Abstract
We recently reported that the endogenous ATP-binding cassette transporter (ABC) A7 strongly associates with phagocytosis, being regulated by sterol regulatory element binding protein 2. We therefore examined the effect of statins on phagocytosis in vitro and in vivo through the SREBP-ABCA7. Phagocytosis was found to be enhanced by pravastatin, rosuvastatin and simvastatin and cyclodextrin in J774 macrophages, as cellular cholesterol was reduced and expressions of the cholesterol-related genes were modulated, including an increase of ABCA7 mRNA and decrease of ABCA1 mRNA. Conversely, knock-down of ABCA7 expression by siRNA ablated enhancement of phagocytosis by statins. In vivo, pravastatin enhanced phagocytosis in wild-type mice, but not in ABCA7-knockout mice. We thus concluded that statins enhance phagocytosis through the SREBP-ABCA7 pathway. These findings provide a molecular basis for enhancement of the host-defense system by statins showing that one of their “pleiotropic” effects is in fact achieved through their reaction to a primary target.
Keywords: HMG-CoA reductase inhibitor, Statin, Phagocytosis, ABCA7, Cholesterol, Atherosclerosis
1. Introduction
Statins, competitive inhibitors of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, have been marketed as cholesterol lowering drugs for more than 20 years. They lower plasma low-density lipoprotein (LDL) by up-regulation of the LDL receptor gene through the sterol regulatory element binding protein (SREBP) system that senses cellular cholesterol levels [1]. The clinical trials for secondary and primary prevention of coronary heart diseases [2, 3], established the benefits of these drugs on mortality and morbidity. While many other studies have also provided evidence for the clinical benefits of lowering LDL by statin treatment to prevent atherosclerotic diseases, a new viewpoint of statin treatment has been introduced by some authors, suggesting that statins have extra beneficial effects beyond lowering LDL [4-7], which are attributed to various types of anti-inflammatory or anti-oxidative effects through regulation of proinflammatory mediators [8, 9]. These effects may be interpreted as the consequence of inhibition of the reactions in the mevalonate cascade, such as isoprenylation of the membrane proteins including Ras [10]. Involvement of Rho GTPases has been proposed for enhancement of phagocytosis by statins [11]. The molecular pharmacological grounds for these effects remain quite ambiguous.
Phagocytosis is one of the fundamental functions of animal cells and is an important responsive reaction to infection, injury and apoptosis. Some ATP-binding cassette transporter (ABC) proteins have been reported to affect the phagocytic function of cells [12-17]. ABCA7, a membrane protein and highly homologous to ABCA1 [18] that mediates biogenesis of high-density lipoprotein (HDL) [19-21], plays no significant role in the generation of HDL [16, 22, 23] and is actively associated with phagocytosis [16, 17]. Interestingly, the ABCA7 gene is regulated by sterol regulatory element binding protein 2 [16] in the opposite direction to the liver X receptor-mediated regulation of ABCA1 with respect to cellular cholesterol [24-26]. We recently demonstrated that helical apolipoproteins of HDL enhanced phagocytosis by inhibiting degradation of ABCA7 [27]. These findings shed light on the question of the relationship between sterol homeostasis and the host defense system. Here, we examine the effect of statins on phagocytosis in vitro and in vivo based on the hypothesis that statin treatment enhances the SREBP-ABCA7 pathway.
2. Methods
2.1. Reagents and antibodies
Pravastatin and rosuvastatin were provided by Daiichi-Sankyo and AstraZeneca, respectively. Simvastatin was purchased from Sigma (S6196) and activated as previously described [28]. Cytochalasin D was purchased from Merck (250255). 2-Hydroxypropyl)-β-cyclodextrin and anti β-actin antibody were purchased from Sigma (C0926 and A5441, respectively). Monoclonal antibodies against mouse ABCA1 (MABI98-4) and ABCA7 (MABI97-17) were generated at the MAB Institute (Yokohama, Japan) as described previously[16, 29]. Apolipoprotein A-I (apoA-I) was isolated from human plasma HDL [30].
2.2 Animals and Cells
ABCA7-knockout mice were generated and backcrossed to a C57BL/6 background [22] and bred on the same background [27]. Mouse resident peritoneal macrophages were obtained as described previously [27]. The animal experimental protocols were approved by the institutional animal welfare committee. J774 cells and Jurkat cells were obtained from the Riken Cell Bank and cultured in 10% fetal calf serum (FCS)/Roswell Park Memorial Institute (RPMI) 1640 medium. Cells were maintained at 37 °C in humidified atmosphere of 5% CO2.
2.3. RNA interference
SiRNAs (Stealth Select RNAi) for ABCA1 and ABCA7 were purchased from Invitrogen. They were transfected by nucleofection (Nucleofector Kit V for J774; AMAXA biosystems). Two different siRNAs were tested and yielded similar results. The data presented represent composite results.
2. 3. Lipid assay
J744 cells were subcultured in a 6-well tray in 10% FCS/RPMI1640 medium for 1 day. The cells were washed with phosphate buffered saline (PBS) and incubated overnight in 1 ml/well of RPMI1640 medium in the presence of either 50 μM pravastatin, 5 μM rosuvastatin or 5 mM cyclodextrin. The doses were found by monitoring increase of ABCA7 expression described below. Cellular lipid was determined by colorimetric enzymatic assay [31]. Cellular protein was dissolved in radioimmunoprecipitation assay (RIPA) buffer, and determined with the BCA Protein Assay Kit (Pierce).
2.4. Quantitative analysis of mRNA
Total RNA was isolated by ISOGEN (Wako) and reverse-transcribed by SuperScript III (Invitrogen) with oligo dT primers. Quantitative expression analysis by real-time reverse transcription polymerase chain reaction (PCR) was performed in a StepOnePlus Real-Time PCR system (Applied Biosystems) using SYBR Green technology. The following PCR primers were used for amplification of mouse RNAs: ABCA7, 5′-GCC AGT ATG GAA TCC CTG AA-3′ (forward) and 5′-ATG GAG ACA CCA GGA ACC AG-3′ (reverse); ABCA1, 5′-AAG GGG TGG TGT TCT TCC TC-3′ (forward) and 5′-CCT CAC ATC CTC ATC CTC GT-3′ (reverse); LXRα, 5′-GAA ACT GAA GCG GCA AGA AGA-3′ (forward) and 5′-AAG TCC TTG AGG AAG GTG ATG C-3′ (reverse); SREBP1, 5′-AAC CAG AAG CTC AAG CAG GA-3′ (forward) and 5′-TCA TGC CCT CCA TAG ACA CA-3′(reverse); SREBP2, 5′-GTG GAG CAG TCT CAA CGT CA-3′(forward) and 5′- TGG TAG GTC TCA CCC AGG AG-3′ (reverse); LDL receptor, 5′-TCC AAT CAA TTC AGC TGT GG-3′(forward) and 5′-GAG CCA TCT AGG CAA TCT CG-3′ (reverse); β- actin, 5′- CTG TAT TCC CCT CCA TCG TG-3′ (forward) and 5′-AGG TGT GGT GCC AGA TCT TC-3′ (reverse).
2.5. Quantitative phagocytosis assay
We used fluorescent polystyrene microspheres, 1 μm diameter, having carboxylate groups on the surface (15702, Polysciences) that could be activated for the covalent coupling of proteins to quantify in vitro phagocytosis [27]. Alternatively, Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), and zymosan A conjugate of Alexa Flour 488 (S23371, E13231, Z23373, Invitrogen, respectively) were used as fluorescent phagocytosis probes [27] and Jurkat cells with apoptosis induced by ultraviolet light were used as physiological phagocytosis probes. Polystyrene bead uptake was estimated in the presence and absence of 50 μM pravastatin or 5 μM rosuvastatin as described in our previous paper [27]. Validity of the method was confirmed by inhibition of the phagocytic reaction by cytochalasin D, a known phagocytosis inhibitor [27]. For S. aureus, E. coli, and zymosan A uptake, J744 cells were cultured in a 24-well tray as above and incubated with 6 or 12 × 106 /well of S. aureus, 6 × 106 /well of E. coli, 2 × 106 /well of zymosan for 1 h. Cells were washed thoroughly and carefully with PBS twice, and treated with 200 μl of 1 mg/ml lysozyme for 25 min at 37°C. After washing cells, fluorescence intensity was measured in a plate reader FL600, BioTek Inc. For the apoptotic cell uptake assay, J774 cells and Jurkat cells were stained with CellTracker (Invitrigen, C7025 and C34552, respectively). Apoptosis was induced in Jurkat cells by 15 minutes of exposure to ultraviolet light [32]. J744 cells were cultured in a 24-well tray as above and incubated with the 2.5 × 106 /well of the apoptosis -induced Jurkat cells for 1 h. Cells were washed with PBS three times, and fixed with 4% paraformaldehyde. Photographs were taken at 2 positions in each well at 40-fold magnification in a fluorescence microscope BZ-800 (Keyence) with a Plan Fluor ELWD 20x lens (Nikon). Total J774 cells and those that engulfed Jurkat cells were counted in the fields, respectively, and the ratio of the cells engulfing Jurkat was calculated as an “uptake” index being standardized for control cells without treatment. Confidence of this method was validated by the apoAI-mediated enhancement as a positive control and cytochalasin D as a negative control [33].
2.6. In vivo phagocytosis assay
Carbon ink (Platinum Carbon Ink Black) was diluted 5-fold with PBS and injected into the mouse peritoneal cavity at 10 μl/g body weight [27]. Pravastatin was given intraperitoneally by mixing the carbon ink solution with 10 μM pravastatin or PBS with 1:1 volume just before injection, or subcutaneously by injecting 10 μM pravastatin at 5 μl/g body weight in the abdominal area. After overnight starvation of mice, peritoneal macrophages were recovered. Over 100 cells of each sample were counted and the phagocytosis index was calculated as the relative number of cells that engulfed carbon ink particles per total cells standardized for the wild type mice without treatment.
2.7. Other methods
Samples for polyacrylamide gel electrophoresis were treated with RIPA buffer containing protease inhibitor cocktail (Sigma P2714) unless described otherwise. Western blotting was performed as described previously [27] by using loading controls of β-actin as a housekeeping cellular protein. The bands were digitally scanned by an LAS- 3000min (Fuji Film) and analyzed by software, Multi Gauge v.3.0 (Fuji Film). Data were statistically analyzed by t-test or one-way ANOVA with Tukey’s test. P < 0.05 was accepted as statistically significant.
3. Results
3.1. Cell cholesterol and phagocytosis
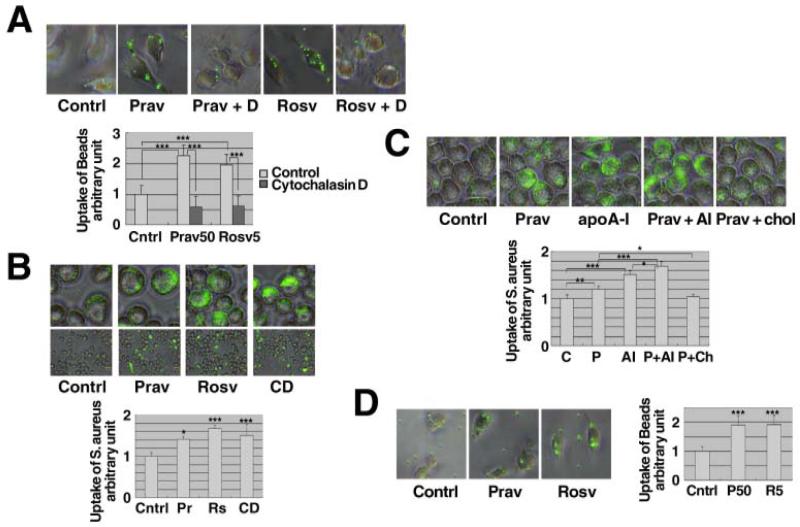
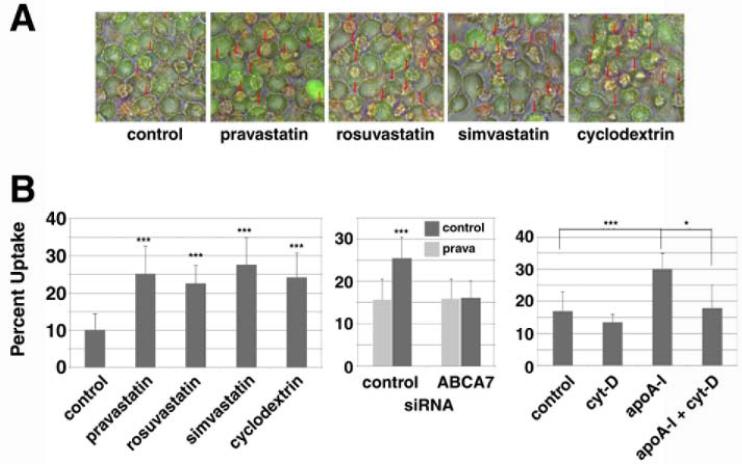
Influence of cholesterol metabolism on phagocytotic uptake was assessed in J774 macrophages by measuring the effect that statin treatment has on the ability of these cells to take up fluorescent microsphere beads. Pravastatin and rosuvastatin significantly enhanced the uptake and these effects were blocked by cytochalasin D, an inhibitor of phagocytosis (Figures 1A). Likewise, statins significantly stimulated phagocytotic uptake of S. aureus, as did depleting cellular cholesterol by treating the cells with cyclodextrin (Figure 1B). Moreover, apoA-I significantly enhanced the phagocytosis as previously reported [27] and this was further increased by pravastatin, effects that were canceled by re-supplying cholesterol to the cells (Figure 1C). Finally, the enhancement of phagocytosis by statins was reproduced in peritoneal macrophages prepared from wild-type mice (Figure 1D).
Figure 1. Pravastatin and rosuvastatin enhance phagocytosis.
A: J774 cells were subcultured as 4 × 103 cells/well for one day. After overnight incubation with and without 50 μM pravastatin (Prav) or 5 μM rosuvastatin (Rosv), quantitative phagocytosis assay for polystyrene beads was performed. The results were expressed as relative phagocytic activity to the blank control (without statins, 0.02 % BSA). Cytochalasin D (D) 10 μM was added to inhibit. B and C: J774 cells were seeded as 5×105 cells/well for one day. After overnight incubation with 50 μM pravastatin, 5 μM rosuvastatin, 5 mM cyclodextrin (CD) or 10 μg/mL apoA-I (apoA-I or AI), and 0.3 mM cholesterol (chol), quantitative phagocytosis assay for 3 × 106 (B) or 6×106 (C) S. aureus was performed. D: Peritoneal macrophages were collected from wild-type mice. The cells were subcultured in a 96-well tray as 5 × 104 cells/well for one day. After overnight incubation with and without 50 μM pravastatin or 5 μM rosuvastatin, quantitative phagocytosis assay for polystyrene beads was performed. The data in the graphs represent the mean ± SD for 8 samples. Statistical significance is indicated as * for P < 0.05, ** for P < 0.01 and *** for P < 0.001 from the control or between the data indicated.
3.2. Expression of ABCA7
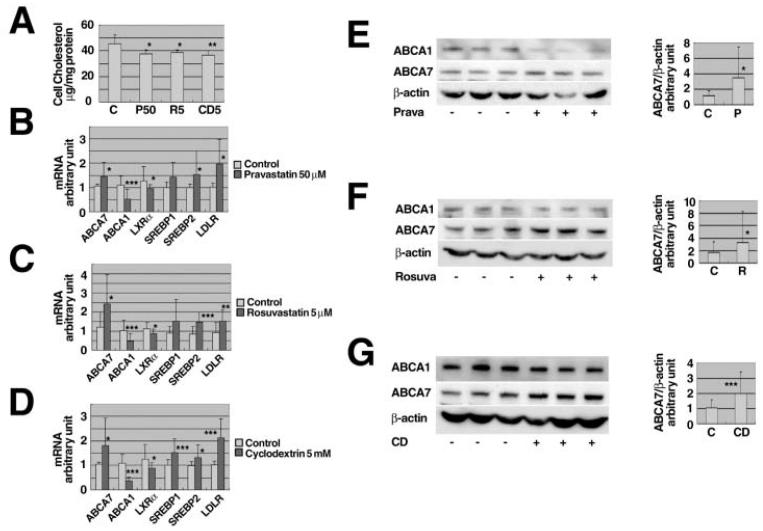
Expression of ABCA7 was down-regulated by specific siRNA in J774 as we previously reported [16, 27] and described in the Methods section. Treatment of the J774 cells with pravastatin, rosuvastatin and cyclodextrin all significantly reduced cell cholesterol, as expected (Figure 2A). A decrease of cell cholesterol was reflected in changes of expression of the genes related to sterol homeostasis shown in Figures 2B, 2C and 2D. As we previously demonstrated [16], expression of the ABCA1 gene was decreased while ABCA7 gene expression was increased, being consistent with the enhancement of phagocytosis by statins and cyclodextrin. Expression of ABCA7 protein was shown increased in these conditions while ABCA1 protein decreased (Figures 2EFG).
Figure 2. Effects of statins on cellular cholesterol level, expression of the sterol-related genes and ABCA7 protein level.
A: J774 cells were subcultured in a 6-well tray as 1 × 106 cells/well for one day, and incubated overnight with and without 50 μM pravastatin (P50), 5 μM rosuvastatin (R5), or 5 mM cyclodextrin (CD5). Cell lipid was extracted and cholesterol was measured. B - G: J774 cells subcultured as above were incubated overnight with and without 50 μM pravastatin (B, E), 5 μM rosuvastatin (C, F), and 5 mM cyclodextrin (D, G). mRNA was extracted and quantitative expression analysis was performed for ABCA1, ABCA7, LXRα, SREBP1, SREBP2, the LDL receptor (LDLR) and standardized for β-actin (B, C, D). Results were expressed as fold change relative to the each control. The protein was extracted and analyzed for ABCA7 by Western blotting (E, F, G). Data represent the mean ± SD for six (A) and eighteen (B - G) samples. Statistical significance is indicated as * for P < 0.05, ** for P < 0.01 and *** for P < 0.001 against each control.
3.3. Phagocytosis and ABCA7
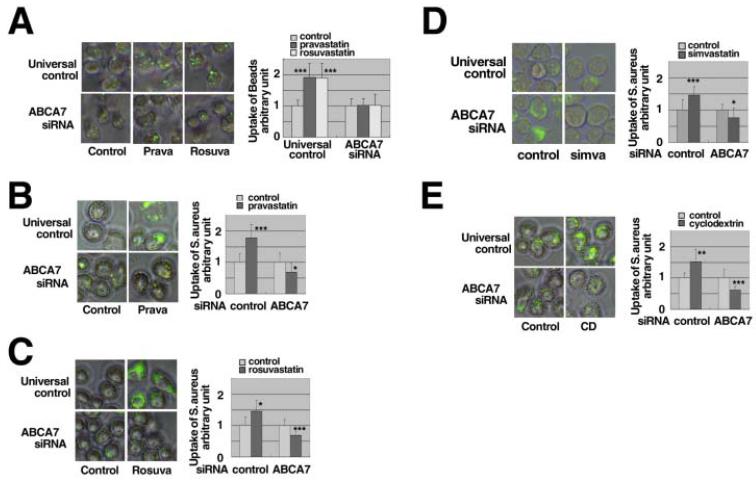
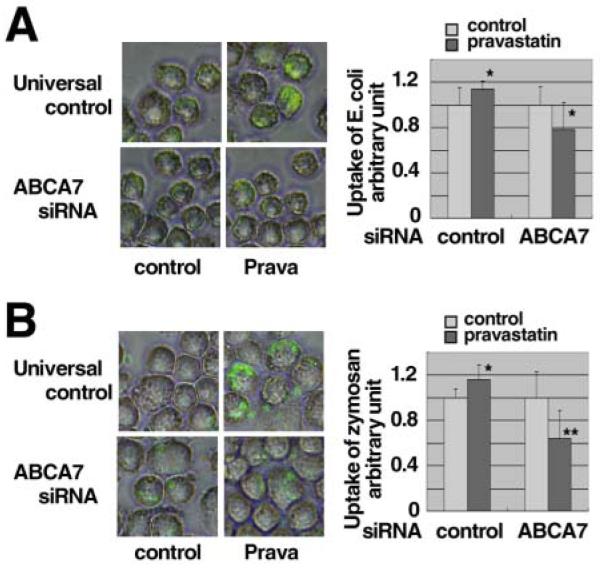
The enhancement of phagocytosis of fluorescent microspheres by pravastatin and rosuvastatin was ablated by knockdown of the ABCA7 gene expression (Figure 3A). The enhancement of phagocytosis of S.aureus by pravastatin, rosuvastatin, simvastatin and cyclodextrin was ablated or rather attenuated in the cells with knockdown of ABCA7 (Figures 3BCDE). Similar results were demonstrated for the uptake of E.coli and zymosan in the presence of pravastatin (Figure 4). Finally, we examined phagocytosis in Jurkat cells with apoptosis induced by ultraviolet light, a physiological model target of apoptotic cells, (Figure 5). Statins and cyclodextrin were shown to enhance phagocytosis of this model target through ABCA7.
Figure 3. Effects of statins on phagocytosis when the ABCA7 gene was knocked-down.
A: SiRNA specific for ABCA7 was transfected into J774 cells of 3.2 × 106 cells/cuvette. The cells were subcultured in a 96-well tray as 1 × 105 cells/well. After overnight incubation with 50 μM pravastatin (Prava) and 5 μM rosuvastatin (Rosv), quantitative phagocytosis for polystyrene beads assay was performed. B, C, D and E: SiRNA specific for ABCA7 was transfected into J774 cells at a density of 8 × 106 cells/cuvette. The cells were subcultured in a 24-well tray as 1 × 106 cells/well. After overnight incubation with and without 50 μM pravastatin (B), 5 μM rosuvastatin (C), 10 μM simvastatin (D) and 5 mM cyclodextrin (E), quantitative phagocytosis assay of 3 × 106 of S.aureus was performed. The results in graphs are shown as phagocytic activity relative to each control (without statins and cyclodextrin, 0.02 % BSA). The data represent the mean ± SD for sixteen (A) and eight (B, C, D, E) samples. Statistical significance is indicated as * for P < 0.05, ** for P < 0.01 and *** for P < 0.001 against each control.
Figure 4. Effects of pravastatin on phagocytosis when the ABCA7 gene was knocked-down.
SiRNAs specific for ABCA7 was transfected into J774 of 8 × 106 cells/cuvette. The cells were subcultured in a 24-well tray as 1 × 106 cells/well. After overnight incubation with and without 50 μM pravastatin, quantitative phagocytosis assay of 3 × 106 for E. coli (A) and 2 × 105 zymosan (B) was performed. The results in graphs were shown as phagocytic activity relative to each control (without pravastatin, 0.02 % BSA). The data represent the mean ± SD for sixteen samples. Statistical significance is indicated as * for P < 0.05 and ** for P < 0.01 against each control.
Figure 5. Statins enhance phagocytosis of apoptosis cells by ABCA7.
A: J774 cells were subcultured as 5 × 105 cells/well for one day. After overnight incubation with 50 μM pravastatin, 5 μM rosuvastatin, 10 μM simvastatin or 5 mM cyclodextrin, a quantitative phagocytosis assay for apoptotic Jurkat cells was performed, being shown as representative photos with phagocytosis indicated by red arrows. B: Left, the results of quantitative analysis for the experiments of Figure 5A. Center, knock-down of ABCA7 and phagocytosis of apoptotic cells. ABCA7-specific siRNA was transfected to J774 cells at a density of 8 × 106 cells/cuvette. The cells were subcultured in a 24-well tray as 1 × 106 cells/well. After overnight incubation with and without 50 μM pravastatin, quantitative phagocytosis assay for apoptotic cells was performed. Right, enhancement of phagocytosis of apoptosis cells by apoAI and its inhibition by cytochalasin D. J774 cells were subcultured as 5 × 105 cells/well for one day. After overnight incubation with 10 μg/ml apoAI, 10 μM cytochalasin D, and both, quantitative phagocytosis assay for apototic cells was performed. The data represent the mean ± SD for six samples. Statistical significance is indicated as * for P < 0.05 and *** for P < 0.001.
3.4. Effect of statins in vivo
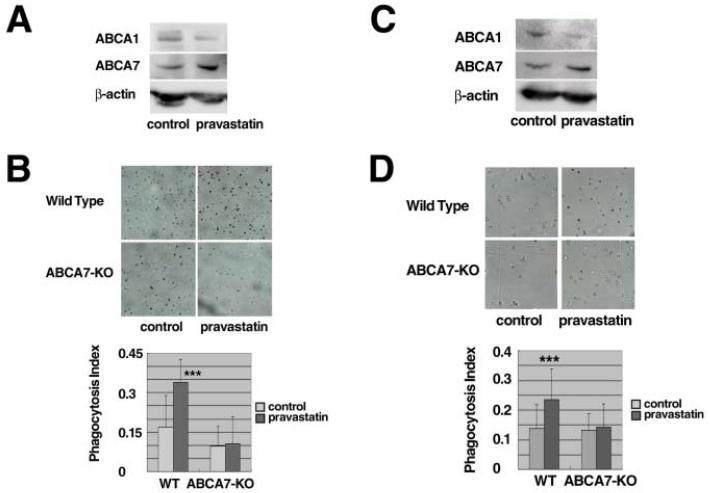
The in vivo role of ABCA7 in the effect of pravastatin on phagocytosis was examined by measuring phagocytic activity of macrophages in the mouse peritoneal cavity. Carbon microparticles were injected into the peritoneal cavity of wild type and ABCA7-knockout mice, with and without pravastatin, given intraperitoneally (Figures 6AB) or subcutaneously (Figures 6CD), and the uptake of the particles was determined microscopically as described in the Methods section. No apparent effect of pravastatin was observed on plasma cholesterol levels, 73.9 ± 9.9 vs. 59.0 ± 15.9 mg/dL for the intraperitoneal dose and 76.1 ± 13.1 vs. 79.0 ± 12.9 mg/dL for the subcutaneous dose in the normal mice, and 49.5 ± 6.8 vs. 48.6 ± 7.6 mg/dL for the intraperitoneal dose and 81.1 ± 9.6 vs. 70.0 ± 10.6 mg/dL for the subcutaneous dose in ABCA7-knockout mice. Pravastatin increased expression of ABCA7 in the peritoneal cells of the wild type mice while it decreased expression of ABCA1 (Figures 6AC). The uptake of the carbon particles by the peritoneal cells was increased in the wild-type mouse by administration of pravastatin. This effect was completely negative in the ABCA7-knockout (Figures 6BD).
Figure 6. Pravastatin enhances phagocytosis of peritoneal cells in vivo.
A, C: Wild type mice were treated with and without pravastatin by peritoneal (A) and subcutaneous (C) injection as described in the text. Peritoneal cells were collected from the mice, two mice for one sample, and analyzed for proteins by Western blotting immediately after collection. B, D: Phagocytic activity was measured directly in the peritoneal cavity of the mice in vivo. Diluted carbon ink with or without pravastatin was given intraperitoneally (B) or subcutaneously (D). After overnight starvation, peritoneal macrophages were recovered and over 100 cells were counted for calculation of the phagocytosis index as the relative number of cells that engulfed carbon ink particles. Data represents mean ± SD of n = 4 for wild type and ABCA7-knockout mice. Statistical significance is indicated as *** for P < 0.001 against each control.
4. Discussion
The findings of this study are summarized as follows: 1) Statins decrease cellular cholesterol and increase expression of ABCA7; 2) Statins enhance cellular phagocytic function against various targets, including apoptotic cells, through the increase of the ABCA7 expression, shown in vitro and in vivo; 3) The results thus indicate direct involvement of statin treatment in the regulation of the host defense system through modulation of the ABCA7 function.
Pharmacological reduction of LDL has been shown to reduce the risk of atherosclerosis; this was initially using bile acid sequestrants [34, 35] and later statins [3, 36]. Reduction of LDL is the primary effect of statins and the main therapy for a decrease of atherosclerosis risk [37]. On the other hand, it has been suggested that statins have additional, off-target effects to LDL-lowering such as anti-inflammatory effects [38], anti-lipid-oxidative effects [39], stabilization of atherosclerotic plaques [40], and regulation of cell proliferation [41]. Many authors have argued that these effects are related to the suppression of the release of proinflammatory mediators caused by inhibition of HMG-CoA reductase [8, 9] or regulation of phagocytosis thorough Rho GTPases [11], but others have proposed that these effects are independent of the main pharmacological action. These experimental findings may have clinical implications such that the extent of the clinical benefits of statins may go beyond the level expected from the magnitude of the LDL reduction. In the Cholesterol and Recurrent Events (CARE) study, for example, pravastatin-mediated lowering of cholesterol and triglycerides appeared to account for most but not all of the benefits [4]. Analysis of the results of other trials, such the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) study [5], the Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) trial [6], and the Heart Protection Study (HPS) [7], suggested that part of the effect of statins in certain subjects may not be directly dependent on lowering of LDL cholesterol. However, molecular grounds for these effects are obscure and based largely on circumstantial evidence. The results of this study, demonstrate that statin therapy to increase hepatic LDL receptor expression also enhances the phagocytotic reaction, not only in vitro but also in vivo, through the SREBP-ABCA7 pathway.
Phagocytosis is one of the most primitive but important host defense reactions. It has been proposed that macrophage apoptosis is associated with diminished cellularity and decreased progression in early atherosclerotic lesions where phagocytic clearance of apoptotic cells appears efficient. In late lesions, however, their phagocytic clearance may be impaired [42], leading to secondary necrosis of these cells and a proinflammatory response.
Thus, lesional phagocytes would safely clear apoptotic cells and play an important preventive role in the development of the unstable necrotic core in atherosclerosis. We discovered that ABCA7, highly homologous to ABCA1 a key protein for HDL biogenesis, is a major regulator for phagocytosis [16]. Expression of the ABCA7 gene is regulated by cellular sterol through the SREBP system in the opposite direction to the LXR-mediated regulation of the ABCA1 gene [16]. On the other hand, ABCA7 is stabilized by helical apolipoproteins similarly to ABCA1, resulting in an increase of phagocytosis [27]. Consequently, it is quite rational to assume that a decrease of cell cholesterol in its regulatory pool enhances phagocytosis and therefore statins increase this reaction. In our in vitro study, statins and cyclodextrin in fact reduced enhanced phagocytosis including the reaction targeting apoptotic cells through the SREBP-ABCA7 pathway [16]. These findings were reproduced in a mouse model indicating activation of this pathway, despite that the effect of statins on plasma cholesterol is limited due to induction of cholesterol biosynthesis and small contribution of LDL to plasma cholesterol. As far as comparing the effects of three statins, the effects do not depend on their physicochemical nature such as hydrophobicity (Figure 3). This is not entirely consistent with some previous reports that the effects of hydrophilic statins are limited on extrahepatic cells [43]. The difference may be resulted from length of exposure of cells to the drugs. Involvement of Toll-like receptors in the SREBP-ABCA7 system is unknown. It should be investigated whether this signaling pathway modulates expression of ABCA7.
Interestingly, statins and cyclodextrin rather suppressed phagocytic reactions against natural targets such as S. aureus, E coli and zymozan when ABCA7 expression was suppressed (Figures 3 and 4). This finding may indicate an additional factor(s) involved in regulation of phagocytosis by sterol homeostasis. The difference may also be due to the use of an artificial probe and natural organisms as a target of phagocytosis. The additional factor may therefore be involved in the processing and digestion of the phagocyted targets that could be up-regulated by cellular cholesterol. In the presence of ABCA7, this factor is overcome by the SREBP-ABCA7 system for overall phagocytotic reaction. Deletion of ABCA7 cancels cholesterol-dependent regulation of phagocytosis but not cholesterol-independent one, indicating a specific contribution of ABCA7 to phagocytosis. Lowering cholesterol reportedly increases membrane fluidity and results in enhancement of phagocytosis [44, 45], so that ABCA7 may contribute to this process.
The present findings confirm a direct relationship between cholesterol homeostasis and phagocytosis, and indicate that pharmacological modulation of cholesterol homeostasis could actually influence the phagocytic function in vitro and in vivo. The finding provides a molecular basis for “pleiotropic” effects of statins.
Acknowledgments
This study was supported by grants-in-aids from The Ministries of Education, Culture, Science, Sports and Technology, and of Health, Welfare and Labor of Japan, and by NIH grants to M.L.F (HL074136).
Footnotes
Present addresses: Noriyuki Iwamoto, Division of Atherosclerosis, Metabolism and Clinical Nutrition, National Cerebral & Cardiovascular Center Hospital, Suita, Osaka 565-8565, Japan; Shinji Yokoyama, Food and Nutritional Sciences, College of Bioscience and Biotechnology, Chubu University, 1200 Matsumoto-cho, Kasugai, 487-8501, Japan
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Rreferences
- [1].Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–8. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].4S, Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- [3].Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- [4].Sacks FM, Moye LA, Davis BR, Cole TG, Rouleau JL, Nash DT, Pfeffer MA, Braunwald E. Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the Cholesterol and Recurrent Events trial. Circulation. 1998;97:1446–52. doi: 10.1161/01.cir.97.15.1446. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. Jama. 2001;285:1711–8. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- [6].Simes RJ, Marschner IC, Hunt D, Colquhoun D, Sullivan D, Stewart RA, Hague W, Keech A, Thompson P, White H, Shaw J, Tonkin A. Relationship between lipid levels and clinical outcomes in the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Trial: to what extent is the reduction in coronary events with pravastatin explained by on-study lipid levels? Circulation. 2002;105:1162–9. doi: 10.1161/hc1002.105136. [DOI] [PubMed] [Google Scholar]
- [7].Collins, MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- [8].Rosenson RS, Tangney CC, Casey LC. Inhibition of proinflammatory cytokine production by pravastatin. Lancet. 1999;353:983–4. doi: 10.1016/S0140-6736(98)05917-0. [DOI] [PubMed] [Google Scholar]
- [9].Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100:2671–9. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6:541–55. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- [11].Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, Xiao YQ, Kench JA, Henson PM, Vandivier RW. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176:7657–65. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- [12].Wu YC, Horvitz HR. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell. 1998;93:951–60. doi: 10.1016/s0092-8674(00)81201-5. [DOI] [PubMed] [Google Scholar]
- [13].Williamson P, Schlegel RA. Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim Biophys Acta. 2002;1585:53–63. doi: 10.1016/s1388-1981(02)00324-4. [DOI] [PubMed] [Google Scholar]
- [14].Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- [15].Bared SM, Buechler C, Boettcher A, Dayoub R, Sigruener A, Grandl M, Rudolph C, Dada A, Schmitz G. Association of ABCA1 with syntaxin 13 and flotillin-1 and enhanced phagocytosis in tangier cells. Mol Biol Cell. 2004;15:5399–407. doi: 10.1091/mbc.E04-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Iwamoto N, Abe-Dohmae S, Sato R, Yokoyama S. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J Lipid Res. 2006;47:1915–27. doi: 10.1194/jlr.M600127-JLR200. [DOI] [PubMed] [Google Scholar]
- [17].Jehle AW, Gardai SJ, Li S, Linsel-Nitschke P, Morimoto K, Janssen WJ, Vandivier RW, Wang N, Greenberg S, Dale BM, Qin C, Henson PM, Tall AR. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol. 2006;174:547–56. doi: 10.1083/jcb.200601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kaminski WE, Orso E, Diederich W, Klucken J, Drobnik W, Schmitz G. Identification of a novel human sterol-sensitive ATP-binding cassette transporter (ABCA7) Biochem Biophys Res Commun. 2000;273:532–8. doi: 10.1006/bbrc.2000.2954. [DOI] [PubMed] [Google Scholar]
- [19].Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–51. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- [20].Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr., Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–45. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- [21].Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–5. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- [22].Kim WS, Fitzgerald ML, Kang K, Okuhira K, Bell SA, Manning JJ, Koehn SL, Lu N, Moore KJ, Freeman MW. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem. 2005;280:3989–95. doi: 10.1074/jbc.M412602200. [DOI] [PubMed] [Google Scholar]
- [23].Linsel-Nitschke P, Jehle AW, Shan J, Cao G, Bacic D, Lan D, Wang N, Tall AR. Potential role of ABCA7 in cellular lipid efflux to apoA-I. J Lipid Res. 2005;46:86–92. doi: 10.1194/jlr.M400247-JLR200. [DOI] [PubMed] [Google Scholar]
- [24].Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–5. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- [26].Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–9. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- [27].Tanaka N, Abe-Dohmae S, Iwamoto N, Fitzgerald ML, Yokoyama S. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J Lipid Res. 2010;51:2591–9. doi: 10.1194/jlr.M006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sadeghi MM, Collinge M, Pardi R, Bender JR. Simvastatin modulates cytokine-mediated endothelial cell adhesion molecule induction: involvement of an inhibitory G protein. J Immunol. 2000;165:2712–8. doi: 10.4049/jimmunol.165.5.2712. [DOI] [PubMed] [Google Scholar]
- [29].Hu W, Abe-Dohmae S, Tsujita M, Iwamoto N, Ogikubo O, Otsuka T, Kumon Y, Yokoyama S. Biogenesis of HDL by SAA is dependent on ABCA1 in the liver in vivo. J Lipid Res. 2008;49:386–93. doi: 10.1194/jlr.M700402-JLR200. [DOI] [PubMed] [Google Scholar]
- [30].Yokoyama S, Tajima S, Yamamoto A. The process of dissolving apolipoprotein A-I in an aqueous buffer. J Biochem. 1982;91:1267–72. doi: 10.1093/oxfordjournals.jbchem.a133811. [DOI] [PubMed] [Google Scholar]
- [31].Abe-Dohmae S, Suzuki S, Wada Y, Aburatani H, Vance DE, Yokoyama S. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry. 2000;39:11092–9. doi: 10.1021/bi0008175. [DOI] [PubMed] [Google Scholar]
- [32].Kiss RS, Elliott MR, Ma Z, Marcel YL, Ravichandran KS. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr. Biol. 2006;16:2252–8. doi: 10.1016/j.cub.2006.09.043. [DOI] [PubMed] [Google Scholar]
- [33].Tanaka N, Abe-Dohmae1 S, Iwamoto N, Fitzgerald ML, Yokoyama S. Helical Apolipoproteins of High-Density Lipoprotein Enhance Phagocytosis by Stabilizing ATP-Binding Cassette Transporter A7. J. Lipid Res. 2010;51:2591–9. doi: 10.1194/jlr.M006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. Jama. 1984;251:351–64. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- [35].The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. Jama. 1984;251:365–74. [PubMed] [Google Scholar]
- [36].Pedersen TR. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- [37].Pedersen TR, Olsson AG, Faergeman O, Kjekshus J, Wedel H, Berg K, Wilhelmsen L, Haghfelt T, Thorgeirsson G, Pyorala K, Miettinen T, Christophersen B, Tobert JA, Musliner TA, Cook TJ. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S) Circulation. 1998;97:1453–60. doi: 10.1161/01.cir.97.15.1453. [DOI] [PubMed] [Google Scholar]
- [38].Bisoendial RJ, Stroes ES, Kastelein JJ, Tak PP. Targeting cardiovascular risk in rheumatoid arthritis: a dual role for statins. Nat Rev Rheumatol. 2010;6:157–64. doi: 10.1038/nrrheum.2009.277. [DOI] [PubMed] [Google Scholar]
- [39].Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–72. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- [40].Mason RP, Walter MF, Jacob RF. Effects of HMG-CoA reductase inhibitors on endothelial function: role of microdomains and oxidative stress. Circulation. 2004;109:II34–41. doi: 10.1161/01.CIR.0000129503.62747.03. [DOI] [PubMed] [Google Scholar]
- [41].Bocan TM. Pleiotropic effects of HMG-CoA reductase inhibitors. Curr Opin Investig Drugs. 2002;3:1312–7. [PubMed] [Google Scholar]
- [42].Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–61. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- [43].Salman H, Bergman M, Djaldetti M, Bessler H. Hydrophobic but not hydrophilic statins enhance phagocytosis and decrease apoptosis of human peripheral bloos cells in vitro. Biomed. Pharmacother. 62:41–5. doi: 10.1016/j.biopha.2007.07.007. 20008. [DOI] [PubMed] [Google Scholar]
- [44].Rabini RA, Polenta M, Staffolani R, Tocchini M, Signore R, Testa I, Mazzanti L. Effect of hydroxymethylglutaryl-CoA reductase inhibitors on the functional properties of erythrocyte membranes. Exp. Mol. Pathol. 1993;59:51–7. doi: 10.1006/exmp.1993.1026. [DOI] [PubMed] [Google Scholar]
- [45].Koter M, Broncel M, Chojnowska-Jezierska J, Klikczynska K, Franiak I. The effect of atorvastatin on erythrocyte membranes and serum lipids in patients with type-2 hypercholesterolemia. Eur. J. Clin. Pharmacol. 2002;58:501–6. doi: 10.1007/s00228-002-0507-9. [DOI] [PubMed] [Google Scholar]