Abstract
Percutaneous renal surgery provides a minimally invasive approach to the kidney for stone extraction in a number of different clinical scenarios. Certain clinical cases present inherent challenges to percutaneous access to the kidney. Herein, we present scenarios in which obtaining and/or maintaining percutaneous access is difficult along with techniques to overcome the challenges commonly encountered. Also, complications associated with these challenging percutaneous renal surgeries are discussed.
Keywords: angiomyolipoma, calyceal diverticulum, horseshoe kidney, nephrolithotomy, obesity, PCNL
Introduction
History of percutaneous renal surgery
Since its inception in the latter half of the 1970s, percutaneous nephrolithotomy (PCNL) has taken a stronghold as a reliable management approach for larger renal stones or those positioned such that ureteroscopic approaches would be less effective. PCNL was developed as a therapeutic extension to the established percutaneous nephrostomy tube insertion which was previously reserved for decompressive purposes [Smith et al. 1979; Karamcheti and O'Donnell, 1977; Fernström and Johansson, 1976]. Since then, indications for PCNL have been well-defined and widely accepted to treat: (1) staghorn calculi or large (>2 cm) intrarenal stones; (2) stones with concomitant ureteropelvic junction obstruction; and (3) large intrarenal stones not amenable to extracorporeal shockwave lithotripsy (SWL) or endoscopic management due to stone composition or anatomic variability. Absolute contraindications to PCNL are active urinary tract infection and uncontrolled coagulopathy.
Limitations and challenges with percutaneous renal access
Herein we present different clinical scenarios in which percutaneous renal surgery is made more difficult due to the challenges with obtaining and/or maintaining percutaneous access. The case scenarios presented include patients with nephrolithiasis and concomitant angiomyolipomas (AMLs) of the kidney, patients with stones in calyceal diverticula, patients with horseshoe or pelvic kidneys, patients who are morbidly obese, and patients with full staghorn calculi. Each clinical scenario is presented with explanations of why each presents challenges to percutaneous surgery along with technical approaches to overcome the associated difficulties with obtaining and maintaining access. Finally, potential complications associated with these challenging cases are discussed.
Cases of challenging percutaneous renal access
Nephrolithiasis and concomitant angiomyolipoma
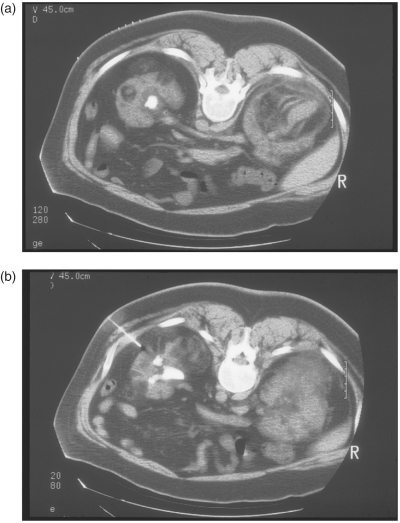
Renal AMLs are benign vascular tumors with a risk of spontaneous hemorrhage and increased risk of bleeding in the setting of any trauma. PCNL in the context of renal AML presents inherent risk of hemorrhage. The literature presents very few reported cases of percutaneous renal surgery with concomitant AML. Although one case report describes PNCL access through an AML without any intraoperative bleeding, complications, or need for postoperative blood transfusion, this approach is anecdotal and carries a theoretical increased risk of bleeding [Kropp et al. 1994]. A more conservative approach has been reported whereby the AML is preoperatively angioembolized if indicated by size parameters and percutaneous access is obtained using three-dimensional CT reconstruction for planning and CT guidance for precise avoidance of AMLs along the tract from the percutaneous entry to the renal collecting system [Eiley et al. 1999]. (Figure 1(a) and (b))
Figure 1.
(a) Preoperative CT imaging of nephrolithiasis in the setting of renal AML. (b) CT guided percutaneous access to the renal collecting system in the setting of renal AML.
Intraoperatively, it is essential to recall the anatomic association of the percutaneous tract to the AML or multiple AMLs present in the kidney for purposes of tract dilation, intraoperative manipulation, and consideration of placing additional percutaneous tracts for access to the stone. For large stone burdens inaccessible by a single percutaneous tract with moderate manipulation, it is prudent to approach the stone at a staged second session with CT assessment of residual stone burden and CT guidance for a second safe percutaneous tract.
Stones in calyceal diverticula
Calyceal diverticula are outpouchings of the pelvicalyceal system that are lined with transitional cells, often communicating with the renal collecting system via a narrowed infundibulum, present in less than 0.5% of patients [Hulbert et al. 1986]. Stone formation within calyceal diverticula is very common, reportedly found in 10–50% of cases, thought to be resultant from the static nature of the urine collected in the cavity that is inherently nonsecretory [Jones et al. 1991b].
Percutaneous access into calyceal diverticula containing stones is challenging due to multiple factors. However, a percutaneous approach to stone extractions has been demonstrated to be more successful than ureteroscopic and SWL [Canales and Monga, 2003]. Since the diverticular neck is often very narrow and the calyceal diverticulum presents as a limited cystic cavity, maintaining access after initial puncture into the diverticulum can be difficult. It is challenging and at times not feasible to advance a guidewire beyond the diverticulum into the pelvicalyceal system to provide additional stability for tract dilation. Furthermore, the size of the stone or stones within the diverticulum further adds challenge to passing adequate wire length into the cavity in preparation for dilation.
To best define the location and caliber of the diverticular neck, preoperative contrast-enhanced, axial imaging with a delayed pyelogram phase should be performed. Then, intraoperative retrograde pyelography via an open-ended ureteral catheter can be performed to correlate diverticular location and plan the best approach. In cases when the wire cannot be traversed into the pelvicalyceal system, a second wire can be introduced though a 10 French dilator prior to serial Amplatz or balloon dilation of the tract to a working caliber. Also, it is imperative to maintain adequate wire length within the diverdiculum beyond the flexible wire tip.
Direct puncture into the calyceal diverticulum and dilation for nephroscope access provides not only better vantage for stone extraction, but also superior visualization and maneuverability for definitive management of the diverticulum, both important in preventing stone recurrence [Shalhav et al. 1998].
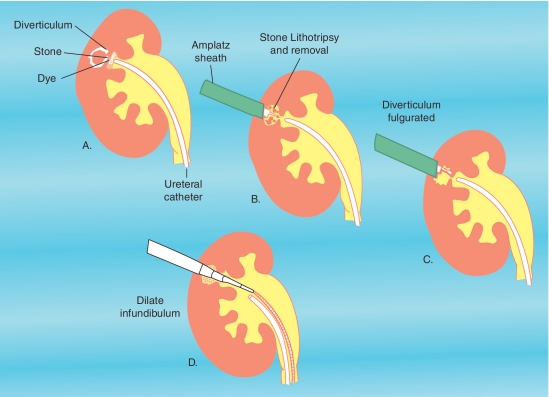
Once access is obtained into the calyceal diverticulum and the stone burden is evacuated, attention is directed toward management of the calyceal diverticulum to help prevent recurrence of stone, infections, and pain (Figure 2). Fulguration of the urothelial lining is one option either alone or in conjunction with dilation of the diverticular neck. The ostium can be identified intraoperatively with retrograde instillation of methylene blue or indigo carmine dye through a ureteral catheter. Once identified the neck can be dilated or incised to provide a patent outflow tract and help prevent stasis within the cavity. A recent study has shown that dilation or incision of the infundibulum does not add benefit when combined with adequate fulguration of the diverticular lining [Krambeck and Lingeman, 2009]. This is particularly important in more anterior calyceal diverticula for which accessing the infundibulum for dilation presents a geometric limitation given the routine posterolateral approach taken for percutaneous nephrostomy access.
Figure 2.
Percutaneous management of stones within calyceal diverticula including fulgration of the diverticular lining and dilation of the infundibulum following stone extraction.
Horseshoe kidneys
Amongst abnormally situated kidneys, horseshoe kidney is the most common with an incidence of 1 in 400, resulting from the incomplete cranial migration and rotation of the kidney [Jones et al. 1991a]. The result is a more inferiorly and medially positioned kidney, renal malrotation, and a high insertion of the ureter into an elongated renal pelvis. Owing to the aberrancy of the ureteropelvic junction and the course of the proximal ureter, a relative ureteral obstruction can result, and if present, give rise urinary stasis and stone formation in up to 70% of patients [Lampel et al. 1996].
For percutaneous access to a horseshoe kidney, the positioning, orientation, and altered blood supply should be accounted for with care. Accessory renal arteries may be present, entering at the renal hilum as with accessory arteries in normally situated kidneys. However, accessory polar arteries may also arise from the aorta, common iliac, or internal iliac arteries. Owing to the malrotation of horseshoe kidneys, a dorsal or dorsolateral puncture should be made to avoid major renal vessels in most cases [Janetschek and Kunzel, 1988].
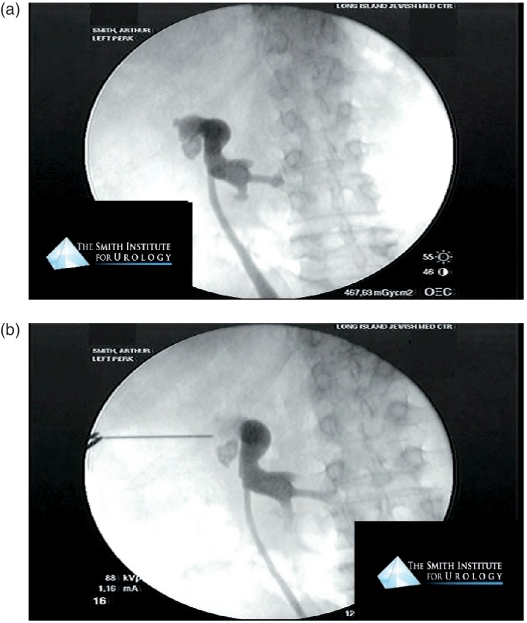
Also, preoperative CT imaging will be imperative for planning access to avoid percutaneous access through a retrorenal colon or other adjacent organs while providing adequate access to involved calyces given the malrotation and aberrant positioning of the collecting system in horseshoe kidneys. Owing to this malrotation placing lower pole calyces angled medially, they are rarely sought for direct access. Hence, the renal collecting system is often accessed through upper pole calyces that are more posterolaterally positioned and necessitate either multiple tracts or flexible nephroscopy to access and adequately clear the stone burden effectively [Raj et al. 2003; Al-Otaibi and Hosking, 1999] (Figure 3(a) and (b)).
Figure 3.
(a) Retrograde pyelogram of a horseshoe kidney with intrarenal calculi. (b) Fluoroscopically guided percutaneous access to a horseshoe kidney via upper pole access.
Pelvic kidneys
Percutaneous access to congenitally ectopic pelvic kidneys presents an anatomic challenge, individually unique due to the variable location and lie of the renal units in the pelvis. The incidence of ectopic pelvic kidneys is rare, estimated at 1 in 3000 [Zafar and Lingeman, 1996]. Ectopic pelvic kidneys are retroperitoneal organs with bowel loops and pelvic reproductive organs interposed between the kidney and the anterior abdominal wall, limiting direct percutaneous access from that approach. Similarly, the posterior and posterolateral approaches are limited due to the lower spine and pelvic bones.
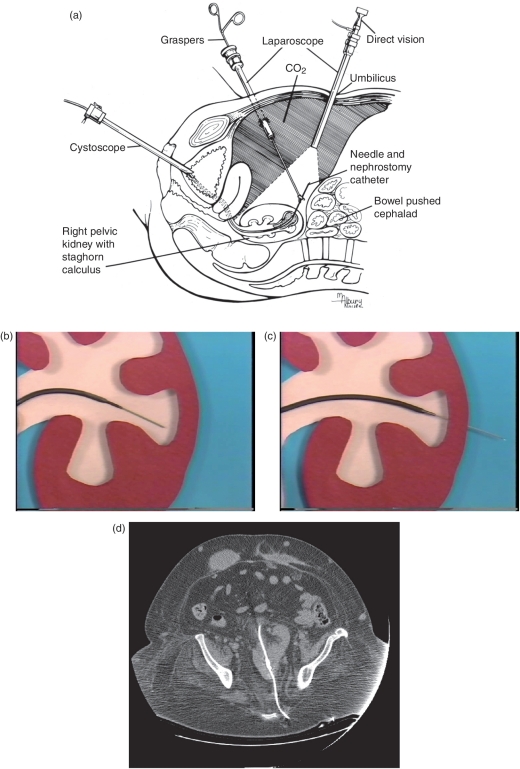
In very thin patients with a pelvic kidney, bowel loops may be displaced under direct visualization with compression by an ultrasound probe allowing for direct puncture percutaneously through the lower anterior abdominal wall [Desai and Jasani, 2000]. Otherwise in most patients with normal or overweight body habitus where the pelvic kidney cannot be directly accessed with applied pressure on the anterior abdominal wall, laparoscopically assisted PCNL is used to allow for a safe anterior approach [Eshghi et al. 1985]. Laparoscopy is performed in the Trendelenburg position to mobilize the bowel and directly visualize the entire tract of percutaneous access from the anterior abdominal wall to the pelvic kidney (Figure 4(a)). The percutaneous tract can be obtained using a standard fashion under biplanar fluoroscopic guidance or via retrograde puncture originating from the renal collecting system outward under laparoscopic visualization to establish needle passage outward through the abdominal wall (Figure 4(b) and (c)). CT planning of a posterior approach performed under fluoroscopy guidance can also be done through the greater sciatic foramen, with caution to avoid possible nerve injury [Cinman et al. 2007] (Figure 4(d)).
Figure 4.
(a) Setup for laparoscopically-guided means for percutaneous access to a pelvic kidney. (b) Coaxial retrograde guidance of a wire and ureteral catheter into a desired calyx. (c) Retrograde puncture through the renal parenchyma with a needle-end wire. (d) Percutaneous access to a pelvic kidney obtained by image guidance through the greater sciatic foramen.
Hypermobile kidney
Percutaneous surgery on hypermobile kidneys presents a unique access dilemma. Often needle puncture into the kidney is not significantly different in these cases. However, after wire access is obtained, dilation and subsequent operative manipulation into a hypermobile kidney is challenging particularly if access is into a lower pole calyx. We propose percutaneous access into a calyx away from the ideal working tract which provides the shortest excursion (Figure 5(a)). This tract needs to be carefully dilated to provide access adequate for passage of a council-tip Foley catheter. Once a council-tipped catheter is passed into the renal collecting system, the balloon can be inflated with dilute contrast material for real-time fluoroscopic confirmation of positioning. Once securely inflated in the renal collecting system, outward tension can be applied to the catheter, which in effect pins the kidney to the lateral abdominal wall and allows for more ease in establishing a second percutaneous working tract (Figure 5(b)). The working tract can be dilated to a larger caliber, and with the kidney secured laterally, it will be able to endure the manipulation of repeated instrument passage and removal.
Figure 5.
(a) Retrograde pyelogram in a hypermobile kidney to identify the calyx away from the ideal working tract which provides the shortest excursion from the skin. (b) Establishing percutaneous access obtained to the calyx of interest after a council-tipped catheter is passed into the renal collecting system through the shortest excursion tract and outward tension on the catheter provides stabilization of the hypermobile kidney.
Obese patients
Obese patients are at increased surgical and anesthetic risk as a result of their multiple comorbidities often associated with obesity [Freedman et al. 2002]. Interestingly, postoperative morbidity following PCNL has been shown in multiple studies to not differ significantly when patients were stratified by weight and body mass index [Koo et al. 2004; Pearle et al. 1998; Carson et al. 1988]. General anesthetic concerns arise for cases of PCNL performed in the prone position, particularly in obese patients since increased restriction of their chest wall expansion can lead to added difficulty with adequate ventilation. For this reason, some prefer flank or supine positioning to facilitate ventilation [Manohar et al. 2007; Gofrit et al. 2002; Hofmann and Stoller, 1992]. However, this approach will increase the length of the percutaneous nephrostomy tract used and alter the anatomy which may decrease maneuverability of the nephroscope during the case and result in a slightly lower stone-free rate [de la Rosette et al. 2008].
Percutaneous access in a morbidly obese patient presents a challenge for three main reasons. First, limitations of imaging alter the effectiveness of real-time imaging necessary for an accurate, targeted calyceal puncture. Second, the increased skin-to-stone distance makes puncture, dilation, and securing a tract more difficult than in a nonobese patient. Finally, maintaining access adds difficulty due to the limitations on length of working sheaths, nephroscopes, and working instruments.
Challenges resulting from obtaining and maintaining access due to the increased girth of obese patients require technical modifications and appropriate instrumentation to facilitate a safe and effective PCNL procedure. Extra long working sheaths, nephroscopes, and working instruments are available to overcome the challenges of obese patients requiring a longer nephrostomy tract for access to the renal collecting system. Also, in cases of PCNL in obese patients, employing a flexible nephroscope can help overcome the limitations of length in addition to preventing the need for multiple percutaneous nephrostomy tracts since torquing manipulation of a rigid nephroscope and working sheath is further limited by a large body habitus.
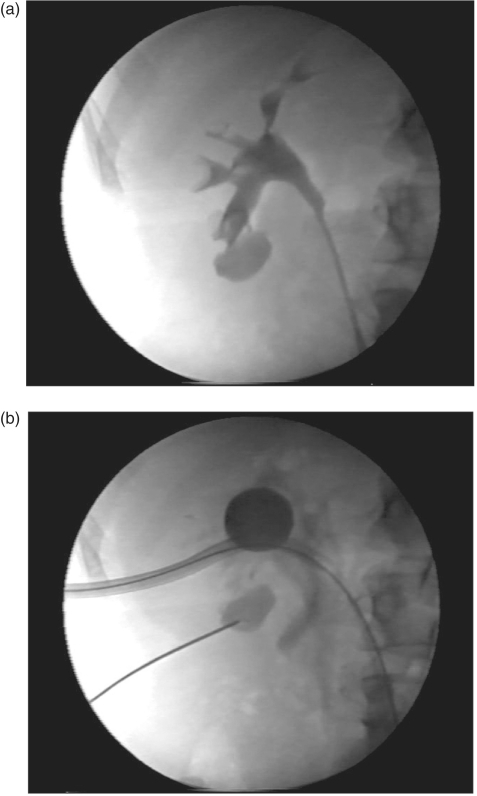
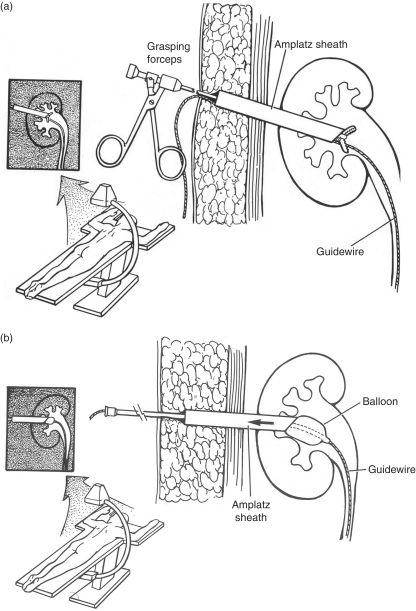
Despite the use of these specialty instruments, PCNL on obese patients often requires added technical modifications. To help prevent losing access, the working sheath can be tagged by placing sutures solely through the sheath to provide retrieval ties for instances when the sheath migrates deep to the level of the skin [Nguyen and Belis, 1998]. In addition to preemptive suture placement in the working sheath, retraction of the sheath can be accomplished using the open jaws of the grasping forceps passed through the sheath or withdrawal of a Foley catheter inserted through the sheath with inflation of the balloon to the diameter to provide distal control of the sheath (Figure 6(a) and (b)).
Figure 6.
(a) Using the grasping forceps as a tool to withdraw the working sheath in cases of obese patients where the sheath is advanced under the level of the skin or subcutaneous tissues. (b) Technique of using a catheter with a balloon inflated in the renal collecting system to control and withdraw a working sheath advanced under the level of the skin or subcutaneous tissues.
Full staghorn calculi
Patients with staghorn calculi present a challenging clinical scenario. Staghorn calculi are not routinely amenable to successful treatment by any means other than percutaneous extraction. Conservative management of staghorn calculi has been associated with a risk of renal loss in up to 30% [Koga et al. 1991]. The best management of staghorn calculi requires complete removal of the stone burden to minimize risk of reformation by the urea-splitting bacteriuria often harbored by residual stone debris in cases of incomplete extraction [Silverman and Stamey, 1983]. Most staghorn calculi are composed of struvite although other crystals including calcium oxalate, uric acid, and cystine can coalesce and form a staghorn calculus [Segura, 1997]. Although PCNL is the preferred treatment approach to staghorn renal calculi, it presents challenges to the urologist, both in terms of access to the collecting system as well as the level of manipulation required to clear the kidney of the large stone burden.
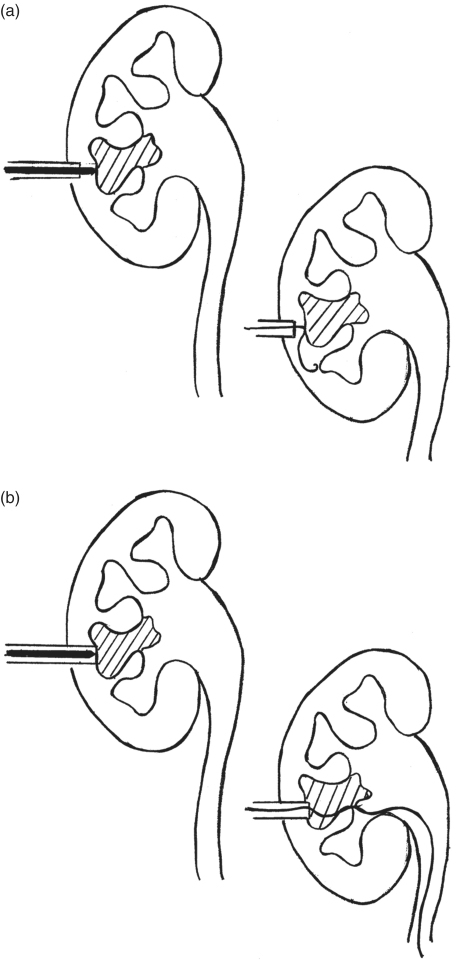
With full staghorn calculi, access to the pelvicalyceal system is limited as there may be limited space surrounding the stone which by definition takes the form-fitting shape of the renal collecting system. If any calyces have a pocket of urine due to obstructive hydronephrosis, this should preferentially be targeted. In cases where a hydronephrotic calyx is not present, percutaneous access into a calyx can be accomplished with a trocar-tipped stylet needle; however, wire passage into the system may be difficult once the stylet is removed from the needle sheath.
One method to overcome this space limitation is to advance the initial stylet needle into the stone to the extent possible. Then, while extracting the inner stylet, maintain forward pressure on the needle sheath to maintain the needle access in the collecting system, even if abutting the stone (Figure 7(a) and (b)). Advancement of the wire through the needle sheath can be done under fluoroscopy, withdrawing the needle sheath as the wire comes into contact with the stone, attempting to get coiling of the wire tip around the calyceal branch of the stone while maintaining access within the collecting system.
Figure 7.
(a) Challenge with advancement of an access wire in cases of full staghorn calculi because the needle sheath remains outside the collecting system. (b) Advancing the needle sheath to the level of the stone while extracting the needle stylet allows for obtaining successful wire access adjacent to the stone in the collecting system.
With large staghorn calculi that involve multiple calyces, stone clearance is important but also very challenging. To adequately clear multiple calyces of stone burden, percutaneous access into a single calyx may be restrictive, depending on the level of collecting system dilation, the angles between different calyces, and the caliber of the calyceal infundibula. At times, these challenges can be overcome using flexible nephroscopy. However, flexible nephroscopy may prolong the procedure and limit efficacy of clearing the stone fragments. An alternative is to plan and obtain multiple percutaneous tracts for access to the entire stone. Although multiple percutaneous tracts increase the risk of hemorrhage and renal parenchymal scarring, excessive manipulation through a single tract to access a full staghorn calculus can also portend bleeding or other complications.
Potential complications with challenging percutaneous renal surgery
Pulmonary complications
Pneumothorax, hydrothorax, and hemothorax are all potential complications of supracostal access in which the pleural fold is compromised and there is introduction of air, irrigation fluid, or blood into the pleural space, respectively. Reports of upper pole access and multiple dilated percutaneous tracts for access increases the risk of pulmonary complications [Munver et al. 2001; Lam et al. 1992]. At the culmination of a PCNL with a supracostal access puncture, examination of the chest with fluoroscopy or a formal chest X-ray should be routinely performed in the operating room to rule out pneumothorax, hydrothorax, and hemothorax. This provides the opportunity for timely intraoperative aspiration with a pigtail drainage catheter which can be removed if the nephrostomy tube does not traverse the pleural space and an indwelling chest tube is otherwise not clinically warranted [Ogan et al. 2003; Ogan and Pearle, 2002]. Prompt recognition and treatment helps reduce the formation of pleural fibrosis and subsequently decreases the risk for a fixed or entrapped lung.
Adjacent organ injury
Injury to adjacent organs (small bowel, colon, spleen, liver, etc) is rarely encountered. Rates of adjacent organ injury are commonly reported as less than 0.5%. Nevertheless, bowel and solid organ injury is always a possible complication with percutaneous renal surgery, which is minimized by operative planning using preoperative CT imaging, anatomic landmarks, and real-time ultrasonographic and/or biplanar fluoroscopic guidance. However, in cases of abnormally situated kidneys including horseshoe and pelvic kidneys, there is an added risk of bowel injury as well as solid visceral organ injury due to the path required for the percutaneous tract to access the kidney. There has been an association reported between horseshoe kidneys and retrorenal colon increasing the risk of a colonic perforation [Skoog et al. 1985]. As described previously, CT guidance for percutaneous access or direct visualization with laparoscopy can help further ensure safe access avoiding adjacent bowel loops and visceral solid organs.
Conclusions
Particular clinical scenarios present challenges for percutaneous renal access. For each anatomic limitation, there are specific imaging modalities, modified surgical techniques, and novel instrumentation to facilitate overcoming the inherent challenges with percutaneous access. Recognition of these challenging scenarios, understanding surgical limitations, and employing methodical approaches to overcome difficulties encountered will help minimize potential complications which may arise more commonly in these complex clinical cases.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
None declared.
References
- Al-Otaibi K., Hosking D.H. (1999) Percutaneous stone removal in horseshoe kidneys. J Urol 162: 674–677 [DOI] [PubMed] [Google Scholar]
- Canales B., Monga M. (2003) Surgical management of the calyceal diverticulum. Curr Opin Urol 13: 255–260 [DOI] [PubMed] [Google Scholar]
- Carson C.C., III, Danneberger J.E., Weinerth J.L. (1988) Percutaneous lithotripsy in morbid obesity. J Urol 139: 243–245 [DOI] [PubMed] [Google Scholar]
- Cinman N.M., Okeke Z., Smith A.D. (2007) Pelvic kidney: associated diseases and treatment. J Endourol 21: 836–842 [DOI] [PubMed] [Google Scholar]
- de la Rosette J.J., Tsakiris P., Ferrandino M.N., Elsakka A.M., Rioja J., Preminger G.M. (2008) Beyond prone position in percutaneous nephrolithotomy: a comprehensive review. Eur Urol 54: 1262–1269 [DOI] [PubMed] [Google Scholar]
- Desai M.R., Jasani A. (2000) Percutaneous nephrolithotripsy in ectopic kidneys. J Endourol 14: 289–292 [DOI] [PubMed] [Google Scholar]
- Eiley D.M., Ozsvath B., Siegel D.N., Smith A.D. (1999) Percutaneous nephrolithotomy with renal angiomyolipomas: a rare challenge. J Endourol 13: 27–30 [DOI] [PubMed] [Google Scholar]
- Eshghi A.M., Roth J.S., Smith A.D. (1985) Percutaneous transperitoneal approach to a pelvic kidney for endourological removal of staghorn calculus. J Urol 134: 525–527 [DOI] [PubMed] [Google Scholar]
- Fernström I., Johansson B. (1976) Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol 10: 257–259 [DOI] [PubMed] [Google Scholar]
- Freedman D.S., Khan L.K., Serdula M.K., Galuska D.A., Dietz W.H. (2002) Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA 288: 1758–1761 [DOI] [PubMed] [Google Scholar]
- Gofrit O.N., Shapiro A., Donchin Y., Bloom A.I., Shenfeld O.Z., Landau E.H., et al. (2002) Lateral decubitus position for percutaneous nephrolithotripsy in the morbidly obese or kyphotic patient. J Endourol 16: 383–386 [DOI] [PubMed] [Google Scholar]
- Hofmann R., Stoller M.L. (1992) Endoscopic and open stone surgery in morbidly obese patients. J Urol 148: 1108–1111 [DOI] [PubMed] [Google Scholar]
- Hulbert J.C., Reddy P.K., Hunter D.W., Castaneda-Zuniga W., Amplatz K., Lange P.H. (1986) Percutaneous techniques for the management of caliceal diverticula containing calculi. J Urol 135: 225–227 [DOI] [PubMed] [Google Scholar]
- Janetschek G., Kunzel K.H. (1988) Percutaneous nephrolithotomy in horseshoe kidneys. Applied anatomy and clinical experience. Br J Urol 62: 117–122 [DOI] [PubMed] [Google Scholar]
- Jones D.J., Wickham J.E., Kellett M.J. (1991a) Percutaneous nephrolithotomy for calculi in horseshoe kidneys. J Urol 145: 481–483 [DOI] [PubMed] [Google Scholar]
- Jones J.A., Lingeman J.E., Steidle C.P. (1991b) The roles of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy in the management of pyelocaliceal diverticula. J Urol 146: 724–727 [DOI] [PubMed] [Google Scholar]
- Karamcheti A., O'Donnell W.F. (1977) Percutaneous nephrolithotomy: an innovative extraction technique. J Urol 118: 671–672 [DOI] [PubMed] [Google Scholar]
- Koga S., Arakaki Y., Matsuoka M., et al. (1991) Staghorn calculi-long-term results of management. Br J Urol 68: 122–124 [DOI] [PubMed] [Google Scholar]
- Koo B.C., Burtt G., Burgess N.A. (2004) Percutaneous stone surgery in the obese: Outcome stratified according to body mass index. BJU Int 93: 1296–1299 [DOI] [PubMed] [Google Scholar]
- Krambeck A.E., Lingeman J.E. (2009) Percutaneous management of caliceal diverticuli. J Endourol 23: 1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp B.P., Dabagia M.D., Scott J.W., Lingeman J.E. (1994) Percutaneous nephrolithotomy directly through an angiomyolipoma. Urology 44: 915–917 [DOI] [PubMed] [Google Scholar]
- Lam H.S., Lingeman J.E., Mosbaugh P.G., et al. (1992) Evolution of the technique of combination therapy for staghorn calculi: A decreasing role for extracorporeal shock wave lithotripsy. J Urol 148: 1058–1062 [DOI] [PubMed] [Google Scholar]
- Lampel A., Hohenfellner M., Schultz-Lampel D., Lazica M., Bohnen K., Thürof W. (1996) Urolithiasis in horseshoe kidneys: Therapeutic management. Urology 47: 182–186 [DOI] [PubMed] [Google Scholar]
- Manohar T., Jain P., Desai M. (2007) Supine percutaneous nephrolithotomy: Effective approach to high-risk and morbidly obese patients. J Endourol 21: 44–49 [DOI] [PubMed] [Google Scholar]
- Munver R., Delvecchio F.C., Newman G.E., Preminger G.M. (2001) Critical analysis of supracostal access for percutaneous renal surgery. J Urol 166: 1242–1246 [PubMed] [Google Scholar]
- Nguyen T.A., Belis J.A. (1998) Endoscopic management of urolithiasis in the morbidly obese patient. J Endourol 12: 33–35 [DOI] [PubMed] [Google Scholar]
- Ogan K., Corwin T.S., Smith T., Watumull L.M., Mullican M.A., Cadeddu J.A. (2003) Sensitivity of chest fluoroscopy compared with chest CT and chest radiography for diagnosing hydropneumothorax in association with percutaneous nephrostolithotomy. Urology 62: 988–992 [DOI] [PubMed] [Google Scholar]
- Ogan K., Pearle M.S. (2002) Oops we got in the chest: Fluoroscopic chest tube insertion for hydrothorax after percutaneous nephrostolithotomy. Urology 60: 1098–1099 [DOI] [PubMed] [Google Scholar]
- Pearle M.S., Nakada S.Y., Womack J.S., Kryger J.V. (1998) Outcomes of contemporary percutaneous nephrostolithotomy in morbidly obese patients. J Urol 160: 669–763 [DOI] [PubMed] [Google Scholar]
- Raj G.V., Auge B.K., Weizer A.Z., Denstedt J.D., Watterson J.D., Beiko D.T. (2003) Percutaneous management of calculi within horseshoe kidneys. J Urol 170: 48–51 [DOI] [PubMed] [Google Scholar]
- Segura J.W. (1997) Staghorn calculi. Urol Clin North Am 24: 71–80 [DOI] [PubMed] [Google Scholar]
- Shalhav A.L., Soble J.J., Nakada S.Y., Wolf Jr J.S., McClennan B.L., Clayman R.V. (1998) Long-term outcome of caliceal diverticula following percutaneous endosurgical management. J Urol 160: 1635–1639 [PubMed] [Google Scholar]
- Silverman D.E., Stamey T.A. (1983) Management of infection stones: The Stanford experience. Medicine (Baltimore) 62: 44–51 [DOI] [PubMed] [Google Scholar]
- Skoog S.J., Reed M.D., Gaudier F.A., Jr, Dunn N.P. (1985) The posterolateral and the retrorenal colon: Implication in percutaneous stone extraction. J Urol 134: 110–112 [DOI] [PubMed] [Google Scholar]
- Smith A.D., Reinke D.B., Miller R.P., Lange P.H. (1979) Percutaneous nephrostomy in the management of ureteral and renal calculi. Radiology 133: 49–54 [DOI] [PubMed] [Google Scholar]
- Zafar F.S., Lingeman J.E. (1996) Value of laparoscopy in the management of calculi complicating renal malformations. J Endourol 10: 379–383 [DOI] [PubMed] [Google Scholar]