Abstract
Background
Ventral tegmental area (VTA) brain-derived neurotrophic factor (BDNF) contributes to time-dependent increases in cue-induced cocaine seeking after withdrawal (incubation of cocaine craving). Here, we studied the role of glial cell line– derived neurotrophic factor (GDNF) in incubation of cocaine craving because, like BDNF, GDNF provides trophic support to midbrain dopamine neurons.
Methods
We first trained rats to self-administer intravenous cocaine for 10 days (6 hours/d, cocaine injections were paired with a tone-light cue). We then manipulated VTA GDNF function and assessed cue-induced cocaine seeking in extinction tests after withdrawal from cocaine.
Results
VTA injections of an adeno-associated virus (AAV) vector containing rat GDNF cDNA (5 ×108 viral genomes) on withdrawal Day 1 increased cue-induced cocaine seeking on withdrawal days 11 and 31; this effect was not observed after VTA injections of an AAV viral vector containing red fluorescent protein (RFP). Additionally, VTA, but not substantial nigra (SN), GDNF injections (1.25 μg or 12.5 μg/side) immediately after the last cocaine self-administration session increased cue-induced drug seeking on withdrawal days 3 and 10; this effect was reversed by VTA injections of U0126, which inhibits the activity of extracellular signal-regulated kinases (ERK). Finally, interfering with VTA GDNF function by chronic delivery of anti-GDNF monoclonal neutralizing antibodies via minipumps (600 ng/side/d) during withdrawal Days 1–14 prevented the time-dependent increases in cue-induced cocaine seeking on withdrawal days 11 and 31.
Conclusions
Our results indicate that during the first weeks of withdrawal from cocaine self-administration, GDNF-dependent neuroad-aptations in midbrain VTA neurons play an important role in the development of incubation of cocaine craving.
Keywords: Drug self-administration, ERK, extinction, glial cell line–derived neurotrophic factor, growth factors, reinstatement, relapse, ventral tegmental area
Relapse to cocaine use in humans can occur after prolonged abstinence periods and is often precipitated by exposure to craving-provoking cocaine-associated cues (1). Gawin and Kleber (2) hypothesized that cue-induced cocaine craving increases over the first several weeks of abstinence and remains high over extended periods. We and others identified an analogous phenomenon in rats: time-dependent increases in cue-induced cocaine seeking over weeks after withdrawal from self-administered cocaine (3–5), a phenomenon termed “incubation of cocaine craving.” This incubation was primarily demonstrated in two procedures used to assess cue-induced reward seeking (6,7): a single extinction session in the presence of cocaine-associated cues (8–10) and discrete-cue-induced reinstatement after extinction of lever presses without these cues (3,11,12).
On the basis of earlier studies (13–15), we previously studied mesolimbic brain-derived neurotrophic factor’s (BDNF’s) role in incubation of cocaine craving. BDNF colocalizes with midbrain (ventral tegmental area [VTA] and substantia nigra [SN]) dopamine neurons (16) and supports their function (17,18). We found that BDNF levels in the VTA (the cell-body region of mesolimbic dopamine neurons), nucleus accumbens, and amygdala progressively increase over the first 90 withdrawal days (11). We also found that a single BDNF injection into the VTA at the end of the cocaine self-administration period enhances cue-induced drug seeking for up to 30 days after withdrawal (19). These findings implicate mesolimbic BDNF in incubation of cocaine craving (for a discussion of BDNF’s role in this incubation see Lu et al.) (19).
Another growth factor that is important for the survival and function of midbrain dopamine neurons is glial cell line–derived neurotrophic factor (GDNF) (20,21), a member of the GDNF-subfamily of ligands (22). Although there is evidence that stimulating mesolimbic BDNF potentiates rodents’ response to cocaine and cocaine cues (13,23–26), opposite effects were found for GDNF (27–30). GDNF VTA injections decrease, whereas local anti-GDNF antibodies injections increase, cocaine’s rewarding effects in a conditioned place preference (CPP) procedure (31). Additionally, striatal transplantation of simian virus-40 glial cells that produce GDNF or local injections of GDNF-conjugated nanoparticles decrease cocaine self-administration (32,33).
Given these findings and those on the inhibitory effect of VTA GDNF on alcohol reward (27,30,34,35), we explored whether VTA GDNF is also a negative modulator of the incubation of cocaine craving. We also studied the role of extracellular signal-regulated kinases (ERK), the activation of which contributes to the behavioral effects of abused drugs (36,37). Most relevant here is that VTA GDNF injections increase local ERK activity and this activity mediates the effect of GDNF on alcohol-taking behavior (34). Additionally, we found that inhibition of VTA ERK activity prevents the potentiation effect of VTA BDNF injections on incubation of cocaine craving (19). Here, we report results suggesting that, contrary to our hypothesis, during the first several weeks of withdrawal from cocaine, GDNF actions in the VTA are necessary for the development of incubation of cocaine craving.
Methods and Materials
Subjects
Subjects were male Long-Evans rats (Charles River, Raleigh, North Carolina; Experiments 1 and 2) and male Sprague-Dawley rats (Laboratory Animal Center, Peking University; Experiments 3–5) weighing 325–375 g. Rats were maintained on a reversed 12-hour light-dark cycle (lights off at 9 or 10 AM) with food and water freely available in the home cage. Procedures followed the “Principles of Laboratory Animal Care” (National Institutes of Health publication No. 86–23, 1996) and were approved by the institutional animal care committees. The self-administration and locomotor-activity chambers are described in the Supplement 1.
Surgical Procedures and Intracranial Injections
The intravenous and intracranial surgical procedures and the VTA or SN coordinates are based on our previous work (19,38) and are provided in the Supplement 1. Supplement 1 also provides the procedures for intracranial injections of AAV-GDNF and AAV- red fluorescent protein (RFP), GDNF, anti-GDNF monoclonal neutralizing antibodies, and U0126.
Construction of the GDNF and the RFP Viruses and Real-Time Polymerase Chain Reaction Measurement of GDNF mRNA Expression
The procedures for the construction of the GDNF and RFP viruses and real-time polymerase chain reactin (PCR) measurements are based on our previous work (39–41) and other reports (42,43), and are described in the Supplement 1.
Experiments
Experiments consisted of three phases: self-administration training, withdrawal, and extinction testing. Procedures were similar to those used in our recent studies (19,44–47) and those of others (8–10,48) in which incubation of craving was assessed in extinction tests. In these tests, rats are exposed to contextual cues previously associated with cocaine availability (e.g., house light), and responding to the previously active lever (or hole) results in contingent presentations of the discrete tone-light cue (which serves as a conditioned reinforcer during testing) (49). The details of the training phase, withdrawal phase, and test phase are provided in Supplement 1.
Experiment 1: Effect of VTA GDNF AAV Injections
We assessed the effect of increasing GDNF VTA levels by an AAV vector expressing rat GDNF on the time-dependent increases in cue-induced cocaine seeking. Two groups of Long-Evans rats (n = 8–10 per group) were used. Rats were infused with the AAV-GDNF or the AAV-RFP into the VTA on withdrawal day 1. Subsequently, the rats underwent repeated 1-hour extinction tests on withdrawal days 4, 11, and 31. In this experiment, and in Experiment 4, the duration of the extinction test was 1 hour to maximize our ability to detect time-dependent increases in extinction responding on withdrawal day 31 after exposing the rats to the cocaine cues in two earlier tests.
Experiment 2: Effect of VTA GDNF Injections: Long-Evans Rats
We assessed the effect of a single GDNF VTA injection, performed within 1–2 hours after the last cocaine self-administration, on the time-dependent increases in cue-induced cocaine seeking. Three groups of rats (n = 8–10 per group) were used. Rats were injected 1–2 hours after the last cocaine self-administration training session with either vehicle or GDNF (1.25 or 12.5 μg/site) into the VTA. After the injections, rats were brought to the animal facility. Subsequently, the rats underwent repeated extinction tests on withdrawal days 3 and 10. Tests consisted of two 1-hour sessions that were separated by 5 min.
Experiment 3: Effect of VTA or SN GDNF Injections: Sprague-Dawley Rats
In Experiment 2, we used Long-Evans rats that were trained to lever press for cocaine infusions and found that a single VTA injection of GDNF increased extinction responding on withdrawal days 3 and 10. Here, we assessed the generality of this effect to Sprague-Dawley rats that were trained to nose poke for cocaine infusions. We also assessed anatomical specificity by injecting GDNF into the nearby SN. Four groups of rats (n = 8–9 per group) were used. Rats were injected 2–4 hours after the last cocaine self-administration training session with either vehicle or GDNF (12.5 μg per site) into the VTA or SN. After the injections, rats were brought to the animal facility. Subsequently, rats underwent repeated extinction tests on withdrawal days 3 and 10. Tests consisted of two 1-hour sessions that were separated by 5 min.
Experiment 4: Effect of Chronic Delivery of Anti-GDNF Antibodies into the VTA
We further accessed the potential role of GDNF in incubation of cocaine craving by determining whether interfering with GDNF function in VTA by chronic delivery of anti-GDNF antibodies would prevent time-dependent increases in cue-induced cocaine seeking. Two groups of Sprague-Dawley rats (n = 8 per group) were used. After the last cocaine self-administration training session, rats were implanted with osmotic minipumps that contain either anti-GDNF monoclonal antibodies (600 ng/side/d) or mouse control immunoglobulin G (IgG). These pumps provided a constant infusion rate of .5-μL/hour for up to 14 days after implantation. The minipumps were removed after 14 days under anesthesia. Rats underwent repeated 1-hour extinction tests on withdrawal days 4, 11, and 31.
Experiment 5: Effect of U0126 VTA Injections on GDNF-Induced Potentiation of Extinction Responding
We assessed the role of the ERK signalling pathway in the potentiation effect of GDNF VTA injections on cue-induced cocaine seeking. For this purpose, we used U0126, which inhibits ERK phosphorylation (50). Four groups of Sprague-Dawley rats (n = 8–9 per group) were used in a 2 (GDNF dose: 0 or 12.5 μg) × 2 (U0126 dose: 0 or 1 μg) factorial design. Rats were injected into the VTA with U0126 or its vehicle (50% dimethylsulfoxide solution [DMSO]) 20 min before injections of GDNF or its vehicle; injections were performed 1–2 hours after the last training session. After the injections, rats were brought to the animal facility. Subsequently, the rats underwent repeated 2-hour extinction tests on withdrawal days 3 and 10. Tests consisted of two 1-hour sessions that were separated by 5 min.
Results
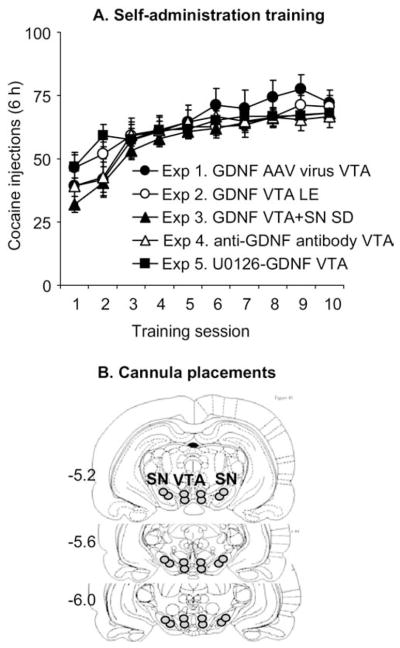
Figure 1 shows mean ± SEM number of infusions during the training phase of Experiments 1–5. These experiments were conducted over 6 years in different institutions with Long-Evans rats using levers (Experiments 1 and 2) or Sprague-Dawley rats using a nose-poke manipulandum (Experiments 3–5). Thus, we analyzed the training data using the between-subjects factor of Experiment (Experiments 1–5) and the within-subjects factor of Training Session (Sessions 1–10). This analysis revealed a main effect of Training Session [F(9,1170) = 63.0, p < .01], reflecting increased cocaine intake over days, and Training Session by Experiment [F(9,1170) = 2.1, p < .01], reflecting different rates of acquisition in the different experiments. However, a main effect of Experiment was not significant (p > .5), reflecting similar overall cocaine intake over the 10 training days in Experiments 1–5. The groups in the different conditions in Experiments 1–5 were matched for cocaine intake during training.
Figure 1.
Cocaine self-administration training in Experiments 1–5. (A) Mean ± SEM number of cocaine injections (.75 mg/kg/injection) over the 10 6-hour daily self-administration sessions. During training, lever presses or nose pokes were reinforced under a fixed-ratio-1 40-sec timeout reinforcement schedule; cocaine injections were paired with a 5-sec tone-light cue. Data are from rats that were subsequently tested for cue-induced cocaine seeking after ventral tegmental area (VTA) injections of an adeno-associated virus (AAV) viral vector containing glial cell line– derived neurotrophic factor (GDNF) cDNA (AAV-GDNF) or red fluorescent protein cDNA (AAV-RFP) (Experiment 1, Long-Evans rats [LE], total n = 18), GDNF in VTA of LE rats (Experiment 2, total n = 28), GDNF in VTA or SN of Sprague-Dawley (SD) rats (Experiment 3, total n = 33), anti-GDNF monoclonal neutralizing antibodies or mouse immunoglobulin G (Experiment 4, SD rats, total n = 16), and U0126+ GDNF in VTA (Experiment 5, SD rats, total n = 34). (B) Schematic illustration of the approximate cannulae placements within the VTA (Experiments 1–5) and SN (Experiment 3) (This figure was published in The Rat Brain Stereotaxic Coordinates by Paxinos and Watson, pp. 18 – 83, copyright Elsevier 2005 [86]); numbers represent millimeters posterior from bregma. SN, substantia nigra.
VTA Injections of AAV-GDNF Potentiated the Time-Dependent Increases in Cue-Induced Cocaine Seeking After Withdrawal (Experiment 1)
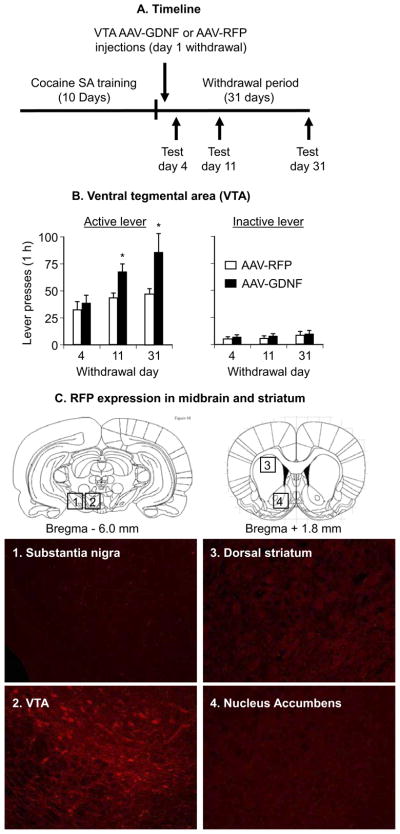
Figure 2B shows mean ± SEM number of nonreinforced presses on the active and inactive levers during the extinction tests conducted 4 days, 11 days, and 31 days after withdrawal from cocaine in rats injected with the AAV-GDNF or the AAV-RFP on withdrawal day 1. The mixed analysis of variance (ANOVA) included the between-subjects factor of Virus Type and the within-subjects factor of Withdrawal Day. Active-lever responding analysis revealed significant effects of virus Type [F(1,16) = 10.6, p < .01] and Withdrawal Day [F(2,32) = 8.0, p < .01]; the interaction between the two factors was not significant (p = .12) because responding increased over time in both groups (incubation). One-way ANOVAs for assessing “incubation” within each group revealed approaching significant (p = .063) or significant (p <.05) effects of Withdrawal Day for the AAV-RFP and AAV-GDNF groups, respectively. The approaching significant effect in the AAV-RFP group is due to a single outlier rat that lever pressed four standard deviations above the mean of the other rats in this group on Day 4. After excluding this rat, the Withdrawal Day effect in the AAV-RFP group was significant (p < .01). No significant effects were found in the analysis of inactive lever responding (p values > .15).
Figure 2.
Ventral tegmental area (VTA) injections of an adeno-associated virus (AAV) viral vector containing rat glial cell line– derived neurotrophic factor (GDNF) cDNA potentiate cue-induced cocaine seeking. Data are mean ± SEM responses per 1 hour on the previously active lever (left column) and on the inactive lever (right column) during the extinction tests for cue-induced cocaine seeking performed on withdrawal days 4, 11, and 31. During the test sessions, cocaine was not available, and lever-presses resulted in the delivery of the tone-light cue previously paired with cocaine injections. AAV viral vector containing rat GDNF cDNA (AAV-GDNF) or red fluorescent protein cDNA (AAV-RFP) was injected bilaterally into the VTA on withdrawal day 1. (A) Timeline of the Experiment, (B) VTA injections and (C) RFP expression in midbrain and striatum (see Supplement 1 for details of the experimental procedures) (This figure was published in The Rat Brain Stereotaxic Coordinates by Paxinos and Watson, pp. 18 – 83, copyright Elsevier 2005 [86]). * Different from AAV-RFP, p <.05 (n = 8 –10, Long-Evans rats per experimental condition). SA, self-administration.
Quantitative RT-PCR measurements of GDNF and RNA polymerase II (a housekeeping gene) mRNA indicated that normalized (deltaCtvirus) GDNF mRNA levels in the VTA of the AAV-GDNF group (n = 7) were 300% higher than those of the AAV-RFP group (n = 9), F(1,14,) = 5.5, p < .05.
A Single VTA, but Not SN, GDNF Injection Potentiated the Time-Dependent Increases in Cue-Induced Cocaine Seeking After Withdrawal (Experiments 2 and 3)
Experiment 2, Long-Evans Rats
Figure 3B shows mean ± SEM number of nonreinforced responses on the active and inactive levers during the extinction tests conducted 3 and 10 days after withdrawal in rats given VTA injections of vehicle or GDNF 1–2 hours after the last training session. The ANOVA included the between-subjects factor of GDNF Dose and the within-subjects factor of Withdrawal Day. Active-lever responding analysis revealed significant effects of GDNF Dose [F(2,25) = 4.6, p < .05] and Withdrawal Day [F(1,25) = 10.0, p < .01]. The interaction between these two factors was not significant. One-way ANOVAs within each group revealed significant (p < .05) Withdrawal Day effects for the vehicle and 1.25-μg GDNF dose groups, but not for the 12.5-μg GDNF dose. The lack of Withdrawal Day effect for the 12.5-μg GDNF dose group was due to high responding on Day 3. Inactive lever responding analysis revealed significant effects of Withdrawal Day [F(1,25) = 13.2, p < .01] but not of GDNF Dose or an interaction between these two factors (p > .5). The modest time-dependent increases in low-rate inactive lever responding has been observed previously in incubation studies; this increase likely reflects response generalization (5,19) (for a discussion of potential interpretations of inactive lever data in extinction and reinstatement studies see Shalev et al. (51).
Figure 3.
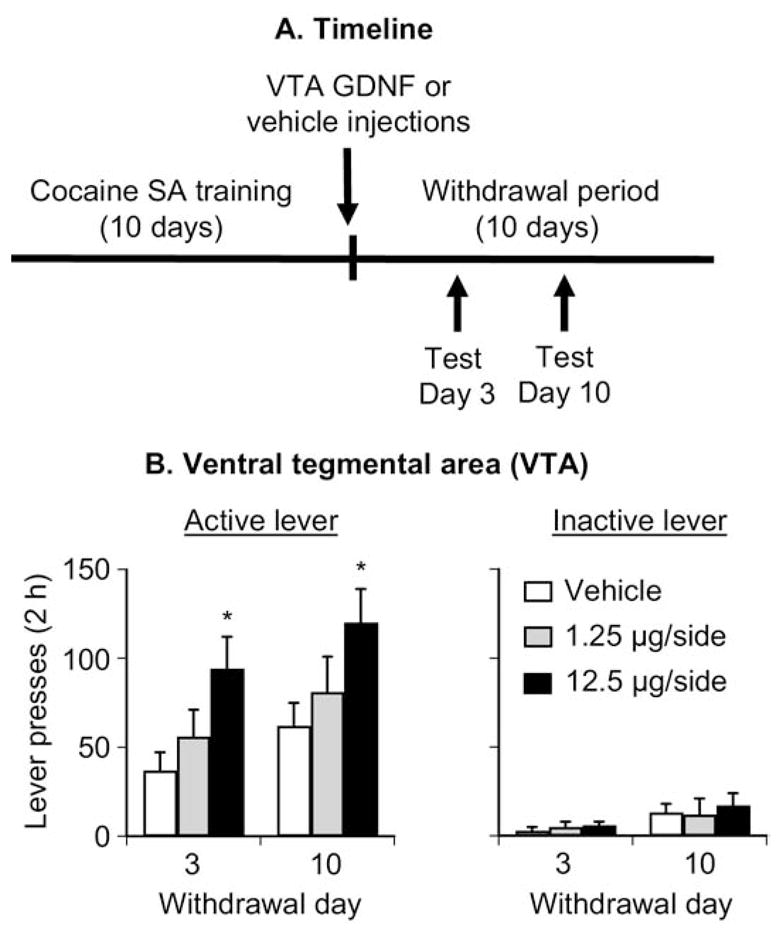
Ventral tegmental area (VTA) injections of glial cell line– derived neurotrophic factor (GDNF) potentiate cue-induced cocaine seeking in Long-Evans rats. Data are mean ± SEM responses per 2 hours (sum of two 1-hour sessions that were separated by 5 min) on the previously active lever (left column) and on the inactive lever (right column) during the extinction tests for cue-induced cocaine seeking performed on withdrawal days 3 and 10. During the test sessions, cocaine was not available, and lever presses resulted in the delivery of the tone-light cue previously paired with cocaine injections. Vehicle or GDNF was injected bilaterally into the VTA 1–2 hours after the last training session. (A) Timeline of the experiment and (B) VTA injections. * Different from vehicle, p < .05 (n = 9 –10 per group). SA, self-administration.
Experiment 3: Sprague-Dawley Rats
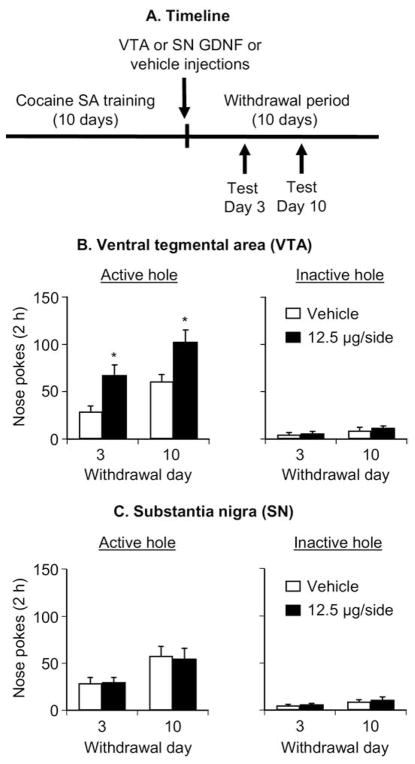
The purpose of Experiment 3 was to assess the generality of the effect of VTA GDNF injections to Sprague-Dawley rats that nose-poked for cocaine infusions and to assess the anatomical specificity of the effect of the GDNF injections. Figure 4(B and C) shows mean ± SEM number of nonreinforced responding in the active and inactive holes during the extinction tests conducted 3 and 10 days after withdrawal in rats given VTA or SN injections of vehicle or GDNF (12.5 μg/side) 2–4 hours after the last training session. The ANOVA included the between-subjects factor of GDNF Dose and Midbrain Site (VTA, SN), and the within-subjects factor of Withdrawal Day. Active nose-poke responding analysis revealed significant effects of Withdrawal Day [F(1,29) = 40.8, p < .01], Midbrain Site [F(1,29) = 10.4, p < .01], GDNF Dose [F(1,29) = 8.1, p < .01], and an interaction between Midbrain Site and GDNF Dose [F(1,29) = 8.8, p < .01]. One-way ANOVAs within each group revealed significant (p < .05) Withdrawal Day effects for all groups. Inactive nose-poke responding analysis revealed significant effects of Withdrawal Day [F(1,29) = 16.4, p < .01) but not of GDNF Dose and Midbrain Site or an interaction between Midbrain Site and GDNF Dose (p > .1). Results on the effect of GDNF VTA injections on locomotor activity on withdrawal days 1, 3, and 10 are provided in Supplement 1.
Figure 4.
Ventral tegmental area (VTA), but not substania nigra (SN), injections of glial cell line– derived neurotrophic factor (GDNF) potentiate cue-induced cocaine-seeking in Sprague-Dawley rats. Data are mean ± SEM responses per 2 hours (sum of two 1-hour sessions that were separated by 5 min) on the previously active hole (left column) and on the inactive hole (right column) during the extinction tests for cue-induced cocaine seeking performed on withdrawal days 3 and 10. During the test sessions, cocaine was not available, and nose pokes resulted in the delivery of the tone-light cue previously paired with cocaine injections. Vehicle or GDNF was injected bilaterally into the VTA or the SN 2– 4 hours after the last training session. (A) Timeline of the Experiment, (B) VTA injections and (C) SN injections. * Different from vehicle, p <.05 (n =8 –9 per group). SA, self-administration.
Chronic Delivery of Anti-GDNF Monoclonal Antibodies into the VTA Prevented the Time-Dependent Increases in Cue-Induced Cocaine Seeking After Withdrawal (Experiment 4)
Figure 5B shows mean ± SEM number of nonreinforced responding in the active and inactive holes during the extinction tests conducted 4, 11, and 31 days after withdrawal. The anti-GDNF antibodies or the mouse IgG control was chronically delivered by Alzet osmotic minipumps during withdrawal days 1–14. The ANOVA included the between-subjects factor of Antibody Type and the within-subjects factor of Withdrawal Day. Active nose-poke responding analysis revealed significant effects of Antibody Type [F(1,14) = 7.3, p < .05] and Withdrawal Day F [(2,28) = 19.3, p < .01] and an interaction between these two factors [F(2,28) = 10.5, p < .01]. The significant Withdrawal Day effect in the anti-GDNF antibodies condition, which is not readily apparent from the data depicted in Figure 5B, is due to significant differences in responding between Days 11 and 31. One-way ANOVAs within each group revealed significant (p < .05) Withdrawal Day effects for both groups. Inactive nose-poke responding analysis revealed significant effects of Withdrawal Day [F(2,28) = 4.4, p < .05], but not of Antibody Type or an interaction between these two factors (p > .5).
Figure 5.
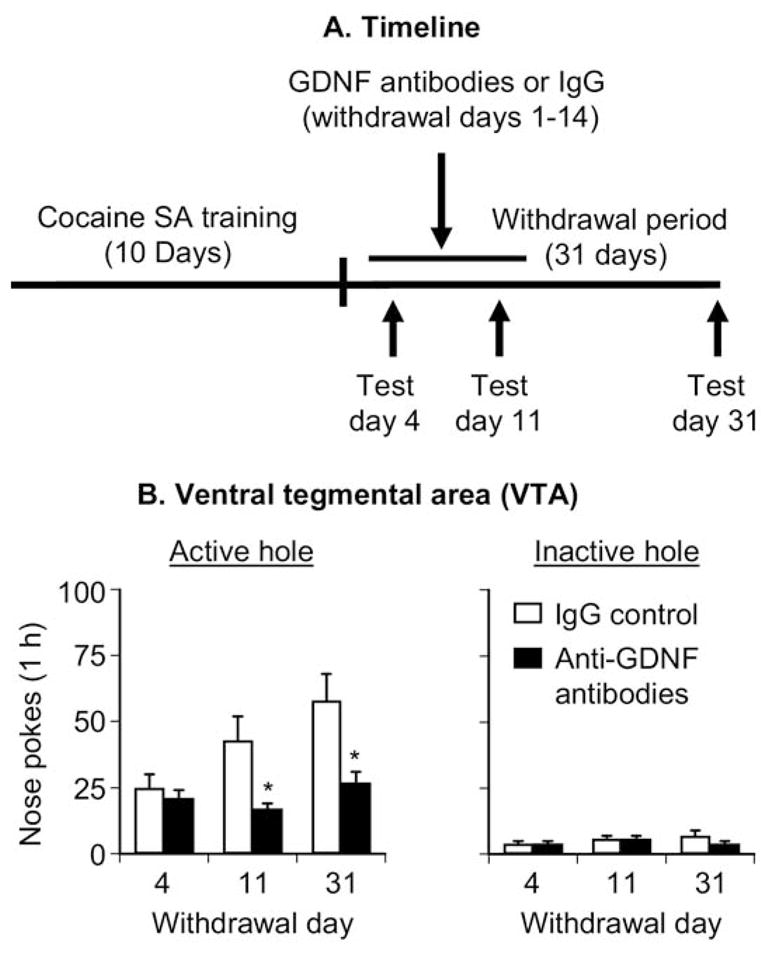
Chronic delivery of anti-glial cell line– derived neurotrophic factor (GDNF) monoclonal neutralizing antibodies into the ventral tegmental area (VTA) prevented the time-dependent increases in cue-induced cocaine seeking. Data are mean ±SEM responses per 1 hour on the previously active hole (left column) and on the inactive hole (right column) during the extinction tests for cue-induced cocaine seeking performed on withdrawal days 4, 11, and 31. During the test sessions, cocaine was not available, and nose pokes resulted in the delivery of the tone-light cue previously paired with cocaine injections. The anti-GDNF monoclonal neutralizing antibodies (600 ng/side/d) or control immunoglobulin G (IgG) was delivered through Alzet minipumps during withdrawal days 1–14. (A) Timeline of the experiment and (B) VTA injections. * Different from IgG control, p <.05 (n =8 Sprague-Dawley rats per experimental condition). SA, self-administration.
Blockade of VTA ERK Activity with U0126 Prevents GDNF-Induced Potentiation of Cue-Induced Cocaine Seeking After Withdrawal (Experiment 5)
Figure 6B shows the mean ± SEM number of nonreinforced responding in the active and inactive holes during the extinction tests conducted 3 and 10 days after withdrawal from cocaine in rats given VTA injections of GDNF (12.5 μg/side) or its vehicle and U0126 (1.0 μg/side) or its vehicle (DMSO) 1–2 hours after the last training session. The ANOVA included the between-subjects factors of GDNF Dose and U0126 Dose and the within-subjects factor of Withdrawal Day. Active nose-poke responding analysis revealed significant effects of Withdrawal Day [F(1,30) = 69.0, p < .01], GDNF dose [F(1,30) = 9.2, p < .01], and U0126 dose [F(1,30) = 5.3, p < .05] and an interaction between GDNF dose and U0126 dose [F(1,30) = 5.1, p < .05]. One-way ANOVAs within each group revealed significant (p < .05) Withdrawal Day effects for all groups. Analysis of inactive nose-poke responding revealed significant effects of Withdrawal Day [F(1,30) = 4.9, p < .03] but not of GDNF Dose, U0126 Dose, or an interaction between these factors (p > .1).
Figure 6.
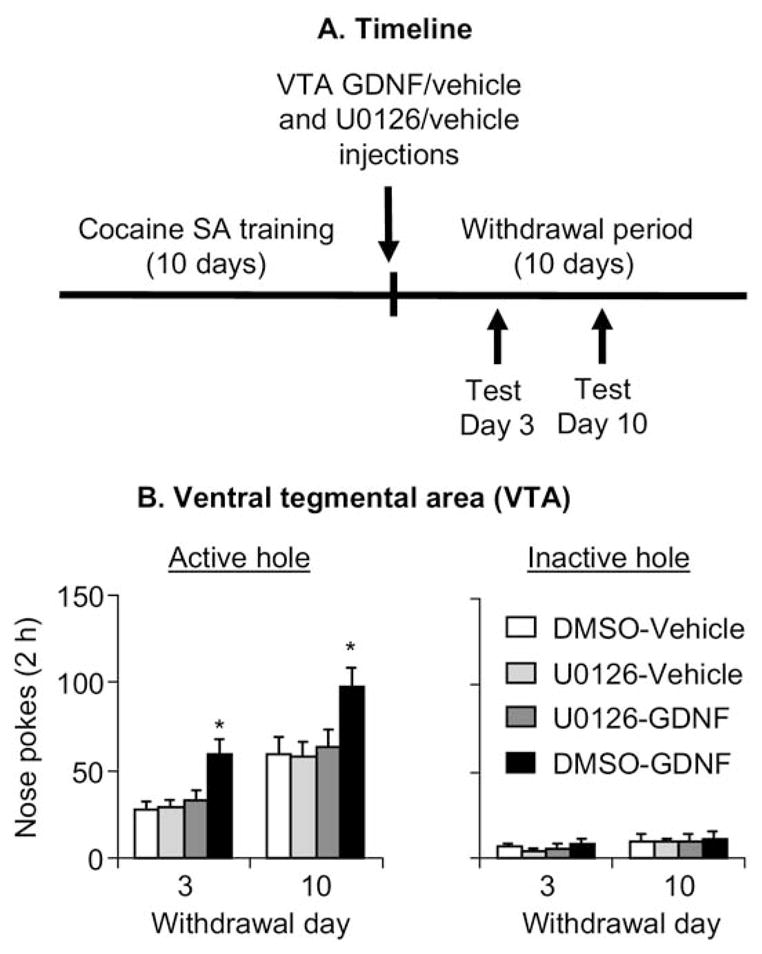
Blockade of extracellular signal-regulated kinases (ERK) activity in ventral tegmental area (VTA) with U0126 prevents glial cell line– derived neurotrophic factor (GDNF)-induced potentiation of cue-induced cocaine-seeking after withdrawal (Experiment 5, Sprague-Dawley rats). Data are mean ± SEM responses per 2 hours (sum of two 1-hour sessions that were separated by 5 min) on the previously active hole (left column) and on the inactive hole (right column) during the extinction tests conducted 3 and 10 days after withdrawal from cocaine. During the test sessions, cocaine was not available, and nose pokes resulted in the delivery of the tone-light cue previously paired with cocaine injections. VTA injections of U0126 (1.0 μg/side) or its vehicle (DMSO), and GDNF (12.5 μg/side) or its vehicle were performed 1–2 hours after the last training session. U0126 or its vehicle was injected 20 min before the injections of GDNF or its vehicle. (A) Timeline of the experiment, and (B) VTA injections. * Different from the other conditions, p < .05 (n = 8 –9 per group). SA, self-administration.
Discussion
We studied the role of GDNF in the VTA in incubation of cocaine craving. We found that VTA injections of an AAV vector containing GDNF cDNA on withdrawal day 1 increased cue-induced cocaine seeking on withdrawal days 11 and 31. We also found that VTA, but not SN, GDNF injections immediately after the last self-administration session increased cue-induced cocaine seeking on withdrawal days 3 and 10; this effect was antagonized by VTA injections of U0126, which inhibits ERK activity. Most important, we found that interfering with local GDNF function by chronic delivery of anti-GDNF neutralizing antibodies during withdrawal days 1–14 prevented the time-dependent increases in cue-induced cocaine seeking on withdrawal days 11 and 31. These results suggest that during the first weeks of withdrawal from cocaine, GDNF-dependent neuroadaptations in the VTA, which involve ERK activity, contribute to the development of incubation of cocaine craving. These findings are unique, because they identify a neurobiological mechanism underlying the development of incubation of cocaine craving. In previous studies, we and others studied mechanisms underlying the expression of the incubated response to the drug cues, only after its development (44,46,52).
Methodological Considerations
Several methodological issues should be considered. First, in studies using intracranial injections, drugs may diffuse away from the injection site and act in adjacent areas (53). This possibility is unlikely, because SN GDNF injections were ineffective. For GDNF and AAV-GDNF VTA injections, an issue to consider is axonal transport to distal projection sites (54,55), which in turn may mediate GDNF effects on cocaine seeking. However, it is unlikely that axonal transport can account for our data, because the AAV-RFP injected into the VTA was not transported to the nucleus accumbens (Figure 2C), a primary projection area of VTA neurons.
Second, midbrain GDNF injections cause long-lasting (several weeks) increases in spontaneous locomotor activity (56). Thus, increased active lever (or hole) responding after VTA GDNF injections may be due to increased activity. However, it is unlikely that nonspecific locomotor activity can account for our data, because spontaneous locomotion in a nondrug context was not altered by VTA GDNF injections (Supplement 1). In this regard, the effect of GDNF on locomotion has been primarily reported after SN injections (56), a manipulation that had no effect on extinction responding. Furthermore, at the dose range used here, VTA GDNF injections decreased lever presses for oral alcohol and had no effect on lever presses for sucrose (34,35). Finally, our experimental manipulations of VTA GDNF had no effect on inactive lever (or hole) responding, a putative measure of nonselected increases in activity and/or response generalization (51).
Third, a potential limitation in our study is the use of Long-Evans rats trained to lever press for cocaine infusions in Experiments 1 and 2 and Sprague-Dawley rats trained to nose poke for cocaine infusions in Experiments 3–5. However, we believe that it is unlikely that strain and type of operant response are potential confounds. Cocaine self-administration during the 10-day training phase was similar at the National Institute on Drug Abuse (Experiments 1–2) and in Beijing (Experiments 3–5). Also, time-dependent increases in cue-induced cocaine seeking (incubation of cocaine craving) that we have been studying since 2001 in Long-Evans rats using lever pressing as the operant response were observed in Sprague-Dawley rats using nose poke as the operant response. Finally, the effect of acute VTA GDNF injection on cue-induced cocaine seeking appears independent of rat strain and type of operant response.
Role of GDNF in Cocaine Reward and Relapse
The findings of similar roles of BDNF and GDNF in cue-induced cocaine seeking correlate with findings showing similar effects of these growth factors on survival and function of midbrain dopamine neurons. However, as mentioned earlier, these similar roles were not predicted from previous studies in the addiction literature. Specifically, whereas Berglind et al. (57) recently reported that BDNF injections into the medial prefrontal cortex decrease cue-induced cocaine seeking, results from studies with BDNF heterozygote knockout mice and those in which BDNF function was augmented in VTA or nucleus accumbens indicate that BDNF promotes rodents’ responses to psychostimulants and psychostimulant-associated cues (13,23–26,58,59). In contrast, results from studies with GDNF heterozygote knockout mice and those in which GDNF function was augmented in VTA or nucleus accumbens suggest that GDNF inhibits the rodents’ responses to psychostimulants and psychostimulant-associated cues (27–29,60). Below, we now discuss results from previous studies on the role of GDNF in psychostimulant reward and relapse with respect to these results.
With respect to cocaine, Messer et al. (31) and Green-Sadan et al. (32,33) studied mesolimbic GDNF’s role in the initial rewarding effects of cocaine, assessed by a CPP procedure or by a limited-access (1-hour) drug self-administration procedure, respectively. Additionally, these investigators exposed rats to low amounts of daily cocaine (1.25–10 mg/kg intraperitoneal or ~12 mg/kg intravenous [IV]), and GDNF function was manipulated before and during drug exposure. In contrast, we assessed VTA GDNF’s role in cue-induced cocaine seeking in rats given extended access to cocaine (6 hours/day), which leads to substantially higher daily cocaine intake (~45 mg/kg IV). Furthermore, we manipulated GDNF function after withdrawal from cocaine rather than before or during exposure to this drug.
In reconciling these previous studies with our results, two issues should be considered. First, there is evidence for differences in the mechanisms underlying the acute initial rewarding effects of cocaine versus those underlying cue-induced drug seeking after withdrawal (51,61). Second, extended daily cocaine exposure leads to drug-taking patterns and drug-induced neuroadaptations that are not observed under conditions of limited access (62–65). Finally, an interpretation issue in Green-Sadan et al. studies (32,33) is that they used a single cocaine dose (1 mg/kg) that is on the descending limb of the cocaine dose-response curve (66). Thus, decreased cocaine self-administration after increasing striatal GDNF levels may reflect a leftward shift in the dose-response curve or increases in cocaine reward.
Our data are also different from those obtained in a self-administration/reinstatement study with GDNF heterozygote knockout mice. Yan et al. (67) reported that in these mice, the dose-response curve for self-administered methamphetamine is shifted upward and to the left and that responding on a progressive-ratio reinforcement schedule is higher, suggesting enhanced methamphetamine reward in GDNF heterozygote knockout mice. These mice also demonstrated enhanced methamphetamine-priming-induced and cue-induced reinstatement. In reconciling the differences between the knockout mice data and our data, two issues should be considered. First, the constituent GDNF knockout manipulation changes GDNF function in the entire brain (and periphery) and affects multiple systems in addition to the VTA neurons that we studied. Second, when a gene is deleted or manipulated throughout development, it is unknown whether the observed behavioral changes indicate the gene’s normal role in behavior or compensatory developmental changes (68). A compensatory change that may be relevant to enhanced methamphetamine reward and reinstatement in GDNF heterozygote mice is increased striatal dopamine levels (69,70).
Mechanisms of GDNF Role in Incubation of Cocaine Craving
GDNF modulation of incubation of cocaine craving likely involves VTA-nucleus accumbens dopamine projections that have been previously implicated in cue-induced drug seeking (71,72). GDNF mRNA is expressed in nucleus accumbens and dorsal striatum, while the mRNA of its binding subunit the GDNF family receptor alpha-1 (GFRalpha-1), and its signaling receptor subunit the RET receptor tyrosine kinase are expressed in VTA and SN (73–76). Striatal GDNF undergoes retrograde transport to the midbrain (55) where its cellular effects are primarily mediated by GFRalpha-1 and RET (22).
In vivo, midbrain GDNF injections cause long-lasting (several weeks) increases in dopamine transmission in both the midbrain and striatum (77–80). In midbrain cell cultures, GDNF enhances synaptic transmission in tyrosine hydroxylase (TH)-positive dopamine neurons through an ERK-dependent mechanism (81,82). GDNF activates the ERK pathway in several brain areas (83,84), including the VTA (34). We previously found that inhibiting VTA ERK activity reverses BDNF-induced potentiation of cue-induced cocaine seeking (19). Here, we found that inhibiting VTA ERK activity reverses GDNF-induced potentiation of cue-induced cocaine seeking. These results indicate that VTA ERK activity, which is increased after chronic cocaine exposure (85), contributes to both BDNF- and GDNF-induced potentiation of cue-induced cocaine seeking after withdrawal.
Concluding Remarks
Our results suggest that during the first weeks of withdrawal from cocaine, GDNF function in the VTA is critical for the development of incubation of cocaine craving. These findings extend previous results on the role of BDNF in the mesolimbic system in incubation of cocaine craving and rodents’ responses to cocaine cues. The integration of our results with the previous findings from the groups of Nestler and Yadid raises the possibility that mesolimbic GDNF’s role in cocaine behavioral effects is dependent on the phase of the addiction cycle: inhibition of initial acute rewarding effects of cocaine versus potentiation of cocaine seeking after withdrawal from the drug.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (National Institutes of Health, U.S. Department of Health and Human Services) and by the National Basic Research Program of China (Grant Nos. 2007CB512302 and 2009CB522004), the National High Technology Research and Development Program of China (863 Program, Grant No. 2006AA02Z4D1), and the Natural Science Foundation of China (Grant Nos. 30,670,713 and 30,725,016). We thank Sarah Gray, Kristina Wihbey, Haifeng Zhai, Katie Zuchowski, and Doug Howard for expert technical assistance and Dr. Dorit Ron for helpful comments.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.O’Brien CP. Anticraving medications for relapse prevention: A possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 2.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 3.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: Different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- 6.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 8.Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology. 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- 9.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: Implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mead AN, Zamanillo D, Becker N, Stephens DN. AMPA-receptor GluR1 subunits are involved in the control over behavior by cocaine-paired cues. Neuropsychopharmacology. 2007;32:343–353. doi: 10.1038/sj.npp.1301045. [DOI] [PubMed] [Google Scholar]
- 13.Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- 15.Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- 16.Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- 17.Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors: From molecule to man. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 21.Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 22.Airaksinen MS, Saarma M. The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 23.Graham DL, Edwards S, Bachtell RK, Di LRJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 24.Pierce RC, Pierce-Bancroft AF, Prasad BM. Neurotrophin-3 contributes to the initiation of behavioral sensitization to cocaine by activating the ras/mitogen-activated protein kinase signal transduction cascade. J Neurosci. 1999;19:8685–8995. doi: 10.1523/JNEUROSCI.19-19-08685.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: A review of preclinical data. Neuropharmacology. 2004;47(suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Bahi A, Boyer F, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology. 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- 27.Ron D, Janak PH. GDNF and addiction. Rev Neurosci. 2005;16:277–285. doi: 10.1515/revneuro.2005.16.4.277. [DOI] [PubMed] [Google Scholar]
- 28.Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromol Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 29.Niwa M, Nitta A, Yamada K, Nabeshima T. The roles of glial cell line-derived neurotrophic factor, tumor necrosis factor-alpha, and an inducer of these factors in drug dependence. J Pharmacol Sci. 2007;104:116–121. doi: 10.1254/jphs.cp0070017. [DOI] [PubMed] [Google Scholar]
- 30.Carnicella S, Ron D. GDNF—A potential target to treat addiction (published online ahead of print December 24) Pharmacol Ther. 2008 doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, et al. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green-Sadan T, Kinor N, Roth-Deri I, Geffen-Aricha R, Schindler CJ, Yadid G. Transplantation of glial cell line-derived neurotrophic factor-expressing cells into the striatum and nucleus accumbens attenuates acquisition of cocaine self-administration in rats. Eur J Neurosci. 2003;18:2093–2098. doi: 10.1046/j.1460-9568.2003.02943.x. [DOI] [PubMed] [Google Scholar]
- 33.Green-Sadan T, Kuttner Y, Lublin-Tennenbaum T, Kinor N, Boguslavsky Y, Margel S, et al. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194:97–105. doi: 10.1016/j.expneurol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–628. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girault JA, Valjent E, Caboche J, Herve D. ERK2: A logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YH, Harvey BK, Hoffman AF, Wang Y, Chiang YH, Lupica CR. MPTP-induced deficits in striatal synaptic plasticity are prevented by glial cell line-derived neurotrophic factor expressed via an adeno-associated viral vector. FASEB J. 2008;22:261–275. doi: 10.1096/fj.07-8797com. [DOI] [PubMed] [Google Scholar]
- 40.Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008;372:24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey BK, Shen H, Chen GJ, Yoshida Y, Wang Y. Midkine and retinoic acid reduce cerebral infarction induced by middle cerebral artery ligation in rats. Neurosci Lett. 2004;369:138–141. doi: 10.1016/j.neulet.2004.07.086. [DOI] [PubMed] [Google Scholar]
- 42.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamagata K, Tagami M, Ikeda K, Tsumagari S, Yamori Y, Nara Y. Differential regulation of glial cell line-derived neurotrophic factor (GDNF) mRNA expression during hypoxia and reoxygenation in astrocytes isolated from stroke-prone spontaneously hypertensive rats. Glia. 2002;37:1–7. doi: 10.1002/glia.10003. [DOI] [PubMed] [Google Scholar]
- 44.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, et al. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 45.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koya E, Uejima JM, Wihbey KA, Bossert JM, Hope BT, et al. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(suppl 1):117–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, et al. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robbins TW. The potentiation of conditioned reinforcement by psychomotor stimulant drugs: A test of Hill’s hypothesis. Psychopharmacologia. 1975;45:103–114. [Google Scholar]
- 50.Sweatt JD. The neuronal MAP kinase cascade: A biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 51.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: A review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, et al. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–13257. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wise RA, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse. 1992;10:247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- 54.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, et al. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A. 1995;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gash DM, Gerhardt GA, Hoffer BJ. Effects of glial cell line-derived neurotrophic factor on the nigrostriatal dopamine system in rodents and nonhuman primates. Adv Pharmacol. 1998;42:911–915. doi: 10.1016/s1054-3589(08)60895-9. [DOI] [PubMed] [Google Scholar]
- 57.Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- 58.Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- 59.Shen F, Meredith GE, Napier TC. Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci. 2006;26:11041–11051. doi: 10.1523/JNEUROSCI.2898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janak PH, Wolf FW, Heberlein U, Pandey SC, Logrip ML, Ron D. BIG news in alcohol addiction: New findings on growth factor pathways BDNF, insulin, and GDNF. Alcohol Clin Exp Res. 2006;30:214–221. doi: 10.1111/j.1530-0277.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- 61.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: A pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 63.Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA. 1985;254:81–83. [PubMed] [Google Scholar]
- 64.Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mutschler NH, Miczek KA, Hammer RP., Jr Reduction of zif268 messenger RNA expression during prolonged withdrawal following “binge” cocaine self-administration in rats. Neuroscience. 2000;100:531–538. doi: 10.1016/s0306-4522(00)00298-0. [DOI] [PubMed] [Google Scholar]
- 66.Yokel RA. Intravenous self-administration: Response rates, the effects of pharmacological challenges, and drug preference. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. New York: Springer-Verlag; 1987. pp. 1–34. [Google Scholar]
- 67.Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. FASEB J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- 68.Routtenberg A. Reverse piedpiperase: Is the knockout mouse leading neuroscientists to a watery end? Trends Neurosci. 1996;19:471–472. doi: 10.1016/S0166-2236(96)20051-7. [DOI] [PubMed] [Google Scholar]
- 69.Airavaara M, Planken A, Gaddnas H, Piepponen TP, Saarma M, Ahtee L. Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci. 2004;20:2336–2344. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- 70.Airavaara M, Tuomainen H, Piepponen TP, Saarma M, Ahtee L. Effects of repeated morphine on locomotion, place preference and dopamine in heterozygous glial cell line-derived neurotrophic factor knockout mice. Genes Brain Behav. 2007;6:287–298. doi: 10.1111/j.1601-183X.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 71.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: A neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feltenstein MW, See RE. The neurocircuitry of addiction: An overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, et al. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 75.Oo TF, Ries V, Cho J, Kholodilov N, Burke RE. Anatomical basis of glial cell line-derived neurotrophic factor expression in the striatum and related basal ganglia during postnatal development of the rat. J Comp Neurol. 2005;484:57–67. doi: 10.1002/cne.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarabi A, Hoffer BJ, Olson L, Morales M. GFRalpha-1 mRNA in dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area. J Comp Neurol. 2001;441:106–117. doi: 10.1002/cne.1400. [DOI] [PubMed] [Google Scholar]
- 77.Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- 78.Hebert MA, Van Horne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: Presynaptic studies using in vivo electro-chemistry and microdialysis. J Pharmacol Exp Ther. 1996;279:1181–1190. [PubMed] [Google Scholar]
- 79.Martin D, Miller G, Cullen T, Fischer N, Dix D, Russell D. Intranigral or intrastriatal injections of GDNF: Effects on monoamine levels and behavior in rats. Eur J Pharmacol. 1996;317:247–256. doi: 10.1016/s0014-2999(96)00756-x. [DOI] [PubMed] [Google Scholar]
- 80.Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- 81.Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 82.Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, et al. GDNF acutely modulates excitability and A-type K(+) channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- 83.Du Y, Li X, Yang D, Zhang X, Chen S, Huang K, et al. Multiple molecular pathways are involved in the neuroprotection of GDNF against proteasome inhibitor induced dopamine neuron degeneration in vivo. Exp Biol Med Maywood. 2008;233:881–890. doi: 10.3181/0712-RM-329. [DOI] [PubMed] [Google Scholar]
- 84.Hauck SM, Kinkl N, Deeg CA, Swiatek-de Lange M, Schoffmann S, Ueffing M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol Cell Biol. 2006;26:2746–2457. doi: 10.1128/MCB.26.7.2746-2757.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Amsterdam: Elsevier Academic; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.