Summary
Stringent control of the NF-κB and type I interferon pathways is critical to effective host immune responses, yet the molecular mechanisms that negatively regulate these pathways are poorly understood. Here we show that NLRC5, a member of the NOD-like protein family, can inhibit the IKK complex and RIG-I/MDA5 function. NLRC5 strongly inhibited NF-κB-dependent responses by interacting with IKKα/IKKβ and blocking their phosphorylation. It also interacted with RIG-I and MDA5, but not with MAVS, to potently inhibit RIG-I-like receptor-mediated type I interferon responses. Consistent with these observations, NLRC5-specific siRNA knockdown not only enhanced the activation of NF-κB and its responsive genes, TNF-α and IL-6, but also promoted type I interferon signaling and antiviral immunity. Our findings identify NLRC5 as a key negative regulator that blocks two central components of the NF-κB and type I interferon pathways, and hence is a pivotal element in the homeostatic control of the innate immune system.
Introduction
The innate immune response, elicited through the detection of pathogen-associated molecular patterns (PAMPs), provides the first line of defense against invading microorganisms. PAMP recognition depends on several classes of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs) (Akira et al., 2006; Honda and Taniguchi, 2006; Inohara et al., 2005; Medzhitov, 2007; Meylan et al., 2006; Ting et al., 2006). Activation of most TLRs leads to the recruitment of a common adaptor, MyD88, and in turn to a series of downstream signaling events that culminate in NF-κB activation (Akira et al., 2006; Chen, 2005; Hayden and Ghosh, 2008). By contrast, activation of RLRs (RIG-I and MDA5) by double- and single-stranded RNAs or certain viruses (Hornung et al., 2006; Kato et al., 2006; Pichlmair et al., 2006) results in recruitment of the MAVS protein (mitochondrial antiviral signalling; also called VISA, IPS-1 and Cardif), which further activates the downstream signaling molecules TBK1/IKKi and IRF3 for type I interferon responses, as well as IKK molecules for NF-κB activation (Meylan et al., 2006). Besides their roles in innate immunity and inflammation, TLR-mediated signaling pathways have been shown to play an important role in the control of regulatory T cell function (Liu et al., 2006; Peng et al., 2005; Peng et al., 2007; Sutmuller et al., 2006).
Because uncontrolled immune responses can be harmful, even fatal, to the host (Liew et al., 2005), NF-κB activation and type I interferon signaling must be tightly regulated to maintain immune balance in the organism. Despite the importance of the IKK complex as a central transducer of signaling from various stimuli, leading to the activation of the NF-κB pathway, and of RLRs as critical receptors in type I interferon signaling (Chen, 2005; Honda and Taniguchi, 2006), the molecular mechanisms responsible for IKK activation and RLR-mediated signaling remain poorly understood.
NLRs represent a large family of intracellular PRRs that are characterized by a conserved nucleotide-binding and oligomerization domain (NOD) and a leucine-rich repeat (LRR) region, and are involved in the activation of diverse signaling pathways (Akira et al., 2006; Inohara et al., 2005; Meylan et al., 2006). Several NLRs, such as NOD1, NOD2 and NALP3, have been extensively studied and shown to activate signaling pathways once they encounter relevant PAMPs (Akira et al., 2006; Chen et al.; Inohara et al., 2005; Meylan et al., 2006). NALP3 inflammasome, for example, functions as a crucial component in the adjuvant effect of aluminium and asbestos (Dostert et al., 2008; Eisenbarth et al., 2008). More recently, NLRX1 was demonstrated to function as a mitochondrial protein that interacts with the mitochondrial adaptor MAVS to inhibit the RIG-I-mediated signaling pathway and triggers the generation of reactive oxygen species as well (Moore et al., 2008; Tattoli et al., 2008). These studies suggest that understanding the function and mechanisms of these innate immune receptors or regulators may aid in developing more effective strategies for the immunological treatment of inflammation-associated diseases (Karin et al., 2006; Wang et al., 2008).
Given that the NLR protein family is involved in many biological processes and functions as proinflammatory receptors as well as negative regulators, we hypothesized that some NLR members may play a critical regulatory role in the control of NF-κB and type I interferon signaling. Here we report the identification of NLRC5 as a potent negative regulator of NF-κB and IRF3 activation. It strongly inhibits NF-κB-dependent responses by interacting with IKKα and IKKβ and blocking their phosphorylation. It also interacts with RIG-I and MDA5, but not with MAVS, to potently inhibit RLR-mediated type I interferon responses. As a key negative regulator of NF-κB and type I interferon signaling, NLRC5 may serve as a useful target for manipulating immune responses against infectious or inflammation-associated diseases, including cancer.
Results
Molecular Cloning and Characterization of NLRC5
As a member of the NLR protein family, NLRC5 contains a CARD-like domain, a central NOD domain and a large LRR region (Fig. 1A), but its biological function remains unknown (Chen et al., 2009; Dowds et al., 2003). To determine the function of NLRC5, we cloned full-length human and murine NLRC5 complementary DNAs (cDNAs) by rapid amplification of cDNA ends (RACE) (Fig. 1A). There was a 64% amino acid sequence identity between the human and murine proteins. Expression of both HA-tagged NLRC5 and mNLRC5 was demonstrated by Western blotting with an anti-HA antibody (Fig. 1B). Both human and murine NLRC5 mRNAs were strongly expressed in spleen, thymus, and lung (Fig. 1C), suggesting that this molecule is biologically conserved in these tissues.
Figure 1. Domain organization, expression and intracellular localization of human and mouse NLRC5.
(A) Domain organization of human and mouse NLRC5 proteins. CARD, caspase recruitment domain; NOD, nucleotide binding domain; LRR, leucine rich repeats.
(B) Western blot analysis of HA-tagged NLRC5 and mNLRC5 protein expression in 293T cells.
(C) Human and mouse NLRC5 mRNAs were determined in different tissues determined by real-time PCR analysis.
D) Expression of mNLRC5 mRNA in RAW264.7 cells was determined by real-time PCR analysis after LPS, intracellular poly (I:C) stimulation or VSV-eGFP infection.
(E) Immunoblot analysis of mNLRC5 protein expression after LPS stimulation or VSV-eGFP infection. The relative protein levels of mNLRC5 were quantified by the band density scanning of Western blot images and plotted against time (h) after LPS treatment or VSV infection.
(F) Both wild-type and MyD88-deficient macrophages were treated with LPS, and mNLRC mRNA expression was determined by real-time PCR analysis.
See also Fig. S1.
To further demonstrate the protein expression of NLRC5 in different cell types, we generated polyclonal antibodies against endogenous NLRC5 and mNLRC5 (Fig. S1A–S1E), which readily detected these proteins in human HEK293T (293T) cells, human THP1 cells or mouse RAW264.7 cells (Fig. S1F). To determine whether NLRC5 expression could be regulated in response to TLR stimulation or virus infection, we treated RAW264.7 cells with LPS, intracellular poly(I:C), or infection with vesticular stomatitis virus-enhanced green fluorescent protein (VSV-eGFP), which are known to activate the NF-κB or type I IFN pathway (Yoneyama and Fujita, 2009). Real-time PCR and Western blot analyses revealed strong upregulation of mNLRC5 at both the mRNA and protein levels, which peaked at 6 h after LPS treatment (Fig. 1D–1E and Fig. S1G). In contrast, we observed only a weak increase of mNLRC5 mRNA and little or no change in mNLRC5 protein after intracellular poly(I:C) treatment or VSV infection (Fig. 1D and 1E), consistent with a previous study showing that VSV infection is a weak NF-κB inducer (Ishikawa and Barber, 2008). To further explore the mechanisms by which NLRC5 is regulated, we performed similar experiments with wild-type and MyD88 knockout (KO) macrophages and found that upregulation of mNLRC5 was completely abolished when MyD88 KO macrophages were stimulated with LPS (Fig. 1F), indicating that the expression of mNLRC5 itself is controlled by the NF-κB signaling pathway.
To determine the cellular localization of NLRC5, we transfected 293T cells with NLRC5-GFP fusion DNA and found that NLRC5-GFP was present in the cytoplasm, but not in the nucleus or mitochondria (Fig. S1H–S1I), suggesting that NLRC5 is a cytosolic protein. No change in the cellular localization of NLRC5 was observed after LPS treatment (Fig. S1I–S1K).
NLRC5 Is a Potent Negative Regulator of NF-κB Activation Induced by TLR Ligands
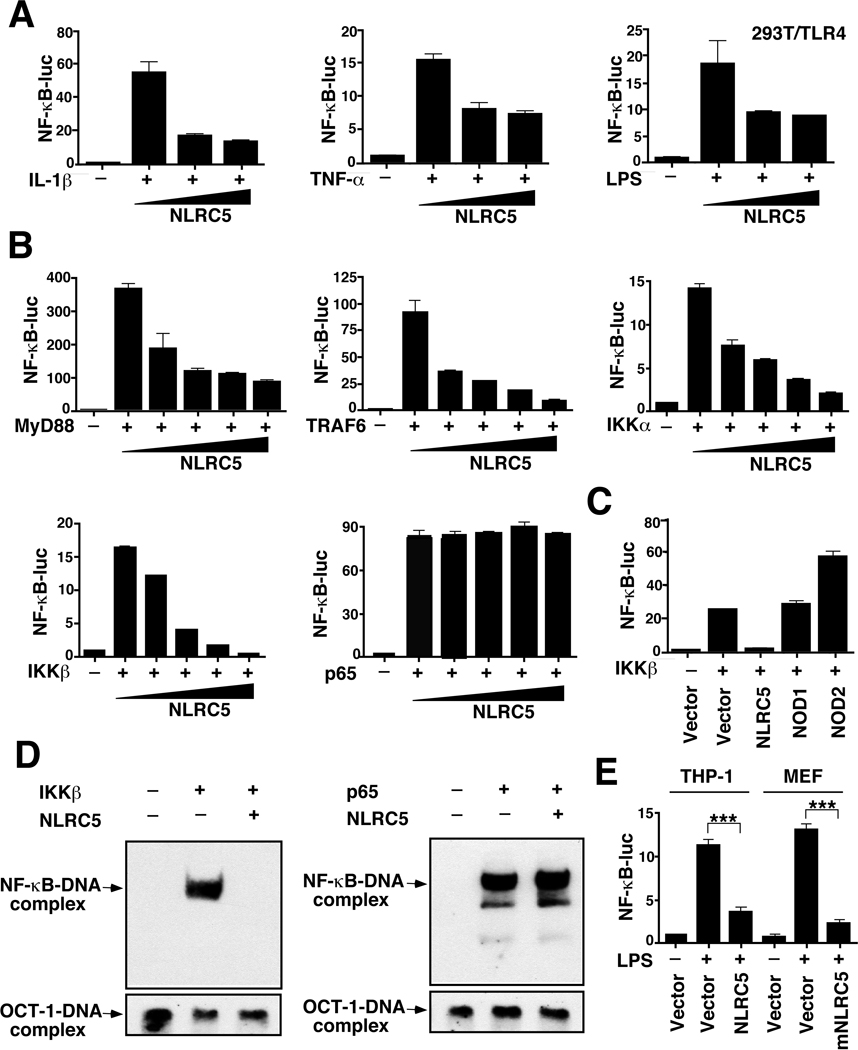
To determine whether NLRC5 is involved in TLR-and/or cytokine-mediated NF-κB activation, we transfected 293T or 293T/TLR4 cells with NF-κB-luc reporter DNA, with or without the NLRC5 plasmid, and then treated them with interleukin (IL)-1β, TNF-α or LPS. Results in Fig. 2A show that NLRC5 potently inhibited NF-κB activation induced by IL-1β, TNF-α or LPS. We next sought to identify potential signaling molecules that activated the NF-κB-luc reporter. Expression of MyD88, TRAF6, IKKα or IKKβ strongly induced NF-κB-luc activity, but such activity was inhibited when NLRC5 was cotransfected at increasing concentrations (Fig. 2B). In contrast, NLRC5 did not inhibit p65-mediated NF-κB-luc activity, suggesting that it may interact with IKK signaling molecules upstream of p65 to block NF-κB activation (Fig. 2B). The control plasmids NOD1 and NOD2 enhanced rather than inhibited NF-κB activity (Fig. 2C), consistent with previous studies (Inohara et al., 1999; Ogura et al., 2001).
Figure 2. NLRC5 inhibits NF-κB activation induced by IL-1β, LPS, TNF-α and their downstream signaling molecules.
(A) 293T cells were transfected with an NF-κB-luc reporter plasmid and TLR4 plasmid (for LPS treatment), together with an empty vector or NLRC5 construct, and analyzed for NF-κB-dependent luciferase activity (fold induction) after treatment with IL-1β, LPS or TNF-α.
(B) 293T cells were transfected with MyD88, TRAF6, IKKα, IKKβ, or p65, along with NF-κB-luc.
(C) 293T cells were transfected with NOD1 or NOD2, along with NF-κB-luc.
(D) Detection of endogenous NF-κB DNA binding activity in a gel-mobility shift assay. Oct-1/DNA-binding complexes served as a loading control for nuclear extracts.
(E) Human THP-1 cells and murine embryonic fibroblasts (MEF) were transfected with the NF-κB-luc reporter plasmid, together with (or without) NLRC5 or mNLRC5 plasmid, and then analyzed for NF-κB-dependent luciferase activity after LPS treatment.
See also Fig. S2.
Since LPS can also activate the type I IFN pathway through an adaptor TRIF molecule, we asked whether NLRC5 can inhibit LPS-induced IFN-β-luc activity (which requires cooperation between IRF3 and NF-κB activation) or interferon-stimulated response element (ISRE)-luc activity (which requires IRF3 activation only) (Zhong et al., 2008). Functional assays showed that NLRC5 inhibited LPS- or TRIF-induced NF-κB-luc and IFN-β-luc activities, but had no effects on ISRE-luc activity (Fig. S2A–S2B). Further experiments demonstrated that NLRC5 strongly inhibited R848-induced NF-κB-luc activity and weakly inhibited IFN-β-luc activity, but did not inhibit ISRE-luc activity (Fig. S2C). Thus, NLRC5 strongly inhibits NF-κB activation induced by different TLR ligands and weakly blocks IFN-β activation mainly due to its requirement for NF-κB activity, but has no apparent effect on ISRE activity.
To determine whether the observed NLRC5-mediated inhibition of NF-κB-luc activity is associated with endogenous NF-κB activity, we performed experiments with a gel-mobility shift assay. IKKβ expression allowed endogenous NF-κB to bind to the biotin-HRP-labeled DNA with NF-κB binding sites, but this activity was completely inhibited when NLRC5 was cotransfected. However, NLRC5 failed to inhibit p65-mediated NF-κB activation (Fig. 2D), consistent with results obtained with the NF-κB-luc reporter assay. To substantiate these findings, we found that like its human homolog, mNLRC5 strongly inhibited NF-κB activation by MyD88, TRAF6, IKKα and IKKβ, but not by p65 (Fig. S2D). Moreover, NLRC5- or mNLRC5-mediated NF-κB inhibition in 293T cells could be extended to THP-1 cells and murine embryonic fibroblasts (MEFs) (Fig. 2E). Taken together, these results suggest that NLRC5 functions as a negative regulator of NF-κB activation induced by TNF-α, IL-1β, LPS or by their downstream signaling molecules, and that its biological function is conserved between humans and mice as well as among multiple cell types.
NLRC5 Interacts with IKKα/IKKβ in Unstimulated and Stimulated Cells
Results presented in Fig. 2B and 2D suggest that NLRC5 may directly interact with IKKα, IKKβ or NEMO to inhibit NF-κB activation. To test this prediction, we transfected 293T cells with HA-tagged NLRC5 together with Flag-tagged IKKα, Flag-tagged IKKβ or Flag-tagged NEMO expression plasmids. Coimmunoprecipitation and Western blot analyses revealed that NLRC5 interacted with IKKα and IKKβ subunits, but not with NEMO, although the corresponding proteins were readily detected in whole cell lysates (Fig. 3A). Notably, NLRC5 did not interact with either TAK1 or MEKK3 in the upstream of IKK (Fig. S3A). To further test whether NLRC5 can interact with endogenous IKKα/β, we immunoprecipitated 293T cell lysates with an isotype IgG or anti-NLRC5 antibody, followed by Western blot analysis with the anti-IKKα/β, anti-NEMO or anti-NLRC5 antibody. As shown in Fig. 3B, the IKKα/β, but not NEMO could be detected in the anti-NLRC5 or anti-mNLRC5-immunoprecipitants. Such an interaction between NLRC5 and IKKα/β was also observed when anti-IKKα/β and anti-NEMO antibodies were used for immunoprecipitation (Fig. S3B). These results suggest that NLRC5 can interact with IKKα/β, but not with NEMO, under physiological conditions.
Figure 3. NLRC5 interacts with IKKα and IKKβ to inhibit their phosphorylation.
A) 293T cells transfected with Flag-IKKα, Flag-IKKβ, Flag-NEMO and HA-NLRC5. HA-tagged NLRC5 protein was immunoprecipitated with anti-HA beads, and blotted with anti-Flag.
(B) 293T and RAW264.7 cell extracts were immunoprecipitated with IgG, anti-NLRC5 or anti-mNLRC5 antibody, respectively, and then analyzed together with whole cell extracts by Western blot with an anti-IKKα/β, anti-NEMO, anti-NLRC5 or anti-mNLRC5 antibody.
(C) Cell extracts of RAW264.7 cells were fractionated on a size-exclusion column (HiPrep 16/60 Sephacryl S-300 HR). The collected factions with an equal volume were used for Western blot analysis with specific antibodies. The elution positions of calibration proteins with known molecular masses (kDa) were used to determine the size of complexes.
(D) 293T cells were transfected with the indicated doses of Flag-NLRC5, HA-IKKβ and HA-NEMO. Whole cell extracts were immunoprecipitated with anti-Flag beads, and blotted with anti-HA.
(E) 293T/TLR4 cells were transfected with empty vector or different doses of HA-NLRC5 (0, 50 or 200 ng) DNA and then treated with LPS. Cell extracts were collected at 30 min poststimulation and prepared for immunoprecipitation with anti-HA and anti-NEMO, followed by immunoblot (IB) with indicated antibodies or kinase assay (KA).
(F) The domain structure of IKKβ. Numbers in parentheses indicate amino acid position in construct. LZ, leucine zipper; HLH, helix-loop-helix.
(G) 293T cells were transfected with HA-NLRC5 and Flag-IKKβ or various Flag-IKKβ mutants. Whole cell extracts were immunoprecipitated with anti-Flag beads, and blotted with anti-HA.
(H and I) 293T cells transfected with IKKα, IKKβ, JNK1, JNK2, and p38 with or without HA-NLRC5 were used to analyze the phosphorylation of IKKα/β, JNK and p38.
(J) RAW264.7 cells were treated with LPS and collected at the indicated time points. Cell extracts were prepared for immunoprecipitation with anti-mNLRC5 or anti-NEMO, followed by immunoblot (IB) to determine the IKK phosphorylation with anti-p-IKK or anti-IKKα/βantibody and kinase activity of IKK.
See also Fig. S3 and S4.
Since IKKα/β generally forms a complex with NEMO, a key question is whether there are two distinct complexes in unstimulated cells: IKKα/β/NEMO and IKKα/β/NLRC5. To address this issue, we used different antibodies (anti-IKKα, anti-Flag and anti-NEMO) to immunoprecipitate the IKK complexes containing either NLRC5 or NEMO, and then determined the components of their complexes. We found NEMO and NLRC5 in the anti-IKKα immunoprecipitants, NLRC5 and IKKα/β (but not NEMO) in the anti-Flag-NLRC5 immunoprecipitants, and IKKα/β and NEMO (but not NLRC5) in the anti-NEMO immunoprecipitants (Fig. S3C), suggesting that the IKKα/β/NEMO and IKKα/β/NLRC5 complexes co-exist in unstimulated cells. To definitively demonstrate the presence of two distinct complexes, we fractionated cell extracts of RAW264.7 on a size-exclusion column (HiPrep 16/60 Sephacryl S-300 HR). Immunoblotting of fractions collected from chromatography showed that IKKα/β mainly coeluted with NEMO in fractions 22–31 (molecular mass about 700 kDa) (Fig. 3C), consistent with a previous report (Rothwarf et al., 1998). In contrast, NLRC5 mainly eluted in fractions 15–18 (molecular mass about 1100 kDa), which also contained IKKα/β but no NEMO (Fig. 3C), suggesting that NLRC5 forms a larger complex with IKKα/β than previously reported for the IKKα/β/NEMO complex, thus confirming the co-existence of IKKα/β/NLRC5 and IKKα/β/NEMO complexes in unstimulated cells.
To determine the dynamics of NLRC5 interaction with IKKα/β after stimulation, we treated RAW264.7 cells with LPS, collected them at different time points and performed immunoprecipitation and Western blot analyses. The interaction between NLRC5 and IKKα/β was reduced at 30 min, but was restored at 60 min after LPS stimulation (Fig. S4A). This oscillating pattern of interaction correlated inversely with IKK phosphorylation (p-IKK) during the first 60 min post-treatment and then diminished or even disappeared after 2 h of treatment (Fig. S4A–C), suggesting that the negative regulatory activity of NLRC5 is signal-dependent, but is not affected exclusively by protein concentration.
NLRC5 Competes with NEMO for IKKα/IKKβ and Inhibits Their Phosphorylation and Kinase Activity
We next tested whether NLRC5 and NEMO compete each other for binding to IKKα/β. 293T cells were transfected with a fixed concentration of Flag-NLRC5 and HA-IKKβ DNAs, together with increasing concentrations of HA-NEMO DNA. Coimmunoprecipitation and immunoblot revealed that the binding between NLRC5 and IKKβ was markedly decreased with increasing concentrations of NEMO (Fig. 3D). Although the phosphorylation and kinase activity of IKKα/β could be detected in the IKKα/β/NEMO complex after LPS stimulation, they decreased with increasing amounts of transfected NLRC5. There were no detectable phosphorylation and kinase activity of IKKα/β in the IKKα/β/NLRC5 complex (Fig. 3E). Importantly, increasing amounts of NLRC5 had more pronouced effects on the phosphorylation and kinase activity of IKKα/β in the total and NEMO-immunoprecipitated IKKα/β fraction than on its changes at the protein level (Fig. 3E). These results suggest that NLRC5 not only competes with NEMO for IKKα/β binding, but also inhibits IKKα/β phosphorylation and its ability to phosphorylate IκB or free IKKα/IKKβ. To test this possibility, we performed experiments with constitutively active IKKα (SS176/180 →EE) or IKKβ (SS177/181→EE) mutants and found that NLRC5 interacted with constitutively active IKKα (EE) and IKKβ (EE) (Fig. S4D). Kinase assays revealed that expression of NLRC5 inhibited the ability of IKKα (EE) and IKKβ (EE) to phosphorylate IκB (i.e. 32p-GST-IκBα), as well as their ability to autophosphorylate IKK (i.e. 32p-IKK) (Fig. S4E). These observations were further supported by NF-κB-luc assays showing that NLRC5 can inhibit NF-κB activation by the constitutively active IKKα (EE) and IKKβ (EE) (Fig. S4F). These results suggest that NLRC5 can inhibit the ability of active IKKα/IKKβ to phosphorylate IκBα or free IKKα/IKKβ.
To determine how NLRC5 inhibits the phosphorylation of IKK, we sought to identify the domain of IKKβ responsible for interacting with NLRC5. Because NEMO is known to bind to the C-terminus of IKKβ (May et al., 2000), we generated deletion mutants encompassing the amino-terminal kinase domain (KD), leucine zipper domain (LZ) and a C-terminal helix-loop-helix (HLH) domain of IKKβ, and tested them for their ability to interact with NLRC5 in an immunoprecipitation assay (Fig. 3F). Like the full-length IKKβ, the IKKβ-KD construct strongly interacted with NLRC5, while neither the IKKβ-LZ nor the IKKβ-HLH construct showed appreciable binding activity with NLRC5 (Fig. 3G), indicating that NLRC5 specifically binds to the IKKβ-KD domain. However, because of the large size of the NLRC5 protein, we considered that its binding to IKKβ might physically block any binding of NEMO to IKK (Fig. 3D and 3E).
We next tested whether the specificity of NLRC5-mediated inhibition of IKK phosphorylation. As shown in Fig. 3H and 3I, NLRC5 markedly inhibited the IKKα/β phosphorylation, but not p38 or JNK phosphorylation, consistent with the observation that NLRC5 did not interact with TAK1 or MEKK3 (Fig. S3A). Finally, we further tested the status of the phosphorylation and kinase activity of IKKα/β in IKKα/β/NLRC5 and IKKα/β/NEMO complexes in RAW264.7 cells under physiological conditions. IKKα/β in the IKKα/IKKβ/NLRC5 (NLRC5-IP) complex was not phosphorylated and showed no kinase activity during LPS stimulation. By contrast, IKKα/β in the IKKα/β/NEMO (NEMO-IP) complex was phosphorylated and exhibited a strong kinase activity after LPS stimulation (Fig. 3J), suggesting that NLRC5 inhibits its phosphorylation and kinase activity in the IKKα/IKKβ/NLRC5 complex under physiological conditions.
LRR Region Is Responsible for NLRC5-mediated Inhibition of IKK Phosphorylation
To identify the functional domains of NLRC5, we generated four deletion constructs: NLRC5-D1, containing the CARD and NOD domains (aa 1–517); NLRC5-D2, containing the LRR-R1 (aa 651–898); NLRC5-D3, containing the linker region and LRR-R2 (aa 900–1329); and NLRC5-D4, containing the CARD domain and LRR-R3 (aa 1–215 plus 1471–1866) (Fig. 4A). While all four NLRC5 deletion constructs could interact with the full-length IKKβ protein as well as IKKβ-KD (Fig. 4B and 4C), NLRC5-D3, like the full-length NLRC5, strongly inhibited NF-κB-luc activity (Fig. 4D). Other NLRC5 deletions showed either partial or no inhibitory effect on NF-κB-luc activity. These results suggest that NLRC5-D3 is required for the observed inhibition of NF-κB activity by NLRC5.
Figure 4. Interaction and functional analysis of NLRC5 deletions in the inhibition of NF-κB activation.
(A) Four NLRC5 deletion constructs were generated.
(B) and (C) 293T cells were transfected with NLRC5 and its mutations constructs, IKKβ or kinase domain of IKKβ and analyzed by coimmunoprecipitation and Western blot.
(D) 293T cells were transfected with NF-κB-luc reporter, together with an empty vector, or with full-length NLRC5 and its deletion constructs, and analyzed for luciferase activity (fold induction).
(E) 293T cells were transfected with various expression plasmids and the phosphorylation of IKKα or IKKβ was determined.
See also Fig. S4G.
To further investigate the molecular mechanism by which NLRC5-D3 inhibits NF-κB activity, we tested whether these deletions can inhibit the phosphorylation of IKKα/β. We found that NLRC5-D3 strongly inhibited IKKα and IKKβ phosphorylation, while NLRC5-D1, -D2 and -D4 produced little or weak inhibition (Fig. 4E). Notably, the inhibitory activity of these NLRC5 deletion mutants on the phosphorylation of IKKα and IKKβ correlated with their ability to inhibit NF-κB activation (Fig. 4D), suggesting that NLRC5-D3 inhibits NF-κB activation by blocking the phosphorylation of IKK complexes. Unlike the full-length NLRC5, however, NLRC5-D3 failed to compete with NEMO for binding to IKKα/IKKβ (Fig. S4G), suggesting that the size of full-length NLRC5 is critical for its ability to physically block NEMO from interacting with IKKα/β.
Knockdown of NLRC5 Enhances NF-κB Activation As Well As the Inflammatory Response
Since NLRC5 specifically interacts with IKKα/β and inhibits NF-κB activation, we reasoned that knockdown of NLRC5 would release IKKα/β for increased NF-κB activation under physiological conditions. To test this prediction, we first demonstrated the knockdown efficiency and specificity of NLRC5 or mNLRC5 at both the mRNA and protein levels in various cell types with at least two corresponding siRNAs, but not scrambled siRNAs (Fig. 5A and S5A–S5C). We next tested the effect of mNLRC5 knockdown on the phosphorylation of IKK, IκB, and other kinases, including JNK, ERK and p38. As shown in Fig. 5B and Fig. S5D, the phosphorylation of IKK (p-IKK) and IκB (p-IκB) in the mNLRC5 knockdown cells was at least 3-fold higher than that in the scrambled siRNA-transfected (control) cells at 30 min after LPS treatment, although the total amounts of IKK and IκB proteins were comparable between mNLRC5 knockdown and control cells. More importantly, we did not observe any appreciable differences in p-JNK, p-ERK and p-p38 between mNLRC5 knockdown and control cells, clearly indicating the specific inhibitory effect of mNLRC5 on the phosphorylation of IKK, but not on JNK, ERK and p38 phosphorylation. Similar results were obtained with human THP-1 cells (Fig. S5E). Collectively, these results suggest that specific knockdown of NLRC5 or mNLRC5 strongly enhances the phosphorylation of the IKK complexes, but does not affect the phosphorylation of JNK, ERK and p38 after LPS stimulation.
Figure 5. Knockdown of NLRC5 can significantly enhance NF-κB activation and inflammatory responses.
(A) Specific knockdown of NLRC5 or mNLRC5 was evaluated in various types of cells transfected with NLRC5/mNLRC5- siRNA, or scrambled siRNA.
(B) RAW264.7 cells were transfected with mNLRC5 siRNA or scrambled siRNA, and then treated with LPS. The cell extracts were harvested at different time points and used for Western blot of various kinases and signaling proteins.
(C) The cell extracts of mNLRC5 knockdown and control RAW264.7 cells after LPS treatment were used for immunoprecipitation to obtain NEMO-associated IKK (IP-1) and NEMO-free IKK (free IKK and mNLRC5 associated IKK (IP-2). The phosphorylation of IKKα/β, the total amount of IKKα/β and NEMO proteins in different fractions were determined by anti-p-IKK, anti-IKKα/β or anti-NEMO antibody.
(D) NLRC5 or mNLRC5 was knocked down in 293T/TLR4 and RAW264.7 cells. NF-κB-luc activity was determined after LPS treatment.
(E) The mNLRC5 knockdown and control AW264.7 cells were treated with LPS for 1 h; the nuclear proteins were harvested for NF-κB binding activity determined by EMSA. Oct-1 DNA-binding complexes were served as a control.
(F) Endogenous NLRC5 was knocked down in THP-1 and RAW264.7 cells and TNF-α and IL-6 production was measured after LPS treatment. Data in panels (D) and (F) are presented as means ± SEM. Asterisks indicate significant differences between groups (** P< 0.01, *** P<0.001 as determined by t-test analysis).
See also Fig. S5.
To further determine the molecular mechanisms by which NLRC5 knockdown affects NF-κB signaling, we performed two-step immunoprecipitations to obtain NEMO-associated IKK (IP-1) and NEMOCui free IKK (i.e., free IKK and NLRC5-associated IKK, IP-2) (Fig. 5C) and then compared the amounts of IKKα/IKKβ in each complex and their phosphorylation (p-IKK) in mNLRC5 knockdown versus control cells. We found that the total IKKα/β proteins in the NEMO-free IKK fraction (i.e., IP-2) of mNLRC5 knockdown cells was slightly lower than that of control cells, while IKKα/β in the IKKα/β/NEMO fraction (i.e. IP-1) in mNLRC5 knockdown cells was slightly higher than that in control cells, suggesting that the reduction of IKKα/β/NLRC5 complex in mNLRC5 knockdown cells decreases the IKKα/β proteins in the NEMO-free IKK fraction, but increases the IKKα/β proteins in the NEMO/IKK complex (Fig. 5C). Consistent with this observation, we found that the phosphorylation of IKK (p-IKK) in the NEMO-containing IP fraction (IP-1) in the mNLRC5 knockdown cells was higher than that in control cells. Importantly, even though the total IKKα/β in the NEMO-free fraction (IP-2) of mNLRC5 knockdown cells was less than that of control cells, its phosphorylation (p-IKK) level in mNLRC5 knockdown cells was higher than that in control cells (Fig. 5C), suggesting that mNLRC5 knockdown reduces the inhibition of phosphorylation of free IKKα/β. This notion agrees with data, showing that NLRC5 interacts with constitutively active IKKα (EE) and IKKβ (EE) and inhibits their phosphorylation and kinase activity (Fig. S4D and S4E).
We next sought to determine whether the enhanced IKK phosphorylation seen with NLRC5 knockdown promotes NF-κB activation and NF-κB-dependent gene expression. Using the NF-κB-luc reporter assay, we found that NLRC5 knockdown markedly increased NF-κB-luc activity in 293T/TLR4 and RAW264.7 cells after LPS treatment (Fig. 5D). To directly demonstrate that mNLRC5 knockdown enhances endogenous NF-κB activity, we examined the DNA-binding activity of endogenous NF-κB. Results in Fig. 5E show that endogenous NF-κB activity in mNLRC5 knockdown cells was at least 2-fold higher than that in control cells. Consistent with this observation, knockdown of NLRC5 or mNLRC5 resulted in markedly increased secretions of NF-κB-responsive cytokines, such as TNF-α and IL-6, in both mNLRC5 knockdown THP-1 and RAW264.7 cells (Fig. 5F). Hence, knockdown of NLRC5 or mNLRC5 enhances the IKKα/β phosphorylation and NF-κB activity, thus increasing NF-κB-dependent cytokine responses under physiological conditions.
NLRC5 Negatively Regulates Type I Interferon Signaling by Interacting with RIG-I and MDA5
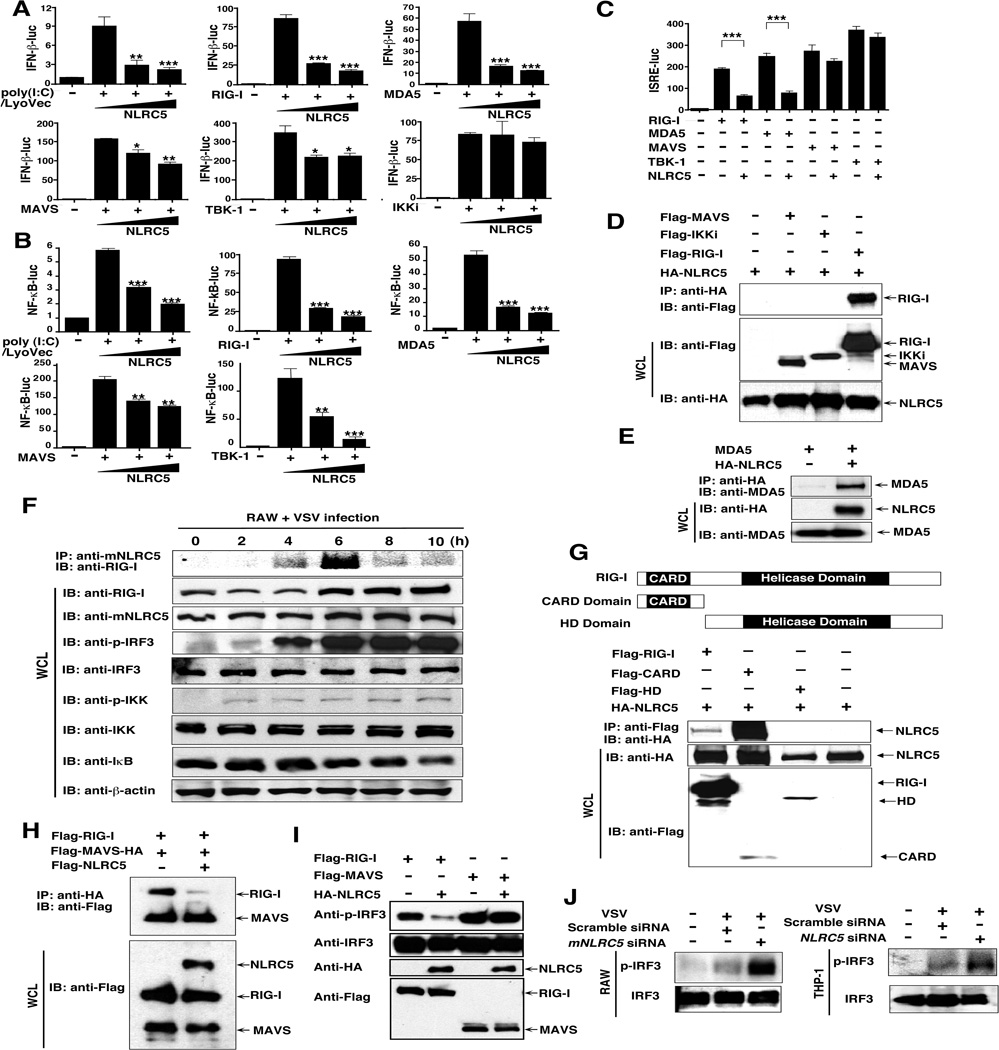
Recent studies show that NLRX1 inhibits the RIG-I-mediated signaling pathway by targeting MAVS (Moore et al., 2008). To determine whether NLRC5 might also be involved in the regulation of type I interferon signaling, we performed functional assays in TLR3-deficient 293T cells and found that intracellular poly(I:C) activated IFN-β signalling, although such activation was strongly inhibited by NLRC5 (Fig. 6A), suggesting that NLRC5 functions as a negative regulator of this antiviral pathway.
Figure 6. NLRC5 negatively regulates IFN-β activation by inhibiting RIG-I and MDA5 function.
(A–C): 293T cells were transfected with NF-κB-luc, INF-β-luc or ISRE-luc, NLRC5 plus poly(I:C)/Lyovec, RIG-I, MDA5, MAVS, TBK1 or IKKi plasmids and analyzed for INF-β or ISRE luciferase activity. Values are means ± SEM of three independent experiments.
(D) 293T cells were transfected with HA-NLRC5 plus RIG-I, MAVS or IKKi. After immunoprecipitation with anti-HA beads, specific proteins were analyzed by Western blot with anti-Flag.
(E) 293T cells were transfected with MDA5 with or without HA-NLRC5. After immunoprecipitation with anti-HA beads, specific proteins were analyzed by Western blot with anti-MDA5.
(F) RAW264.7 cells were infected with VSV-eGFP, and cell extracts were harvested at different time points, immunoprecipitated with anti-mNLRC5 antibody and analyzed by Western blot with anti-RIG-I.
(G) NLRC5 binds to the CARD domain of RIG-I. 293T cells were transfected with HA-NLRC5 plus Flag-RIG-I, Flag-RIG-I CARD domain and Helicase domain (HD). After immunoprecipitation with anti-Flag beads, specific proteins were analyzed by Western blot with anti-HA.
(H) NLRC5 competitively binds to RIG-I with MAVS. HEK293T cells were transfected with Flag-MAVS-HA plus Flag-RIG-1, or Flag-NLRC5. After immunoprecipitation with anti-HA beads, specific proteins were analyzed by Western blot with anti-Flag.
(I) 293T cells were transfected with Flag-RIG-I and Flag-MAVS, with or without HA-NLRC5, and used for Western blot analysis with anti-phospho-IRF3 and IRF3 antibodies.
(J) RAW264.7 and THP-1 cells were transfected with NLRC5/mNLRC5-specific siRNA or scrambled siRNA, respectively, and then infected with VSV-eGFP. The cell extracts were harvested for Western blot with anti-phospho-IRF3 and IRF3 antibodies.
See also Fig. S6.
To determine the molecular mechanisms by which NLRC5 could inhibit the IFN-β response, we performed similar experiments with different signaling molecules and found that RIG-I- and MDA5-induced IFN-β-luc activities could be markedly inhibited by increasing concentrations of NLRC5. However, NLRC5 weakly inhibited MAVS- and TBK1-induced IFN-β-luc activity and did not inhibit IKKi-induced IFN-β luciferase activity (Fig. 6A and Fig. S6A). Furthermore, NLRC5 markedly inhibited NF-κB-luc activity induced by intracellular poly(I:C), RIG-I, MDA5, MAVS or TBK1 (Fig. 6B). To determine whether the weak inhibition of MAVS- and TBK1-induced IFN-β-luc activity by NLRC5 might be due to the inhibitory effect of NLRC5 on IKK complexes, but not due to any direct effect on MAVS or TBK1, we transfected 293T cells with ISRE-luc, together with RIG-I, MDA5, MAVS or TBK1 in the presence or absence of NLRC5. Both RIG-I- and MDA5-induced ISRE-luc activities were potently inhibited, while MAVS- and TBK1-induced ISRE-luc activities were not (Fig. 6C), suggesting that NLRC5 inhibits IFN-β activation by directly interacting with RIG-I and MDA5, but not with MAVS or TBK1. Indeed, we found that NLRC5 was strongly associated with RIG-I and MDA5, but did not interact with MAVS, IKKi, TBK1, TRIF, TRAF3, or IRF3 (Fig. 6D, 6E and Fig. S6B). Thus, NLRC5 specifically binds to the RIG-I and MDA5 proteins to inhibit the IFN-β response.
To further define the interaction between RIG-I and mNLRC5 under physiological conditions, we infected RAW264.7 cells with VSV-eGFP and performed immunoprecipitation with anti-mNLRC5 at different time points after VSV-eGFP infection. We detected only a weak RIG-I protein band in anti-mNLRC5 immunoprecipitants at 4 h postinfection; however, it peaked 6 h and then became weak at 8 and 10 h postinfection (Fig. 6F). Notably, RIG-I protein expression was also increased at 6 h postinfection (Fig. 6F), consistent with previous observations (Honda and Taniguchi, 2006). Importantly, IRF3 phosporylation was observed at 4 h and further increased at 6 h postinfection, indicating that VSV-eGFP infection activated the IRF3-dependent signaling pathway. However, IKK phosphorylation and IκB degradation were weak after VSV-eGFP infection (Fig. 6F). These results suggest that interaction between NLRC5 and RIG-I is inducible after VSV-eGFP infection..
Because both the RIG-I protein level and IRF3 phosphorylation peaked at 6 h postinfection, coincident with the peak interaction between mNLRC5 and RIG-I in RAW264.7 cells, we considered that this interaction might critically rely on activating signal. To test this possibility, we generated RIG-I-CARD and RIG-I-helicase constructs and tested their ability to interact with NLRC5. Interestingly, NLRC5 strongly bound to the CARD domain of RIG-I, but not to the helicase domain, while inding of NLRC5 to the CARD domain of RIG-I markedly exceeded that to the full-length RIG-I (Fig. 6G), suggesting that the CARD domain becomes accessible only when RIG-I is activated by virus-derived ligands. Furthermore, competition experiments revealed that NLRC5 strongly inhibited the ability of MAVS to bind to RIG-I (Fig. 6H). Thus, it appears that the accessibility of the CARD domain of RIG-I is critical for a productive interaction between NLRC5 and RIG-I, and that such interaction may also be influenced by the relative concentrations of MAVS and NLRC5.
To confirm the inhibitory effect of NLRC5 on the IRF3 phosphorylation, we assessed the phosphorylation states of IRF3 by overexpressing NLRC5 in 293T cells and found that NLRC5 potently blocked the phosphorylation of endogenous IRF3 induced by RIG-I, but not by MAVS (Fig. 6I). In contrast, NLRC5 or mNLRC5 knockdown in THP-1 and RAW264.7 cells markedly increased IRF3 phosphorylation (Fig. 6J). Thus, NLRC5 can inhibit IFN-β signaling by blocking the binding of MAVS to RIG-I and the IRF3 phosphorylation.
Knockdown of NLRC5 Enhances Innate and Antiviral Immunity
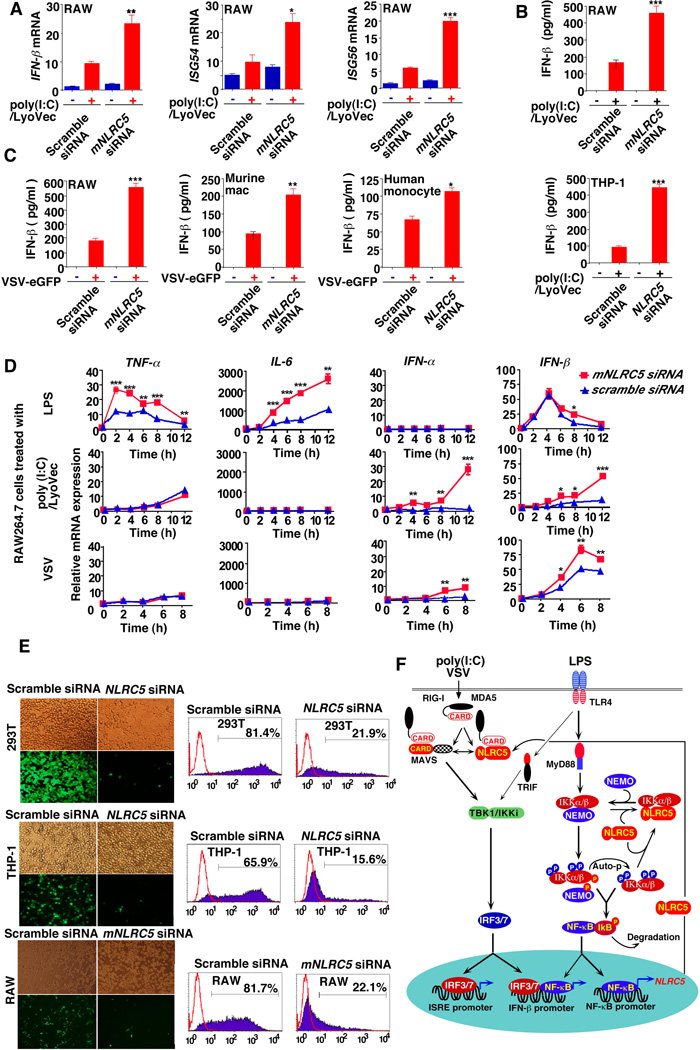
To further demonstrate the effects of NLRC5 knockdown on the expression of IFN-responsive genes, we knocked down endogenous NLRC5 or mNLRC5 and then treated the cells with poly(I:C)/Lyovec or infected them with VSV-eGFP. Real-time PCR analysis revealed that poly(I:C)/Lyovec treatment or infection with VSV-eGFP strongly increased mRNA levels of IFN-β and the interferon-stimulating genes ISG54 and ISG56 in cells transfected with mNLRC5- or NLRC5-specific siRNAs (Fig. 7A and Fig. S 7A), consistent with a previous study showing the upregulation of IFN-β mRNA in A549 cells with NLRC5 knockdown (Opitz et al., 2006). Furthermore, we found that poly(I:C)/Lyovec treatment or VSV-eGFP infection led to a large increase in the production of IFN-β protein in THP-1, RAW264.7, primary murine macrophages and primary human monocytes transfected with mNLRC5 or NLRC5-specific siRNA (Fig. 7B, 7C and Fig. S7B). We next determined their expression patterns over time after stimulation or VSV infection, and found that the NF-κB-responsive genes TNF-α and IL-6 in mNLRC5-knockdown cells were upregulated as early as 2–4 h after LPS treatment, and IL-6 continued in that state for another 10 h (Fig. 7D). No difference was found between mNLRC5 knockdown and control cells, with the exception of IFN-β expression at 8 h after LPS treatment (Fig. 7D). Similar experiments with intracellular poly(I:C) treatment or VSV infection showed strong upregulation of IRF3-responsive IFN-α and IFN-β expression, but little or no effect on TNF-α and IL-6 expression (Fig. 7D). Consistent with these observations, we found that LPS treatment resulted in more IL-6 and TNF-α than IFN-β protein, while VSV-eGFP infection led to more IFN-β than TNF-α and IL-6 proteins in mNLRC5 knockdown RAW264.7 cells (Fig. S7C). It appears that stimulation with LPS leads to more pronounced effects on NF-κB-regulated genes than does either poly(I:C) treatment or VSV-eGFP infection, while the converse is seen for IRF-3- regulated genes.
Figure 7. Knockdown of NLRC5 enhances cytokine response and antiviral immunity.
(A and B) RAW264.7 cells or THP-1 cells were transfected with mNLRC5/NLRC5-specific siRNA or scrambled siRNA, followed by poly(I:C)/Lyovec treatment. ISG-54, ISG-56, IFN-β mRNA and IFN-β protein were determined by real-time RT-PCR or ELISA.
(C) NLRC5 or mNLRC5 knockdown and control cells were infected with VSV-eGFP. Cell supernatants were used to measure IFN-β protein secretion by ELISA.
(D) RAW264.7 cells were transfected with mNLRC5 siRNA or scrambled siRNA for 36 h and then treated with LPS, poly (I:C)/LyoVec (1 µg/ml) or VSV-eGFP infection. Total RNAs from the treated cells were harvested at different time points and used for real-time PCR analysis to determine the expression of TNF-α, IL-6, IFN-α and IFN-β. The data in (A–D) are reported as means + SEM of three independent experiments. Asterisks indicate significant differences between groups (* P< 0.05, ** P<0.01, *** P<0.001 determined by t-test analysis).
(E) 293T cells, THP-1 cells and RAW264.7 cells were transfected with NLRC5-, mNLRC5-specific siRNA or scrambled siRNA, and then infected with VSV-eGFP. Viral infections were analyzed by fluorescence microscopy (with phase contrast as a control) as well as FACS analysis.
(F) Proposed model illustrating how NLRC5 negatively regulates both NF-κB and type I IFN signaling pathways. Auto-p, autophosphorylation.
See also Fig. S7.
To demonstrate a link between increased innate cytokine responses and antiviral immunity in NLRC5-silenced cells, we showed that knockdown of endogenous NLRC5 or mNLRC5 rendered cells remarkably resistant to viral infection and reduced the levels of VSV-eGFP-positive cells among 293T, THP-1, and RAW264.7 cells, as well as MEFs and human monocytes (Fig. 7E and Fig. S7D). To monitor VSV-eGFP propagation in both mNLRC5 knockdown and control cells, we performed time course experiments and showed that GFP expression from VSV-eGFP could be observed at 8 h postinfection with rapid propagation at 10 h in control cells. More than 90% of the cells were GFP positive at 12 h. By contrast, GFP expression from VSV-eGFP viruses could rarely be observed at 10 h, became visible at 12–14 h, and remained limited to a few cells even at 18 h in mNLRC5-silenced cells (Fig. S7E). At 15 h, the morphology of the cells was markedly changed in control cells, but not in mNLRC5 knockdown cells (Fig. S7F). At 20 h, most of the cells in control group had died with a striking reduction in the GFP signal, while the cells treated with mNLRC5 siRNA remained alive and appeared normal (Fig. S7G). We conclude that NLRC5 knockdown can significantly increase innate cytokine production and antiviral immunity against VSV-eGFP infection and propagation.
Discussion
Activation of innate immune receptors (TLRs, NLRs and RLRs) by their corresponding ligands initiates several key signaling pathways, leading to the production of proinflammatory cytokines, such as IL-6 and TNF-α, which in turn induce profound positive feedback for adaptive immune responses (Akira et al., 2006; Honda and Taniguchi, 2006). Increasing evidence indicates that many inflammation-associated diseases may result from dysregulated innate immunity (Inohara et al., 2005; Ting et al., 2006). More recently, IL-1, IL-6 and TNF-α produced by innate immune cells in chronic inflammation conditions have been shown to promote cancer development and progression (Karin et al., 2006). Thus, an understanding of the molecular mechanisms by which innate immunity is held in check through negative regulators appears critical for developing novel and more effective treatments for inflammation-induced autoimmune diseases and cancer (Wang et al., 2008).
Both NF-κB and type I interferon signaling are controlled at multiple levels by distinct mechanisms, whose regulatory proteins may themselves be direct transcriptional targets of NF-κB and type I interferon signaling, contributing to a negative regulatory feedback loop (Komuro et al., 2008). For example, expression of the A20, CYLD and DUBA negative regulators is controlled by NF-κB activity, while the RIG-I and MDA5 genes are transcriptionally regulated by type I interferon signaling (Komuro et al., 2008). We similarly observed the upregulation of NLRC5 or mNLRC5 at both the mRNA and protein levels after 6 h of treatment with LPS. Such upregulation was abolished in MyD88-deficient macrophages, suggesting that expression of NLRC5 itself is under the control of MyD88-NF-κB pathways and forms a negative regulatory feedback loop.
It has been demonstrated that the deubiquitinating enzymes A20 and CYLD inhibit NF-κB signaling by targeting TRAF6 upstream of IKK (Kovalenko et al., 2003; Liew et al., 2005; Trompouki et al., 2003; Wertz et al., 2004), while the deubiquitinating protein DUBA inhibits type I interferon activity by targeting TRAF3 (Kayagaki et al., 2007). Despite the importance of IKK as a central transducer of signaling from cytokines, TLRs and RLRs, leading to NF-κB activation, relatively little is known about its negative regulation. Our findings show that NLRC5 blocks IKK phosphorylation and thus NF-κB activation by interacting with IKKα and IKKβ, but not with the regulatory subunit NEMO. To elucidate the mechanism(s) by which NLRC5 inhibits IKK function, we provide evidence that NLRC5 forms a large complex with IKKα/β, in addition to the previously described stable IKKα/β/NEMO complex that seems to be dominant in unstimulated cells (Rothwarf et al., 1998). The interaction between NLRC5 and IKKα/β appears to be dynamic during the early phase (1–2 h) of LPS stimulation and correlates inversely with the phosphorylation of IKKα/β (p-IKK). This oscillation pattern diminishes or even disappears with time after LPS stimulation, suggesting the importance of this activating signal in the regulation of the interaction between NLRC5 and IKKα/β. NLRC5 knockdown experiments showed increased cytokine responses in NLRC5-silenced cells compared with control cells, suggesting that the NLRC5 protein concentration is also an important factor in the regulation of the IKK activity. Overall, it appears that both the LPS-induced activating signals and the relative protein concentration of NLRC5 influence the interaction between NLRC5 and IKKα/β, as well as the phosphorylation of IKK. Although NLRC5 is ubiquitinated at about 30–40 min after LPS stimulation (data not shown), further studies are needed to determine whether the ubiquitination of NLRC5 plays a role in the interaction between NLRC5 and IKKα/β.
A recent study shows that CUEDC2 interacts with IKKα/β, but not NEMO, and recruits PP1c to deactivate the IKK complex by dephosphorylating IKKα/β (Li et al., 2008). In resting cells, CUEDC2 binds to IKKα/β (but not NEMO) and undergoes transient disassociation and reassociation steps after TNF-α treatment (Li et al., 2008), similar to the interaction we described between NLRC5 and IKKα/β after LPS stimulation. We also show that IKKα/β in the IKKα/β/NLRC5 complex is not phosphorylated and lacks kinase activity, in contrast to IKKα/β in the IKKα/IKKβ/NEMO complex (Fig. 3E and 3J). These studies clearly indicate that besides NEMO, other important regulatory proteins such as NLRC5 can control the phosphorylation and kinase activity of IKK complex through sequestering active IKKα/β.
Although the kinase domain of IKKβ specifically recognized by NLRC5 differs from the NEMO-binding site at the C-terminus of IKKβ (May et al., 2000), we show that NLRC5 physically blocks the binding of NEMO to IKKα/β due to its large size (Fig. 3E and Fig. S4G). Our results also show that NLRC5 can interact with constitutively active forms of IKKα (EE) and IKKβ (EE) and inhibit their ability to phosphorylate IκBα, as well as their ability to autophosphorylate IKK (Fig. S4D and S4E). Importantly, experiments with two-step immunoprecipitation provide further evidence that mNLRC5 knockdown has striking effects on IKK and NF-κB signalling (Fig. 5C), probably through a modulating dynamic balance between /IKKα/β/NLRC5 and /IKKα/β/NEMO complexes as well as sequestering the active IKKα/β to form an inactive IKKα/β/NLRC5 complex. Furthermore, it has been suggested that the phosphorylation of Ser740 in IKKβ and Ser68 in NEMO by active IKK may disrupt the interaction between IKK and NEMO (Hayden and Ghosh, 2008), thus allowing other proteins, including NLRC5 or phosphatases, to interact with active IKK and terminate NF-κB signalling. These studies suggest that NLRC5 plays a critical role in inhibiting the phosphorylation and kinase activity of IKKα/β in NF-κB activation after LPS stimulation.
RIG-I and MDA-5 are key receptors for triggering type I interferon signaling pathways, and are controlled by positive and negative regulators, such as TRIM25 and RNF125, through ubiquitination (Arimoto et al., 2007; Gack et al., 2007). To test the hypothesis that other proteins are involved in the regulation of RLRs, we present evidence showing that NLRC5 can bind to both RIG-I and MDA5 but not to their downstream signaling molecules such as MAVS, TBK1, IKKi, TRAF3 or IRF3. These findings support a dual regulatory role for NLRC5 that encompasses both NF-κB and type I interferon signaling. Importantly, our results show that NLRC5 binds more strongly to the CARD domain of RIG-I than to the full-length RIG-I, suggesting that NLRC5 specifically recognizes the CARD domain when it becomes accessible after viral infection or stimulation by its ligands. This interpretation agrees with the proposed model for RIG-I action, in which the CARD domain of inactive RIG-I is masked by its intramolecular binding with the repressor domain in the C-terminus (Cui et al., 2008; Yoneyama and Fujita, 2009). Once RIG-I is activated, the CARD domain is exposed for binding to MAVS or NLRC5.
Based on the experimental data discussed above, we propose a model to illustrate how NLRC5 could negatively control both NF-κB and type I interferon signalling (Fig. 7F). First, the expression of NLRC5 itself is controlled by NF-κB activation, thus forming a negative regulatory feedback loop. Second, the key to modulating the activation of NF-κB by NLRC5 in stimulated cells lies in the dynamic balance between NLRC5/IKKα/IKKβ and NLRC5/IKKα/IKKβ complexes, which is controlled by signaling stimulation and relative protein (NLRC5 vs. NEMO) concentrations, as well as the ability of NLRC5 to inhibit IKK phosphorylation and kinase activity by sequestering active IKKα/IKKβ during signaling amplification after LPS stimulation. Thus, the striking effects of NLRC5 on NF-κB signalling and its downstream cytokine target genes cannot be explained by only a small fraction of the NLRC5/IKKα/IKKβ complex being in an unstimulated state. Third, in contrast to its regulation of NF-κB activation, NLRC5 competes with MAVS for binding to the CARD domain of RIG-I or MDA5 only after it is exposed by ligand stimulation of these receptors, leading to dampened activation of IRF3. The interaction between NLRC5 and RIG-I is inducible after poly (I:C) treatment or VSV viral infection. Although TLR activation, cytokine stimulation and viral infection can activate both NF-κB and type I signaling pathways, but they tend to have a much more pronounced effect on either NF-κB-regulated genes (e.g., LPS stimulation) or IRF3-regulated genes (poly(I:C) or VSV infection). This selectivity appears to reflect the different mechanisms by which NLRC5 regulates IKK activity or RIG-I/MDA5 proteins. The ultimate outcome of NLRC5 inhibition is determined by whether target gene promoters require NF-κB, IRF3, or both transcription factors for gene expression.
Finally, we show that specific knockdown of NLRC5 not only enhances NF-κB and type I interferon signaling and expression of their target genes, but also increases antiviral immunity in multiple cell lines and primary cells. Because of the conserved biological function of NLRC5 in humans and mice, as well as in various cell types, it appears to play a physiologically important role in the maintenance of immune homeostasis, especially in with regard to regulation of the innate immune responses. Hence, NLRC5 may provide a useful therapeutic target for enhancing immunity against microbial infections and inflammation-associated diseases.
Experimental Procedures
Molecular Cloning of Full-Length Human and Mouse NLRC5
A full-length NLRC5 cDNA was obtained from human PBMC cDNA by two-step PCR and was then cloned into pcDNA3.1Z with HA tag sequence. A similar strategy was used to clone mouse NLRC5. Both pcDNA-HA-NLRC5 and pcDNA-HA-mNLRC5 were sequenced to verify the correct DNA sequence and their open reading frames.
Expression Profile and Antibody Production
The expression profile of NLRC5 and mNLRC5 in different tissues was evaluated by reverse transcriptase (RT)-PCR analysis. NLRC5 and mNLRC5 peptides were used to generate polyclonal antibodies by standard methods.
Luciferase Assays, Immunoblot, Immunoprecipitation and Kinase Assay
HEK293 cells were transfected with IFN-β, NF-κB or ISRE luciferase plasmids and HA-NLRC5. TNF-α, IL-1β, LPS as well as exogenous MyD88, TRAF6, IKKα, IKKβ, NEMO, p65 (NF-κB), RIG-I, MDA5, MAVS and IKKi plasmids were used as stimulators. Dual-luciferase kits (Promega) were used for subsequent analysis. For kinase assay, a fusion of glutathione S-transferase and amino acids of 1–54 of IκBα (GST-IκBα) was used as the substrate. To determine the kinase activity of immuoprecipitated IKKα (EE)and IKKβ (EE) to autophosphorylate IKK, we added 32p-ATP to immunoprecipitatants and incubated the mixture at 37° C for 30 min. 32p-GST-IκBα (*p-GST-IκBα) and 32p-IKK (*p-IKK) were detected by autoradiography.
Protein Fractionation by Size-exclusion Column
Cell extracts prepared from RAW264.7 cell with lysis buffer were centrifuged at 20,000 rpm for 20 min at 4°C. Fifteen mg of protein in a volume of 1.5 ml was loaded onto a size-exclusion column (HiPrep 16/60 Sephacryl S-300 HR) with the capacity to separate the large protein complexes. Samples were fractionated with a flow rate of 0.5 ml per min, and collected as 0.5 ml fractions after passage through the void volume. Protein fractions were separated by SDS-PAGE and detected by western blotting with antibodies against mNLRC5, IKKα/β, or NEMO.
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assays were performed by using the LightShift Chemiluminescent EMSA kit from Pierce Biotechnology according to the manufacturer’s standard protocol.
Real-time PCR Analysis
First-strand cDNA was generated from total RNA using oligo-dT primers and reverse transcriptase (Invitrogen). Real-time PCR was conducted with the QuantiTect SYBR Green PCR Master Mix (Qiagen) and specific primers on an ABI Prism 7000 analyzer (Applied Biosystems).
Knockdown of NLRC5 and mNLRC5 by RNA Interference
NLRC5-specific, mNLRC5-specific and control (2-scramble mix) siRNA oligonucleotides were obtained from Invitrogen and Integrated DNA Technologies, and transfected into 293T, THP-1, RAW264.7 and primary cells with use of Lipofactamine 2000 (Invitrogen) and various Nucleofector kits (one for each cell type) according to the manufacturer’s instruction.
Statistical Analysis
The results of all quantitative experiments are reported as mean ± SEM of three independent experiments. Comparisons between groups for Statistical Significance were performed with a two-tailed paired Student’s t test.
Supplementary Material
Acknowledgements
We would like to thank Drs. Michael Karin and Bing Su for IKKα, IKKβ, NEMO and p38 constructs, Yinling Hu and Feng Zhu for constitutively active IKKα/β (EE) mutants and GST-IkB (1–54) constructs, Jae U Jung for RIG-I construct, Hongbin Shu for TRAF6, Kate Fitzgerald for TBK1 and IKKi constructs, and John Hiscott for pISRE-luc construct. We would also like to thank Drs Shao-Cong Sun, Xin Lin, and John Gilbert for their critical reading of this manuscript. This work was supported in part by grants from National Cancer Institute, National Institutes of health and Cancer Research Institute (to R.F.W). J.C. and L.Z. were partially supported by the China Scholarship Council. Z. J. C is an investigator of Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Data
Supplemental information includes Extended Experimental Procedures and seven figures and can be found with this article online.
REFERENCES
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A. 2007;104:7500–7505. doi: 10.1073/pnas.0611551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Eisenacher K, Kirchhofer A, Brzozka K, Lammens A, Lammens K, Fujita T, Conzelmann KK, Krug A, Hopfner KP. The C-terminal regulatory domain is the RNA 5'-triphosphate sensor of RIG-I. Mol Cell. 2008;29:169–179. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowds TA, Masumoto J, Chen FF, Ogura Y, Inohara N, Nunez G. Regulation of cryopyrin/Pypaf1 signaling by pyrin, the familial Mediterranean fever gene product. Biochem Biophys Res Commun. 2003;302:575–580. doi: 10.1016/s0006-291x(03)00221-3. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, et al. 5'-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Inohara, Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Phung Q, Chan S, Chaudhari R, Quan C, O'Rourke KM, Eby M, Pietras E, Cheng G, Bazan JF, et al. DUBA: a deubiquitinase that regulates type I interferon production. Science. 2007;318:1628–1632. doi: 10.1126/science.1145918. [DOI] [PubMed] [Google Scholar]
- Komuro A, Bamming D, Horvath CM. Negative regulation of cytoplasmic RNA-mediated antiviral signaling. Cytokine. 2008;43:350–358. doi: 10.1016/j.cyto.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- Li HY, Liu H, Wang CH, Zhang JY, Man JH, Gao YF, Zhang PJ, Li WH, Zhao J, Pan X, et al. Deactivation of the kinase IKK by CUEDC2 through recruitment of the phosphatase PP1. Nat Immunol. 2008;9:533–541. doi: 10.1038/ni.1600. [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- Opitz B, Vinzing M, van Laak V, Schmeck B, Heine G, Gunther S, Preissner R, Slevogt H, N'Guessan PD, Eitel J, et al. Legionella pneumophila induces IFNbeta in lung epithelial cells via IPS-1 and IRF3, which also control bacterial replication. J Biol Chem. 2006;281:36173–36179. doi: 10.1074/jbc.M604638200. [DOI] [PubMed] [Google Scholar]
- Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang R-F. Toll-like receptor 8 mediated-reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo K, Wang R-F. Tumor-infiltrating gamma-delta T cells suppress T and dendritic cell function via mechanisms controlled by a unique Toll-like receptor signaling pathway. Immunity. 2007;27:334–338. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattoli I, Carneiro LA, Jehanno M, Magalhaes JG, Shu Y, Philpott DJ, Arnoult D, Girardin SE. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 2008;9:293–300. doi: 10.1038/sj.embor.7401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- Wang RF, Miyahara Y, Wang HY. Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene. 2008;27:181–189. doi: 10.1038/sj.onc.1210906. [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.