Abstract
Objectives
People with severe mental illness (SMI) may be at increased risk for several adverse health conditions, including HIV/AIDS. This disproportionate disease burden has been studied primarily at the individual rather than community level, in part due to the rarity of data sources linking individual information on medical and mental health characteristics with community-level data. We demonstrated the potential of Medicaid data to address this gap.
Methods
We analyzed data on Medicaid beneficiaries with schizophrenia from eight states that account for 66% of cumulative AIDS cases nationally.
Results
Across 44 metropolitan statistical areas (MSAs), the treated prevalence of HIV among adult Medicaid beneficiaries diagnosed with schizophrenia was 1.56% (standard deviation = 1.31%). To explore possible causes of variation, we linked claims files with a range of MSA social and contextual variables including local AIDS prevalence rates, area-based economic measures, crime rates, substance abuse treatment resources, and estimates of injection drug users (IDUs) and HIV infection among IDUs, which strongly predicted community infection rates among people with schizophrenia.
Conclusions
Effective strategies for HIV prevention among people with SMI may include targeting prevention efforts to areas where risk is greatest; examining social network links between IDU and SMI groups; and implementing harm reduction, drug treatment, and other interventions to reduce HIV spread among IDUs. Our findings also suggest the need for research on HIV among people with SMI that examines geographical variation and demonstrates the potential use of health-care claims data to provide epidemiologic insights into small-area variations and trends in physical health among those with SMI.
A 2006 report of the National Association of State Mental Health Program Directors (NASMHPD) called on the federal government to designate the population with serious mental illness as a health disparities group as part of an effort to slow an accelerating “epidemic of premature death” and morbidity among people with serious mental illness.1 The NASMHPD based these conclusions partly on estimates that in the public mental health system in eight states, patients die an average of 25 years earlier than their general population counterparts. Suicide and injury are estimated to account for about one-third of excess mortality, with most of the excess associated with chronic medical illnesses,2 particularly for diseases whose etiology implicates behavioral and environmental contributions, including cardiovascular and metabolic conditions, as well as infectious diseases.3–5 The report concluded that the elevated rates of disease and death in this population should be made a policy priority, requiring significant efforts to develop data sources necessary for meaningful surveillance, and that cooperative efforts between Medicaid and public health agencies could provide one important source for these efforts.1
The rarity with which the elevated morbidity and mortality among people with severe mental illness (SMI) has been described as evidence of health disparities is striking, both because many of the social structural processes that produce the disadvantages commonly tied to health disparities are associated with SMI and because the medical risks faced by people with SMI are arguably a paradigm case of “falling through the cracks” in a poorly configured public health and health-care system. Despite mention of definitions that include disabilities (presumably including those associated with psychiatric illness) as a disparities category alongside race/ethnicity, socioeconomic status, and others, neither of two recent reviews of health disparities does more than mention the drastic differences in disease burden found in this population.66,7
BACKGROUND
Strategies for surveillance and research on SMI and medical illness
The challenges of monitoring morbidity and treatment patterns for this population are complex. Many of the same factors that make it hard to provide quality health care to people with SMI also make it hard to study these conditions. Recruitment and retention over time are difficult because many patients seek care erratically and across multiple settings, are alienated and distrustful of the medical community, and are wary of the stigma associated with SMI. Subgroups of people with cognitive impairment may not be able to give consent or participate fully in interviews. Recruitment is easiest for patients with a stable source of care, which contributes to bias. Aggregating data from major, multisite clinical trials can increase numbers and introduce economies, but these studies typically screen out unstably housed people and people with comorbidities that may complicate outcomes. Organizationally, the clinical sites that are most willing and able to allow data collection may not be representative of poorly resourced community settings, where recruitment may be less easily accommodated (or welcomed). Even when expensive, time-consuming epidemiologic studies can be conducted, they tend to provide a snapshot in time, which can be misleading when considering dynamic disease processes such as the spread of human immunodeficiency virus (HIV) into new populations.
One issue is the familiar quantitative challenge of case finding for rare conditions. The most severe mental disorders, such as schizophrenia and bipolar disorder, are relatively uncommon, which means that studies of uncommon medical conditions require screening of large numbers of individuals. Epidemiologic studies of mental illness typically collect relatively limited information on medical illness, and when they do, data collection tends to neglect less common infectious diseases and relies on self-report rather than provider-assigned diagnoses and/or medical records.
Further challenges are posed by variations over time and across locations. Disease burden for a population is not static, as it is subject to changes over time in risk, protective factors, and treatment resources. Rapid changes can accompany the introduction of infectious diseases or development and rapid diffusion of efficacious treatments. Slower changes follow from factors such as trends in lifestyle, reconfiguration of resources, and other improvements in public health and the public health infrastructure. The fundamentally dynamic quality of disease burden profiling requires data that can be updated regularly and reliably, allowing longitudinal monitoring.
Geographic variation reflects variations in environmental risks, housing stock, economic environments, and cultural/behavioral norms, as well as prevention and treatment resources. While small-area variation studies have pioneered methods for identifying apparently unexplained variations in medical practice, data are needed on geographic variations in disease burden and care for relatively uncommon, high-impact conditions, such as SMI. Among the factors contributing to geographic variations are the sharp differences in services systems and funding levels across states and localities. Services for mental health, physical illness, and substance abuse are often separate, and local areas and states differ from one another in how they combine them. (For example, substance abuse and mental health functions may be funded by one agency or different agencies.)
No single data source can optimally address all of the important unanswered questions about disparities in medical conditions and physical health for people with SMI, but our experience has pointed to the potential value of datasets created by linking Medicaid claims to community-level data relevant to the disease group under study. In this article, we explore this possibility using the example of elevated rates of HIV among people with SMI. Medicaid claims files have already played some role in examining these comorbidities in several studies by our group in New Jersey and by University of Pennsylvania researchers in Philadelphia,8–12 and we extend that work by examining HIV disease patterns in a large group of cities for which data are available.
Surveillance of HIV and acquired immunodeficiency syndrome (AIDS) has provided essential information in understanding disease epidemiology, combating spread, promoting access to care, and contributing to the policy and planning process. It has consistently been viewed from a dynamic perspective, using multiple complementary and overlapping data sources, and adapting over time to new information needs associated with evolving understanding of HIV, treatments, and epidemiology.13 It is now clear that we need to know more about HIV infection outside the most studied, typically urban, locales, and about infection rates among groups that were not commonly described in early surveillance studies, such as those with SMI. Longer life spans produced by improved antiretroviral therapy also mean that service delivery systems now provide individual patients with many more years of care14 (the Centers for Disease Control and Prevention's [CDC's] Medical Monitoring Project is one response to this need15). However, existing surveillance systems have significant limitations, including limited ability to identify and follow those with comorbidity of HIV/AIDS and other conditions and limited ability to provide denominator data for estimating prevalence rates in subpopulations of concern, such as those with SMI. Thus, it is important to consider complementary sources of information that can provide insight into the distribution of diagnosed HIV/AIDS among such subpopulations in communities. In this context, Medicaid claims files have the data needed to identify diagnostically defined subgroups whose profile across large geographic areas is poorly understood, and to cost-efficiently use a full range of encounter-based information to follow incident diagnosis, treated prevalence, and patterns of utilization over time.
Research on HIV and people with SMI
During the first few years of the HIV epidemic, the issue of HIV infection among people with severe psychiatric illness received relatively little attention. The potential significance attached to possible comorbidity between HIV and SMI was initially so limited that some investigators using structured psychiatric assessment instruments opted to drop a psychosis screen to save interview time.16,17 However, beginning in the late 1980s, several studies drew attention to this challenge, including the pioneering work of the research group led by Francine Cournos, MD, at Columbia University in New York City (NYC), which showed high rates of HIV in psychiatric inpatient settings in the city. From 1989 to 1991, the group conducted five seroprevalence studies using anonymous blood collected in various hospital settings in NYC, totaling 1,116 inpatients, 5.2% of whom tested positive for HIV. An additional seven studies were soon published using various other methods, all but two also from NYC. This group of 12 early studies formed a widely cited core and provided the basis for a range estimate of 4.0% to 22.9% HIV seroprevalence among people with SMI.18
In 1996, the Office of AIDS at the National Institute of Mental Health held a working conference to examine clinical, scientific, and policy issues associated with this issue,19 and spelled out a research agenda on HIV epidemiology, epidemiology of sexual and substance-related risk, risk reduction/prevention, and treatment.20
Compared with these early published estimates from disease epicenters, subsequent HIV prevalence data on psychiatric patients from clinical settings have generally suggested rates that, while still elevated, are lower and somewhat more variable: 1.2% in a study of general psychiatric outpatients;21 5.0% at metropolitan sites and 1.7% in nonmetropolitan sites in community treatment settings;22 and 1.0% in a national group of Veterans Administration (VA) patients with SMI (schizophrenia, bipolar, and other nonorganic psychoses) compared with 0.5% among non-SMI patients in the VA system.23 Using Medicaid claims data linked to welfare records, a study in Philadelphia reported a rate of 1.8% for patients with SMI (vs. 0.6% for non-SMI patients).24 Research using Medicaid claims in New Jersey for the late 1990s found rates of schizophrenia and bipolar disorder that were much higher among people living with HIV/AIDS than in the general population.12 Other studies using medical records and administrative data have also found variably elevated HIV rates in large populations of community-dwelling people with SMI.8,25
Despite cautions voiced by early investigators on the probable contribution of the social circumstances of people with SMI to elevated HIV rates, studies of risk26–33 and risk reduction34 have tended to emphasize, to varying degrees, combinations of individual psychopathology, substance abuse, impulsivity, and behavior control. Results have varied, but increasing evidence implicates a central role for substance abuse, possibly acting on sexual risk behavior, although responsible mechanisms continue to be investigated.31 Interventions to reduce HIV risk have found that producing and sustaining large behavioral changes is challenging, particularly in the presence of substance abuse.34–37
Evidence regarding the important role played by substance abuse in the spread of HIV among people with SMI highlights the extent to which HIV risk can be understood in the context of variations in the characteristics of local HIV epidemics, such as the spread of HIV/AIDS specifically among substance abusers, and in light of multiple risk factors, such as the combination of psychiatric illness with substance abuse.38–41 In institutionally based studies, the highest rates of HIV infection have been found in settings that treated both substance abuse and psychiatric illness.42 In the VA study cited previously, increased HIV risk was limited to those SMI patients who also had a substance abuse diagnosis.23 Among patients with schizophrenia without a substance abuse diagnosis, rates of HIV diagnosis were lower, not higher, than the comparison group. Less is known about rates of diagnosed HIV infection among the broader range of patients and treatment settings represented by Medicaid, which is the payer for a high proportion of the population with severe and chronic mental illness, as well as the medical costs of care for many substance abusers.43,44
While much research on HIV infection risk traditionally focused on individual-level clinical and behavioral factors, recent prevention research has stressed the importance of environmental and social factors external to the individual in producing an “HIV risk environment” in which influences interact to increase vulnerability to HIV.45 Risk environments vary considerably across different urban areas, although few data have been available in the past on these geographical variations in relation to individuals with SMI.
We combined information available from public and published data sources potentially relevant to the risk-environment construct with information from Medicaid claims files on HIV diagnosis rates among people with schizophrenia in metropolitan areas of eight large states (California, Florida, New Jersey, New York, Texas, Illinois, Georgia, and Ohio), from 2002 and 2003. Because Medicaid pays for both psychiatric and infectious disease care, the data provide information on treated prevalence for both categories of diagnosis. Geographical location of beneficiaries was identified at the ZIP-code level for residents of the eight states during 2002–2003. The more than 45 million individuals receiving Medicaid in these states represented 51% of Medicaid beneficiaries nationally. In 2001, these eight states included 66% of the cumulative AIDS cases in the U.S.46
Using these claims files, we investigated two questions: (1) To what extent do claims-based estimates of HIV vary across metropolitan areas for one group of patients with SMI—people with schizophrenia—and (2) does this variation across cities show associations with community-level characteristics that, the existing literature suggests, may impact local epidemic dynamics?
Specifically, based on evidence indicating associations between SMI and substance abuse in inpatient samples with HIV/AIDS,18 as well as findings regarding injection drug use by people with SMI,47 we examined community-level evidence regarding the scope of the HIV epidemic among injection drug users (IDUs), and we hypothesized that communities with more HIV infection among IDUs would have higher rates of HIV among people with schizophrenia (beyond the influence of community-level AIDS rates on HIV among people with schizophrenia). Similarly, we examined whether community-level substance-abuse comorbidity among patients with schizophrenia was associated with community-level HIV rates among patients with schizophrenia. The developing literature on the HIV risk environment45 has argued that HIV risk along with local HIV epidemic dynamics are associated with community-level factors such as poverty, social disorder, policing practices, criminal justice activities, and availability of treatment and harm-reduction opportunities.48,49 We looked for evidence that this hypothesis holds for HIV among people with schizophrenia by including in our model publicly available measures of a set of these variables.
METHODS
Data sources and inclusion criteria
We used data from the Medicaid Analytic eXtract (MAX), provided by the Centers for Medicaid and Medicare Services, to identify individuals diagnosed with schizophrenia and with HIV disease. Details on MAX are available elsewhere.50 Schizophrenia was chosen because it is generally associated with significant functional disability and a chronic course, and it can be effectively ascertained in claims.51,52 This work builds on our extensive experience using Medicaid claims data for research on treatment of both schizophrenia and HIV/AIDS.10,12,53–56
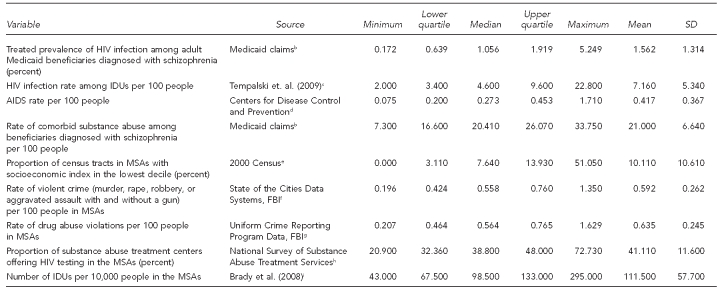
The unit of analysis was the metropolitan statistical area (MSA), a geographical area unit defined by the Office of Management and Budget. To ensure sufficient sample sizes for planned analyses, we excluded MSAs with fewer than 2,000 adult Medicaid beneficiaries. As state Medicaid program characteristics differ, we further excluded MSAs that cross state borders. In addition, some MSAs were excluded due to the unavailability of major environmental-level variables, particularly HIV infection rates among IDUs. To calculate the treated prevalence of HIV infection among Medicaid beneficiaries in these states, we included residents aged 18–64 years with at least one full year of Medicaid eligibility, no comprehensive health maintenance organization coverage, no days in a long-term care facility, and who were classified as having schizophrenia based on the diagnoses recorded on the Medicaid claims. Individuals with managed care participation were excluded because medical encounter data for these individuals may not be complete. For analyses of the association of these rates of HIV among people with schizophrenia with the rate of HIV infection among IDUs, data were available for 44 of the MSAs (Table 1).
Table 1.
Quartiles, ranges, means, and SDs for study constructs in a study of community rates of treated prevalence of HIV infection among adult Medicaid beneficiaries diagnosed with schizophrenia in 44 MSAs, 2002–2003a
aNumber of MSAs included in the analyses = 44; averages are across MSAs.
bDepartment of Health and Human Services (US), Centers for Medicare and Medicaid Services. Medicaid Analytic eXtract (MAX) general information [cited 2008 Jan 10]. Available from: URL: http://www.resdac.org/Medicaid/data_available.asp
cTempalski B, Lieb S, Cleland CM, Cooper H, Brady JE, Friedman SR. HIV prevalence rates among injection drug users in 96 large US metropolitan areas, 1992–2002. J Urban Health 2009;86:132-54.
dCenters for Disease Control and Prevention (US). HIV/AIDS surveillance report, 2003. Vol. 15. 2004 [cited 2009 Dec 18]. Available from: URL: http://www.cdc.gov/hiv/stats/hasrlink.htm
eCensus Bureau (US). American FactFinder [cited 2008 Apr 3]. Available from: URL: http://factfinder.census.gov/home/saff/main.html?_lang=en
fDepartment of Housing and Urban Development (US). State of the cities data systems (SOCDS) [cited 2009 Aug 27]. Available from: URL: http://socds.huduser.org/SOCDS_Home.htm
gDepartment of Justice (US), Federal Bureau of Investigation. Uniform Crime Reporting program data [United States]: county-level detailed arrest and offense data. Washington: Department of Justice, FBI; 2009.
hDepartment of Health and Human Services (US), Substance Abuse and Mental Health Services Administration. DASIS [Drug & Alcohol Services Information System]. National Survey of Substance Abuse Treatment Services (N-SSATS) [cited 2008 Feb 1]. Available from: URL: http://wwwdasis.samhsa.gov/dasis2/nssats.htm
iBrady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the U.S. and in large U.S. metropolitan areas from 1992 to 2002. J Urban Health 2008;85:323-51.
SD = standard deviation
HIV = human immunodeficiency virus
MSA = metropolitan statistical area
IDU = injection drug user
AIDS = acquired immunodeficiency syndrome
FBI = Federal Bureau of Investigation
Measures
Primary outcome.
We calculated treated prevalence of HIV infection among adult Medicaid beneficiaries diagnosed with schizophrenia from Medicaid claims. Each claim provided information on specific health-care services utilized, category of service, dates of service, and up to five diagnosis codes conforming to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Using an algorithm validated in prior research,51 we categorized individuals with at least one inpatient claim and/or two or more outpatient claims with ICD-9-CM code 295 during a two-year period (2002–2003) as diagnosed with schizophrenia. For identification of Medicaid beneficiaries with HIV/AIDS, we utilized another algorithm adapted from prior work.57 Individuals were categorized as having an HIV/AIDS diagnosis based on having at least one inpatient claim and/or two or more outpatient claims with ICD-9-CM codes 042, 043, and 044 during the two-year period (2002–2003). The proportion of individuals with schizophrenia who were also diagnosed with HIV was calculated for each MSA.
Explanatory variables.
We examined associations of prevalence of diagnosed HIV/AIDS among people with schizophrenia with community characteristics that prior research suggests might influence exposure to HIV/AIDS in the SMI population. Characteristics used were chosen based on availability of public or published data sources and their ability to operationalize elements derived from the published literature on “HIV risk environment.”45
For community-level epidemiologic data, we utilized results of previously published studies. CDC maintains national AIDS surveillance data through receipt of AIDS case reports submitted by individual state and local health departments.58 The cumulative number of AIDS cases in each MSA through 2003 was available from the CDC surveillance report.59 (HIV estimates were not used because, as discussed in the technical notes of this CDC surveillance report, various issues regarding data collection and representativeness suggest caution in interpretation.) These surveillance data and population data from the U.S. Census60 were used to calculate the AIDS rate in each MSA.
The estimated rates of HIV infection among IDUs by MSA in 2002 were obtained from published estimates by Tempalski and colleagues.61 These estimates were derived from (1) research-based HIV prevalence rate estimates, (2) CDC voluntary HIV counseling and testing data, (3) data on the number of people living with AIDS compiled by CDC, and (4) estimates of HIV prevalence in the U.S. Data on rates of violent crimes in 2003 within the MSAs were obtained from the State of the Cities Data Systems, Federal Bureau of Investigation (FBI) crime data.62 Figures on arrests for drug sales and possession in 2003 were available from FBI data.63 Data on the number of substance abuse centers within the MSAs in 2003, and the number of these centers that offered HIV testing in 2003, were obtained from the National Survey of Substance Abuse Treatment Services.64 The estimated rates of injection drug use within MSAs (per 10,000 people aged 15–64 years) in 2002 were obtained from Brady et al.65 Rates of comorbid substance abuse among beneficiaries diagnosed with schizophrenia were calculated using the same methods we used to calculate proportion of individuals with schizophrenia who were also diagnosed with HIV, using data from MAX for 2002–2003. The ICD-9-CM codes used to identify substance abuse are available from the authors.
Environmental-level socioeconomic measures.
We created an area-based summary measure of socioeconomic status for each MSA's population (the socioeconomic position [SEP] index) using methods developed by the Public Health Disparities Geocoding Project group at Harvard University.66–68 (For details of these methods, see http://www.hsph.harvard.edu/-thegeocodingproject.) This measure was calculated using 2000 Census data at the census tract level and consisted of a standardized z-score combining data on six components: percent working class, percent unemployed, percent below poverty, percent with education less than high school, percent expensive homes, and median household income. The groups were created from 2000 Census summary tables 1 and 3.60 To identify concentrations of poor people, census tracts belonging to the lowest decile of the index were operationalized as socioeconomically disadvantaged areas. For each MSA, we reported the ratio of socially disadvantaged tracts to the total number of tracts in the MSA.
Table 3.
Sensitivity analysesa with transformed dependent variableb in a study of community rates of treated prevalence of HIV infection among adult Medicaid beneficiaries diagnosed with schizophrenia in 44 MSAs, 2002–2003
aAdjusted R-squared = 0.7914
bDependent variable is log(p/(1–p)), where p represents HIV prevalence as rate, potentially ranging between 0 and 1.
HIV = human immunodeficiency virus
MSA = metropolitan statistical area
AIDS = acquired immunodeficiency syndrome
Statistical analysis
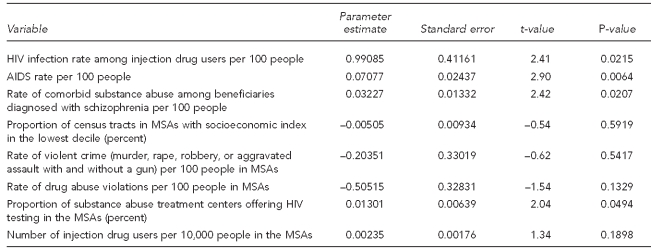
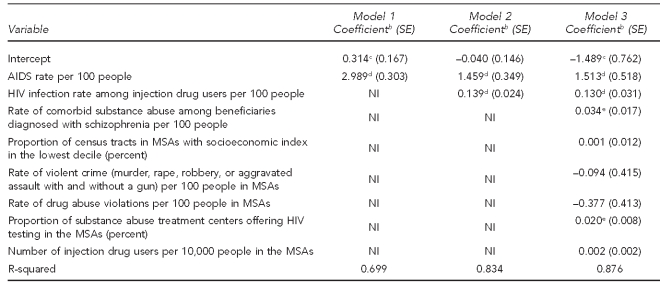
We modeled treated prevalence rates of HIV among Medicaid patients diagnosed with schizophrenia with ordinary least squares (OLS) regression models, including a range of explanatory variables potentially relevant to the risk-environment construct. Basic statistics for environmental-level constructs are presented in Table 1. To investigate the association between MSA-level HIV rates among patients with schizophrenia and environmental factors, we estimated three nested models (Table 2). The first model included as predictors an intercept and overall rate of AIDS in the community, as reported by CDC, as an explanatory variable, given its a priori logical priority. The second model introduced MSA-level HIV rates among IDUs along with the AIDS rates reported by CDC to investigate how the effect size in model 1 (i.e., the general HIV epidemic in the city) was impacted by controlling for the HIV epidemic within the IDU population. In the final model, other environmental-level constructs were included. We used SAS® version 9.269 for data management and used the regression procedure in Stata® version 9.2.70 This research was approved by the Rutgers University Institutional Review Board.
Table 2.
Ordinary least squares regression models predicting community rates of treated prevalence of HIV infection among adult Medicaid beneficiaries diagnosed with schizophrenia in 44 MSAs, 2002–2003a
aDependent variable is HIV prevalence, expressed in percentage points, potentially ranging between 0 and 100.
bReported coefficients are parameter estimates from ordinary least squares regressions.
cp<0.10
dp<0.01
ep<0.05
HIV = human immunodeficiency virus
MSA = metropolitan statistical area
SE = standard error
AIDS = acquired immunodeficiency syndrome
NI = not included in model
Sensitivity analyses.
As the prediction of values based on OLS estimates has the potential to produce negative prevalence numbers, we ran an additional model in which the dependent variable was transformed with the logit function. The model is expressed as follows:
where X1 to Xk measure the value of each of the k explanatory variables in our model, and b1 to bk measure their estimated effect on the HIV treated prevalence rate.
RESULTS
The weighted mean (mean weighted to population size) for treated prevalence of HIV infection among adult Medicaid beneficiaries diagnosed with schizophrenia across sites was 1.56% in these 44 MSAs (Table 1). Claims-based estimates of HIV among people diagnosed with schizophrenia varied appreciably across MSAs with a standard deviation of 1.31%. Comorbid substance abuse among beneficiaries with schizophrenia was common (mean = 21.00%). Table 1 provides a list of variables used for the multivariate analysis for the 44 communities.
Table 2 shows OLS regressions predicting treated prevalence of HIV infection among adult Medicaid beneficiaries diagnosed with schizophrenia in the 44 communities. In model 1, we observed the anticipated strong association between HIV rates among people with schizophrenia and overall rates of AIDS in the community (our proxy for overall HIV/AIDS rates). An increase of one percentage point in the AIDS rate was associated with a 2.989 (standard error [SE] = 0.303) percentage point increase in treated prevalence of HIV among people diagnosed with schizophrenia. In model 2, we added the HIV rate among IDUs as an explanatory variable. Results for this model indicated that even when overall community rates of HIV/AIDS were taken into account, the more specific measure of HIV infection rate among IDUs was strongly predictive of HIV infection among Medicaid beneficiaries with schizophrenia. A one percentage point increase in the HIV rate among IDUs resulted in a 0.139 percentage point increase in the HIV rate among people with schizophrenia (SE=0.024). Controlling for the HIV rate among IDUs reduced the strength of the association between MSA AIDS rates and MSA HIV rates among people with schizophrenia. (In model 1, the coefficient was 2.989 [SE=0.303], which was reduced to 1.459 [SE=0.349] in model 2, and 1.513 [SE=0.518] when other covariates were added in model 3.) Model 2 results indicated that together, the overall community AIDS rate and the rate of HIV among IDUs explained 83% of the variance in the MSA-level HIV infection rate among people with schizophrenia.
This association between HIV rates among IDUs and HIV rates among those with schizophrenia was robust when we controlled for other environmental characteristics (Model 3). The effect of the HIV infection rate among IDUs was only slightly reduced from Model 2, with a one percentage point increase in the HIV infection rate among IDUs resulting in a 0.130 percentage point (SE=0.031) increase in treated prevalence of HIV among people with schizophrenia. In Model 3, a one percentage point increase in overall AIDS rates was associated with a 1.489 percentage point (SE=0.762) increase in treated prevalence of HIV among people with schizophrenia. With two exceptions, other covariates were not associated with the outcome. These exceptions were (1) the community-level rates of comorbid substance abuse among beneficiaries diagnosed with schizophrenia (beta coefficient = 0.034, SE=0.017) and (2) the percentage of substance abuse treatment centers offering HIV testing in the MSA. In MSAs where the proportion of substance abuse treatment centers that offer HIV testing was higher, the prevalence of HIV among people diagnosed with schizophrenia was also higher. This association might reflect the fact that the presence of HIV testing sites in substance abuse treatment centers helps to more effectively identify HIV among people with schizophrenia, or this association might have resulted from the impact of unmeasured MSA characteristics on both elevated HIV prevalence among people with schizophrenia in an MSA and MSA-level HIV testing policies and practices.
The sensitivity analysis confirmed our findings, with both the OLS (Table 2) and the model in which the dependent variable was transformed with the logit function (Table 3) revealing similar results.
DISCUSSION
These analyses were intended to explore the potential value of data sources and a research strategy for clarifying the contribution of contextual and social influences on patterns of infectious disease spread among people with SMI. Applying existing research tools to Medicaid data from eight states, we found that rates of HIV among patients with schizophrenia differed sharply across MSAs.
Not surprisingly, it appears that one important source of the variability of HIV prevalence among people with schizophrenia was the underlying variability in prevalence of HIV across the MSAs. As suggested by findings in the literature, we found an association between an MSA's estimated rate of HIV among IDUs within the MSA and the MSA-level treated prevalence rates for Medicaid patients diagnosed with schizophrenia, even after controlling for underlying AIDS prevalence. This association was not eliminated after controlling for a range of environmental covariates. Multiple MSA-level predictors suggested by the -literature (e.g., crime rates and poverty) did not appear to contribute to an explanation of variation in the treated prevalence of HIV among people with schizophrenia over and above the association with HIV among IDUs and HIV in the general population.
There have been some efforts to explain geographical variation in HIV. One study found higher rates of HIV among IDUs in East Coast vs. West Coast cities, but found that this difference could not be adequately explained by differences in self-reported injection or sexual practices.71 Pioneering work by Holmberg calculated figures for HIV incidence and prevalence, and sizes of populations at risk, for 96 MSAs in the early 1990s.72 More recently, the National Development and Research Institutes group has produced a body of work examining associations between various social and structural characteristics and variations in local HIV epidemic characteristics among IDUs. Community-level associations have been found between HIV prevalence among IDUs and the presence of laws against over-the-counter purchasing of syringes and income inequality.73 The population density of IDUs in metropolitan areas may correlate with poverty rates or other indicators of socioeconomic distress and/or laws limiting syringe access in a locality.74 It seems possible that differences in MSA-level approaches to the medical, psychiatric, and social welfare needs of people with SMI will influence the risk environment they encounter and, ultimately, incidence and prevalence of HIV. Future research using ethnographic and social network methods can examine the nature and extent of overlap and contact between IDUs and people with SMI. Given the evidence for possible linkages between drug use and sexual risk,31 it may be valuable to extend work on existing sexual networks of people with SMI.75,76
Additional quantitative comparisons across MSAs are needed but will be challenging. Prior research, particularly in the 1990s, demonstrated that it is feasible to estimate rates of SMI, including schizophrenia, as well as major mood disorders among both in-treatment and out-of-treatment IDUs.77–80 These studies suggest that ongoing research with IDUs, including work with populations using syringe-exchange programs, can be an important potential source of information on major psychiatric illness and HIV dynamics, particularly when they permit comparisons across multiple MSAs.
Additional sources of data may increase the explanatory power of models. Data sources with the potential to provide additional insights into the co-occurrence of SMI and HIV may include Medicare and private insurance files, data from CARE Act81-funded programs such as the AIDS Drug Assistance Programs,82 and possibly enhanced and/or linked HIV/AIDS surveillance data. MSAs may also differ in the proportion of the -population with an SMI that spends some time in prison or local jails, which may pose HIV risk, suggesting that estimates of these figures may hold promise.
Heuristically, research in this area can benefit from examination of the ways in which the epidemic of HIV among people with SMI is embedded in local epidemics and reflects their dynamics, as well as the distinctive features of psychiatric illnesses. Much research has understandably focused on identifying and describing this group; assessing its needs; adapting clinical practices; and developing, testing, and diffusing psychosocial programs able to accommodate its distinctive needs.83 However, searching for ways in which people with SMI do not differ from others can also call attention to fruitful areas of investigation. For example, multiple studies have already found that contrary to early expectations, people with SMI can often achieve levels of antiretroviral therapy adherence at least as good as others with HIV.56,57,84,85 Similarly, it may be the case that community-level changes relevant to HIV prevention have a significant impact on those with SMI. For example, declines in prevalence rates of HIV among IDUs in a number of cities that have been noted by some authors61,86,87 may have implications for HIV incidence among people with SMI. Generally, our results suggest that harm-reduction and other policies that address the spread of HIV among drug abusers may not only affect those people with SMI who abuse drugs, but also serve as effective strategies for limiting new infections in other “downstream” populations who may interact with drug-abusing populations, such as people with SMI.
Limitations
In evaluating the suitability of Medicaid claims for examination of SMI morbidity/mortality issues, it is important to be aware of both the general limitations of claims-based research, as well as the specific limitations of our study. Regarding general limitations, our impression is that although claims-based research was once viewed with intense suspicion, its potential is now more widely recognized. However, this new acceptance should not tempt researchers or policy makers to oversell what can be accomplished. Diagnoses found in administrative claims are of uneven quality, although most research finds high rates of concordance with chart diagnosis for more severe disorders such as schizophrenia; some studies have reported 100% agreement.52
While psychiatric diagnoses based on standardized interviews typically produce high-quality diagnoses, clinicians are aware that diagnoses based on these standardized interviews do not possess every advantage over the provider-based diagnoses found in medical charts and administrative claims. Often, the treating clinician has observed the patient over time, knows relatives and other providers who can give clinically important information, and is able to solicit information from a patient based on a treatment alliance, which can motivate disclosures not provided in a paid interview.
Claims provide no information about undiagnosed conditions, so interpretations must gauge the scope of missed cases. In one of the few studies of its kind, we estimated concordance between claims indicative of HIV and state HIV and AIDS registry information.57 We found sensitivity was generally high, but particularly so in groups that use services frequently. While we suspect that this is a good rule of thumb, further research on validity issues is needed.
A further significant limitation was dependence on the availability of additional data sources. We were fortunate to be able to rely on the publications by Tempalski and colleagues61 of their high-quality, carefully constructed estimates of MSA-level rates of HIV among IDUs. Had these not been available, impressions regarding links between MSA-level rates of HIV among IDUs and HIV among people with SMI could not have been explored empirically.
Our findings may also have implications for an expanded view of the role of research development and diffusion. To date, much research on HIV infection rates among people with SMI has been conducted in major medical centers in urban areas and has performed a vital sentinel function, calling the attention of local providers to the emerging, overlooked clinical and public health challenge of HIV among people with SMI, and spurring the development and diffusion of prevention-focused technologies to local communities. But improved understanding and effective response require strengthening the information and influence flow from multiple local communities to the research enterprise and developing community- and provider-based partnerships able to contextualize local disease risks.
CONCLUSIONS
Numerous studies have documented that HIV risk behaviors and HIV rates are elevated in some groups of people with SMI, such as those with schizophrenia or bipolar disorder. Our findings suggest that, even among those who are impoverished and rely on Medicaid, HIV infection rates among patients with schizophrenia vary considerably from one location to another. Program planning and delivery of interventions and services can benefit from considering the risk for people with schizophrenia in the context of local patterns of HIV infection. Although universal education on HIV risks has many benefits, it is also important to consider the very different risks existing in different communities in prioritizing the use of limited resources for HIV prevention efforts in populations at risk, including those with SMI.
Evidence that HIV risks among people living with severe psychiatric illness are closely linked to local epidemiologic patterns of HIV among IDUs underscores the need for information more closely linked to real time, which can inform prevention initiatives for both populations. Properly used, Medicaid claims data may provide timely findings needed by local and state policy makers and planners, if state health agencies are able to collaborate with their state's Medicaid programs to utilize state data for this purpose on a relatively current basis.
Linkages between subgroup epidemics suggest that interventions aimed at reducing HIV spread among IDUs, including harm-reduction interventions such as syringe-exchange programs, may be one of the best ways of protecting other vulnerable groups such as those with SMI. Counseling and behavior change technologies adapted for people with SMI are valuable but labor intensive, and so may have their greatest impact if they are targeted to geographical areas where risks are higher, rather than being spread out thinly across the whole population. A range of methods, including seroprevalence studies, ethnographic studies, surveys, and tracking of administrative datasets, should be used to identify locally specific transmission links between drug-using and SMI communities (e.g., co-location of these communities in downtown single-room--occupancy hotels with few services and active drug use) to inform intervention.
More broadly, important learning can result from these research efforts, although many of the most significant results may be specific to the challenges of HIV or infectious diseases. Other types of data and analytic strategies may need to be adapted to conduct equivalent research on, for example, the impact of dietary risks on the health of people with SMI. We do believe, however, that a focus on geographical variation can be a powerful analytic approach for many aspects of morbidity and mortality among this population.
Footnotes
This work was supported by the National Institute of Mental Health (NIMH) (R01 MH076206), with additional support from the Agency for Healthcare Research and Quality (AHRQ) through a cooperative agreement for the Center for Research and Education on Mental Health Therapeutics at Rutgers (U18HS016097), as part of AHRQ's Centers for Education and Research on Therapeutics program.
The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of NIMH or AHRQ.
REFERENCES
- 1.Parks J, Svendsen D, Singer P, Foti ME. Morbidity and mortality in people with serious mental illness. Thirteenth in a series of technical reports. Alexandria (VA): National Association of State Mental Health Program Directors; 2006. [Google Scholar]
- 2.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- 3.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–31. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 4.Weber NS, Cowan DN, Millikan AM, Niebuhr DW. Psychiatric and general medical conditions comorbid with schizophrenia in the National Hospital Discharge Survey. Psychiatr Serv. 2009;60:1059–67. doi: 10.1176/ps.2009.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 5.von Hausswolff-Juhlin Y, Bjartveit M, Lindstrom E, Jones P. Schizophrenia and physical health problems. Acta Psychiatr Scand Suppl. 2009;438:15–21. doi: 10.1111/j.1600-0447.2008.01309.x. [DOI] [PubMed] [Google Scholar]
- 6.Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health. 2006;27:167–94. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- 7.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–52. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 8.Blank MB, Mandell DS, Aiken L, Hadley TR. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53:868–73. doi: 10.1176/appi.ps.53.7.868. [DOI] [PubMed] [Google Scholar]
- 9.Crystal S, Akincigil A, Bilder S, Walkup JT. Studying prescription drug use and outcomes with Medicaid claims data: strengths, limitations, and strategies. Med Care. 2007;45(10 Suppl 2):S58–65. doi: 10.1097/MLR.0b013e31805371bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoover DR, Sambamoorthi U, Walkup JT, Crystal S. Mental illness and length of inpatient stay for Medicaid recipients with AIDS. Health Serv Res. 2004;39:1319–39. doi: 10.1111/j.1475-6773.2004.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walkup J, Wei W, Sambamoorthi U, Crystal S. Antidepressant treatment and adherence to combination antiretroviral therapy among patients with AIDS and diagnosed depression. Psychiatr Q. 2008;79:43–53. doi: 10.1007/s11126-007-9055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkup J, Crystal S, Sambamoorthi U. Schizophrenia and major affective disorder among Medicaid recipients with HIV/AIDS in New Jersey. Am J Public Health. 1999;89:1101–3. doi: 10.2105/ajph.89.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakashima AK, Fleming PL. HIV/AIDS surveillance in the United States, 1981–2001. J Acquir Immune Defic Syndr. 2003;32(Suppl 1):S68–85. [PubMed] [Google Scholar]
- 14.Sullivan PS, McKenna MT, Janssen RS. Progress toward implementation of integrated systems for surveillance of HIV infection and morbidity in the United States. Public Health Rep. 2007;122(Suppl 1):1–3. doi: 10.1177/00333549071220S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNaghten AD, Wolfe MI, Onorato I, Nakashima AK, Valdiserri RO, Mokotoff E, et al. Improving the representativeness of behavioral and clinical surveillance for persons with HIV in the United States: the rationale for developing a population-based approach. PLoS One. 2007;2:e550. doi: 10.1371/journal.pone.0000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes WC, Bix B, Meritz M, Turner J, Hutelmyer C. Human immunodeficiency virus (HIV) infection and quality of life: the potential impact of Axis I psychiatric disorders in a sample of 95 HIV seropositive men. Psychosom Med. 1997;59:187–92. doi: 10.1097/00006842-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Williams JB, Rabkin JG, Remien RH, Gorman JM, Ehrhardt AA. Multidisciplinary baseline assessment of homosexual men with and without human immunodeficiency virus infection: II. Standardized clinical assessment of current and lifetime psychopathology. Arch Gen Psychiatry. 1991;48:124–30. doi: 10.1001/archpsyc.1991.01810260032005. [DOI] [PubMed] [Google Scholar]
- 18.Cournos F, McKinnon K. HIV seroprevalence among people with severe mental illness in the United States: a critical review. Clin Psychol Rev. 1997;17:259–69. doi: 10.1016/s0272-7358(97)00018-4. [DOI] [PubMed] [Google Scholar]
- 19.Carey MP, Cournos F. HIV and AIDS among the severely mentally ill: introduction to the special series. Clin Psychol Rev. 1997;17:241–5. doi: 10.1016/s0272-7358(97)00016-0. [DOI] [PubMed] [Google Scholar]
- 20.McKinnon K, Carey MP, Cournos F. Research on HIV, AIDS, and severe mental illness: recommendations from the NIMH National Conference. Clin Psychol Rev. 1997;17:327–31. doi: 10.1016/s0272-7358(97)00022-6. [DOI] [PubMed] [Google Scholar]
- 21.Beyer JL, Taylor L, Gersing KR, Krishnan KR. Prevalence of HIV infection in a general psychiatric outpatient population. Psychosomatics. 2007;48:31–7. doi: 10.1176/appi.psy.48.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SD, Goodman LA, Osher FC, Swartz MS, Essock SM, Butterfield MI, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91:31–7. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himelhoch S, McCarthy JF, Ganoczy D, Medoff D, Dixon LB, Blow FC. Understanding associations between serious mental illness and HIV among patients in the VA Health System. Psychiatr Serv. 2007;58:1165–72. doi: 10.1176/ps.2007.58.9.1165. [DOI] [PubMed] [Google Scholar]
- 24.Blank MB, Mandell DS, Aiken L, Hadley TR. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53:868–73. doi: 10.1176/appi.ps.53.7.868. [DOI] [PubMed] [Google Scholar]
- 25.Stoskopf CH, Kim YK, Glover SH. Dual diagnosis: HIV and mental illness, a population-based study. Community Ment Health J. 2001;37:469–79. doi: 10.1023/a:1017577827658. [DOI] [PubMed] [Google Scholar]
- 26.Carey MP, Carey KB, Kalichman SC. Risk for human immunodeficiency virus (HIV) infection among persons with severe mental illnesses. Clin Psychol Rev. 1997;17:271–91. doi: 10.1016/s0272-7358(97)00019-6. [DOI] [PubMed] [Google Scholar]
- 27.Carey MP, Carey KB, Maisto SA, Gleason JR, Gordon CM, Brewer KK. HIV risk behavior among outpatients at a state psychiatric hospital: prevalence and risk modeling. Behavior Ther. 1999;30:389–406. doi: 10.1016/S0005-7894(99)80017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinnon K, Cournos F, Herman R. A lifetime alcohol or other drug use disorder and specific psychiatric symptoms predict sexual risk for HIV infection among people with severe mental illness. AIDS Behav. 2001;5:233–40. [Google Scholar]
- 29.Meade CS, Sikkema KJ. Psychiatric and psychosocial correlates of sexual risk behavior among adults with severe mental illness. Community Ment Health J. 2007;43:153–69. doi: 10.1007/s10597-006-9071-6. [DOI] [PubMed] [Google Scholar]
- 30.Gottesman II, Groome CS. HIV/AIDS risks as a consequence of schizophrenia. Schizophr Bull. 1997;23:675–84. doi: 10.1093/schbul/23.4.675. [DOI] [PubMed] [Google Scholar]
- 31.Meade CS, Weiss RD. Substance abuse as a risk factor for HIV sexual risk behavior among persons with severe mental illness: review of evidence and exploration of mechanisms. Clin Psychol. 2007;14:23–33. [Google Scholar]
- 32.Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev. 2005;25:433–57. doi: 10.1016/j.cpr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Tucker JS, Kanouse DE, Miu A, Koegel P, Sullivan G. HIV risk behaviors and their correlates among HIV-positive adults with serious mental illness. AIDS Behav. 2003;7:29–40. doi: 10.1023/a:1022557222690. [DOI] [PubMed] [Google Scholar]
- 34.Kelly JA. HIV risk reduction interventions for persons with severe mental illness. Clin Psychol Rev. 1997;17:293–309. doi: 10.1016/s0272-7358(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 35.Berkman A, Pilowsky DJ, Zybert PA, Herman DB, Conover S, Lemelle S, et al. HIV prevention with severely mentally ill men: a randomised controlled trial. AIDS Care. 2007;19:579–88. doi: 10.1080/09540120701213989. [DOI] [PubMed] [Google Scholar]
- 36.Castor D, Pilowsky DJ, Hadden B, Fuller C, Ompad DC, de Leon CL, et al. Sexual risk reduction among non-injection drug users: report of a randomized controlled trial. AIDS Care. 2010;22:62–70. doi: 10.1080/09540120903012510. [DOI] [PubMed] [Google Scholar]
- 37.Berkman A, Pilowsky DJ, Zybert PA, Leu CS, Sohler N, Susser E. The impact of substance dependence on HIV sexual risk-reduction among men with severe mental illness. AIDS Care. 2005;17:635–9. doi: 10.1080/09540120412331291797. [DOI] [PubMed] [Google Scholar]
- 38.Carey MP, Carey KB, Maisto SA, Schroder KE, Vanable PA, Gordon CM. HIV risk behavior among psychiatric outpatients: association with psychiatric disorder, substance use disorder, and gender. J Nerv Ment Dis. 2004;192:289–96. doi: 10.1097/01.nmd.0000120888.45094.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carey MP, Carey KB, Kalichman SC. Risk for human immunodeficiency virus (HIV) infection among persons with severe mental illnesses. Clin Psychol Rev. 1997;17:271–91. doi: 10.1016/s0272-7358(97)00019-6. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg SD, Trumbetta SL, Mueser KT, Goodman LA, Osher FC, Vidaver RM, et al. Determinants of risk behavior for human immunodeficiency virus/acquired immunodeficiency syndrome in people with severe mental illness. Compr Psychiatry. 2001;42:263–71. doi: 10.1053/comp.2001.24576. [DOI] [PubMed] [Google Scholar]
- 41.Krakow DS, Galanter M, Dermatis H, Westreich LM. HIV risk factors in dually diagnosed patients. Am J Addict. 1998;7:74–80. [PubMed] [Google Scholar]
- 42.Silberstein C, Galanter M, Marmor M, Lifshutz H, Krasinski K, Franco H. HIV-1 among inner city dually diagnosed inpatients. Am J Drug Alcohol Abuse. 1994;20:101–13. doi: 10.3109/00952999409084060. [DOI] [PubMed] [Google Scholar]
- 43.Frank RG, McGuire TG, Regier DA, Manderscheid R, Woodward A. Paying for mental health and substance abuse care. Health Aff (Millwood) 1994;13:337–42. doi: 10.1377/hlthaff.13.1.337. [DOI] [PubMed] [Google Scholar]
- 44.Mark TL, Coffey RM, Vandivort-Warren R, Harwood HJ, King EC. MHSA Spending Estimates Team. U.S. spending for mental health and substance abuse treatment, 1991–2001. Health Aff (Millwood) 2005;(Suppl Web Exclusives):W5-133–W5-142. doi: 10.1377/hlthaff.w5.133. [DOI] [PubMed] [Google Scholar]
- 45.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61:1026–44. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention (US). HIV/AIDS surveillance report, 2001. Vol. 13. Atlanta: CDC; 2001. [Google Scholar]
- 47.Susser E, Miller M, Valencia E, Colson P, Roche B, Conover S. Injection drug use and risk of HIV transmission among homeless men with mental illness. Am J Psychiatry. 1996;153:794–8. doi: 10.1176/ajp.153.6.794. [DOI] [PubMed] [Google Scholar]
- 48.Roberts ET, Friedman SR, Brady JE, Pouget ER, Tempalski B, Galea S. Environmental conditions, political economy, and rates of injection drug use in large US metropolitan areas 1992–2002. Drug Alcohol Depend. 2010;106:142–53. doi: 10.1016/j.drugalcdep.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tempalski B, McQuie H. Drugscapes and the role of place and space in injection drug use-related HIV risk environments. Int J Drug Policy. 2009;20:4–13. doi: 10.1016/j.drugpo.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Department of Health and Human Services (US), Centers for Medicare and Medicaid Services. Medicaid Analytic eXtract (MAX) general information. [cited 2008 Jan 10]. Available from: URL: http://www.resdac.org/Medicaid/data_available.asp.
- 51.Lurie N, Popkin M, Dysken M, Moscovice I, Finch M. Accuracy of diagnoses of schizophrenia in Medicaid claims. Hosp Community Psychiatry. 1992;43:69–71. doi: 10.1176/ps.43.1.69. [DOI] [PubMed] [Google Scholar]
- 52.Walkup JT, Boyer CA, Kellermann SL. Reliability of Medicaid claims files for use in psychiatric diagnoses and service delivery. Adm Policy Ment Health. 2000;27:129–39. doi: 10.1023/a:1021308007343. [DOI] [PubMed] [Google Scholar]
- 53.Bagchi A, Sambamoorthi U, McSpiritt E, Yanos P, Walkup J, Crystal S. Use of antipsychotic medications among HIV-infected individuals with schizophrenia. Schizophr Res. 2004;71:435–44. doi: 10.1016/j.schres.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Sambamoorthi U, Walkup J, McSpiritt E, Warner L, Castle N, Crystal S. Racial differences in end-of-life care for patients with AIDS. AIDS Public Policy J. 2000;15:136–48. [PubMed] [Google Scholar]
- 55.Walkup J, Akincigil A, Bilder S, Rosato NS, Crystal S. Psychiatric diagnosis and antiretroviral adherence among adolescent Medicaid beneficiaries diagnosed with human immunodeficiency virus/acquired immunodeficiency syndrome. J Nerv Ment Dis. 2009;197:354–61. doi: 10.1097/NMD.0b013e3181a208af. [DOI] [PubMed] [Google Scholar]
- 56.Walkup J, Sambamoorthi U, Crystal S. Incidence and consistency of antiretroviral use among HIV-infected Medicaid beneficiaries with schizophrenia. J Clin Psychiatry. 2001;62:174–8. doi: 10.4088/jcp.v62n0307. [DOI] [PubMed] [Google Scholar]
- 57.Walkup JT, Wei W, Sambamoorthi U, Crystal S. Sensitivity of an AIDS case-finding algorithm: who are we missing? Med Care. 2004;42:756–63. doi: 10.1097/01.mlr.0000132749.20897.46. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention (US). AIDS public information data set [manual] [cited 2009 Aug 26]. Available from: URL: http://www.cdc.gov/hiv/topics/surveillance/resources/software/apids/manual/pdf/pids2002.pdf.
- 59.Centers for Disease Control and Prevention (US). [cited 2009 Dec 18];HIV/AIDS surveillance report. 2003 Vol. 15 Available from: URL: http://www.cdc.gov/hiv/stats/hasrlink.htm. [Google Scholar]
- 60.Census Bureau (US). American FactFinder. [cited 2008 Apr 3]. Available from: URL: http://factfinder.census.gov/home/saff/main.html?_lang=en.
- 61.Tempalski B, Lieb S, Cleland CM, Cooper H, Brady JE, Friedman SR. HIV prevalence rates among injection drug users in 96 large US metropolitan areas, 1992–2002. J Urban Health. 2009;86:132–54. doi: 10.1007/s11524-008-9328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Department of Housing and Urban Development (US). State of the cities data systems (SOCDS) [cited 2009 Aug 27]. Available from: URL: http://socds.huduser.org/SOCDS_Home.htm.
- 63.Department of Justice (US), Federal Bureau of Investigation. Uniform Crime Reporting program data [United States]: county-level detailed arrest and offense data. Washington: Department of Justice, FBI; 2009. [Google Scholar]
- 64.Department of Health and Human Services (US), Substance Abuse and Mental Health Services Administration. DASIS [Drug & Alcohol Services Information System]. National Survey of Substance Abuse Treatment Services (N-SSATS) [cited 2008 Feb 1]. Available from: URL: http://wwwdasis.samhsa.gov/dasis2/nssats.htm.
- 65.Brady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the U.S. and in large U.S. metropolitan areas from 1992 to 2002. J Urban Health. 2008;85:323–51. doi: 10.1007/s11524-007-9248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312–23. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krieger N, Waterman PD, Chen JT, Soobader MJ, Subramanian SV. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures—the Public Health Disparities Geocoding Project (US) Public Health Rep. 2003;118:240–60. doi: 10.1093/phr/118.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 69.SAS Institute, Inc. SAS®: Version 9.2. Cary (NC): SAS Institute, Inc.; 2008. [Google Scholar]
- 70.StataCorp LP. Stata®: Version 9.2. College Station (TX): StataCorp LP; 2006. [Google Scholar]
- 71.Garfein RS, Monterroso ER, Tong TC, Vlahov D, Des Jarlais DC, Selwyn P, et al. Comparison of HIV infection risk behaviors among injection drug users from East and West Coast US cities. J Urban Health. 2004;81:260–7. doi: 10.1093/jurban/jth112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holmberg SD. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86:642–54. doi: 10.2105/ajph.86.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedman SR, Lieb S, Tempalski B, Cooper H, Keem M, Friedman R, et al. HIV among injection drug users in large US metropolitan areas, 1998. J Urban Health. 2005;82:434–45. doi: 10.1093/jurban/jti088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedman SR, Tempalski B, Cooper H, Perlis T, Keem M, Friedman R, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004;81:377–400. doi: 10.1093/jurban/jth125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perry BL, Wright ER. The sexual partnerships of people with serious mental illness. J Sex Res. 2006;43:174–81. doi: 10.1080/00224490609552312. [DOI] [PubMed] [Google Scholar]
- 76.Wright ER, Wright DE, Perry BL, Foote-Ardah CE. Stigma and the sexual isolation of people with serious mental illness. Soc Problems. 2007;54:78–98. [Google Scholar]
- 77.Dinwiddie SH, Cottler L, Compton W, Abdallah AB. Psychopathology and HIV risk behaviors among injection drug users in and out of treatment. Drug Alcohol Depend. 1996;43:1–11. doi: 10.1016/s0376-8716(96)01290-2. [DOI] [PubMed] [Google Scholar]
- 78.Lipsitz JD, Williams JB, Rabkin JG, Remien RH, Bradbury M, el Sadr W, et al. Psychopathology in male and female intravenous drug users with and without HIV infection. Am J Psychiatry. 1994;151:1662–8. doi: 10.1176/ajp.151.11.1662. [DOI] [PubMed] [Google Scholar]
- 79.Rabkin JG, Johnson J, Lin SH, Lipsitz JD, Remien RH, Williams JB, et al. Psychopathology in male and female HIV-positive and negative injecting drug users: longitudinal course over three years. AIDS. 1997;11:507–15. doi: 10.1097/00002030-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 80.Kidorf M, Disney ER, King VL, Neufeld K, Beilenson PL, Brooner RK. Prevalence of psychiatric and substance use disorders in opioid abusers in a community syringe exchange program. Drug Alcohol Depend. 2004;74:115–22. doi: 10.1016/j.drugalcdep.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 81.1990. Ryan White Comprehensive AIDS Resources Emergency (CARE) Act. Pub. L. No. 101-381, 104 Stat. 576.
- 82.The Henry J. Kaiser Family Foundation. HIV/AIDS policy fact sheet: AIDS Drug Assistance Programs (ADAPs), April 2008. [cited 2010 Jun 1]. Available from: URL: http://www.kff.org/hivaids/upload/1584_09.pdf.
- 83.Walkup JT, Crystal S. Mental health and the changing contexts of HIV. In: Scheid TL, Brown TN, editors. A handbook for the study of mental health: social contexts, theories, and systems. 2nd ed. New York: Cambridge University Press; 2009. pp. 548–70. [Google Scholar]
- 84.Wagner GJ, Kanouse DE, Koegel P, Sullivan G. Adherence to HIV antiretrovirals among persons with serious mental illness. AIDS Patient Care STDS. 2003;17:179–86. doi: 10.1089/108729103321619782. [DOI] [PubMed] [Google Scholar]
- 85.Wagner GJ, Kanouse DE, Koegel P, Sullivan G. Correlates of HIV antiretroviral adherence in persons with serious mental illness. AIDS Care. 2004;16:501–6. doi: 10.1080/09540120410001683420. [DOI] [PubMed] [Google Scholar]
- 86.Des Jarlais DC, Arasteh K, Perlis T, Hagan H, Abdul-Quader A, Heckathorn DD, et al. Convergence of HIV seroprevalence among injecting and non-injecting drug users in New York City. AIDS. 2007;21:231–5. doi: 10.1097/QAD.0b013e3280114a15. [DOI] [PubMed] [Google Scholar]
- 87.Des Jarlais DC, Perlis T, Arasteh K, Hagan H, Milliken J, Braine N, et al. “Informed altruism” and “partner restriction” in the reduction of HIV infection in injecting drug users entering detoxification treatment in New York City, 1990–2001. J Acquir Immune Defic Syndr. 2004;35:158–66. doi: 10.1097/00126334-200402010-00010. [DOI] [PubMed] [Google Scholar]