Abstract
Objectives
We compared invasive pneumococcal disease (IPD) incidence by race/ethnicity and neighborhood poverty level and assessed their relative utility to describe disparities in IPD in 1998–1999 and again in 2007–2008, after introduction of the 7-valent pneumococcal conjugate vaccine (PCV7).
Methods
We conducted laboratory surveillance for pneumococcal isolates from sterile body sites and serotyped the isolates. Home address was geocoded to the census-tract level. Census-tract data on the percentage of people below poverty were grouped into three categories. The difference in the magnitude of incidence by race/ethnicity and by census-tract socioeconomic status (SES) (high poverty minus low poverty) was compared for 1998–1999 and 2007–2008 for PCV7 and non-PCV7 serotypes.
Results
In 1998–1999, incidence difference (all per 100,000 population) for PCV7 serotypes for black people compared with white people was 14.3 and by poverty level was 13.9. The highest rate was among white people in high-poverty tracts (77.3). By 2007–2008, there were only slight differences between rates for black and white people (0.7) and SES (1.4). In 1998–1999, the incidence difference for non-PCV7 serotypes was 4.7 between black and white people and 6.0 by SES. By 2007–2008, the differences were 11.6 and 11.7, respectively. Among those living in the highest-poverty tracts, white people had the highest rate (42.9).
Conclusions
In the absence of vaccine, IPD incidence is higher among people living in higher-poverty census tracts and among black people. Emerging serotypes also follow this trend. Differences in neighborhood poverty levels reveal disparities in rates of IPD as large as those seen by race/ethnicity and could be used to routinely describe disparities and target prevention.
Racial/ethnic disparities in pneumococcal disease occurrence in the United States have long been recognized, with black people having higher incidence of invasive pneumococcal disease (IPD) in all age groups compared with white people, and with variable incidence among other racial/ethnic groups, generally falling between the lower rates among white people and the higher rates among black people.1 These disparities were among those highlighted in Healthy People 2010, in which there was a specific objective to eliminate pneumococcal disease racial/ethnic disparities in the two age groups targeted by vaccine recommendations: adults ≥65 years of age for whom the 23-valent pneumococcal polysaccharide vaccine had long been recommended, and children <5 years of age for whom a new 7-valent pneumococcal conjugate vaccine (PCV7) was licensed in 2000.2
Following the introduction in 2000 and subsequent widespread use of PCV7, there was a dramatic shift in the incidence and epidemiology of the most severe and easily monitored form of pneumococcal disease, IPD.3 Not only did incidence decrease in the target group of children <5 years of age, but there also was evidence of community-level or “herd” immunity, with reductions in disease caused by the serotypes covered by PCV7 among all age groups.3,4 By 2004, racial/ethnic disparities had been greatly reduced and practically eliminated.1 Shortly after, however, it became clear that several non-vaccine serotypes of pneumococci, particularly serotype 19A, were beginning to fill the niche vacated by those covered by PCV7. Disease caused by these non-vaccine serotypes began to steadily increase.5,6 Increases were noted particularly among people who were immunosuppressed and Alaska Native children.7,8
The use of race/ethnicity as the sole social descriptive variable for analysis to determine disparities has recently been called into question.9 For most diseases with racial/ethnic disparities, including pneumococcal disease, there does not appear to be a biological basis for incidence to be different, and for the most part, race/ethnicity is a surrogate for other social determinants of health that are factors within and across racial/ethnic groups such as poverty, crowding, low education level, and reduced health-care access. This finding has led to a call to begin to use other available measures of socioeconomic status (SES) to monitor disparities in addition to or in place of race/ethnicity.9 After more than five years of work to systematically identify the best available SES measure for disparities monitoring across a wide range of conditions of public health interest, the Public Health Disparities Geocoding Project recommended use of a neighborhood measure, the percentage of people living below the federal poverty level (FPL) in their census tract.10 In 1999, the FPL for a family of four was $17,029.11 The choice of a census tract-level measure rather than an individual one was made for two major reasons: individual SES is usually not available in most routinely collected public health morbidity data, and neighborhood of residence is, in itself, a powerful independent factor in determining morbidity.10
Connecticut has participated in the Emerging Infections Program network since it was created in 1995 and has contributed data to help elucidate racial/ethnic disparities in IPD.12 Given the dynamics of pneumococcal disease incidence when examined by race/ethnicity, we sought to describe pre-PCV7 disparities by SES, using neighborhood poverty, as well as by race/ethnicity, and examine trends in reduction of PCV7 serotypes and emergence of replacement disease by these variables. To the extent that use of this SES measure described disparities that were as large as those described by race/ethnicity, we felt it might be an appropriate measure to use routinely for future monitoring.
METHODS
The Connecticut Department of Public Health (CDPH) participates in Active Bacterial Core Surveillance as part of the Emerging Infections Program of Connecticut. As part of this surveillance project, active surveillance for IPD is conducted for the entire state.
A case of IPD was defined by the isolation of Streptococcus pneumoniae from a normally sterile body site of a Connecticut resident. Cases were reported to the CDPH by laboratories and clinicians. To ensure completeness of reporting, surveillance personnel performed routine audits of laboratory data of all 32 acute care hospital laboratories and two outpatient reference laboratories. Case data were collected through medical chart review for hospitalized patients and physician interview for outpatients. A standardized questionnaire was used to collect demographic, clinical syndrome, underlying risk factor, and outcome data.
As part of laboratory reporting, pneumococcal isolates were sent to the Centers for Disease Control and Prevention's Streptococcus Laboratory for confirmation and serotyping. Pneumococcal serotypes included in PCV7 were defined as “vaccine serotypes.” This included serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. PCV7-related serotypes included all strains not in the vaccine but that cross-react with vaccine strains, including 6A, 9A, 9L, 9N, 18A, 18B, 18F, 19B, 19C, 23A, and 23B. All other serotypes were characterized as “non-vaccine serotypes.”
Records were geocoded to the census-tract level using ArcGIS® version 9.1 software.13 Home address at the time of culture was used for geocoding. Cases with missing or invalid home addresses (post office box as address, incarcerated, or no match in U.S. Postal Service database) were excluded. Cases that had documented addresses that were unable to be matched during the geocoding process were matched to neighboring addresses that fell within the same census tract. Census tract-level poverty data were determined for each case by matching the census tract of residence to 2000 Census data. Cases were characterized as living in low-poverty (0% to <10% of residents living below FPL), medium-poverty (10% to <20% of residents living below FPL), or high-poverty (20% to 100% of residents living below FPL) census tracts. The high-poverty category is consistent with the standard federal definition of a “poverty area.”
Incidence rates by year using 2000 Census data were calculated for total IPD, PCV7 serotypes, non-PCV7 serotypes, and serotype 19A. For each pneumococcal category, incidence rates by year were also calculated for race/ethnicity and SES. To assess the relative importance of race/ethnicity and SES to describe disparities, the difference in the magnitude of the incidence rate by race/ethnicity and by SES (high poverty vs. low poverty) were calculated for the two years before the introduction of the vaccine (1998–1999) compared with the two most recent post-vaccine-era years (2007–2008) for each of the pneumococcal categories defined previously. In addition, relative incidence was calculated using incidence among white people and in the lowest-poverty neighborhoods as the reference groups. To explore the effect of neighborhood poverty level on rates within racial/ethnic groups during the highest disparity periods, we examined rates of PCV7 serotype-associated disease in 1998–1999 and rates of non-vaccine serotype disease in 2007–2008 by poverty level within racial/ethnic groups. We conducted all data analyses using SAS® version 9.2.14
RESULTS
We identified 5,600 cases of IPD from 1998 to 2008; isolate serotype data were available for 5,023 (89.7%) cases. A total of 5,422 (96.8%) cases were able to be geocoded. Cases unable to be geocoded (n=178) were excluded due to invalid address (59.6%), post office box (25.8%), homeless (9.6%), missing address (3.9%), and incarcerated (1.1%). The percentage able to be geocoded to the census-tract level did not vary significantly by year (range: 93.2% to 98.8%).
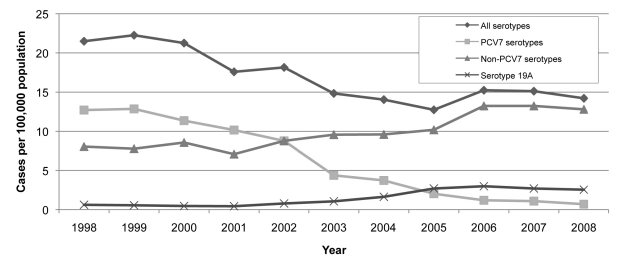
The rates of IPD from 1998 to 2008 declined from a mean high of 21.9 per 100,000 population in 1998–1999 to a mean low of 13.7 per 100,000 population in 2005 and a mean of 14.7 per 100,000 population in 2007–2008 (Figure 1). The overall decrease in IPD incidence was primarily driven by the decrease in incidence in PCV7 strains, which went from 12.8 per 100,000 population in 1998–1999 to 0.9 per 100,000 population in 2007–2008. During this time period, there was an increase in non-PCV7 strains from 6.8 per 100,000 population in 1998–1999 to 12.1 per 100,000 population in 2007–2008, and in serotype 19A from 0.6 per 100,000 population in 1998–1999 to 2.6 per 100,000 population in 2007–2008.
Figure 1.
Invasive pneumococcal disease incidence, overall and by selected serotypes, by year: 1998–2008, Connecticut
PCV7 = 7-valent pneumococcal conjugate vaccine
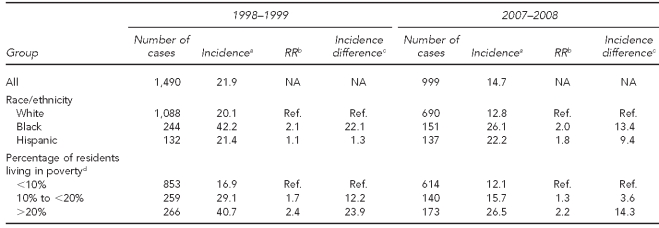
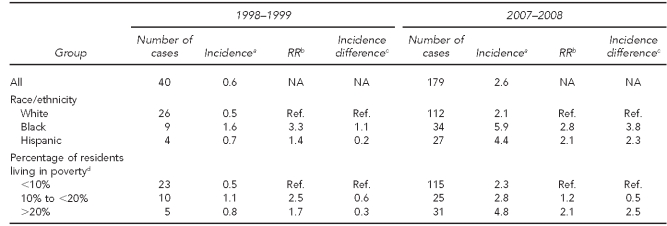
Table 1 shows the incidence of IPD for all serotypes by race/ethnicity and by SES in the pre-vaccine (1998–1999) and post-vaccine (2007–2008) eras. Before the introduction of PCV7, rates among black people were 2.1 times those among white people, with an incidence difference of 22.1 per 100,000 population. Rates among people living in the highest-poverty tracts showed a similar magnitude of disparity, being 2.4 times higher for those living in the poorest census tracts compared with the lowest-poverty tracts, with an incidence difference of 23.9 per 100,000 population. Seven and eight years after the introduction of PCV7, the incidence difference between black and white people dropped to 13.4 per 100,000 population, while the difference between high- and low-poverty tracts was of a similar magnitude, dropping to 14.3 per 100,000 population. Of interest, incidence of IPD among Hispanic people was not affected by the introduction of PCV7.
Table 1.
Invasive pneumococcal disease, all serotypes, incidence per 100,000, and changes over time, by race/ethnicity and poverty level: 1998–1999 to 2007–2008, Connecticut
aMean annual incidence per 100,000 people for the two-year period
bRelative incidence vs. reference group
cIncidence difference compared with reference group
dPercentage of people in census tract with income below the federal poverty level, 2000 Census
RR = rate ratio
NA = not applicable
Ref. = reference group
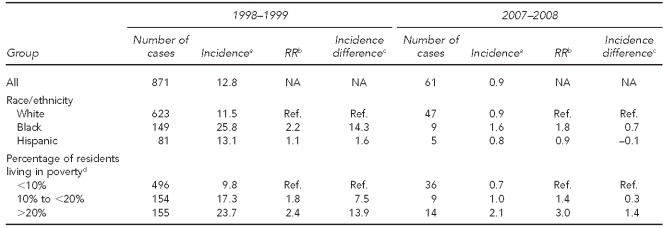
Table 2 shows similar data for IPD attributable to PCV7 serotypes. In 1998–1999, there were marked disparities by race/ethnicity and neighborhood poverty level that were of a similar magnitude to each other. Incidence among black people was 2.2 times that of white people, with an incidence difference of 14.3 per 100,000 population. The incidence among residents of high-poverty neighborhoods was 2.4 times that of residents in low-poverty neighborhoods, and the incidence difference was 13.9 per 100,000 population. Seven and eight years after the introduction of PCV7, these disparities were practically eliminated. The incidence difference between black and white people was reduced to 0.7 per 100,000 population and the difference between higher- and lower-poverty neighborhoods was reduced to 1.4 per 100,000 population.
Table 2.
Invasive pneumococcal disease, PCV7 serotypes, incidence per 100,000, and changes over time, by race/ethnicity and poverty level: 1998–1999 to 2007–2008, Connecticut
aMean annual incidence per 100,000 people for the two-year period
bRelative incidence vs. reference group
cIncidence difference compared with reference group
dPercentage of people in census tract with income below the federal poverty level, 2000 Census
PCV7 = 7-valent pneumococcal conjugate vaccine
RR = rate ratio
NA = not applicable
Ref. = reference group
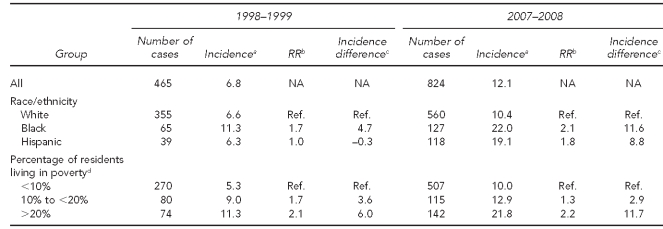
Table 3 shows the emergence of non-vaccine serotypes from 1998–1999 to 2007–2008. While there were initial disparities of comparable magnitude by race/ethnicity and poverty level (incidence difference of 4.7 per 100,000 population between black and white people, and 6.0 per 100,000 population between higher and lower neighborhood poverty levels), both disparities grew as incidence of IPD due to non-vaccine serotypes increased. By 2007–2008, the incidence difference between black and white people was 11.6 per 100,000 population and by neighborhood poverty level was 11.7 per 100,000 population. Of interest, while the incidence differences compared with white people increased over time for Hispanic people, too, the incidence differences did not increase for the mid-level poverty group compared with the lowest-level poverty group.
Table 3.
Invasive pneumococcal disease, all non-vaccine serotypes, incidence per 100,000, and changes over time, by race/ethnicity and poverty level: 1998–1999 to 2007–2008, Connecticut
aMean annual incidence per 100,000 people for the two-year period
bRelative incidence vs. reference group
cIncidence difference compared with reference group
dPercentage of people in census tract with income below the federal poverty level, 2000 Census
RR = rate ratio
NA = not applicable
Ref. = reference group
We also examined whether disparities by race/ethnicity and neighborhood SES were heightened during the emergence of the most prominent non-vaccine serotype, 19A (Table 4). Although the number of cases was much lower, we observed a similar widening of incidence differences between black and Hispanic people vs. white people, and between people living in neighborhoods with high poverty levels vs. those living in neighborhoods with lower poverty levels.
Table 4.
Invasive pneumococcal disease, serotype 19A, incidence per 100,000, and changes over time, by race/ethnicity and poverty level: 1998–1999 to 2007–2008, Connecticut
aMean annual incidence per 100,000 people for the two-year period
bRelative incidence vs. reference group
cIncidence difference compared with reference group
dPercentage of people in census tract with income below the federal poverty level, 2000 Census
RR = rate ratio
NA = not applicable
Ref. = reference group
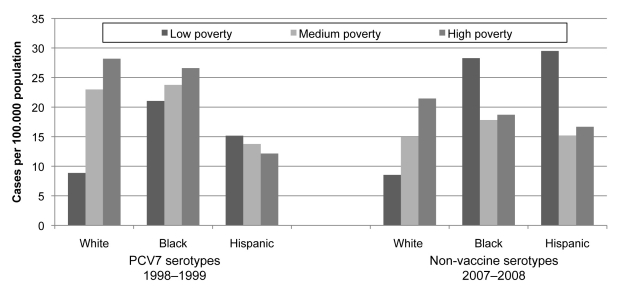
Figure 2 shows the impact of neighborhood poverty level by racial/ethnic group for two distinct groups of IPD serotypes for which there were wide disparities by race/ethnicity and poverty level: PCV7 serotypes before PCV7 introduction and non-vaccine serotypes that have emerged since PCV7 introduction. For white people, there was a strong gradient by poverty for both groups, and white people living in high-poverty areas had the single highest rate of all people living in high-poverty areas. By contrast, trends for black and Hispanic people were not consistent and had no systematic association with poverty level. Among residents of the neighborhoods with the least poverty, incidence of IPD was consistently higher among black and Hispanic people than among white people.
Figure 2.
Invasive pneumococcal disease incidence by neighborhood poverty level within racial/ethnic groups, all serotypes, 1998–1999, and non-vaccine serotypes, 2007–2008, Connecticut
PCV7 = 7-valent pneumococcal conjugate vaccine
DISCUSSION
The reasons behind racial/ethnic, but particularly racial, disparities in the incidence of IPD—whether and to what extent they are social, economic, environmental, medical (underlying disease), or genetic—have not been clear. Our study helps to shed light on factors that may contribute to racial/ethnic disparities by demonstrating that there is a strong socioeconomic component to them and that socioeconomic factors appear to play a role within individual racial/ethnic groups, particularly among white people. Before the introduction of PCV7, there were strong disparities in IPD incidence by neighborhood poverty level, as well as race/ethnicity, and these disparities were largely eliminated with high coverage levels of vaccine. When serotypes not covered by the vaccine emerged, they increased most among people living in poorer neighborhoods, mirroring the disease distribution by SES before PCV7 introduction. Of particular note, white people showed the largest and most consistent gradient of IPD incidence by neighborhood SES, and white people living in the highest-poverty neighborhoods consistently had the highest rates of disease among people living in those neighborhoods.
By contrast, neighborhood poverty levels had a much weaker, if any, association with IPD incidence among black and Hispanic people. The reason for this finding is not clear. One possible explanation is that the numbers of people in these groups in Connecticut are relatively small—particularly the number of cases among black and Hispanic people in the least impoverished neighborhoods—and the incidence is highly variable from year to year. However, it is also possible that there are other social factors operating in these groups. For example, social networks likely play a role in the probability of exposure to pneumococci, and it is possible that social networks among these groups in Connecticut are less hierarchical by SES than they are for the much larger white group. In addition, it is possible that racial/ethnic segregation could result in a more socioeconomically heterogeneous group of black or Hispanic people living in each poverty-level neighborhood. This could result in smaller neighborhood poverty differences in IPD incidence within racial/ethnic minority groups than among white people.
The main reason we decided to examine disparities by neighborhood SES was to find out whether disparities described by the SES measure recommended by the Public Health Disparities Geocoding Project, the percentage of people living below the FPL, described disparities that were as large as those measured by race/ethnicity.9 We identified disparities of a similar magnitude, whether using relative rates or incidence difference as the outcome measure. In addition, we observed large disparities within groups of white people categorized by this measure and found that white people living in poor census tracts had as high or higher rates of IPD as black and Hispanic people. Further, we were able to describe a gradient of incidence by neighborhood poverty level, not just all-or-nothing extremes. Given these observations, we believe that future efforts to monitor disparities in IPD incidence should routinely include this measure and be used to target and monitor intervention and prevention measures, including education and vaccination, to the populations and geographic areas with the highest disease burden.
Limitations
Our study had several notable limitations. Most importantly, the number of people and cases among black and Hispanic people in census tracts with lower poverty were small, reducing our ability to find differences within these racial/ethnic groups by neighborhood poverty level. It also prevented us from being able to meaningfully examine our data by age group. Second, we only attempted to use one neighborhood SES measure. One of the mechanisms by which poverty could affect IPD incidence is household crowding. Indoor crowding and time spent indoors likely underlie high levels of IPD and replacement disease in Alaska Native people8 and may also provide additional insight into trends in IPD in Connecticut. However, our purpose in using the measure we chose was to use the currently recommended standard measure for assessing disparities by SES. Third, our measure only looked at neighborhood SES. Personal SES, if known, likely would further contribute to the potential for disparities in disease incidence related to poverty. Finally, our denominators for all years were 2000 Census denominators, the only intercensal denominators available to use for census tract-level data. However, the main effect of using 2000 denominators for later years would be to slightly overestimate IPD incidence rates, assuming the population in each census-tract poverty category grew over time.
CONCLUSIONS
In the absence of vaccine, IPD incidence is higher among people living in higher-poverty census tracts and among black people. Emerging serotypes also follow this trend. Differences in neighborhood poverty levels reveal disparities in rates of IPD as large as those seen by race/ethnicity and could be used to routinely describe disparities and target prevention efforts.
Acknowledgments
The authors thank the staff of the Centers for Disease Control and Prevention's Streptococcus Laboratory for serotyping the invasive pneumococcal isolates.
REFERENCES
- 1.Flannery B, Schrag S, Bennett NM, Lynfield R, Harrison LH, Reingold A, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004;291:2197–203. doi: 10.1001/jama.291.18.2197. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services (US). Healthy People 2010. Immunization and infectious diseases. [cited 2011 Apr 8]. Available from: URL: http://www.healthypeople.gov/2010/Document/HTML/Volume1/14Immunization.htm.
- 3.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 4.Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 5.Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196:1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 6.Moore MR, Gertz RE, Jr, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016–27. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 7.Flannery B, Heffernan RT, Harrison LH, Ray SM, Reingold AL, Hadler J, et al. Changes in invasive pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med. 2006;144:1–9. doi: 10.7326/0003-4819-144-1-200601030-00004. [DOI] [PubMed] [Google Scholar]
- 8.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 9.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of U.S. socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312–23. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the Public Health Disparities Geocoding Project. Am J Public Health. 2003;93:1655–71. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Census Bureau (US). Poverty thresholds 1999. [cited 2011 Feb 1]. Available from: URL: http://www.census.gov/hhes/www/poverty/data/threshld/thresh99.html.
- 12.Schuchat A, Hilger T, Zell E, Farley MM, Reingold A, Harrison L, et al. Active Bacterial Core Surveillance of the Emerging Infections Program network. Emerg Infect Dis. 2001;7:92–9. doi: 10.3201/eid0701.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ESRI, Inc. ArcGIS®: Version 9.1. Redlands (CA): ESRI, Inc.; 2006. [Google Scholar]
- 14.SAS Institute, Inc. SAS®: Version 9.2. Cary (NC): SAS Institute, Inc.; 2008. [Google Scholar]