Abstract
The cell wall of Candida albicans is central to the yeasts ability to withstand osmotic challenge, to adhere to host cells, to interact with the innate immune system and ultimately to the virulence of the organism. Little is known about the effect of culture conditions on the cell wall structure and composition of C. albicans. We examined the effect of different media and culture temperatures on the molecular weight (Mw), polymer distribution and composition of cell wall mannan and mannoprotein complex. Strain SC5314 was inoculated from frozen stock onto yeast peptone dextrose (YPD), blood or 5% serum agar media at 30 or 37°C prior to mannan/mannoprotein extraction. Cultivation of the yeast in blood or serum at physiologic temperature resulted in an additive effect on Mw, however, cultivation media had the greatest impact on Mw. Mannan from a yeast grown on blood or serum at 30°C showed a 38.9 and 28.6% increase in Mw, when compared with mannan from YPD-grown yeast at 30°C. Mannan from the yeast pregrown on blood or serum at 37°C showed increased Mw (8.8 and 26.3%) when compared with YPD mannan at 37°C. The changes in Mw over the entire polymer distribution were due to an increase in the amount of mannoprotein (23.8–100%) and a decrease in cell wall mannan (5.7–17.3%). We conclude that C. albicans alters the composition of its cell wall, and thus its phenotype, in response to cultivation in blood, serum and/or physiologic temperature by increasing the amount of the mannoprotein and decreasing the amount of the mannan in the cell wall.
Keywords: C. albicans, mannan, mannoprotein, molecular weight, temperature
Introduction
The commensal fungus, Candida albicans, is an opportunistic pathogen in the immunocompromised host. As one of the leading causes of nosocomial infections (Wenzel 1995; Wisplinghoff et al. 2004), C. albicans has several traits that allow it to survive and infect its host. These include the secretion of proteolytic enzymes, production of adhesins and the ability to undergo morphogenic shift from a yeast morphology to a hyphal morphology (Kobayashi et al. 1991; Braun and Johnson 2000; Fu et al. 2002; Monod and Borg-von 2002). In addition, a major contributor to C. albicans virulence is the composition and phenotype of the cell wall. The cell wall of C. albicans has a network of chitin (a polymer composed of N-acetylglucosamine) to which is attached a matrix of (1 → 3)-β-d-glucan. The (1 → 3)-β-d-glucan polymer has a number of (1 → 6)-β-d-glucan branch points that play a role in linking to other (1 → 3)-β-d-glucan chains or can serve as binding sites via a glycophosphatidylinositol remnant and internal repeat moieties for N-and O-linked mannosylated protein attachment (Chauhan et al. 2002).
It has been suggested that C. albicans masks underlying cell wall (1 → 3)-β-glucan with a dense layer of mannan and/or mannoprotein (Netea et al. 2008). Glucan is the primary fungal pathogen-associated molecular pattern (PAMP) (Brown and Gordon 2003; Brown and Williams 2009; Klippel et al. 2010). Therefore, “masking” or covering the cell wall glucan with mannan/mannoprotein is thought to be a means of preventing recognition of the yeast by antifungal innate immune mechanisms, such as via recognition by Dectin-1 (Brown et al. 2003). However, the role of mannan/mannoprotein as a “mask” for fungal cell wall glucan is controversial (Brown and Gordon 2005). Some reports indicate that glucan is clearly detectable on the cell surface, where it is recognized by Dectin-1 or antibody, while others indicate that glucan is not exposed to the cell surface or that it is exposed in restricted areas, i.e. bud/birth scars (Torosantucci et al. 2000, 2005, 2009; Gantner et al. 2005). The cell wall of Candida has been extensively studied, but most of these investigations have focused on defining the cell wall structure following cultivation in medium such as yeast peptone dextrose (YPD) or Sabouraud agar (Kobayashi et al. 1989, 1990, 1991, 1992, 1994; Kruppa et al. 2003, 2004; Li et al. 2009). While these data have advanced our knowledge, they have not addressed the question of what changes occur in the cell wall of Candida in response to cultivation in complex biological media, such as blood or serum. Indeed, little is known about the changes that occur in the cell wall composition and architecture as a result of cultivating fungi on different media and under different environmental conditions, such as growth at physiologic temperature, i.e. 37°C.
In this study, we compared and contrasted the molar mass, composition, distribution and solution conformation of N-linked mannan/mannoprotein in the cell wall of C. albicans strain SC5314 grown in blood, serum or YPD. In addition, we also compared and contrasted the effect of cultivation temperature on molar mass, distribution, composition and solution conformation of mannan/mannoprotein. We found that growth of the cells on media containing either blood or serum resulted in an overall increase in the high-molecular-weight (Mw) mannoprotein peak, but not in the lower Mw peak which accounts for ∼70% of total mannan component of the cell wall. Interestingly, the effect of cultivation at physiologic temperature (37°C) was less dramatic, but the combination of cultivation in complex biologic media and physiologic temperature exerted an additive effect on the mannoprotein component in the cell wall. To the best of our knowledge, this is the first in-depth description of how growth conditions impact the phenotype of mannan/mannoprotein component of the cell wall of C. albicans. When considered as a whole, our results indicate that the cell wall of C. albicans is complex, dynamic and highly adaptable. We speculate that changing the phenotype of the cell wall may confer a survival advantage to the organism.
Results
Cultivation of C. albicans SC5314 in blood or serum at 37°C results in an overall increase in Mw of cell wall mannan/mannoprotein
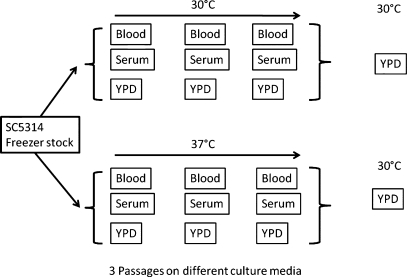
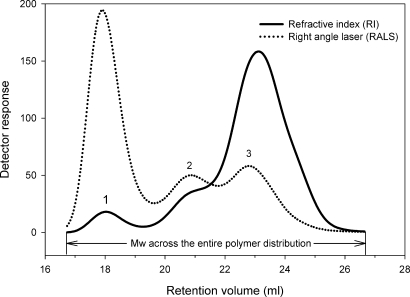
C. albicans were precultivated for 6 days, with a passage every 48 h, on the YPD, serum, or blood agar at 30 or 37°C before growth at 30°C in a liquid YPD (Figure 1). In agreement with previous reports from our laboratory (Li et al. 2009), the mannan samples have a trimodal polymer distribution (Figure 2). Initially the data were analyzed using a single peak assignment in order to obtain an average Mw for the entire polymer distribution (Figure 2 and Table I). Cultivation of C. albicans in blood or serum at 30°C resulted in a 21.9–27.5% increase in Mw, when compared with YPD (Table I). Cultivation of C. albicans in blood or serum at 37°C resulted in an additive effect on Mw, however, growth in blood or serum had the greatest impact on Mw (Table I). An important question was whether there were significant differences in the amount of mannan/mannoprotein produced under different growth conditions. We did not detect any substantive differences in the amount of extractable material, irrespective of growth conditions (data not shown). We also confirmed that the extractable material was mannan/mannoprotein using one-dimensional and two-dimensional nuclear magnetic resonance (NMR) (data not shown) as previously described by our laboratory (Li et al. 2009).
Fig. 1.
Scheme for growth of C. albicans SC5314 for mannan/mannoprotein extraction. C. albicans SC5314, from a frozen stock, was cultured on blood, serum and YPD agar plates and passaged in succession a total of three times before growth in liquid YPD culture.
Fig. 2.
GPC analysis of bulk mannan/mannoprotein isolated from C. albicans SC5314. The refractive index detector (solid line) displays the sample concentration as a function of elution volume, which provides information on polymer distribution. The light scattering detector (dotted line) indicates the molar mass, i.e. Mw, across the polymer distribution. The cell wall mannan/mannoprotein of C. albicans shows a trimodal distribution. The peaks are identified as high-Mw mannoprotein (1), middle-Mw mannan (2) and low-Mw mannan (3). Initially data were analyzed using a single peak assignment in order to obtain an average Mw across the entire polymer distribution. Subsequently, the data were analyzed using multiple peak settings, i.e. peaks 1, 2 and 3, in order to more closely examine the effect of growth temperature and conditions on the mannoprotein versus mannan components.
Table I.
Cultivation media and temperature result in increased molar mass of the mannan/mannoprotein complex in C. albicans cell walla
| Sample description | Mw (×105 Da) | Polydispersity (Mw/Mn) | α Mark-Houwink |
|---|---|---|---|
| Reference mannans | |||
| S. cerevisiae (Sigma)b | 0.5 | 1.1 | 0.64 |
| C. albicans strain (CCY29-3-109 S-2164322)c | 1.0 | 1.2 | 0.45 |
| C. albicans strain (CCY 29-3-102 S-2162322)c | 0.9 | 1.8 | 0.60 |
| Mannan from C. albicans SC5314 grown at 30°C | |||
| YPD | 5.0 | 3.3 | 0.60 |
| Blood | 6.9 (↑27.5%)d | 3.0 | 0.64 |
| Serum | 6.4 (↑21.9%)d | 3.8 | 0.57 |
| YPD + pronase | 2.4 (↓52.0%)e | 1.9 | 0.73 |
| Blood + pronase | 2.4 (↓65.2%)e | 1.9 | 0.71 |
| Serum + pronase | 2.4 (↓62.5%)e | 1.9 | 0.71 |
| Mannan from C. albicans SC5314 grown at 37°C | |||
| YPD | 5.4 (↑ 7.4%)f | 3.7 | 0.61 |
| Blood | 7.5 (↑28.0%)d | 4.6 | 0.58 |
| Serum | 8.1 (↑33.3%)d | 4.7 | 0.55 |
| YPD + pronase | 2.2 (↓59.2%)e | 1.9 | 0.72 |
| Blood + pronase | 2.6 (↓65.3%)e | 1.9 | 0.70 |
| Serum + pronase | 2.6 (↓68.0%)e | 1.9 | 0.71 |
aThe data were calculated using a single peak assignment, which encompassed the entire polymer distribution (see Fig. 1).
bS. cerevisiae mannan (batch # M7504) obtained from Sigma Chemical Co., St. Louis, MO.
cMannan isolated by the method of Jones and Stoodley (1965) was provided by Profs. Bystricky and Machova, Institute of Chemistry, Slovak Academy of Sciences, Bratislava, Slovakia.
dPercent change when compared with the mannan from YPD-grown cells at the same temperature.
ePercent change when compared with the nonpronase-treated mannan grown at the same temperature.
fPercent change when compared with mannan from YPD-grown SC5314 at 30°C.
Pronase treatment eliminates the high-molecular-weight mannoprotein complex in the mannan polymer distribution
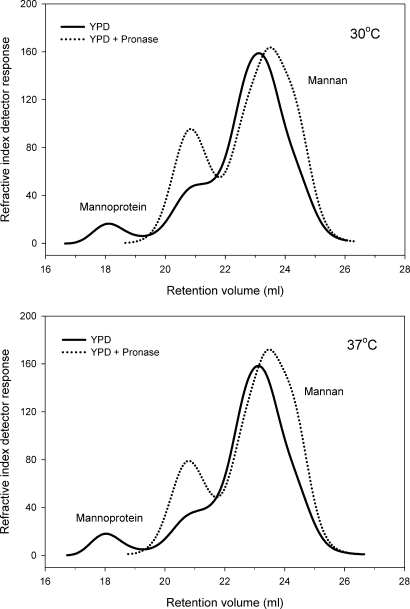
We have previously reported that pronase treatment cleaves mannoproteins, thus releasing the mannans from the mannoprotein complex (Li et al. 2009). The mannans, which have been released from the protein, then redistribute according to their Mw (Li et al. 2009). We have established that the high-Mw peak is predominantly mannoprotein (Figure 3) (Li et al. 2009). We have also established by NMR that peaks 2 and 3 are predominantly mannan after pronase treatment (Li et al. 2009). In the current study, samples grown in YPD, blood or serum, at 30 and 37°C, were treated overnight at 37°C with pronase that had been previously heated to eliminate any glycosidic activity (Li et al. 2009). As expected, pronase treatment effectively eliminated the high-Mw portion of the polymer distribution resulting in a significant reduction in average Mw and polydispersity (Table I). This can be seen as a redistribution of the mannan polymers to the middle- and low-Mw peaks (Figure 3). Specifically, pronase treatment reduced Mw by 52–65% in yeast grown at 30°C and 59–68% in 37°C grown yeast.
Fig. 3.
Pronase treatment eliminates the high-Mw mannoprotein peak. It was also noted that after pronase treatment (dotted line), there was a redistribution of the mannan polymers into two lower-Mw peaks. The same effect of pronase was observed for all growth media and temperatures tested. Representative data for YPD-grown yeast at 30 and 37°C are shown. Refractive index detector data are shown.
In accordance with our previous observations (Li et al. 2009), after pronase treatment, the mannan polymers redistributed into two peaks (Figure 3). The first mannan component has an Mw of 5.2–5.9 × 105 Da and the second mannan component has an Mw of ∼1.1 × 105 Da (Table II). It is the lower-Mw mannan that accounts for the bulk of mannan polymers in the cell wall of C. albicans, i.e. ≥70%, irrespective of growth conditions (Table III). It was also noted that the Mw of samples analyzed in this study was substantially greater than that observed for commercially available Saccharomyces cerevisiae mannan as well as C. albicans CCY29-3-109 S-2164322 and CCY 29-3-102 S-2162322 mannans (Table I). This can be attributed to differences in the yeast and/or strain as well as the mannan isolation methodology (Jones and Stoodley 1965). Polydispersity, the ratio of high-to-low-Mw polymers, was reduced by 39–60% following pronase treatment. Interestingly, after pronase treatment, all of the samples showed a polydispersity of 1.9 irrespective of growth conditions. We speculate that this reflects the polydispersity of cell wall mannan of C. albicans that is not complexed with protein.
Table II.
Cultivation media and temperature do not dramatically influence the Mw of the individual mannoprotein and mannan peaksa
| Sample description | Peak 1 |
Peak 2 |
Peak 3 |
|---|---|---|---|
| Mw × 105 Da |
|||
| 30°C cultivation | |||
| SC5314 YPD | 50.1 | 7.2 | 1.6 |
| SC5314 blood | 51.5 | 6.5 | 1.6 |
| SC5314 serum | 46.4 | 6.6 | 1.4 |
| SC5314 YPD + pronase | n.d.b | 5.6 (↓22.2%) | 1.1 (↓31.2%) |
| SC5314 blood + pronase | n.d.b | 5.2 (↓20.0%) | 1.2 (↓25.0%) |
| SC5314 serum + pronase | n.d.b | 5.5 (↓16.7%) | 1.2 (↓14.3%) |
| 37°C cultivation | |||
| SC5314 YPD | 53.4 | 7.7 | 1.5 |
| SC5314 blood | 50.0 | 6.4 | 1.5 |
| SC5314 serum | 50.6 | 6.3 | 1.6 |
| SC5314 YPD + pronase | n.d.b | 5.9 (↓23.4%) | 1.1 (↓26.7%) |
| SC5314 blood + pronase | n.d.b | 5.4 (↓15.6%) | 1.2 (↓20.0%) |
| SC5314 serum + pronase | n.d.b | 5.2 (↓17.5%) | 1.2 (↓25.0%) |
aPeak 1 is identified as the mannoprotein peak, while peak 3 is predominantly mannan.
bNot detectable.
Table III.
Cultivating C. albicans in blood or serum at 37°C results in an increase in cell wall mannoprotein and a decrease in mannana
| YPDb | % Polymerc | Bloodb | % Polymerc | %ΔYPDd | Serumb | % Polymerc | %ΔYPDd | |
|---|---|---|---|---|---|---|---|---|
| 30°C cultivation | ||||||||
| Peak 1 | 21.5 | 4.8 | 32.2 | 6.3 | ↑23.8 | 46 | 8.4 | ↑75.0 |
| Peak 2 | 62.7 | 14.0 | 93.5 | 18.3 | ↑49.1 | 116 | 21.1 | ↑50.7 |
| Peak 3 | 363.6 | 81.2 | 385.8 | 75.4 | ↓ 5.7 | 387.3 | 70.5 | ↓13.2 |
| 37°C cultivation | ||||||||
| Peak 1 | 23.5 | 5.6 | 50.3 | 10.4 | ↑85.7 | 55.4 | 11.2 | ↑100.0 |
| Peak 2 | 46.2 | 11.0 | 89.7 | 18.5 | ↑68.2 | 97.9 | 19.8 | ↑80.0 |
| Peak 3 | 351.4 | 83.4 | 344.2 | 71.1 | ↓14.7 | 340.8 | 69.0 | ↓17.3 |
aPeak 1 is identified as the mannoprotein peak, while peak 3 is predominantly mannan.
bEach peak was quantified and the data expressed as area under the refractive index curve—adjusted for calculated concentration.
cPercentage that each peak contributes to the total polymer distribution based on a total of 100%.
dPercent change when compared with YPD% polymer at the same temperature.
Solution conformation of mannan/mannoprotein complex
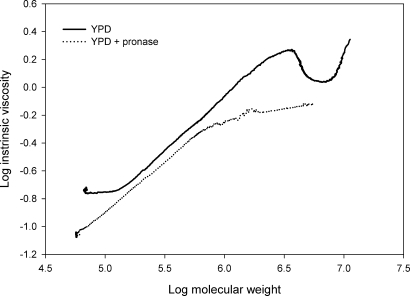
The slope of the linear relationship between log intrinsic viscosity and log molecular mass ([η] = KαMα) is known as the Mark-Houwink α-value for a polymer system (Mueller et al. 2000; Li et al. 2009). Establishing α can provide insights into the solution conformation of the mannan and mannoprotein (Muller et al. 1995; Mueller et al. 2000). The α-values for the mannan and mannoprotein samples are shown in Table I. Mark-Houwink or α-values of 0.4–0.8 are indicative of a flexible polymer system (Muller et al. 1995). Therefore, all of the values obtained in this study indicate that the mannan/mannoprotein complex is flexible. However, closer inspection of the data clearly shows a complex mixture containing multiple solution conformations and/or macromolecular structures in the mannan/mannoprotein complex. This can be seen in Figure 4, where the mannoprotein component, i.e. Mw greater than 1 × 106 Da, shows a Mark-Houwink scaling relationship that is different from that observed for the mannan component, i.e. Mw ≤1 × 106 Da. Following pronase treatment, the α-values remained within the flexible polymer range, although pronase treatment did result in a modest increase in α (Table I). This is due to the fact that the software calculates an average α based on the entire polymer distribution which includes all of the conformations. Following pronase treatment, we found that the α-values for the mannan component were quite similar for all growth conditions, i.e. 0.70–0.72 (Table I and Figure 4). We speculate that this represents the α-value for C. albicans mannan that is not complexed with protein. In addition, we found that the α-values for the high-Mw mannoprotein component exceeded 0.8, suggesting a more ordered or rigid solution conformation for the mannoprotein (data not shown). This was true for all growth conditions, but the highest α-values were obtained for mannoprotein grown in blood or serum at 37°C. It was also noted that the mannan polymers released after pronase treatment showed a higher α-value than the reference mannans (Table I).
Fig. 4.
Mark-Houwink (α) plot comparing YPD-grown SC5314 mannan/mannoprotein extracts in the presence or absence of pronase treatment. Multiple solution conformations are evident in the mannoprotein component. The high-molar-mass mannoprotein component was eliminated by pronase treatment. This resulted in a single solution conformation, which we interpret to be the solution conformation of C. albicans SC5314 cell wall mannan that is not complexed with protein.
Cultivation media and temperature did not substantially alter the Mw of the individual mannoprotein and mannan components
We next sought to determine which component(s) of the mannan/mannoprotein complex accounted for the change in Mw under varying growth conditions. The data were reanalyzed using multiple peak settings (Figure 2 and Table II) in order to more closely examine the effect of growth temperature and conditions on the mannan versus mannoprotein components. Specifically, we established the Mw for the (1) high-, (2) middle- and (3) low-Mw peaks (Figure 2). The mannoprotein peak (peak 1 in Table II; also see Figure 3) exhibited Mw ranging from 4.6 to 5.3 × 106 Da, indicating a very high molar mass for the mannoprotein complex. The middle-range peak (peak 2 in Figure 3) showed an Mw ranging from 6.4 to 7.7 × 105 Da, while the low-Mw peak (peak 3 in Figure 3) had an Mw ranging from 1.4 to 1.6 × 105 Da. Surprisingly, we found that the Mw for all of the peaks in the blood- or serum-grown mannan was either unchanged or decreased when compared with the YPD control at either growth temperature (Table II). This was an unexpected finding, which indicated that the increased Mw observed over the entire mannan/mannoprotein polymer distribution (Table I) is not due to increases in the Mw of the individual mannan or mannoprotein components.
We also evaluated the effect of pronase treatment on the Mw of the peaks (Table II). As expected, the high-Mw mannoprotein peak was eliminated by pronase treatment. The middle-Mw peak showed a 16.7–22% decrease in Mw at 30°C and a 15.6–23.4% decrease in Mw at 37°C resulting from pronase treatment. The low-Mw peak, previously identified as the mannan peak (Li et al. 2009), decreased by 14–31% across all groups and growth conditions. Interestingly, after pronase treatment, the low-Mw peak, which accounts for ≥70% of the polymer distribution, showed a remarkably uniform Mw of 1.1–1.2 × 105 Da. We interpret this to mean that the majority of the mannan found in C. albicans SC5314 cell wall has an Mw of 1.1–1.2 × 105 Da, when it is not complexed with the cell wall protein. In support of this conclusion, the 1.1–1.2 × 105 Da Mw is consistent with that for mannan isolated from C. albicans strains CCY29-3-109 S-2164322 and CCY 29-3-102 S-2162322, neither of which show a mannoprotein component (Table I).
Cultivating C. albicans in complex biological media at 37°C results in an increase in the amount of high-Mw cell wall mannoprotein, but not the lower-Mw mannan
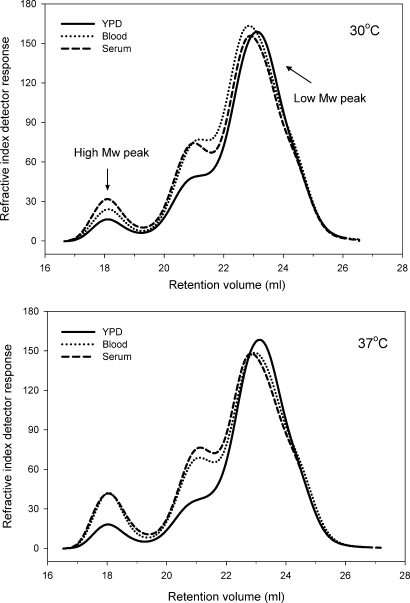
The data in Table II indicated that the increased Mw observed over the entire mannan/mannoprotein polymer distribution (Table I) is not due to increases in the Mw of the individual components. Therefore, we asked the question of whether there could be a shift in the polymer distribution which favors a higher Mw. To critically evaluate this question, we compared and contrasted the polymer distributions (Figure 5). We found that yeast grown in blood or serum showed substantial increases in the proportion of mannoprotein (peak 1) as well as peak 2, when compared with 30°C grown cells (Table III). A comparison of yeast grown in the various media at 30 and 37°C revealed that blood- and serum-grown cells also show a greater increase in the amount of the mannoprotein, when compared with YPD (Figure 5 and Table III). In addition, each peak (1–3) was quantified and the area under the curve, corrected for calculated concentration, was determined (Table III). The percentage that each peak contributed to the total mannan polymer distribution, based on a total of 100%, was then calculated. We found that in YPD-grown yeast, the high-Mw mannoprotein peak accounts for 4.8 and 5.6% of the total polymer distribution at 30 and 37°C, respectively. The middle-Mw peak accounts for 14 and 11% of the polymer distribution, while the low-Mw peak, which is predominantly mannan, accounts for 81.2 and 83.4% of the polymer distribution at 30 and 37°C, respectively. When the yeast was cultured in blood, the amount of the high-Mw mannoprotein peak increased to 6.3 and 10.4% of the polymer distribution at 30 and 37°C, respectively. In serum-cultured yeast, the mannoprotein peak accounted for 8.4 and 11.2% of the polymer distribution. In other words, culturing C. albicans in blood or serum, at physiologic temperature, increased the amount of mannoprotein in the cell wall by 23.8–100.0%. The greatest effect was observed for blood- or serum-grown cells at 37°C (Table III). We also found that the second peak increased by 49–80% when cultured in blood or serum versus YPD. Growth on blood or serum at 37°C had the greatest effect on peak 2 (Table III). In contrast, the low-Mw peak, which accounts for the bulk of the polymer distribution and is predominantly mannan, showed a 5.7–17.3% decrease when cultured in blood or serum (Table III). Again, the greatest effect was observed in blood- or serum-grown cells at 37°C. When taken together, these data indicate that culturing C. albicans in a complex biological media, at physiologic temperature, results in a shift in the polymer distribution whereby there is an increased amount of the very-high-Mw mannoprotein peak, the mid-range peak and a subtle, but detectable, decrease in the low-molar-mass mannan peak.
Fig. 5.
Growth of C. albicans SC5314 in complex biological media and/or at elevated temperature results in a shift in the polymer distribution which favors a higher Mw. Specifically, growth in blood, serum and/or at physiologic temperature resulted in an increased amount of the high-Mw mannoprotein peak and a decrease in the low-Mw peak, which is predominantly mannan. The chromatogram at the top compares C. albicans grown in YPD, blood or serum at 30°C. The chromatograms at the bottom compare yeast grown in YPD, blood or serum at 37°C. The refractive index data are presented adjusted for calculated concentration.
Discussion
Several important observations have emerged from this study. Our data indicate that the cell wall mannan of C. albicans is not upregulated in response to cultivation in blood, serum or physiological temperature. Instead, there is a shift in the polymer distribution whereby the high-Mw mannoprotein portion of the polymer distribution increases by as much as 100%, while the lower Mw mannan portion decreases. The high-Mw mannoprotein is a macromolecular structure which has a Mw of ∼5 × 106 Da. Even though the high-Mw mannoprotein represents only a small portion of the polymer distribution, i.e. 4.8–11.2%, it has a very high molar mass which exerts a substantial effect on the overall Mw of the entire polymer system. It is also important to point out that we did not detect any substantive difference in the total amount of extractable material, irrespective of growth conditions (data not shown). Thus, we conclude that the overall change in Mw observed in the mannan/mannoprotein complex isolated from C. albicans under varying growth conditions is primarily due to an increase in the high-Mw mannoprotein component with a concomitant decrease in the lower Mw predominantly mannan component.
It has been proposed that C. albicans upregulates cell wall mannan and/or mannoprotein as a defense mechanism (Netea et al. 2008; Klippel et al. 2010). It is thought that C. albicans, in response to environmental conditions, increases cell wall mannan/mannoprotein in order to “mask” the (1 → 3)-β-glucan, which is the primary fungal PAMP (Klippel et al. 2010). We did not detect any appreciable increase in low-Mw cell wall mannan. In fact, we observed a decrease in the relative amount of low-Mw mannan, but there was a concomitant increase in the amount of mannoprotein in the cell wall of C. albicans. We have previously reported that the Mw, polymer distribution and composition of cell wall mannan/mannoprotein in the histidine kinase mutant chk1 (Li et al. 2009) produce a cell wall mannan with a uniform polydispersity, but it does not produce a mannoprotein component. In the current study, we also cultivated the chk1 mutant in the presence of blood or serum. Cultivation of chk1 in biological media, at physiologic temperature, did not result in expression of the mannoprotein. Therefore, changing the culture conditions does not induce mannoprotein in the chk1 mutant (data not shown). Recently, Klippel et al. have reported that cell wall (1 → 3)-β-glucan is more exposed in the C. albicans chk1 mutant and, of potentially greater significance, this mutant is more readily recognized by host phagocytes (Klippel et al. 2010). We speculate that this may be due to the lack of mannoprotein in the chk1 cell wall. When considered as whole, our data and the published data (Li et al. 2009; Klippel et al. 2010) suggest that it is the mannoprotein component that is required to mask the underlying cell wall (1 → 3)-β-glucan of C. albicans, thus decreasing innate immune recognition. The fact that cultivation of C. albicans in blood or serum at physiologic temperature results in increased cell wall mannoprotein suggests that this may be an adaptive mechanism to prevent innate immune recognition. However, these data raise an interesting question. Even though cultivation of C. albicans in blood or serum at 37°C increases mannoprotein, it is important to note that the mannoprotein represents only a small portion of the polymer distribution, i.e. ≤11%. It is not clear as to how such a small amount of mannoprotein could effectively mask all of the cell wall (1 → 3)-β-glucan from antifungal innate immune responses. Additional studies will be required to address this question.
As previously reported, pronase treatment disrupts the mannoprotein complex, thus allowing us to conclusively identify the high-Mw peak as the mannoprotein portion of the polymer distribution (Figure 3) (Li et al. 2009). We also noted that after pronase treatment, there was a redistribution of the mannan polymers to lower Mw. Specifically, we observed that the high-Mw peak was eliminated and the mannan polymers redistributed to two peaks. This indicates that there are, in general, two molecular species of mannan in the cell wall of C. albicans, i.e. a mannan component which has an Mw of 5.2–5.9 × 105 Da and a second mannan component which has an average Mw of ∼1.1 × 105 Da. It is the lower-Mw mannan that accounts for the bulk of mannan polymers in the cell wall of C. albicans, i.e. ≥70%. It is also important to point out that the lower-Mw mannan does contain protein, albeit at very low levels. We examined the entire polymer distribution by fractionation with gel permeation chromatography (GPC) as well as on-line UV monitoring. We could detect protein across the entire polymer distribution but the levels were at or very near the limit of detectability (data not shown). Therefore, it is reasonable to conclude that the high-Mw peak, which is readily dissociated with pronase, is a predominant mannoprotein complex. The lower-Mw peak, which accounts for ≥70% of the polymer distribution, also contains protein but it is present in very low concentrations. Thus, the low-Mw peak is predominantly mannan, but at this point we cannot say how the mannan and protein are configured in this peak. It is also worth noting that we isolated mannan/mannoprotein according to the method previously described (Li et al. 2009). It was necessary to isolate mannan/mannoprotein so that we could critically analyze the polymers by GPC. However, the isolation methodology may have influenced the amount of protein present in the isolated material.
Analysis of the Mark-Houwink data clearly shows that mannoprotein has a different solution conformation than free mannan. In fact, we confirmed that there are multiple solution conformations in the mannan/mannoprotein extract. Adams et al. (2008), Adachi et al. (2004) and Palma et al. (2005) have reported on the structure–activity relationships of fungal cell wall carbohydrates and how this impacts recognition by antifungal pattern recognition receptors in the innate immune system. We have found that subtle changes in the structure and/or conformation of fungal carbohydrates can have a significant impact on innate immune receptor recognition (Adams et al. 2008). These data suggest that a more thorough understanding of the structure and conformation of the mannan and mannoprotein from C. albicans is warranted. We are currently investigating whether C. albicans alters the primary and secondary structure of the mannan/mannoprotein complex in response to different environmental conditions (manuscript submitted).
Over the years, a number of models of the fungal cell wall have been proposed. These models were based on the general concept that the fungal cell wall was a relatively static environment that did not substantially change. However, our data clearly challenge this view. Herein, we demonstrate that the cell wall of C. albicans is a complex, dynamic and highly adaptable organelle. Specifically, C. albicans alters the phenotype of its cell wall by changing the mannoprotein and mannan content and polymer distribution in response to changing environmental conditions. Our data suggest that in the future we should consider the fungal cell wall in the context of its environment. We must also consider how the macromolecules within the fungal cell wall, i.e. mannans, glucans, chitins, proteins and lipids, are linked and/or interconnected and how this complex macromolecular structure changes in response to environmental conditions. We speculate that altering the fungal cell wall phenotype may have important implications for host–pathogen interactions.
Materials and methods
C. albicans strain and growth conditions
C. albicans strain SC5314 was taken directly from the frozen stock and passaged onto YPD (1% yeast extract, 2% peptone, 2% dextrose, 2% agar), blood (5% sheep blood, 4% Tryptic soy broth, 2% agar) and serum (5% serum, 2% agar) plate media at 30 and 37°C. Cells were passaged every 48 h for three times (Figure 1). For mannan extraction, cells were inoculated from the third passage of 30 and 37°C plates into 2 L of YPD for growth at 30°C for 18 h.
Mannan/mannoprotein extraction and pronase treatment
Mannan was prepared from cells using a modified extraction, which we have previously utilized (Li et al. 2009). Briefly, cells were delipidated with acetone, and the pellet disrupted by bead beating in distilled H2O. The extracted cells were then autoclaved for 3 h, and then the extract centrifuged. The supernatant was split into half for each sample with one half treated with 100 mg of pronase (heated to 70°C for 30 min to kill glycosidic activity) for 18 h at 37°C. The supernatant was subjected to Fehling precipitation (Li et al. 2009). The resulting precipitate from the pronase-treated and untreated sample fractions were then dissolved in 3 M HCl and the carbohydrate precipitated and copper removed with an 8:1 methanol:acetic acid solution. The solution was washed several times until the remaining precipitate was white to colorless. The precipitate was dried, resuspended in dH2O and the pH adjusted to 6.5–7.0 after which the solution was lyophilized for storage at −20°C. From our cell preparations, we generally recover 100–250 mg of material from 10 to 15 g of cell wet-weight.
Gel permeation chromatographic analysis of cell wall mannan/mannoprotein of C. albicans
The Mw, polydispersity, polymer distribution and Mark-Houwink (α) values were derived from GPC with a Viscotek/Malvern GPC system consisting of a GPCMax autoinjector fitted to a TDA 305 detector (Viscotek, Houston, TX). The TDA contains a refractive index detector, a low-angle laser light scattering detector, a right-angled laser light scattering detector, an intrinsic viscosity detector and a UV detector (λ = 254 nm). Three Waters Ultrahydrogel columns, i.e. 1200, 500 and 120, were fitted in series (Waters Corp., Milford, MA). The columns and detectors were maintained at 40°C within the TDA 305. The system was calibrated using Shodex P-82 pullulan standards (5000–800,000 Da) in mobile phase (Showa Denko distributed by Waters Corp.). Mannan samples were dissolved (3 mg mL−1) in mobile phase (50 mM sodium nitrite, pH 7.6). The samples were incubated for ∼15 min at ambient temperature, followed by sterile filtration (0.2 μm) and injected into the GPC (200 μL). Sample recovery was routinely ≥95%. The data were analyzed using Viscotek OmniSec software v. 4.6.1.354. Dn/dc was calculated using the OmniSec software (v. 4.6.1.354). Dn/dc for the mannan samples was determined to be 0.185. Initially data were analyzed using a single peak assignment in order to obtain an average Mw for the entire polymer distribution (Figure 2). Subsequently, the data were analyzed using multiple peak settings in order to more closely examine the effect of growth temperature and conditions on the mannan versus mannoprotein components (Figure 2). Each peak was quantified and the data expressed as area under the refractive index curve—adjusted for calculated concentration.
The percentage that each peak contributed to the total polymer distribution was calculated based on a total of 100%. Replicate analysis of calibration standards indicated reproducibility of ±3%, which is well within the limits of the technique.
Funding
This work was supported in part by National Institutes of Health (GM53552) to D.L.W. and (F32CA132546-03) to R.G.; ETSU faculty startup funding to M.D.K. and Deutsche Forschungsgemeinschaft NO 928/1-1 to I.N.
Conflict of interest statement
None declared.
Abbreviations
GPC, gel permeation chromatography; Mw, molecular weight; NMR, nuclear magnetic resonance; PAMP, pathogen-associated molecular pattern; YPD, yeast peptone dextrose.
References
- Adachi Y, Ishii T, Ikeda Y, Hoshino A, Tamura H, Aketagawa J, Tanaka S, Ohno N. Characterization of beta-glucan recognition site on C-type lectin, Dectin 1. Infect Immun. 2004;72:4159–4171. doi: 10.1128/IAI.72.7.4159-4171.2004. doi:10.1128/IAI.72.7.4159-4171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams EL, Rice PJ, Graves B, Ensley HE, Yu H, Brown GD, Gordon S, Monteiro MA, Papp-Szabo E, Lowman DW, et al. Differential high affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side chain branching. J Pharmacol Exp Ther. 2008;325:115–123. doi: 10.1124/jpet.107.133124. doi:10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- Braun BR, Johnson AD. TUP1, CPH1, and EFG1 make independent contributions to filamentation in Candida albicans. Genetics. 2000;155:57–67. doi: 10.1093/genetics/155.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Gordon S. Fungal β-glucans and mammalian immunity. Immunity. 2003;19:311–315. doi: 10.1016/s1074-7613(03)00233-4. doi:10.1016/S1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- Brown GD, Gordon S. Immune recognition of fungal beta-glucans. Cell Microbiol. 2005;7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. doi:10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- Brown GD, Herre J, Williams DL, Willment JA, Marshall ASJ, Gordon S. Dectin-1 mediates the biological effects of β-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. doi:10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Williams DL. (1,3)-β-Glucans in innate immunity: mammalian systems. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, Biochemistry and Biology of (1,3)-Beta Glucans and Related Polysaccharides. San Diego, CA: Academic Press, Elsevier, Inc; 2009. [Google Scholar]
- Chauhan N, Li D, Singh P, Calderone R, Kruppa M. The cell wall of Candida spp. In: Calderone RA, editor. Candida and Candidiasis. 1st ed. Washington, DC: ASM Press; 2002. [Google Scholar]
- Fu Y, Ibrahim AS, Sheppard DC, Chen YC, French SW, Cutler JE, Filler SG, Edwards JE., Jr Candida albicans Als1p: An anhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002;44:61–72. doi: 10.1046/j.1365-2958.2002.02873.x. doi:10.1046/j.1365-2958.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. doi:10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JKN, Stoodley RJ. Fractionation using copper complexes. In: Westphal KJ, Whistler RL, editors. Methods in Carbohydrate Chemistry: General Polysaccharides. New York: Academic Press; 1965. [Google Scholar]
- Klippel N, Cui S, Groebe L, Bilitewski U. Deletion of Candida albicans histidine kinase CHK1 improves recognition by phagocytes through an increased exposure of cell wall β-1,3-glucans. Microbiology. 2010;156:3432–3444. doi: 10.1099/mic.0.040006-0. doi:10.1099/mic.0.040006-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Giummelly P, Takahashi S, Ishida M, Sato J, Takaku M, Nishidate Y, Shibata N, Okawa Y, Suzuki S. Candida albicans serotype A strains grow in yeast extract-added Sabouraud liquid medium at pH 2.0, elaborating mannans without beta-1,2 linkage and phosphate group. Biochem Biophys Res Commun. 1991;175:1003–1009. doi: 10.1016/0006-291x(91)91664-x. doi:10.1016/0006-291X(91)91664-X. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Mitobe H, Takahashi K, Yamamoto T, Shibata N, Suzuki S. Structural study of a cell wall mannan–protein complex of the pathogenic yeast Candida glabrata IFO 0622 strain. Arch Biochem Biophys. 1992;294:662–669. doi: 10.1016/0003-9861(92)90739-j. doi:10.1016/0003-9861(92)90739-J. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Shibata N, Mitobe H, Ohkubo Y, Suzuki S. Structural study of phosphomannan of yeast-form cells of Candida albicans J-1012 strain with special reference to application of mild acetolysis. Arch Biochem Biophys. 1989;272:364–375. doi: 10.1016/0003-9861(89)90230-0. doi:10.1016/0003-9861(89)90230-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Shibata N, Nakada M, Chaki S, Mizugami K, Ohkubo Y, Suzuki S. Structural study of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch Biochem Biophys. 1990;278:195–204. doi: 10.1016/0003-9861(90)90248-w. doi:10.1016/0003-9861(90)90248-W. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Takahashi S, Shibata N, Miyauchi M, Ishida M, Sato J, Maeda K, Suzuki S. Structural modification of cell wall mannans of Candida albicans serotype A strains grown in yeast extract-Sabouraud liquid medium under acidic conditions. Infect Immun. 1994;62:968–973. doi: 10.1128/iai.62.3.968-973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruppa M, Goins T, Cutler JE, Lowman D, Williams D, Chauhan N, Menon V, Singh P, Li D, Calderone R. The role of the Candida albicans histidine kinase [CHK1] gene in the regulation of cell wall mannan and glucan biosynthesis. FEMS Yeast Res. 2003;3:289–299. doi: 10.1111/j.1567-1364.2003.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Kruppa M, Jabra-Rizk MA, Meiller TF, Calderone R. The histidine kinases of Candida albicans: Regulation of cell wall mannan biosynthesis. FEMS Yeast Res. 2004;4:409–416. doi: 10.1016/S1567-1356(03)00201-0. doi:10.1016/S1567-1356(03)00201-0. [DOI] [PubMed] [Google Scholar]
- Li D, Williams D, Lowman DW, Monteiro MA, Tan X, Kruppa M, Fonzi W, Roman E, Pla J, Calderone R. The Candida albicans histidine kinase Chk1p: Signaling and cell wall mannan. Fungal Genet Biol. 2009;46:731–741. doi: 10.1016/j.fgb.2009.06.008. doi:10.1016/j.fgb.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod M, Borg-von ZM. Secreted aspartic proteases as virulence factors of Candida species. Biol Chem. 2002;383:1087–1093. doi: 10.1515/BC.2002.117. doi:10.1515/BC.2002.117. [DOI] [PubMed] [Google Scholar]
- Mueller A, Raptis J, Rice PJ, Kalbfleisch JH, Stout RD, Ensley HE, Browder W, Williams DL. The influence of glucan polymer structure and solution conformation on binding to (1→3)-β-d-glucan receptors in a human monocyte-like cell line. Glycobiology. 2000;10(→):339–346. doi: 10.1093/glycob/10.4.339. doi:10.1093/glycob/10.4.339. [DOI] [PubMed] [Google Scholar]
- Muller A, Pretus HA, McNamee RB, Jones EL, Browder IW, Williams DL. Comparison of the carbohydrate biological response modifiers Krestin, schizophyllan and glucan phosphate by aqueous size exclusion chromatography with in-line argon-ion multi-angle laser light scattering photometry and differential viscometry detectors. J Chromatogr B Biomed Appl. 1995;666:283–290. doi: 10.1016/0378-4347(94)00575-p. doi:10.1016/0378-4347(94)00575-P. [DOI] [PubMed] [Google Scholar]
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. doi:10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- Palma AS, Feizi T, Zhang Y, Stoll MS, Lawson AM, Diaz-Rodrigues E, Campanero-Rhodes MA, Costa J, Gordon S, Brown GD, et al. Ligands for the β-glucan receptor, Dectin-1, assigned using ‘designer’ microarrays of oligosaccharides (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2005;281:5771–5779. doi: 10.1074/jbc.M511461200. doi:10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Constantino P, et al. A novel gluco-conjugate vaccine against fungal pathogens. J Environ Monit. 2005;202:597–606. doi: 10.1084/jem.20050749. doi:10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A, Chiani P, Bromuro C, De Bernardis F, Palma AS, Liu Y, Mignogna G, Maras B, Colone M, Stringaro A, et al. Protection by anti-β-glucan antibodies is associated with restricted β-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE. 2009;4:e5392. doi: 10.1371/journal.pone.0005392. doi:10.1371/journal.pone.0005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A, Chiani P, Cassone A. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the β-1,6 glucan of the fungal cell wall. J Leukoc Biol. 2000;68:923–932. [PubMed] [Google Scholar]
- Wenzel RP. Nosocomial candidemia: Risk factors and attributable mortality. Clin Infect Dis. 1995;20:1531–1534. doi: 10.1093/clinids/20.6.1531. doi:10.1093/clinids/20.6.1531. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. doi:10.1086/421946. [DOI] [PubMed] [Google Scholar]