Abstract
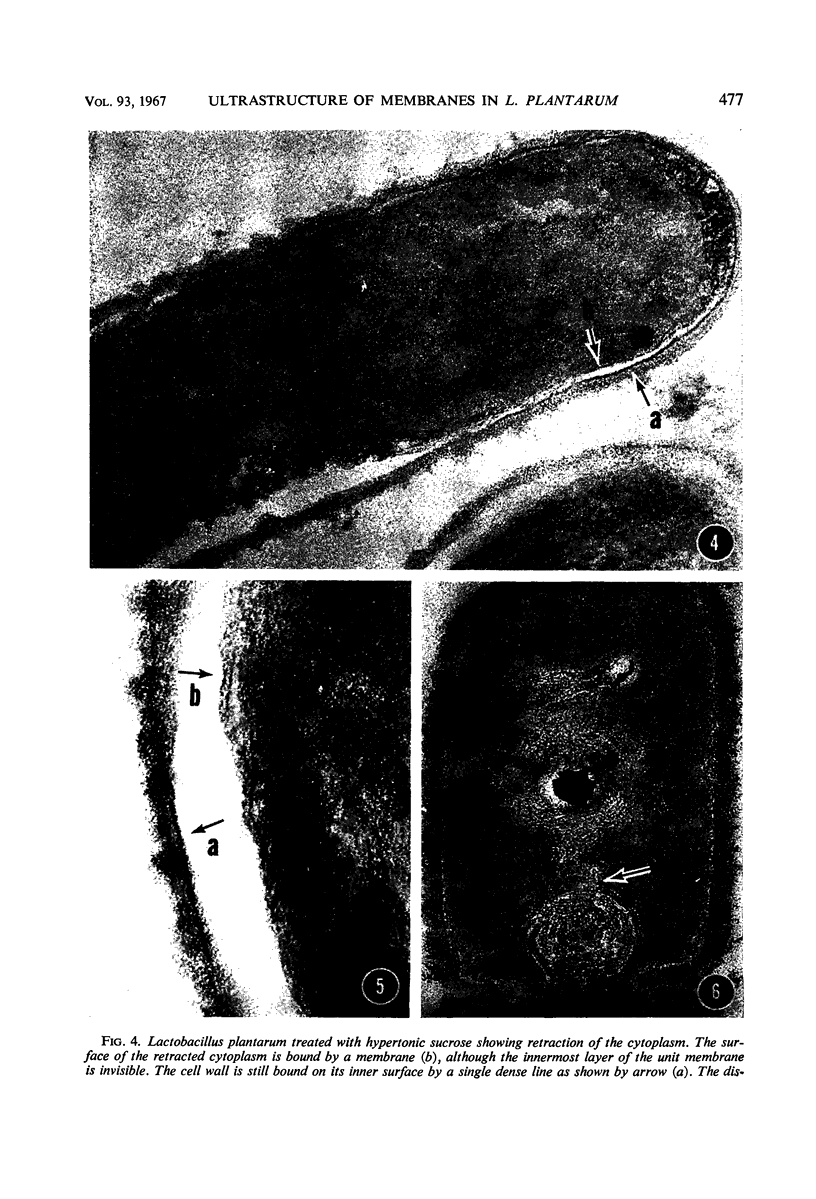
Electron microscopic study of Lactobacillus plantarum revealed mesosomes in different stages of maturation and structural relation with other cell organelles. Small, immature mesosomes were bounded by a prominent electron-dense layer with another extremely faint layer on the outside. This corresponds to the appearance of the cytoplasmic membrane. Large mature mesosomes were surrounded by a triple-layered unit membrane having electron-opaque layers of approximately equal density, suggesting that the composition of the boundary membrane alters during development of this structure. Three-dimensional observations derived from serial sections indicated that mesosomes always maintain a connection between the cytoplasmic membrane and the comparable layers of their boundary. The cytoplasmic membrane also consisted of a triple-layered unit membrane, the innermost layer of which was less electron-opaque and was usually hidden by the relatively dense background of the cytoplasm. The innermost layer of the cytoplasmic membrane was most clearly seen in plasmolyzed cells. Only mature mesosomes made distinct contacts with, or were partially immersed in, the nucleoplasm. The boundary of such mesosomes frequently seemed to be discontinuous, suggesting that the mesosome interior was in direct contact with the nucleoplasm. Mesosomes involved in cross-wall formation at a division plane increased in size and passed through a sequence of positions which led ultimately to an association with the nucleoplasms of the daughter cells. The inner surface of the cell wall was lined by a thin, electron-dense layer whose composition and function are unknown. Under the cultural conditions used, this organism regularly contained a polyphosphate granule.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHIBALD A. R., ARMSTRONG J. J., BADDILEY J., HAY J. B. Teichoic acids and the structure of bacterial walls. Nature. 1961 Aug 5;191:570–572. doi: 10.1038/191570a0. [DOI] [PubMed] [Google Scholar]
- EDWARDS M. R., STEVENS R. W. FINE STRUCTURE OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 Sep;86:414–428. doi: 10.1128/jb.86.3.414-428.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. DISCUSSION. Bacteriol Rev. 1965 Sep;29(3):293–298. doi: 10.1128/br.29.3.293-298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. MORPHOLOGY OF SPORE DEVELOPMENT IN CLOSTRIDIUM PECTINOVORUM. J Bacteriol. 1962 Jul;84(1):104–114. doi: 10.1128/jb.84.1.104-114.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. Electron microscopy of Bacillus megaterium undergoing isolation of its nuclear bodies. J Bacteriol. 1964 May;87(5):1202–1210. doi: 10.1128/jb.87.5.1202-1210.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M. The fine structure of bacteria. Br Med Bull. 1962 Sep;18:245–250. doi: 10.1093/oxfordjournals.bmb.a069988. [DOI] [PubMed] [Google Scholar]
- Green D. E., Murer E., Hultin H. O., Richardson S. H., Salmon B., Brierley G. P., Baum H. Association of integrated metabolic pathways with membranes. I. Glycolytic enzymes of the red blood corpuscle and yeast. Arch Biochem Biophys. 1965 Dec;112(3):635–647. doi: 10.1016/0003-9861(65)90107-4. [DOI] [PubMed] [Google Scholar]
- HOLDEN J. T., HOLMAN J. Accumulation of freely extractable glutamic acid by lactic acid bacteria. J Biol Chem. 1959 Apr;234(4):865–871. [PubMed] [Google Scholar]
- HOLDEN J. T., VANBALGOOY J. N. EFFECT OF NUTRITIONAL AND PHYSIOLOGICAL FACTORS ON THE REACTION BETWEEN LACTOBACILLUS PLANTARUM AND MURAMIDASE. Biochem Biophys Res Commun. 1965 May 3;19:401–406. doi: 10.1016/0006-291x(65)90136-1. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHYE D. F., MURRELL W. G. Formation and structure of the spore of Bacillus coagulans. J Cell Biol. 1962 Jul;14:111–123. doi: 10.1083/jcb.14.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERMAN J. R., PINE L. ELECTRON MICROSCOPY OF CYTOPLASMIC STRUCTURES IN FACULTATIVE AND ANAEROBIC ACTINOMYCES. J Bacteriol. 1963 Oct;86:656–665. doi: 10.1128/jb.86.4.656-665.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINOW C. F. On the plasma membrane of some bacteria and fungi. Circulation. 1962 Nov;26:1092–1104. doi: 10.1161/01.cir.26.5.1092. [DOI] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH I. W., WILKINSON J. F., DUGUID J. P. Volutin production in Aerobacter aerogenes due to nutrient imbalance. J Bacteriol. 1954 Oct;68(4):450–463. doi: 10.1128/jb.68.4.450-463.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERWINKEL E., MURRAY R. G. [Bacterial intracytoplasmic organelles and the site of oxidation-reduction activity]. J Ultrastruct Res. 1962 Aug;7:185–199. doi: 10.1016/s0022-5320(62)80035-5. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. PLASMOLYSIS IN BACILLUS MEGATERIUM. J Bacteriol. 1965 Apr;89:1151–1154. doi: 10.1128/jb.89.4.1151-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W. Symposium on the fine structure and replication of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol Rev. 1965 Sep;29(3):299–325. doi: 10.1128/br.29.3.299-325.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]