplasms. World J Urol 28: 143–149 [DOI] [PubMed] [Google Scholar]
- Barber N., Muir G. (2004) High-power KTP laser prostatectomy: the new challenge to trans-urethral resection of the prostate. Curr Opin Urol 14: 21–25 [DOI] [PubMed] [Google Scholar]
- Becker H.C., Miller J., Nöske H.D., Klask J.P., Weidner W. (1995) Transurethral laser urethrotomy with argon laser: experience with 900 urethrotomies in 450 patients from 1978 to 1993. Urol Int 55: 150–153 [DOI] [PubMed] [Google Scholar]
- Busby J.E., Pettaway C.A. (2005) What’s new in the management of penile cancer? Curr Opin Urol 15: 350–357 [DOI] [PubMed] [Google Scholar]
- Chiang P.H., Chen C.H., Kang C.H., Chuang Y.C. (2010) GreenLight HPS laser 120-W versus diode laser 200-W vaporization of the prostate: comparative clinical experience. Lasers Surg Med 42: 624–629 [DOI] [PubMed] [Google Scholar]
- Cinman N.M., Andonian S., Smith A.D. (2010) Lasers in percutaneous renal procedures. World J Urol 28: 135–142 [DOI] [PubMed] [Google Scholar]
- Cooper C.S., Schwartz I.P., Suh D., Kirsch A.J. (2001) Optimal solder and power density for diode laser tissue soldering. Lasers Surg Med 29: 53–61 [DOI] [PubMed] [Google Scholar]
- Cowles R.S., III, Kabalin J.N., Childs S., Lepor H., Dixon C., Stein B., et al. (1995) A prospective randomized comparison of trans urethral resection to visual laser ablation of the prostate for the treatment of benign prostatic hyperplasia. Urology 46: 155–160 [DOI] [PubMed] [Google Scholar]
- Daehlin L., Frugârd J. (2007) Interstitial laser coagulation in the management of lower urinary tract symptoms suggestive of bladder outlet obstruction from benign prostatic hyperplasia: long term follow up. BJU Int 100: 89–93 [DOI] [PubMed] [Google Scholar]
- Dretler S.P., Watson G., Parrish J.A., Murray S. (1987) Pulsed dye laser fragmentation of ureteral calculi: initial clinical experience. J Urol 137: 386–389 [DOI] [PubMed] [Google Scholar]
- Dubosq F., Pasqui F., Girard F., Beley S., Lesaux N., Gattegno B., et al. (2006) Endoscopic lithotripsy and the FREDDY laser: initial experience. J Endourol 20: 296–299 [DOI] [PubMed] [Google Scholar]
- Einstein A. (1917) Zur Quantentheorie der Strahlung. Phys Z 18: 121–128 [Google Scholar]
- Elzayat E.A., Elhilali M.M. (2006) Laser treatment of benign prostatic hyperplasia. World J Urol 24: 410–417 [DOI] [PubMed] [Google Scholar]
- Floratos D.L., de la Rosette J.J.M.C.H. (1999) Lasers in urology. BJU Int 84: 204–211 [DOI] [PubMed] [Google Scholar]
- Fried N.M. (2001) Potential applications of the erbium:YAG laser in endourology. J Endourol 15: 889–894 (abstract) [DOI] [PubMed] [Google Scholar]
- Geusic J.E., Marcos H.W., Van Uitert L.G. (1964) Laser oscillations in Nd-doped yttrium aluminum, yttrium gallium, and gadolinium garnets. Appl Phys Lett 4: 182–184 [Google Scholar]
- Gilling P.J., Cass C., Cresswell M.D., Malcolm A., Frauendorfer M.R. (1996) The use of the holmium laser in the treatment of benign prostatic hyperplasia. J Endourol 10: 459–461 [DOI] [PubMed] [Google Scholar]
- Gilling P.J., Frauendorfer M.R. (1998) Holmium laser prostatectomy: a technique in evolution. Curr Opin Urol 8: 11–15 [DOI] [PubMed] [Google Scholar]
- Gordon J.P., Zeiger H.J., Townes C.H. (1954) Molecular microwave oscillator and new hyperfine structure in the microwave spectrum of NH3. Phys Rev 95: 282–284 [Google Scholar]
- Gross A.J., Herrmann T.R. (2007) History of lasers. World J Urol 25: 217–220 [DOI] [PubMed] [Google Scholar]
- Hecht J. (2010) A short history of laser development. Appl Opt 49: F99–F122 [DOI] [PubMed] [Google Scholar]
- Heinrich E., Wendt-Nordahl, Honeck P., Alken P., Knoll T., Michel M.S., et al. (2010) 120 W Lithium triborate laser for photoselective vaporization of the prostate: comparison with 80 W potassium-titanyl-phosphate laser in an ex-vivo model. J Endourol 24: 75–79 [DOI] [PubMed] [Google Scholar]
- Hofstetter A., Alvarez A. (1993) Treatment of prostatic tumors with interstitial thermocoagulation with neodymium-YAG (a new treatment in minimally invasive surgery). Arch Esp Urol 46: 317–319 (Abstract) [PubMed] [Google Scholar]
- Kirsch A.J., Miller M.I., Hensle T.W., Chang D.T., Shabsigh R., Olsson C.A., et al. (1995) Laser tissue soldering in urinary tract reconstruction: first human experience. Urology 46: 261–266 [DOI] [PubMed] [Google Scholar]
- Kristo B., Phelan M.W., Gritsch H.A., Schulam P.G. (2003) Treatment of renal transplant ureterovesical anastomotic strictures using antegrade balloon dilation with or without holmium:YAG laser endourethrotomy. Urology 62: 831–834 [DOI] [PubMed] [Google Scholar]
- Kuntz R. (2006) Current role of lasers in the treatment of benign prostatic hyperplasia. Eur Urol 49: 961–969 [DOI] [PubMed] [Google Scholar]
- Lee J., Gianduzzo T.R.J. (2009) Advances in laser technology in urology. Urol Clin N Am 36: 189–198 [DOI] [PubMed] [Google Scholar]
- Lee L.K., Dinneen M.D., Ahmad S. (2001) The urologist and the patient infected with human immunodeficiency virus or with acquired immunodeficiency syndrome. BJU Int 88: 500–510 [DOI] [PubMed] [Google Scholar]
- Leveillee R.J., Lobik L. (2003) Intracorporeal lithotripsy: which modality is best? Curr Opin Urol 13: 249–253 [DOI] [PubMed] [Google Scholar]
- Lindner U., Lawrentschuk N., Trachtenberg J. (2010) Focal laser ablation of localized prostate cancer. J Endourol 24: 791–797 [DOI] [PubMed] [Google Scholar]
- Maiman T.H. (1960) Stimulated optical radiation in ruby. Nature 187: 493–494 [Google Scholar]
- Marks A.J., Teichman J.M. (2007) Lasers in clinical urology: state of the art and new horizons. World J Urol 25: 227–233 [DOI] [PubMed] [Google Scholar]
- McAllister W.J., Gilling P.J. (2004) Vaporization of the prostate. Curr Opin Urol 14: 31–34 [DOI] [PubMed] [Google Scholar]
- Norris J.P., Norris D.M., Lee R.D., Rubenstein M.A. (1993) Visual laser ablation of the prostate: clinical experience in 108 patients. J Urol 150: 1612–1614 [DOI] [PubMed] [Google Scholar]
- Pearle M.S., Sech S.M., Cobb C.G., Riley J.R., Clark P.J., Preminger G.M., et al. (1998) Safety and efficacy of the Alexandrite laser for the treatment of renal and ureteral calculi. Urology 51: 33–38 [DOI] [PubMed] [Google Scholar]
- Perlmutter A.P., Muschter R. (1998) Interstitial laser prostatectomy. Mayo Clinic Proc 73: 903–907 [DOI] [PubMed] [Google Scholar]
- Phillips C.K., Landman J. (2007) Lasers in the upper urinary tract for non-stone disease. World J Urol 25: 249–256 [DOI] [PubMed] [Google Scholar]
- Pierre S., Preminger G.M. (2007) Holmium laser for stone management. World J Urol 25: 235–239 [DOI] [PubMed] [Google Scholar]
- Pierre S.A., Albala D.M. (2007) The future of lasers in urology. World J Urol 25: 275–283 [DOI] [PubMed] [Google Scholar]
- Pinthus J.H., Bogaards A., Weersink R., Wilson B., Trachtenberg J. (2006) Photodynamic therapy for urological malignancies: past to current approaches. J Urol 175: 1201–1207 [DOI] [PubMed] [Google Scholar]
- Pomer S., Grashev G., Sinn H., Kalble T., Staehler G. (1995) Laser induced fluorescence diagnosis and photodynamic therapy of human renal cell carcinoma. Urol Int 55: 197–201 [DOI] [PubMed] [Google Scholar]
- Sachse H. (1978) Direct vision cold knife internal urethrotomy. Urology 17: 177–181 [PubMed] [Google Scholar]
- Sarf, I., Thüroff, J., Cheng, C., Gross, A. and Kuntz, R. (2010) Plenery on male voiding dysfunction. Société Internationale D’Urologie (SIU) World Meeting on Lower Urinary Tract Dysfunction, Marrakech, Morocco, 13–16 October.
- Stables G.I., Stringer M.R., Robinson D.J., Ash D.V. (1999) Erythroplasia of Queyrat treated by topical aminolaevulinic acid photodynamic therapy. Br J Dermatol 140: 514–517 [DOI] [PubMed] [Google Scholar]
- Stein B.S. (1986) Laser treatment of condylomata accuminata. J Urol 136: 593–594 [DOI] [PubMed] [Google Scholar]
- Teichmann H.O., Herrmann T.R. (2007) Technical aspects of lasers in urology. World J Urol 25: 221–225 [DOI] [PubMed] [Google Scholar]
- Wang L., Wang Z., Yang B., Yang Q., Sun Y. (2010) Thulium laser urethrotomy for urethral stricture: a preliminary report. Lasers Surg Med 42: 620–623 [DOI] [PubMed] [Google Scholar]
- Windahl T., Andersson S.O. (2003) Combined laser treatment for penile carcinoma: results after long term follow up. J Urol 169: 2118–2121 [DOI] [PubMed] [Google Scholar]
- Windahl T., Andersson S.O., Lofgren L. (1990) Photodynamic therapy of localized prostate cancer. Lancet 336: 1139–1139 [DOI] [PubMed] [Google Scholar]
- Wosnitzer M.S., Rutman M.P. (2009) KTP/LBO laser vaporization of the prostate. Urol Clin N Am 36: 471–483 [DOI] [PubMed] [Google Scholar]
- Xia S.J., Zhang Y.N., Lu J., Sun X.W., Zhang J., Zhu Y.Y., et al. (2005) Thulium laser resection of prostate – tangerine technique in treatment of benign prostatic hyperplasia. Zhonghua Yi Xue Za Zhi 85: 3225–3228 (Abstract) [PubMed] [Google Scholar]
- Yates J., Zabbo A., Pareek G. (2007) A comparison of the FREDDY and holmium lasers during ureteroscopic lithotripsy. Lasers Surg Med 39: 637–640 [DOI] [PubMed] [Google Scholar]