Abstract
The acoustic startle response (ASR) is a reflexive contraction of skeletal muscles in response to a loud, abrupt acoustic stimulus. ASR magnitude is reduced if the startle stimulus is preceded by a weaker acoustic or non-acoustic stimulus, a phenomenon known as prepulse inhibition (PPI). PPI has been used to test various aspects of sensory discrimination in both animals and humans. Here we show that PPI of the ASR is an advantageous method of assessing frequency discrimination. We describe the apparatus and its performance testing frequency discrimination in young CD1 mice. Compared to classical conditioning paradigms, PPI of the ASR is less time consuming, produces robust results, and can be used without training even in young animals. This approach can be used to investigate the neuronal mechanisms underlying frequency discrimination, its maturation during development, and its relationship to tonotopic organization.
Keywords: acoustic startle response, prepulse inhibition, frequency discrimination, mice, auditory system, behavior
1. Introduction
The ability to discriminate between frequencies is a key function of the auditory system. Vocal communication in particular depends on the ability to detect and encode fast changes in the spectral characteristics of sound. In humans, frequency discrimination skills have been linked to good phonological processing and literacy (Halliday and Bishop, 2005), while patients with specific reading disability (dyslexia) often show impairments in frequency discrimination (Ahissar et al., 2000). People with sensorineural hearing loss also show difficulty discriminating between pure tones (Gengel, 1973; Halliday and Bishop, 2005; Nelson and Freyman, 1986), which may contribute to accompanying problems with speech perception and language development.
Due to the ease of genetic manipulation, mice have become a preferred animal model for understanding auditory processing disorders. Many hearing abilities and auditory functions are also well understood in the mouse (Dooling and Hulse, 1989; Willott, 1983, 2001). As a result, establishing a reliable behavioral test for a particular auditory function in mice offers the possibility to link a specific neuronal substrate to that function. Furthermore, it provides the opportunity to investigate how defined changes in auditory circuitry influence a particular auditory function, or how they might ameliorate identified hearing deficits.
Frequency discrimination is easily assessed in humans, even in young children and patient groups like those with dyslexia, cochlear implants, and hearing loss. However, current methods of assessing frequency discrimination in rodents depend on intricate and time-consuming conditioning paradigms (Kurt and Ehret, 2010; Ono et al., 2006) that are also influenced by confounding issues related to learning and motivation. These conditioning paradigms cannot be utilized in very young animals due to the training required, and may be difficult to use in animals with rapidly changing auditory thresholds, such as models of progressive hearing loss.
To circumvent these issues, we developed a method of assessing frequency discrimination in mice based on prepulse inhibition of the acoustic startle response (ASR). The acoustic startle is a reflexive motor response to an unexpected loud auditory stimulus that manifests behaviorally as a swift contraction of skeletal muscles (Hoffman and Ison, 1980). In humans, the ASR is usually quantified via eyeblink measures (Ison and Pinckney, 1983), while in rodents it is measured by placing the animal on a platform that transmits the downward force produced by the reflex. In rats, the ASR is typically elicited by stimuli >80 decibels (dB) above auditory thresholds (Pilz et al., 1987).
The ASR is attenuated when a weak pre-stimulus, or prepulse, is presented just prior to the startle-eliciting stimulus, a phenomenon known as prepulse inhibition (PPI; Ison et al., 1973). Because inhibition depends solely on the ability to detect and process the prepulse, PPI provides a useful means of assessing acoustic detection and discrimination. Since it is reflexive, PPI also largely eliminates confounds due to motivation and attention. Finally, as no training is required, PPI can also be used on young animals as soon as they can hear (Parisi and Ison, 1979), providing a useful tool for developmental studies.
In the past, PPI paradigms have been successfully used to assess several aspects of complex auditory processing in rodents, including gap detection (Turner et al., 2006), speech sounds (Floody et al., 2010), and tone saliency (Carlson and Willott, 1996). Here we describe and characterize a PPI-based method of assessing frequency discrimination in mice, using a change in the frequency of a background tone as the prepulse stimulus. This approach will be useful not only for understanding this particular aspect of auditory perception, but also for understanding its neural mechanisms.
2. Materials and Methods
2.1. Animals
Experiments utilized fourteen CD1 female mice (Charles River, Wilmington, MA), ten for testing frequency discrimination and four for auditory brainstem responses (ABRs). Mice were received at 4 weeks of age and allowed to acclimate to the animal facility for ~one week before being tested at 5 weeks of age. Mice were maintained on a 12 hour light/dark cycle and provided food and water ad libitum. All experimental procedures were in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
2.2. Apparatus and hardware
The production of precise acoustic stimuli is essential for testing auditory function and behavior. However, we found that commercial acoustic startle equipment introduces unexpected sounds and/or silent gaps. Such acoustic artifacts usually arise when the software transitions between stimuli and reinitializes the sound card. To avoid such artifacts, we “compose” and save the complete sequence of acoustic stimuli in a single audio file. When played back during an experiment, the sound card is initialized only once, at the beginning of each testing session, thereby eliminating sound card initialization-induced acoustic transients during actual testing. The audio file is created using software written in the LabView® programming language1 (National Instruments, TX) and saved as a waveform audio file (WAV).
A schematic of the experimental setup is illustrated in Figure 1A. Channel L of the sound card (LynxTwo-A, Lynx Studio Technology, CA) is connected to an audio amplifier (AMP110, AudioSource, OR) to drive the loudspeaker. Channel R is connected to the analog-to-digital converter to trigger data acquisition (NI USB-6211, National Instruments, TX). The loudspeaker is a planar isodynamic tweeter (RT2H-A, HiVi, CA) that has high-power output (60 W maximum), relatively flat frequency response (1.7-25 kHz), and uniformly distributed sound energy. To minimize changes in sound intensity at the animals’ ears due to changes in its position, a custom-made housing was constructed using a frame of Lego® parts covered by a fine plastic mesh in order to constrain animal motion. The housing rests upon a load-cell platform (E45-11, Coulbourn Instruments, PA) whose output is filtered and amplified (LP122, Grass Technologies, RI), and then digitized before being sent to the computer for storage and analysis. The speaker is placed in front of the animal to minimize standing-wave resonances. The speaker, housing, and platform are placed inside an anechoic chamber (ENV-022SD, Med Associates, VT) along with a webcam and infrared light used for animal monitoring.
Fig. 1.
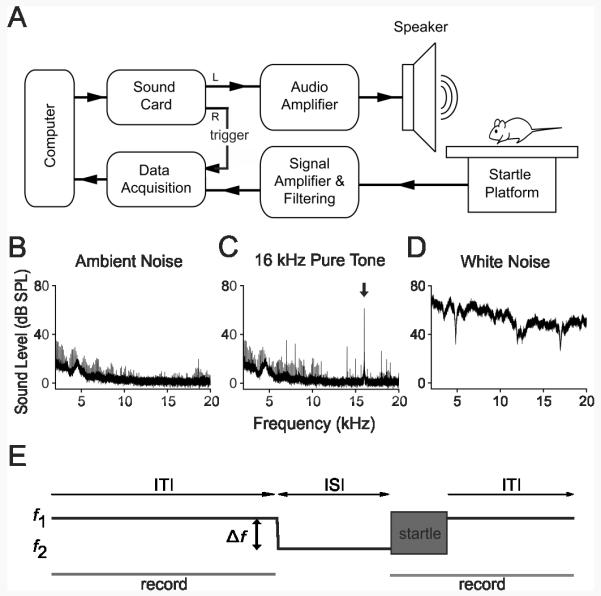
Acoustic startle apparatus for frequency discrimination. (A) Schematic of apparatus. (B-D) Frequency spectra measured at the animal’s ear inside the housing within the anechoic chamber. (B) Ambient noise, (C) 16 kHz tone at 70 dB SPL used as the background frequency, and (D) white noise at 100 dB SPL. To protect the speaker during the 10 s sound delivery required for acquisition of the frequency spectra, white noise was delivered at 100 dB SPL, rather than the 120 dB SPL used to elicit the ASR. Arrow in (C) indicates the 70 dB SPL peak at 16 kHz. (E) Trial schematic. ITI, inter-trial interval; ISI, inter-stimulus interval; f1, background frequency; f2, prepulse frequency; Δf, frequency change.
All acoustic stimuli were calibrated with a ¼” diameter microphone (4939, Brüel & Kjær, Denmark) placed at the level of the animals’ ear within the housing. Microphone signals were sent to a signal conditioning amplifier (Nexus 2690-A-OS1, Brüel & Kjær, Denmark) and analyzed using commercial software (SoundCheck, Listen, MA, USA). Figure 1 shows the frequency spectra of ambient noise within the chamber (B), the 16 kHz pure tone used as the background during frequency discrimination testing (C), and the high-intensity white-noise stimulus used to elicit the ASR (D). A 96 kHz sampling frequency was used for sound production.
2.3. Testing frequency discrimination
At the start of each session, the mouse was placed in the housing and allowed to acclimate to a constant background tone (f1: 16 kHz, 70 dB SPL) for five minutes. The acclimation period was followed by “prepulse” and “startle only” trials. In prepulse trials (Fig. 1E), the prepulse stimulus comprised a frequency change consisting of a 1 ms linear ramp from the background tone, f1, to the prepulse tone, f2, also at 70 dB SPL. The frequency change took place in variable steps of size Δf, such that f2 = f1- Δf. f2 was maintained during the 80 ms inter-stimulus interval (ISI), then followed by the startle stimulus, a 40 ms white noise burst at 120 dB SPL. Following the startle stimulus, f1 was presented again until the prepulse of the next trial. In startle only trials, the prepulse consisted of a 1 ms ramp from f1 to f1, and thus maintained the ramp command while not actually introducing a frequency change. All trials were separated randomly by an inter-trial interval (ITI) ranging from 8-25 seconds.
Trials were divided into three blocks. The first block comprised a series of startle only trials to allow for short-term habituation to the startle stimulus. The second block contained prepulse trials randomly interleaved with an equal number of startle only trials. Prepulse trials were made up of seven classes, where f2 = 15.92, 15.68, 15.47, 15.2, 14.4, 13.34, and 12.0 kHz, corresponding to frequency changes of 0.5, 2.0, 3.3, 5.0, 10, 16.6, and 25%, respectively. Negative frequency changes were used because mice show greater inhibition to downward frequency sweeps than to upward sweeps (Allen et al., 2008). The last block consisted of a final series of startle only trials to test for habituation over the course of the session.
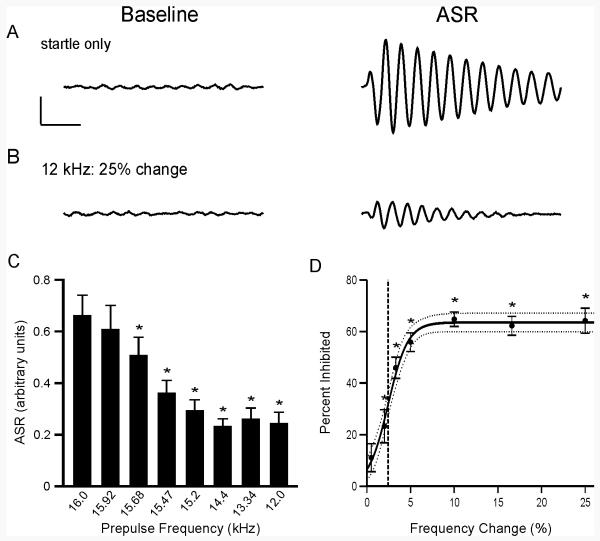
The vertical force exerted by the animal on the platform was measured during two 500 ms recording periods within each trial (Fig. 1E). The first recording period occurred immediately before the prepulse and provided a gauge of the animal’s baseline activity (Fig. 2A-B “Baseline”). The second recording period began at startle stimulus onset and measured the ASR (Fig. 2A-B “ASR”).
Fig. 2.
Inhibition of the ASR by a preceding frequency change. Traces represent the force measured on the platform during the first recording period, “baseline,” and second recording period, “ASR”. Examples of (A) startle only and (B) prepulse trials. For each trial, the maximum force recorded during the second recording period, positive or negative, was reported as the ASR. Scale bars, 0.4 arbitrary units of force, 100 ms. (C) Mean ASR for each trial type. Asterisks indicate that ASR magnitude differed significantly from that of the startle only ASR (p < 0.05, corrected for multiple comparisons). (D) Inhibition of the ASR by a frequency change of various magnitudes. Asterisks indicate frequency changes that caused significant inhibition of the ASR (p < 0.05, one-sample t-test against zero). Line represents a three-parameter sigmoid fit to the data (R2 = 0.99). Dotted lines represent the 95% confidence interval of the sigmoidal fit. Dashed line indicates discrimination threshold. Data show mean ± s.e.m; n = 10 animals.
2.4. Data analysis
The ASR for each trial was defined as the maximum force exerted during the startle recording period minus the background force, defined as root-mean-square of the force exerted during that trial’s background recording period (Fig. 2A,B).
Using the real-time webcam, trials in which the animal showed spontaneous movement or had its feet off the floor of the housing were excluded from analysis. Trials in which the background force exceeded two standard deviations of the session’s mean background force were also excluded.
For each animal, ASRs were averaged across startle only trials and each class of prepulse trials. The mean percent inhibition was then calculated for each class of prepulse trial by normalizing the difference between the mean startle only response amplitude and the mean prepulse response amplitude to the mean startle only response amplitude. A one-way repeated measures ANOVA (Sigmastat, Systat Software, Chicago, IL) was used to determine if the magnitude of the prepulse frequency change had a significant effect on ASR amplitude. Dunnett’s method of multiple comparisons was used to identify prepulse classes in which ASR magnitude differed significantly from the startle only ASR. Frequency changes that caused significant inhibition of the ASR were also identified using a one sample t-test against zero. Discrimination threshold was defined as the frequency change that elicited 50% of the maximum inhibition, determined by a three-parameter sigmoid fit to the frequency change-PPI curve. An alpha level of 0.05 was used to determine statistical significance for all tests.
3. Results
CD1 mice showed a reliable ASR, with an average response of 0.66 ± 0.08 arbitrary units when the startle stimulus was presented alone, without a preceding prepulse (Fig. 2A, C). Preceding the startle stimulus with a shift in frequency caused robust inhibition of the ASR (Fig. 2), with the magnitude of the change having a significant effect on ASR amplitude [One-way repeated measures ANOVA; F(7,79) = 24.25, p < 0.001]. As the difference between the background and prepulse frequencies increased, so did the amount of inhibition elicited, attaining a maximum of 65 ± 3% inhibition for a frequency shift of 10%. The degree of inhibition reached a plateau around 63%, indicating a limit on the amount of inhibition possible. The relationship between frequency change magnitude and extent of PPI is consistent with previous findings that the degree of inhibition is related to the ease with which the prepulse is detected (Ison and Hoffman, 1983; Leitner et al., 1993).
The smallest frequency change, 0.5%, did not significantly inhibit the ASR (one-sample t-test against zero, p > 0.05), suggesting this change is below discrimination threshold, at least to the extent required to inhibit the ASR. Accordingly, the frequency discrimination threshold in 5 week-old female CD1 mice was 2.4% when defined as the frequency change that caused 50% of the maximum inhibition (Fig. 2D).
4. Discussion
To use PPI to assess frequency discrimination, the frequency change must be the only change in the acoustic environment preceding the startle stimulus. Any additional cues (e.g. acoustic transients or intensity change) could also function as a prepulse, making it impossible to attribute attenuation of the ASR solely to the frequency change. We avoided acoustic transients by pre-composing our stimuli and using a ramp that was long enough to allow a smooth transition between frequencies, but still steep enough to produce robust PPI. Nevertheless, during the frequency transitions, the frequency of the pure tone does broaden. The width of broadening is determined by the reciprocal of the frequency transition time. For the 1 ms ramp used in our experiments, the width would be at most 1 kHz. Most importantly however, because length of the ramp is constant, this frequency broadening does not vary with the magnitude of the frequency change. Because PPI increased as the magnitude of the frequency change increased in our experiments, it is highly unlikely that frequency broadening during the ramp is responsible for the observed results.
To ensure that sound intensity remained constant, even within the startle chamber’s enclosed environment, we prevented intensity changes resulting from standing-wave resonances through positioning of the loudspeaker and by constraining the animals’ position. We also compensated for slight differences in loudspeaker sensitivity at different frequencies by precisely calibrating our pure tones at the level of the animals’ ear. Any residual intensity changes occurred only during the ramp and corresponded to at most ~6 dB SPL, which occurred when the frequency shifted from 16 to 12 kHz. Similarly-sized intensity changes have been shown to be too small to function as a prepulse in mice, especially over a 1 ms time scale (Aubert et al., 2006; Pietropaolo and Crusio, 2009). In addition, we observed robust PPI to small frequency changes in which sound intensity during the ramp changed very little. For example, we observed ~45% inhibition for a frequency change of 3.3%, during which the intensity during the ramp changed by only 2.8 dB SPL. We therefore conclude that intensity changes also do not contribute to PPI, but that the observed inhibition reflects the detection of frequency changes. Finally, we used ABRs (n = 4) to confirm that hearing thresholds in our CD1 mice were frequency-independent over the range of frequencies used for discrimination testing (10-18 kHz; data not shown), as reported previously (Shone et al., 1991). Thus, we are confident that a change in tone audibility and therefore perceived intensity, also did not contribute to the observed inhibition.
Our results demonstrate that frequency discrimination can be efficiently assessed in mice using PPI. By comparing the inhibition elicited by frequency changes of various magnitudes, we could determine discrimination thresholds. Using PPI, we found a frequency discrimination threshold of 2.4% in 5 week-old female CD1 mice. In a previous operant conditioning study of frequency discrimination, 16-20 week-old male NMRI mice showed discrimination thresholds of ~0.8% for a 15 kHz reference frequency at 70 dB, with threshold defined as a 66.6% correct response rate (Ehret, 1975). However, this procedure required ~20 days of training followed by 4 days of testing. Almost half of the animals also had to be excluded due to an inability to perform the task. In contrast, our PPI results from each animal were obtained in a single testing session, in less than one hour. Although differences in method and definition of threshold make direct comparisons difficult, our discrimination threshold using PPI is thus comparable to, or only slightly larger than that of mice (0.8%; Ehret, 1975) and cats (1.25-5%; Butler et al., 1957) tested using operant conditioning, as well as humans tested with an adaptive two-interval forced-choice procedure (~0.9% for an 8 kHz reference frequency at ~63.5 dB; Wier et al., 1977). The 64 ± 4% inhibition to a 25% frequency change we measured is also consistent with the 60% inhibition reported in a previous PPI study that used a comparably-sized transition between one octave-wide bands of random noise (Stitt et al., 1974), though this study did not test any additional frequency changes.
As a test of frequency discrimination, PPI provides a useful tool to investigate the neuronal mechanisms underlying pitch discrimination, its maturation during development, and its relationship to tonotopic organization. The straightforward design of the apparatus, together with the robustness of the reflexive behavioral response, provides an efficient and robust method of assessing frequency discrimination, accessible to those who do not specialize in animal training.
Acknowledgements
We thank Courtney Pedersen for help with ABRs. This work was supported by the National Institute on Deafness and Other Communication Disorders Grant RO1 04199 (K.K.), a National Institutes of Health Basic Neuroscience Predoctoral Training Grant T32 NS007433 (A.C.), and a National Science Foundation IGERT Training Grant DGE 0549352 (A.C.).
Role of the funding source
The NIH and NSF provided financial support for the research, but were not involved in study design, data collection, analysis, and interpretation, writing of the manuscript, or in the decision to submit the work for publication.
Footnotes
Source-code available upon request.
Disclosure Statement
A.C., T.N. and K.K. declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahissar M, Protopapas A, Reid M, Merzenich MM. Auditory processing parallels reading abilities in adults. Proc Natl Acad Sci U S A. 2000;97:6832–7. doi: 10.1073/pnas.97.12.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P, Housel N, Yee S, Zenczak C, Ison J. Abstract #365: Response to sweeping frequency changes in the CBA/CaJ mouse model of presbycusis; Thirty-first Annual Midwinter Research Meeting of the Association for Research in Otolaryngology: Phoenix; AZ. 2008. [Google Scholar]
- Aubert L, Reiss D, Ouagazzal AM. Auditory and visual prepulse inhibition in mice: parametric analysis and strain comparisons. Genes Brain Behav. 2006;5:423–31. doi: 10.1111/j.1601-183X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Butler RA, Diamond IT, Neff WD. Role of auditory cortex in discrimination of changes in frequency. J Neurophysiol. 1957;20:108–20. doi: 10.1152/jn.1957.20.1.108. [DOI] [PubMed] [Google Scholar]
- Carlson S, Willott JF. The behavioral salience of tones as indicated by prepulse inhibition of the startle response: relationship to hearing loss and central neural plasticity in C57BL/6J mice. Hear Res. 1996;99:168–75. doi: 10.1016/s0378-5955(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Hulse SH. The Comparative psychology of audition: perceiving complex sounds. L. Erlbaum Associates; Hillsdale, N.J.: 1989. [Google Scholar]
- Ehret G. Frequency and intensity difference limens and nonlinearities in the ear of the housemouse (Mus musculus) Journal of Comparative Physiology. 1975;102:321–36. [Google Scholar]
- Floody OR, Ouda L, Porter BA, Kilgard MP. Effects of damage to auditory cortex on the discrimination of speech sounds by rats. Physiol Behav. 2010;101:260–8. doi: 10.1016/j.physbeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengel RW. Temporal effects in frequency discrimination by hearing-impaired listeners. J Acoust Soc Am. 1973;54:11–5. doi: 10.1121/1.1913550. [DOI] [PubMed] [Google Scholar]
- Halliday LF, Bishop DV. Frequency discrimination and literacy skills in children with mild to moderate sensorineural hearing loss. J Speech Lang Hear Res. 2005;48:1187–203. doi: 10.1044/1092-4388(2005/083). [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–89. [PubMed] [Google Scholar]
- Ison JR, Hoffman HS. Reflex modification in the domain of startle: II. The anomalous history of a robust and ubiquitous phenomenon. Psychol Bull. 1983;94:3–17. [PubMed] [Google Scholar]
- Ison JR, McAdam DW, Hammond GR. Latency and amplitude changes in the acoustic startle reflex of the rat produced by variation in auditory prestimulation. Physiol Behav. 1973;10:1035–9. doi: 10.1016/0031-9384(73)90185-6. [DOI] [PubMed] [Google Scholar]
- Ison JR, Pinckney LA. Reflex inhibition in humans: sensitivity to brief silent periods in white noise. Percept Psychophys. 1983;34:84–8. doi: 10.3758/bf03205900. [DOI] [PubMed] [Google Scholar]
- Kurt S, Ehret G. Auditory discrimination learning and knowledge transfer in mice depends on task difficulty. Proc Natl Acad Sci U S A. 2010;107:8481–5. doi: 10.1073/pnas.0912357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner DS, Hammond GR, Springer CP, Ingham KM, Mekilo AM, Bodison PR, Aranda MT, Shawaryn MA. Parameters affecting gap detection in the rat. Percept Psychophys. 1993;54:395–405. doi: 10.3758/bf03205275. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Freyman RL. Psychometric functions for frequency discrimination from listeners with sensorineural hearing loss. J Acoust Soc Am. 1986;79:799–805. doi: 10.1121/1.393470. [DOI] [PubMed] [Google Scholar]
- Ono K, Kudoh M, Shibuki K. Roles of the auditory cortex in discrimination learning by rats. Eur J Neurosci. 2006;23:1623–32. doi: 10.1111/j.1460-9568.2006.04695.x. [DOI] [PubMed] [Google Scholar]
- Parisi T, Ison JR. Development of the acoustic startle response in the rat: ontogenetic changes in the magnitude of inhibition by prepulse stimulation. Dev Psychobiol. 1979;12:219–30. doi: 10.1002/dev.420120305. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Crusio WE. Strain-Dependent Changes in Acoustic Startle Response and its Plasticity Across Adolescence in Mice. Behav Genet. 2009 doi: 10.1007/s10519-009-9291-y. [DOI] [PubMed] [Google Scholar]
- Pilz PK, Schnitzler HU, Menne D. Acoustic startle threshold of the albino rat (Rattus norvegicus) J Comp Psychol. 1987;101:67–72. [PubMed] [Google Scholar]
- Stitt CL, Hoffman HS, Marsh R, Boskoff KJ. Modification of the rat’s startle reaction by an antecedent change in the acoustic environment. J Comp Physiol Psychol. 1974;86:826–36. doi: 10.1037/h0036419. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–95. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Wier CC, Jesteadt W, Green DM. Frequency discrimination as a function of frequency and sensation level. J Acoust Soc Am. 1977;61:178–84. doi: 10.1121/1.381251. [DOI] [PubMed] [Google Scholar]
- Willott JF. The Auditory psychobiology of the mouse. C.C. Thomas; Springfield, Ill.: 1983. [Google Scholar]
- Willott JF. Handbook of mouse auditory research : From behavior to molecular biology. CRC Press; 2001. [Google Scholar]