Abstract
Background
Gastro-oesophageal reflux disease (GERD) is a common chronic condition effectively treated in most patients with proton pump inhibitors (PPI) or H2-receptor antagonists (H2RA). There appears to be a significant placebo response rate in clinical trials for GERD. Despite this, little is known about the determinants and the circumstances associated with placebo response in the treatment of GERD.
Aim
The purpose of this study is to estimate the magnitude of the placebo response rate in randomized controlled trials (RCTs) for GERD and to identify factors that influence this response.
Methods
We performed a meta-analysis of published, English language, randomized, double-blind, placebo-controlled trials that included 20 or more patients with GERD who were treated with either a proton-pump inhibitor or H2-receptor antagonist for at least 2 weeks. Medline, Cochrane and EMBASE databases were searched. We considered only studies that reported a global response for ‘heartburn’. Eligible studies were synthesized using meta-analysis methods, including cumulative meta-analysis. Heterogeneity and study quality issues were explored.
Results
24 studies were included with 9,989 total patients with GERD. The pooled odds ratio (OR) for response to active treatment versus placebo was 3.71 [95% CI: 2.78-4.96]. The pooled estimate of the overall placebo response was 18.85% [range 2.94% - 47.06%]. Patients with erosive esophagitis had a non-significantly lower placebo response rate than patients without it (11.87% and 18.31%, respectively; p = 0.246). Placebo response was significantly lower in studies of PPI therapy versus studies of H2-receptor antagonists (14.51% vs. 24.69%, respectively; p=0.05).
Conclusion
The placebo response rate in randomized controlled trials for GERD is substantial, although lower than that reported in other disorders of gastrointestinal function. A lower placebo response was associated with the testing of proton pump inhibitors, but not with the underlying presence of erosive esophagitis.
Keywords: GERD or GORD, Functional GI diseases, Acidity (oesophageal), Meta-analyses
Objectives
Placebo controls are considered the standard for evaluating the effectiveness of a therapeutic intervention in clinical trials. However, patients enrolled in the placebo arm of clinical trials often report a measurable improvement in symptoms. Factors such as the quality of patient-physician relationship 1,2, route of administration3,4,5, dosage6, frequency7,8 of the placebo and even the color and the size of the pill have been reported to affect patient responses. Proper understanding of the placebo response and the variables contributing to a placebo response can significantly improve to the designs of clinical trials by allowing better selection and classification of responders and by improving definition and differentiation of a true therapeutic effect of experimental treatments.
Recent research has begun to determine factors that influence the placebo response in gastrointestinal diseases. An analysis of the placebo effect in 79 trials investigating healing of duodenal ulcer demonstrated increased healing rates with administration of placebo four times daily when compared to administration of placebo twice daily7. Studies of inflammatory bowel disease reported more frequent office visits, longer duration of study, less strict definition of response, and more severe disease activity at entry of study as factors associated with higher placebo response rates9,10,11. In contrast, systematic analyses of trials investigating irritable bowel syndrome revealed lower placebo response rates in association with more frequent office visits, fulfillment of the Rome criteria, and less frequent interventions12,8. The placebo response in functional dyspepsia is lower in patients who symptom pattern does not fluctuate over time, and in those who have lower body mass index13. A large study of irritable bowel patients found that patient characteristics such as extroversion, agreeableness, and openness to new experience was associated with placebo response to an enhanced patient-physician relationship but not a business-like relationship14 and individuals with tendencies towards reclusiveness were especially sensitive to the enhanced relationship15.
The magnitude of the placebo response in clinical trials investigating acute treatment of gastroesophageal reflux disease (GERD) has not been systematically evaluated. Approximately 10-20% of adults report at least one episode of GERD per week, a figure consistent with roughly 40% of the general population monthly16. Medications, that suppress gastric acid secretion such as proton pump inhibitors (PPIs) and H2-receptor antagonists(H2RA), remain the mainstay of therapy. However, the placebo response rates reported in clinical trials vary considerably. This meta-analysis aims to determine the magnitude of the placebo response rate in randomized controlled trials (RCTs) investigating PPIs and H2-receptor antagonists for acute treatment of GERD and to identify the variables that influence the placebo response.
Methods
This meta-analysis was conducted and is presented in adherence with the QOROM statement recommendations17.
Search strategy
We conducted a comprehensive literature search of MEDLINE (1966-2009), Cochrane Controlled Trial Register (1997-2009), and EMBASE (1985-2009) databases. The search terms included combinations of the following keywords: GERD, NERD, gastro-oesophageal reflux disease, double blind, placebo controlled, trial, and randomized controlled trials (RCTs) as well as common drug names for the treatment of GERD, including esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, cimetidine, famotidine, nizatidine, ranitidine and dexlansoprazole. A manual search of the references listed by studies retrieved from the online databases, after abstract selection, was subsequently performed to identify additional studies of interest. The search was limited a priori to studies that were double-blinded, placebo-controlled, parallel group, RCTs of English language.
Selection of studies
In the current meta-analysis, we included studies with at least 20 subjects enrolled with duration of 2 weeks or longer, that aimed to investigate treatment of GERD in the acute setting, and which reported relief of heartburn as one of the primary outcomes. Specific inclusion criteria were the following: 1) explicit definition of “clinical response” of heartburn, 2) report of heartburn relief on the final day of the study, 3) report of the number of responders, 4) restriction of concomitant reflux medications during the trial (except rescue medications, excluding antisecretory drugs). The definition for relief of heartburn was heterogeneous among the studies and varied from “complete relief or resolution of 24-heartburn” to “sufficient or substantial control of heartburn” to “complete relief of upper GI symptoms”.
For reference purposes, we also performed a meta-analysis of placebo response rates of trials of endoscopic procedures in GERD. In such studies, the control group is typically treated with sham injection of the esophageal mucosa. The same outcome criteria as for the other studies were used for defining response.
Data extraction
Three investigators (DZ, HC, FC) independently extracted data. A fourth independent investigator (AJL) reviewed the data abstraction and resolved any discrepancies. The overall frequency of agreement was over 95%. Detail of the extracted data is presented in Table 2. Study variables were grouped in the following categories: study design, demographics, heartburn response, adverse events, and quality measures. The methodological quality of the included studies was assessed by the Jadad18 scoring system. Additional factors as the frequency of office visits, the frequency of treatment, and the duration of treatment, all factors that have been identified in earlier studies, as potential predictors of the placebo response in other gastrointestinal disorders9,11,12,7 were also investigated in this study. In order to avoid the inclusion of duplicated data that may lead to an overestimation of placebo effects in final analysis, retrieved studies were carefully appraised and examined by comparison of geographic locations, author names, and period of study, as discussed in the Cochrane Handbook for Systematic Reviews of Interventions19. If the doubt persisted that publications referred to the same or overlapping data sets, we then included the results from the study with the larger population.
Table 2. Characteristics of studies included in the meta-analysis.
| Author, year [reference] | Active study medication | Type of drug* | Definition of response | N of responders in active arms (N of patients in active arms) | N of placebo responders in placebo arm (N of patients in placebo) | Placebo Response Rate (+/-SD)$ | Jadad score | NERD or EE£ | Duration of active treatment (days) | Number of visits during active treatment | Frequency of treatment# | Length of run in period (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fass, 2009 34 | Dexlansoprazole MR | PPI | Complete resolution of heartburn at the end of the study | 318 (630) | 44 (317) | 14 (6.8-21.1) | 5 | NERD | 28 | 2 | QD | 0 |
| Uemura, 200835 | Omeprazole | PPI | Complete resolution of heartburn during the 4th week | 55 (189) | 11 (92) | 12 (5.3-18.6) | 3 | NERD | 28 | 1 | QD | 0 |
| Johnson, 200536 | Esomeprazole | PPI | Complete resolution of 24h-heartburn at the end of the study | 160 (429) | 11 (221) | 5.0 (2.1-7.8) | 5 | Both | 28 | 2 | QD | 0 |
| Kahrilas, 200537 | Rabeprazole | PPI | Complete relief of heartburn during the 4th week | 34 (126) | 4 (126) | 3.2 (0.1-6.2) | 5 | NERD | 28 | 4 | QD | 14 |
| Katz, 200338 | Esomeprazole | PPI | Complete resolution of heartburn at the end of the study | 172 (475) | 31 (242) | 12.8 (8.6-17.0) | 5 | NERD | 28 | 2 | QD | 0 |
| Johnson, 200339 | Esomeprazole | PPI | Complete relief of heartburn symptoms at the 2nd visit | 235 (347) | 15 (92) | 16.3 (10.1-25.3) | 3 | Both | 14 | 2 | QD | 3 |
| Laheij, 200340 | Rabeprazole | PPI | Complete relief of upper GI symptoms at the end of the study | 34 (73) | 30 (70) | 42.9 (31.8-54.6) | 5 | Both | 28 | 1 | QD | 0 |
| Miner, 200241 | Rabeprazole | PPI | Complete relief of heartburn during the 4th week | 38 (131) | 2 (68) | 2.9 (0.7-11.0) | 3 | NERD | 28 | 3 | QD | 14 |
| Richter, 200042 | Pantoprazole | PPI | Persistently free of all GERD-associated symptoms at the end of the study | 289 (521) | 13 (82) | 16 (9.7-26.2) | 3 | EE | 56 | 4 | QD | 0 |
| Richter, 200043 | Omeprazole | PPI | Complete resolution of heartburn at the last day of the study | 145 (236) | 28 (123) | 22.8 (16.2-31.0) | 3 | NERD | 28 | 2 | QD | 0 |
| Carlson, 199844 | Omeprazole | PPI | Complete relief from upper GI symptoms during 4th week | 172 (450) | 17 (88) | 19.3 (11.1-27.6) | 3 | NERD | 28 | 2 | QD | 0 |
| Cloud, 199845 | Rabeprazole | PPI | Complete resolution of upper GI symptoms at the end of the study | 44 (77) | 1 (25) | 4.0 (0.6-23.5) | 2 | EE | 56 | 3 | QD | 0 |
| Galmiche, 199846 | Ranitidine (arm 1); Cimetidine (arm 2) | H2RA | 75% relief of heartburn at the end of the study | 403 (1019) | 77 (270) | 28.5 (22.6-34.5) | 3 | NERD | 15 | 2 | On Demand | 0 |
| Hallerback, 199847 | Ranitidine | H2RA | Complete resolution of upper GI symptoms at the end of the study | 86 (210) | 68 (213) | 31.9 (26.0- 38.5) | 3 | Both | 14 | 2 | BID | 14 |
| Lind, 199748 | Omeprazole | PPI | Complete absence of heartburn at the end of the study | 156 (404) | 14 (105) | 13.3 (6.8-19.8) | 5 | NERD | 28 | 2 | QD | 0 |
| Bate, 199649 | Omeprazole | PPI | Complete relief from upper GI symptoms during the 4th week | 56 (98) | 21 (111) | 18.9 (11.6-26.2) | 3 | NERD | 28 | 1 | QD | 0 |
| Rush, 199550 | Ranitidine | H2RA | Complete relief of heartburn at the end of the study | 193 (301) | 136 (289) | 47.1 (41.4-52.8) | 5 | NERD | 42 | 3 | BID | 0 |
| Simon, 199451 | Famotidine | H2RA | Sustained relief of heartburn at the end of the study | 81 (230) | 6 (58) | 10.3 (4.7-21.2) | 4 | EE | 84 | 2 | BID | 0 |
| Sabesin, 199152 | Famotidine | H2RA | Global relief of GERD symptoms (moderate or excellent improvement) at the end of the study | 191 (272) | 31 (66) | 46.9 (40.3-53.4) | 4 | Both | 84 | 3 | QHS BID |
0 |
| Riemann, 199153 | Cimetidine | H2RA | Complete relief of heartburn symptoms at the end of the study | 27 (60) | 12 (65) | 18.5 (9.0-27.9) | 4 | NERD | 14 | 2 | QID | 5 |
| Robinsen, 199154 | Famotidine | H2RA | Complete disappearance (free) of symptoms at the end of the study | 110 (313) | 19 (76) | 25.0 (15.3-34.7) | 2 | NERD | 42 | 2 | QHS BID |
7 |
| Quik, 199055 | Nizatidine | H2RA | Complete disappearance (free) of symptoms at the end of the study | 47 (218) | 16 (107) | 15.0 (8.2-21.7) | 4 | EE | 84 | 4 | QHS BID |
0 |
| Sontag, 198756 | Ranitidine | H2RA | Complete remission of 24h-heartburn at the end of the study | 38 (119) | 14 (118) | 11.9 (7.2-19.0) | 3 | Both | 42 | 6 | BID | 7 |
| Wesdorp, 198357 | Ranitidine | H2RA | Complete relief of heartburn at the end of the study | 6 (19) | 2 (17) | 11.8 (3.0-36.8) | 3 | EE | 42 | 2 | BID | 0 |
PPI: proton pump inhibitor; H2RA=H2 receptor antagonist.
QD: once daily; BID: twice daily; QID: four times daily; QHS: once at bedtime.
NERD: Non-erosive reflux disease, EE: Erosive esophagitis; GI: gastro-intestinal; GERD: gastro-oesophageal reflux disease
SD: Standard deviation
Meta-analysis
All statistical analyses were performed using SPSS v.17 and Comprehensive Meta-Analysis 2.2. First, the odds ratio (OR) and 95% confidence interval (CI) of responders versus non-responders to GERD treatment were calculated for each study. Placebo response rate was calculated by dividing the number of responders by the total number of subjects assigned to the placebo arm. Secondly, the pooled odds ratio (OR) (pooled placebo response to treatment) with the 95% confidence interval (CI) was calculated using the random effects (RE) model20. Statistical heterogeneity across the various studies was tested with the use of Q-statistic21. A pQ-value <0.10 indicated a significant statistical heterogeneity across studies, allowing for the use of random effects model. Heterogeneity was quantified with the I2 metric, which is independent of the number of studies included in the meta-analysis22. I2 measures values between 0% and 100% with higher values denoting a greater degree of heterogeneity (I2 = 0-25%: no heterogeneity; I2=25%-50%: moderate heterogeneity; I2 = 50-75%: large heterogeneity; I2 = 75-100%: extreme heterogeneity). When there is lack of heterogeneity the random effects (RE) model coincides with the fixed effects model since random effect modeling assumes a genuine diversity in the results of various studies incorporating study variance. In addition to the main (overall) analysis, a subgroup analysis for the two different types of drugs (PPI and H2RA) was also performed.
Meta-regression
Potential associations between the placebo response and several variables were determined by calculating contingency coefficients in the meta-regression. The rate of placebo response was the dependent variable in the meta-regression model, and the independent variables that used as continuous moderators for the meta-regression analysis included: a) the quality of study estimated by the Jadad score, b) the duration of active treatment, c) the number of visits during the active treatment, d) the frequency of treatment, and e) the length of run-in period. Coefficients that reflect the percent increase in placebo response for each unit increase of the independent variable and the 95% confidence interval (CI) for the respective coefficients were calculated.
Cumulative meta-analysis
A cumulative meta-analysis was also carried out23. Cumulative meta-analysis provides a framework for updating the effect from all studies and a measure of how much the effect changes as evidence accumulates. Thus, cumulative meta-analysis indicates the trend in estimated risk effect. In cumulative meta-analysis, studies were chronologically ordered by publication year and subsequently the pooled ORs were obtained at the end of each year, i.e. at each information step.
Assessment of publication bias
Publication bias was determined by the funnel plot of Beggs & Mazumbar's rank correlation test24 and Egger's intercept test25. Rosenthal's Classic Fail-Safe N is also calculated to determine the missing number of null-studies to nullify this bias.
Results
Eligible studies
The literature search identified 116 articles in Cochrane Controlled Trial Register, 123 articles in EMBASE and 166 articles in MEDLINE that met the search criteria.
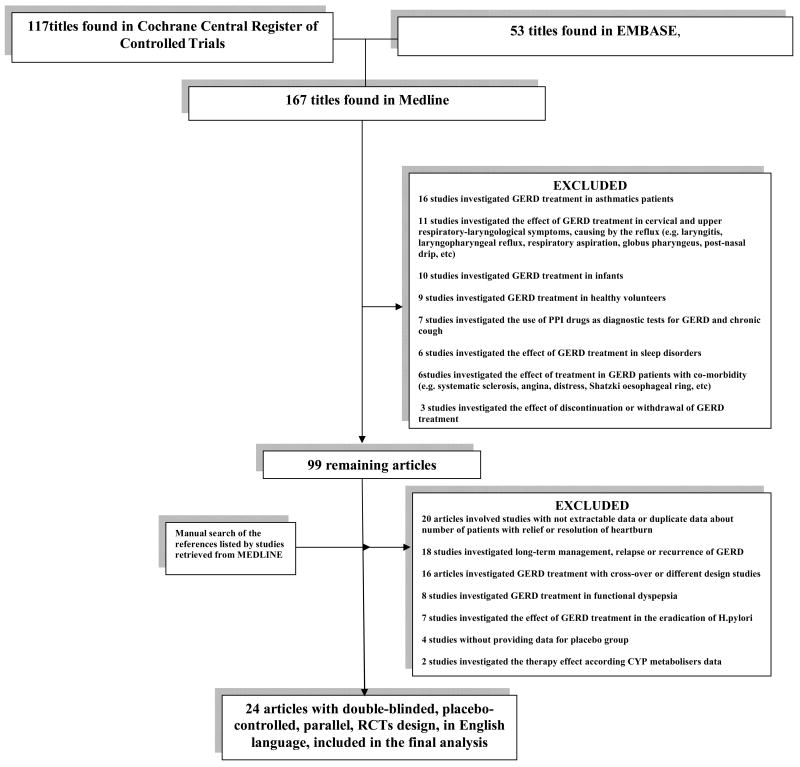
The majority of identified articles in Cochrane (96/117, 82%) and in EMBASE (41/53, 77.3%) were also identified in MEDLINE. Figure 1 shows the flow chart of retrieved studies in MEDLINE and studies excluded, with specification of reasons for exclusion. At the end of the screening process, 24 that involved double-blinded, placebo-controlled, parallel-design, randomized, controlled studies published in English language were included. The studies were published between 1983-2009.
Figure 1.
Flow chart of the meta-analysis, summarizing retrieved, included and excluded studies, with specification of reasons for exclusion.
Demographic, clinical and design characteristics of the studies included in this meta-analysis are summarized in table 2. Patients' median age was 48.8 years (range 18-85, 53.1% female). Fourteen trials tested proton-pump inhibitors (PPI) therapy; ten used H2–receptor antagonists (H2RA). Thirteen trials included patients with non-erosive reflux disease (NERD), five trials included patients with erosive esophagitis and six trials included patients with both NERD and erosive esophagitis. Generally, the populations included were Caucasians (13 studies), East Asians (one study) and a mixed combination of ethnic groups (10 studies).
In fourteen studies, the definition of ‘responder’ was complete relief or resolution of heartburn at the end (n=11) or during the last week (n=3) of the study. In one study the response was defined based on the 75% relief of heartburn , while in the remaining 13 studies included, no individual data for complete relief of 24h heartburn were available, and a ‘responder’ was a patient who experienced complete relief of overall GERD-associated or upper GI symptoms.
Combined, the studies included in the meta-analysis enrolled 9987 patients, of whom 3041 received placebo. The raw numbers of responders in placebo group and in active arms were 623 and 3081, respectively. There was significant heterogeneity across studies (pQ=0.0001, I2:83.1%). Ethnicity appeared to be a factor of heterogeneity in the response to treatment; however there were not sufficient data on ethnicities to allow a formal estimate of relative contribution of this factor to response.
Overall placebo response and subgroup analyses
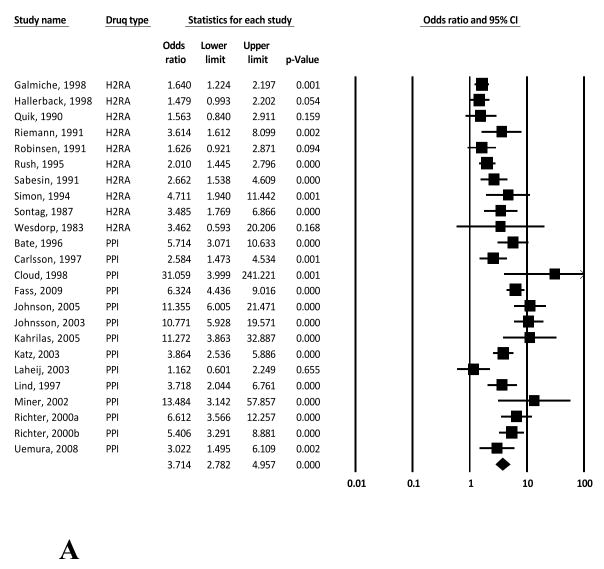
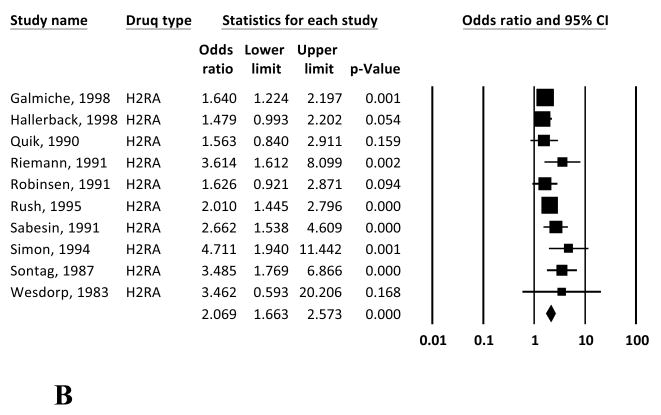
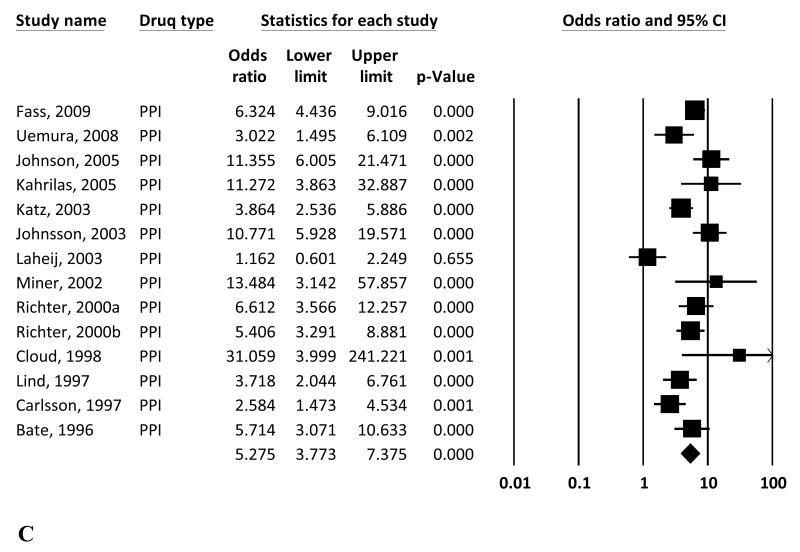
The overall study-weighted mean placebo response was 18.85% (SD: 12.72%), ranging from 2.94% to 47.06%. The overall study-weighted mean response among patients who received active treatment for GERD was 43.49±13.80% (range 21.59-70.22%). The pooled odds ratio (OR) for response to active treatment from the random-effect model was 3.71 [95% CI: 2.78-4.96] (Figure 2a). The OR of response to active treatment with H2RA and PPIs were 2.07 [1.66-2.57] and 5.27 [3.77-7.37] respectively (Figures 2b and 2c).
Figure 2.
Random effects (RE) odds ratio (OR) estimates with the corresponding 95% confidence interval (CI) for (a) the response to treatment in GERD (placebo vs active treatment), (b) the response to treatment in GERD (placebo vs H2RA), and (c) the response to treatment in GERD (placebo vs PPI). The OR estimate of each study is marked with a solid black square. The size of the square represents the weight that the corresponding study exerts in the meta-analysis. The confidence intervals of pooled estimates are displayed as a horizontal line through the diamond; this line might be contained within the diamond if the confidence interval is narrow.
Endoscopic procedures' studies
The mean placebo response rate to sham injection in three endoscopic procedure trials of GERD was 29.46% (range: 23.61-40.63%). In these 3 articles, the response to treatment was defined as daily relief of heartburn symptoms in the one of them 26 and as no need/cessation of anti-secretory drugs after the sham injection in the two of them 27,28. No significant difference was found in comparison to the mean overall placebo response in medical therapies studies (29.46% vs., 18.85% respectively; p =0.178).
Placebo response and its modifiers
Patients with erosive esophagitis had a lower placebo response rate than patients without erosive esophagitis (11.87% and 18.31%, respectively; p = 0.246) although the difference was not significant (Figure 3).
Figure 3.
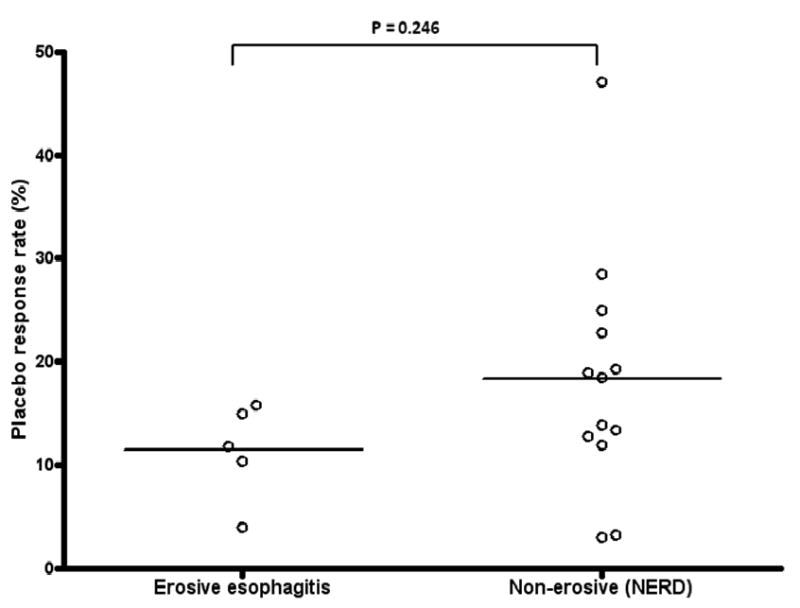
Placebo response rates in trials studying erosive esophagitis patients versus trials with non-erosive esophagitis patients. The mean placebo response rate was non-significant lower in patients with erosive esophagitis than in patients without erosive esophagitis (11.87% and 18.31%, respectively; p = 0.246).
Among studies that included only patients with non-erosive esophagitis, there were positive correlations between the placebo response rate and PPI treatment (r=0.668; p=0.013) and between the placebo response rate and the frequency of treatment (r=0.631; p=0.021). There was no correlation between type of active treatment and placebo response among studies enrolling only patients with erosive esophagitis.
The placebo response rate was significantly lower in studies of PPIs than in studies of H2RA than (14.51% vs. 24.69%, respectively; p=0.05, Figure 4). Among studies of PPIs, no significant association was found between placebo response rate and any of the aforementioned study design variables.
Figure 4.
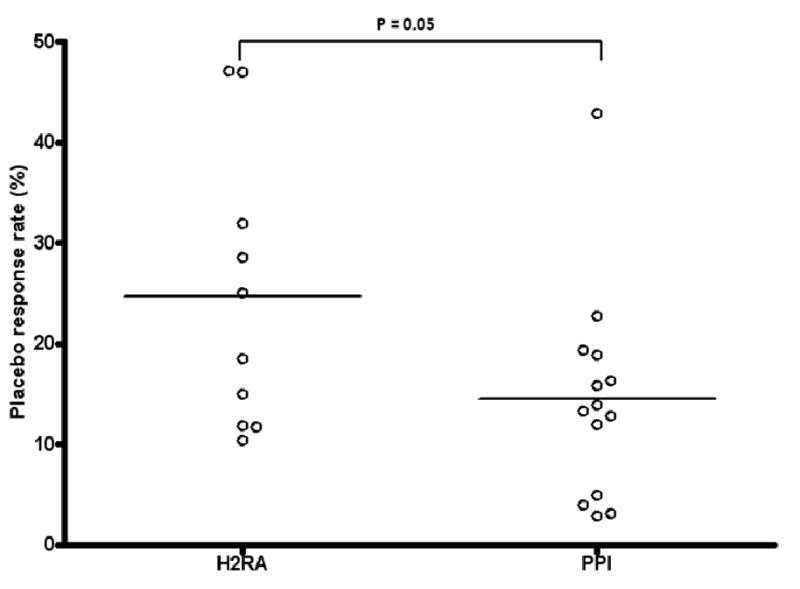
Placebo response rates in trials using proton pump inhibitors versus trials using H2-receptor antagonists. The placebo response rate among patients receiving PPI therapy was borderline significantly lower than the response rate among patients taking H2-receptor antagonists (14.5% vs. 24.7%, respectively; p =0.05).
Meta-regression analysis
The placebo response rate for the 24 studies did not significantly correlate with Jadad score, duration of active treatment, frequency of treatment, number of visits during active treatment, and length of run-in period. Meta-regression analysis for the selected variables regarded as potential sources of heterogeneity did not identify any significant variables (P>0.05) (Table 3).
Table 3.
Meta-regression with placebo response rate as the dependent variable.
| Independent variables | Correlation coefficient | p value |
|---|---|---|
| Jadad score | 0.01 | 0.744 |
| Treatment type | 0.07 | 0.160 |
| Erosive esophagitis | 0.004 | 0.896 |
| Duration of active treatment | 0.00 | 0.341 |
| Number of visit during active treatment | -0.02 | 0.415 |
| Frequency of treatment | 0.01 | 0.382 |
| Length of run-in period | 0.00 | 0.413 |
Cumulative meta-analysis
Cumulative meta-analysis for response to treatment in GERD trials through time of study publication (active treatment vs. placebo) showed that the OR declined from 3.462 in 1983 to 2.295 in 1991 and then increased to 3.714 in 2009 (Figure 5). With the exception of the first year since the earliest publication, the association remained significant throughout the whole study.
Figure 5.
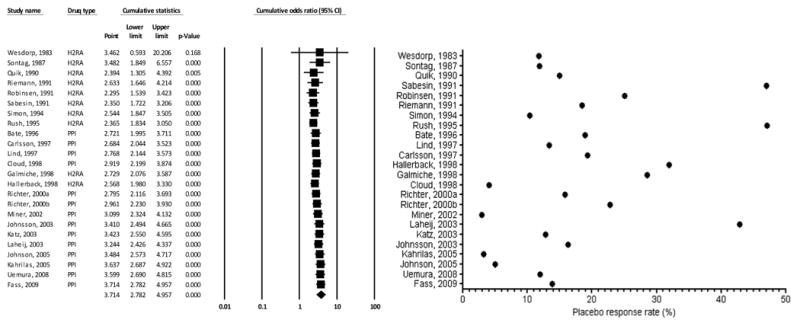
Cumulative meta-analysis for the response to treatment in GERD (placebo vs. active treatment). On the left panel, the random effects odds ratio (OR) with the corresponding 95% confidence interval (CI) according to year-information step is shown. On the right panel, descriptive statistics of actual placebo response rate in the placebo arm of each trial is reported.
Publication bias
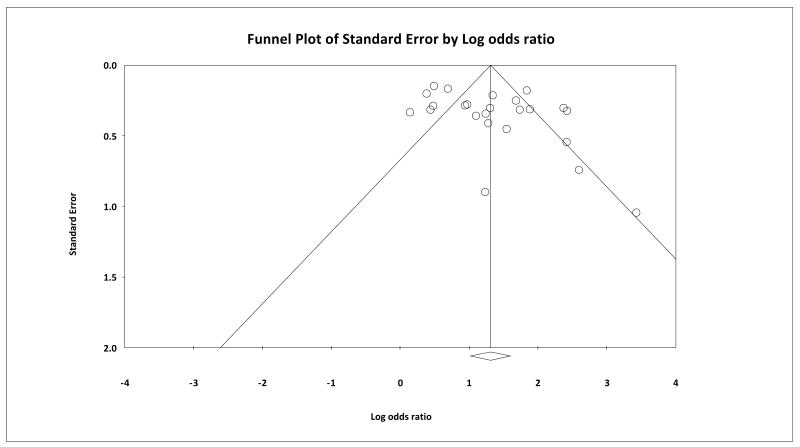
Funnel plot analysis, Beggs & Mazumbar's rank correlation test (Kendall's tau = 0.192, 1-tailed p=0.094), and Egger's intercept test (1-tailed p=0.020 and the 2-tailed p=0.041) indicate a lack of publications that reported negative results, thus confirming the presence of a negative publication bias (Figure 6).
Figure 6.
Begg's funnel plot: A funnel plot is a plot of SE (effect estimate) or SE (log(effect estimate)) vs. effect estimate for each study under the outcome. Each study is represented by a single dot. In this case, log odds ratio (OR) was used as the effect estimate. The overall effect estimate is indicated by the vertical dotted line. The two diagonal lines represent 95% CI. The existence of studies lying outside of the inverted funnel indicates a publication bias.
Conclusions
In this meta-analysis of 24 GERD clinical trials using H2-receptor antagonists and PPIs, we found an overall placebo response rate of approximately 19%. We also identified treatment with PPIs as factors potentially associated with decreased placebo response. A non-significant trend for a lower placebo response rate was present in patients with erosive esophagitis compared to those with non-erosive esophagitis.
The placebo response rate of 19% among GERD clinical trials is comparably lower than placebo response rates reported in other gastrointestinal disorders. Placebo response rates have been reported as 36-40.2% in irritable bowel syndrome trials12,8, 30-40% in dyspepsia trials13,29, 13-30% in inflammatory bowel disease trials9,10, 11, and 36.2% in peptic ulcer trials7.
Multiple reasons can account for this lower rate of placebo response. First, in this analysis we used stringent inclusion criteria. Most of the studies included in this meta-analysis were moderate-to-large clinical trials that were well designed. Likewise, patients enrolled in the trials may have had more severe reflux disease and therefore may be less likely to improve.
Second, only studies reporting “complete relief” of heartburn, the cardinal symptom of reflux disease, as their primary outcome, were included in the meta-analysis. This inclusion criterion was selected to better tease out a “true” placebo response, rather than partial symptom responses which could due to the fluctuating presentation and the evolving natural history of the disease. While the determination of complete relief is still based on the patients' subjective report, including only patients reporting complete relief would not lead to an overestimation of the placebo effect as opposed to using any improvement of symptoms as outcome. Whilst the subjective nature of the outcome can be seen as a limitation, the use of physiologic endpoints such as pH changes would not be appropriate for the aims of this meta-analysis, which is based on symptoms, and would have been of cumbersome interpretation given the relatively small numbers in physiologic studies and the limited correlation between pH changes and symptom reporting in a proportion of patients. As weakly acidic and non-acid reflux can play a role in the generation of symptoms of GERD, adherence to the physiological endpoint of changes in acid concentration would not have been reflective of the several underlying pathophysiological entities.
Also, we recognize that the term “complete relief of heartburn” is reflective of more heterogeneous endpoints and that this endpoint is the result of multiple ones. If it is reasonable to believe that patients with both dyspepsia and GERD could have been included, it is also likely that such patients would have fallen under the definition of what used to be defined reflux-like dyspepsia, which bears many affinities to GERD.
Third, as acid reflux is a key component in the generation of heartburn, a complete symptom remission without alteration of the acid content is less likely than in other disorders, such as dyspsepsia and IBS, where there is no predominant, well identified and treatable, pathogenic factor.
In our study, the placebo response rate was slightly (but not significantly) higher in patients with non-erosive esophagitis compared to patients who had erosive esophagitis by endoscopy prior to treatment. Since esophageal mucosal healing has been shown to correlate with complete heartburn symptom relief30, we expected patients with active esophagitis to respond less to placebo31. In addition, non-erosive reflux disease (NERD) is believed to overlap with other functional gastrointestinal disorders such as functional dyspepsia and IBS32, which generally have high placebo response rates.
A second predictor of the placebo response in GERD trials is the class of antisecretory medication. Studies with PPI therapy had a pooled placebo response rate of 14.68%, whereas the placebo response rate of H2-receptor antagonist studies was 24.68%. This finding was unexpected, as more effective interventions, like PPIs, are commonly associated with higher placebo responses1, presumably due to increased expectation of efficacy. Indeed, cumulative meta-analysis investigating the effect of time on response rates shows a decline in the OR for response rates to active treatment around 1998. This is the time when newer treatments became available in the form of multiple PPIs, potentially reflecting an effect of expectancy and a less demarcated difference of active drugs versus placebo. However, the overall time trends do not support dramatic changes in response rates from the first to the last study included. More likely, the differences in placebo response rate according to drug class could be accounted for by patient selection bias among studies investigating PPI drugs. All of the PPI studies were published after 1996, when both prescription and generic H2-receptor antagonists were widely available. Subjects who enrolled in PPI clinical trials were likely to have tried H2-receptor antagonists and to have failed to respond completely. Thus, these subjects may represent a population of patients with more symptomatic reflux disease, making them less likely to respond to placebo interventions.
Variables that influence the placebo response in other gastrointestinal disorders include frequency of drug dosing7, duration of treatment11, frequency of office visits during the study period12,9,11, severity of disease activity10, and body mass index13, and study duration33. Each factor affects the placebo response to varying degrees and may produce opposing effects in different disease states. For instance, more frequent office visits is associated with higher placebo response among studies of Crohn's disease11, but lower placebo response rate among studies of irritable bowel syndrome 12.
In this study, meta-regression analysis found no correlation between these variables and placebo response in GERD clinical trials (Table 3). Evidence from a randomized controlled trial in IBS suggests patient-practitioner relationship is the most robust predictor of placebo response in that condition1. None of studies included in our analysis, however, provided data to compare the effects of different types of patient-physician relationship on placebo responses.
The dissimilar pathophysiology of GERD and other gastrointestinal disorders may preclude direct comparison between studies. As discussed above, what is relevant to other gastrointestinal conditions may not be applicable to GERD.
There are a number of limitations to this analysis. First, there was significant heterogeneity across the 24 studies included in this analysis, in spite of efforts to minimize this confounding effect by establishing stringent inclusion criteria. We performed a detailed meta-regression analysis which was unable to identify the source of such heterogeneity. The use of random-effect models in data analysis can limit the effects of such heterogeneity by providing more conservative estimates; however fundamental differences across trials remain. Second, as in most meta-analysis we must face an element of publication bias. This bias was identified by funnel plot analysis, by means of the Beggs & Mazumbar's rank correlation test and with the Egger's intercept test, all of which confirmed a lack of publications that reported negative results (Figure 6). Ultimately, this might have deflated our estimate of placebo responses. In fact, studies of interventions whose efficacy proves to be not superior to placebo are believed to be less likely to be published. Having had access for analysis to these unpublished data, if they existed, would have likely driven our pooled placebo response calculations to higher rates. Third, we have not formally included studies testing all the treatment modalities available for reflux disease. Exclusively for reference purpose, we have additionally performed analyses on three recent RCTs that investigated the effect of sham injection versus endoscopic procedures in GERD26,27,28. In these trials, the mean response to sham treatment was 29.46%, somewhat higher than the placebo response seen with H2-blockers and PPI, but not significantly different. While the lack of significant difference suggests the placebo response is in the same range and is proper to GERD as a condition rather than to the procedure or treatment used, one cannot exclude lack of statistical power in this comparison, given the small number of endoscopic studies available.
We have chosen not to consider or discuss studies of surgical anti-reflux procedure as they are not designed with what could be consider a placebo arm for the purpose of our meta-analysis. We also have chosen not to include studies of treatment other than PPI or H2-blockers, as these are the current standard of care for anti-reflux medical therapy.
Lastly, we could not abstract from most of the reports data regarding the effect of tobacco, alcohol, body mass index, hiatal hernia and other comorbidities on the placebo response. It is likely, however, that even with the large amount of individual patients included in the studies, there would have been limited power to identify the separate contributions of these variables to placebo response, as little is still known of the effects of these factors even on the symptomatic response to active treatments.
In conclusion, the placebo response rate is substantial among GERD clinical trials, yet lower than that observed in other functional and inflammatory disorders of the gastrointestinal tract. The placebo response in GERD appears to be independent of the presence/absence of erosive esophagitis but related to the class of acid suppression treatment studied.
Future studies with potentially more efficacious treatment than those available to date, especially for NERD, might have to face potentially higher placebo response rates. The understanding of factors involved in the placebo response in gastro-oesophageal reflux trials should continue to be pursued to guide the design_of such future clinical trials. Further clarification of what variables drive the placebo response could lead the clinician to manipulate these variables when dealing with patients non-responsive to multiple treatments to maximize response rate and satisfaction with treatment.
Table 1. Variables assessed and recorded for each gastro-oesophageal reflux disease (GERD) trial.
| Study design | Study drug |
| Randomized, double-blinded study | |
| Frequency of dosing | |
| Study duration | |
| Oesophageal pH measurement before enrollment | |
| Endoscopic evaluation before enrollment | |
| Presence of erosive esophagitis | |
| Presence of run-in period | |
| Frequency of office visits during active treatment | |
| Frequency of office visits throughout entire study | |
| Study population | Total number of subjects in |
| Placebo arm | |
| Active treatment arm | |
| Total number of subjects who | |
| Completed placebo arm | |
| Completed active treatment arm | |
| Mean age | |
| Gender distribution | |
| Heartburn response | Reported relief of heartburn |
| Defined relief of heartburn as “complete relief of heartburn” | |
| Quality measures (Jadad score) | Was study described as randomized? |
| Was study described as double-blinded? | |
| Were data on withdrawals and dropouts provided? | |
| Was the method of randomization described and appropriate? | |
| Was the method of blinding described and appropriate? |
Acknowledgments
This work was supported in part by the NIH grant K24 AT004095 (To TJK).
References
- 1.Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. Bmj. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Craen AJ, Roos PJ, Leonard de Vries A, et al. Effect of colour of drugs: systematic review of perceived effect of drugs and of their effectiveness. Bmj. 1996;313:1624–6. doi: 10.1136/bmj.313.7072.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaptchuk TJ, Goldman P, Stone DA, et al. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786–92. doi: 10.1016/s0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 4.de Craen AJ, Tijssen JG, de Gans J, et al. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol. 2000;247:183–8. doi: 10.1007/s004150050560. [DOI] [PubMed] [Google Scholar]
- 5.Kaptchuk TJ, Stason WB, Davis RB, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. Bmj. 2006;332:391–7. doi: 10.1136/bmj.38726.603310.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackwell B, Bloomfield SS, Buncher CR. Demonstration to medical students of placebo responses and non-drug factors. Lancet. 1972;1:1279–82. doi: 10.1016/s0140-6736(72)90996-8. [DOI] [PubMed] [Google Scholar]
- 7.de Craen AJ, Moerman DE, Heisterkamp SH, et al. Placebo effect in the treatment of duodenal ulcer. Br J Clin Pharmacol. 1999;48:853–60. doi: 10.1046/j.1365-2125.1999.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitz M, Cheang M, Bernstein CN. Defining the predictors of the placebo response in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:237–47. doi: 10.1016/s1542-3565(04)00626-3. [DOI] [PubMed] [Google Scholar]
- 9.Ilnyckyj A, Shanahan F, Anton PA, et al. Quantification of the placebo response in ulcerative colitis. Gastroenterology. 1997;112:1854–8. doi: 10.1053/gast.1997.v112.pm9178676. [DOI] [PubMed] [Google Scholar]
- 10.Su C, Lewis JD, Goldberg B, et al. A meta-analysis of the placebo rates of remission and response in clinical trials of active ulcerative colitis. Gastroenterology. 2007;132:516–26. doi: 10.1053/j.gastro.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Su C, Lichtenstein GR, Krok K, et al. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn's disease. Gastroenterology. 2004;126:1257–69. doi: 10.1053/j.gastro.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Patel SM, Stason WB, Legedza A, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332–40. doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Locke GR, Lahr BD, et al. Predictors of the placebo response in functional dyspepsia. Aliment Pharmacol Ther. 2006;23:923–36. doi: 10.1111/j.1365-2036.2006.02845.x. [DOI] [PubMed] [Google Scholar]
- 14.Kelley JM, Lembo AJ, Ablon JS, et al. Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med. 2009;71:789–97. doi: 10.1097/PSY.0b013e3181acee12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conboy L, Macklin EA, Kelly J, et al. Which patients improve: characteristics increasing sensitivity to a supportive patient-practitioner relationship. Soc Sci Med. 2009 doi: 10.1016/j.socscimed.2009.10.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–7. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Lundh A, Gotzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8:22. doi: 10.1186/1471-2288-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Lau J, Antman EM, Jimenez-Silva J, et al. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med. 1992;327:248–54. doi: 10.1056/NEJM199207233270406. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corley DA, Katz P, Wo JM, et al. Improvement of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlled trial. Gastroenterology. 2003;125:668–76. doi: 10.1016/s0016-5085(03)01052-7. [DOI] [PubMed] [Google Scholar]
- 27.Deviere J, Costamagna G, Neuhaus H, et al. Nonresorbable copolymer implantation for gastroesophageal reflux disease: a randomized sham-controlled multicenter trial. Gastroenterology. 2005;128:532–40. doi: 10.1053/j.gastro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Rothstein R, Filipi C, Caca K, et al. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: A randomized, sham-controlled trial. Gastroenterology. 2006;131:704–12. doi: 10.1053/j.gastro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Moayyedi P, Delaney BC, Vakil N, et al. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329–37. doi: 10.1053/j.gastro.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Sharma N, Donnellan C, Preston C, et al. A systematic review of symptomatic outcomes used in oesophagitis drug therapy trials. Gut. 2004;53 4:iv58–65. doi: 10.1136/gut.2003.034371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pace F, Maconi G, Molteni P, et al. Meta-analysis of the effect of placebo on the outcome of medically treated reflux esophagitis. Scand J Gastroenterol. 1995;30:101–5. doi: 10.3109/00365529509093245. [DOI] [PubMed] [Google Scholar]
- 32.Tack J. Is there a unifying role for visceral hypersensitivity and irritable bowel syndrome in non-erosive reflux disease? Digestion. 2008;78 1:42–5. doi: 10.1159/000151254. [DOI] [PubMed] [Google Scholar]
- 33.Kamm MA. Review article: the complexity of drug development for irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:343–51. doi: 10.1046/j.1365-2036.2002.01185.x. [DOI] [PubMed] [Google Scholar]
- 34.Fass R, Chey WD, Zakko SF, et al. Clinical trial: the effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with non-erosive reflux disease. Aliment Pharmacol Ther. 2009;29:1261–72. doi: 10.1111/j.1365-2036.2009.04013.x. [DOI] [PubMed] [Google Scholar]
- 35.Uemura N, Inokuchi H, Serizawa H, et al. Efficacy and safety of omeprazole in Japanese patients with nonerosive reflux disease. J Gastroenterol. 2008;43:670–8. doi: 10.1007/s00535-008-2214-5. [DOI] [PubMed] [Google Scholar]
- 36.Johnson DA, Orr WC, Crawley JA, et al. Effect of esomeprazole on nighttime heartburn and sleep quality in patients with GERD: a randomized, placebo-controlled trial. Am J Gastroenterol. 2005;100:1914–22. doi: 10.1111/j.1572-0241.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 37.Kahrilas PJ, Miner P, Johanson J, et al. Efficacy of rabeprazole in the treatment of symptomatic gastroesophageal reflux disease. Dig Dis Sci. 2005;50:2009–18. doi: 10.1007/s10620-005-3000-3. [DOI] [PubMed] [Google Scholar]
- 38.Katz PO, Castell DO, Levine D. Esomeprazole resolves chronic heartburn in patients without erosive oesophagitis. Aliment Pharmacol Ther. 2003;18:875–82. doi: 10.1046/j.1365-2036.2003.01771.x. [DOI] [PubMed] [Google Scholar]
- 39.Johnsson F, Hatlebakk JG, Klintenberg AC, et al. Symptom-relieving effect of esomeprazole 40 mg daily in patients with heartburn. Scand J Gastroenterol. 2003;38:347–53. doi: 10.1080/00365520310002157. [DOI] [PubMed] [Google Scholar]
- 40.Laheij RJ, Van Rossum LG, Jansen JB, et al. Proton-pump inhibitor therapy for acetylsalicylic acid associated upper gastrointestinal symptoms: a randomized placebo-controlled trial. Aliment Pharmacol Ther. 2003;18:109–15. doi: 10.1046/j.1365-2036.2003.01656.x. [DOI] [PubMed] [Google Scholar]
- 41.Miner P, Jr, Orr W, Filippone J, et al. Rabeprazole in nonerosive gastroesophageal reflux disease: a randomized placebo-controlled trial. Am J Gastroenterol. 2002;97:1332–9. doi: 10.1111/j.1572-0241.2002.05769.x. [DOI] [PubMed] [Google Scholar]
- 42.Richter JE, Bochenek W. Oral pantoprazole for erosive esophagitis: a placebo-controlled, randomized clinical trial. Pantoprazole US GERD Study Group. Am J Gastroenterol. 2000;95:3071–80. doi: 10.1111/j.1572-0241.2000.03254.x. [DOI] [PubMed] [Google Scholar]
- 43.Richter JE, Peura D, Benjamin SB, et al. Efficacy of omeprazole for the treatment of symptomatic acid reflux disease without esophagitis. Arch Intern Med. 2000;160:1810–6. doi: 10.1001/archinte.160.12.1810. [DOI] [PubMed] [Google Scholar]
- 44.Carlsson R, Dent J, Watts R, et al. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119–24. [PubMed] [Google Scholar]
- 45.Cloud ML, Enas N, Humphries TJ, et al. Rabeprazole in treatment of acid peptic diseases: results of three placebo-controlled dose-response clinical trials in duodenal ulcer, gastric ulcer, and gastroesophageal reflux disease (GERD). The Rabeprazole Study Group. Dig Dis Sci. 1998;43:993–1000. doi: 10.1023/a:1018822532736. [DOI] [PubMed] [Google Scholar]
- 46.Galmiche JP, Shi G, Simon B, et al. On-demand treatment of gastro-oesophageal reflux symptoms: a comparison of ranitidine 75 mg with cimetidine 200 mg or placebo. Aliment Pharmacol Ther. 1998;12:909–17. doi: 10.1046/j.1365-2036.1998.00384.x. [DOI] [PubMed] [Google Scholar]
- 47.Hallerback B, Glise H, Johansson B, et al. Gastro-oesophageal reflux symptoms--clinical findings and effect of ranitidine treatment. Eur J Surg Suppl. 1998:6–13. doi: 10.1080/11024159850191175. [DOI] [PubMed] [Google Scholar]
- 48.Lind T, Havelund T, Carlsson R, et al. Heartburn without oesophagitis: efficacy of omeprazole therapy and features determining therapeutic response. Scand J Gastroenterol. 1997;32:974–9. doi: 10.3109/00365529709011212. [DOI] [PubMed] [Google Scholar]
- 49.Bate CM, Griffin SM, Keeling PW, et al. Reflux symptom relief with omeprazole in patients without unequivocal oesophagitis. Aliment Pharmacol Ther. 1996;10:547–55. doi: 10.1046/j.1365-2036.1996.44186000.x. [DOI] [PubMed] [Google Scholar]
- 50.Rush DR, Stelmach WJ, Young TL, et al. Clinical effectiveness and quality of life with ranitidine vs placebo in gastroesophageal reflux disease patients: a clinical experience network (CEN) study. J Fam Pract. 1995;41:126–36. [PubMed] [Google Scholar]
- 51.Simon TJ, Berenson MM, Berlin RG, et al. Randomized, placebo-controlled comparison of famotidine 20 mg b.d. or 40 mg b.d. in patients with erosive oesophagitis. Aliment Pharmacol Ther. 1994;8:71–9. doi: 10.1111/j.1365-2036.1994.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 52.Sabesin SM, Berlin RG, Humphries TJ, et al. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations. Results of a multicenter, placebo-controlled, dose-ranging study. USA Merck Gastroesophageal Reflux Disease Study Group. Arch Intern Med. 1991;151:2394–400. [PubMed] [Google Scholar]
- 53.Riemann JF, Hobel W. Cimetidine suspension in patients with stage 0 gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1991;5:191–7. doi: 10.1111/j.1365-2036.1991.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 54.Robinsen M, Decktor DL, Stone RC, et al. Famotidine (20 mg) b.d. relieves gastrooesophageal reflux symptoms in patients without erosive oesophagitis. Famotidine/GERD Investigation Group. Aliment Pharmacol Ther. 1991;5:631–43. doi: 10.1111/j.1365-2036.1991.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 55.Quik RF, Cooper MJ, Gleeson M, et al. A comparison of two doses of nizatidine versus placebo in the treatment of reflux oesophagitis. Aliment Pharmacol Ther. 1990;4:201–11. doi: 10.1111/j.1365-2036.1990.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 56.Sontag S, Robinson M, McCallum RW, et al. Ranitidine therapy for gastroesophageal reflux disease. Results of a large double-blind trial. Arch Intern Med. 1987;147:1485–91. [PubMed] [Google Scholar]
- 57.Wesdorp IC, Dekker W, Klinkenberg-Knol EC. Treatment of reflux oesophagitis with ranitidine. Gut. 1983;24:921–4. doi: 10.1136/gut.24.10.921. [DOI] [PMC free article] [PubMed] [Google Scholar]