Abstract
Pain often accompanies antigen-specific immune-related disorders though little is known of the underlying neural mechanisms. A common feature among these disorders is the elevated level of antigen-specific immunoglobulin (Ig) G in the serum and the presence of IgG immune complex (IC) in the affected tissue. We hypothesize that IC may directly activate the Fc-gamma receptor type I (FcγRI) expressed in nociceptive dorsal root ganglion (DRG) neurons and increase neuronal excitability thus potentially contributing to pain. Immunofluorescent labeling indicated that FcγRI, but not FcγRIIB or FcγRIII, was expressed in a subpopulation of rat DRG neurons including those expressing nociceptive markers. Calcium imaging revealed that the IC, but neither of the antibody (IgG) or antigen alone, produced an increase in intracellular calcium. This effect was abolished by the removal of the IgG Fc portion in the IC or the application of an anti-FcγRI antibody, suggesting a key role of the FcγRI receptor. Removal of extracellular calcium or depletion of intracellular calcium stores prevented the IC-induced calcium response. In whole-cell current-clamp recordings, IC depolarized the resting membrane potential, decreased the rheobase, and increased the number of action potentials evoked by a depolarizing current at 2X rheobase. In about half of the responsive neurons, IC evoked action potential discharges. These results suggest that a subpopulation of nociceptive neurons expresses functional FcγRI and that the activation of this receptor by IC increases neuronal excitability.
Keywords: Immunoglobulin G, immune complex, Fc-gamma receptor type I, dorsal root ganglion, pain
Introduction
Pain often accompanies antigen-specific immune-related disorders that include autoimmune diseases such as Guillain-Barre Syndrome (Moulin, 1998; Moulin et al., 1997) and multiple sclerosis (Kenner et al., 2007), allergic diseases such as atopic and allergic contact dermatitis (Valks and Conde-Salazar, 2003; Wittkowski et al., 2007), and infectious diseases such as herpes zoster (Oaklander, 2008). However, there is insufficient information about the underlying neuronal mechanisms by which an antigen might act to produce pain. A common feature of these diseases is the elevated level of antigen-specific immunoglobulins (Ig), especially IgG in the serum and the presence of immune complex (IC) formed by IgG bound to an antigen (IgG-IC) in the affected tissue (Ferguson and Salinas, 1984; Haanpaa et al., 1998; Stinissen et al., 1997; Yuki et al., 1992).
Fc-gamma receptor (FcγR) is the receptor that binds to the Fc portion of IgG. FcγRs are typically expressed on immune cells. There are two functionally different classes of FcγRs: the activating and inhibitory receptors (Nimmerjahn and Ravetch, 2006, 2008). The activation of FcγR by IgG-IC triggers a wide range of biological functions including phagocytosis, antibody-dependent cell-mediated cytotoxicity and the release of cytokines (Nimmerjahn and Ravetch, 2008). FcγR type I (FcγRI) is the only high-affinity activating receptor, and has been found critically involved in a number of inflammatory and immune responses (Barnes et al., 2002; Ioan-Facsinay et al., 2002) including certain immune-related disorders in the central nervous system (Okun et al., 2010). Treatments such as intravenous immunoglobulin that potentially block FcγRI or reduce the IgG-IC were found to ameliorate symptoms, including pain, in a number of immune-related diseases such as multiple sclerosis (Humle Jorgensen and Sorensen, 2005), systemic lupus erythematosus (Zandman-Goddard et al., 2009) and complex regional syndromes (Goebel et al., 2010).
IC induced cutaneous hyperalgesia after the injection of a foreign antigen to the hindpaw of mice (Verri et al., 2008) and rats (Ma et al., 2009) immunized with the same antigen and exhibiting an elevated level of serum IgG. Although it is well known that IgG-IC may induce pain and hyperalgesia via the activation of certain immune cells and the release of pro-inflammatory cytokines (Pinto et al., 2010; Verri et al., 2007; Verri et al., 2008), a direct effect of the IgG-IC on the excitability of nociceptive primary sensory neurons has not been determined. As suggested in a previous report by Andoh and Kuraishi (2004), functional FcγRI may be expressed on dissociated mouse DRG neurons. However, the expression levels of FcγRI in the intact DRG are unknown. In addition, it is not clear whether the neurons activated by IgG-IC are nociceptive and whether the activation leads to an increase in excitability.
We hypothesized that certain nociceptive neurons express FcγRI and become more excitable when the receptor is activated by IgG-IC. We verified this hypothesis in rat DRG neurons with the use of immunofluorescent labeling, calcium imaging, and whole-cell patch clamp recording. Preliminary results from this study were presented in abstract form (Qu et al., 2010a; Qu et al., 2010b). Our findings reveal a novel mechanism of nociception driven by antigen-specific immune responses, and may provide potential therapeutic strategies for the treatment of pain related to immune disorders.
Methods
Animals
The adult Sprague-Dawley rats (120-180 g) used in this study were all female to maintain consistency with our previous studies on dissociated rat DRG neurons (Ma and LaMotte, 2005; Ma et al., 2006). Animals were housed in groups of three or four under a 12 hr light/dark cycle. The use and handling of animals were approved by Institutional Animal Care and Use Committee of the Yale University School of Medicine and were in accordance with guidelines provided by National Institute of Health and the International Association for the Study of Pain.
Immunohistochemistry
Immunofluorescent labeling of the following markers was performed on naïve rat lumbar DRG cryosections using the methods as previously described (White et al., 2005): IgG receptors FcγRI-III, glutamine synthetase (GS, as a marker for satellite glial cells (Hanani, 2005; Zhang et al., 2009)), isolectin B4 (IB4), transient receptor potential vanilloid 1 (TRPV1), substance P (SP) and calcitonin gene-related peptide (CGRP). Briefly, the L4 and L5 DRGs (n = 12) were harvested from adult Sprague-Dawley rats (n = 6) transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde, post-fixed in the same fixative for 4 hrs, and then cryoprotected in 30% sucrose overnight. The tissue was frozen and sectioned at 10 μm thick on a cryostat. Tissue sections were incubated with blocking buffer (10% normal horse serum and 0.2% Triton X-100 in PBS) for 1 hr, followed by overnight incubation with the primary antibodies (goat-anti-FcγRI, -FcγRIIB, or -FcγRIII, 1:200, Santa Cruz Biotechnology; and one of the following: rabbit-anti-GS, 1:200, Abcam; rabbit-anti-TRPV1, 1:1000, Neuromics; rabbit-anti-SP, 1:1000, Chemicon; rabbit-anti-CGRP, 1:1000; Sigma-Aldrich) at room temperature, and then with the proper secondary antibodies (Alexa Fluor 555-conjugated donkey-anti-goat, 1:500; Alexa Fluor 488-conjugated donkey-anti-rabbit, 1:500, Invitrogen) for 1 hr. FITC conjugated IB4 (10 μg/ml, Sigma-Aldrich) was added with the secondary antibodies. TO-PRO-3 iodide (Invitrogen) was used to stain nuclear profiles. The slides were then washed in PBS and cover-slipped with ProLong Gold antifade reagent (Invitrogen). The cells were visualized and the images were captured using a laser confocal microscopic imaging system (LMS 510, Carl Zeiss MicroImaging). The number of immunofluorescence positive cells was counted using ImagePro Plus 5.0 (Media Cybernetics). DRG neurons were classified according to their cross-sectional areas as small- (area < 636 μm2), medium- (area 637~1431 μm2) and large-sized (area > 1431 μm2). This classification is based on the criteria for dissociated small- (diameter < 30 μm), medium- (diameter 30~45 μm) and large-sized (diameter > 45 μm) neurons in adult rats and taking into account the approximately 10% decrease in size due to the fixation procedure (Fukuoka et al., 2002; White et al., 2005). Only neurons with nucleus profile in the cross-section were counted. Control staining was performed using the normal goat IgG (1:200, Santa Cruz) to replace the goat-anti-FcγRI, or adding blocking peptide (CD64 (N-19) P, 1:100, Santa Cruz) with the primary antibody to test its specificity. In a separate experiment, we also compared a monoclonal FcγRI antibody from a different vendor (MAB20741, 1:200, R&D Systems) with the FcγRI antibody used in this study (sc-7642, 1:200, Santa Cruz).
Cell dissociation and culture
DRG neurons were cultured from 53 adult female Sprague-Dawley rats as previously described (Dib-Hajj et al., 2009; Ma and LaMotte, 2005; Yao et al., 2003). Briefly, bilateral L4 and L5 lumbar DRGs were removed and placed in oxygenated complete saline solution (CSS) for cleaning and mincing. The CSS contained (mM): NaCl 137, KCl 5.3, MgCl2 1, CaCl2 3, Sorbitol 25, and HEPES 10, adjusted to pH 7.2 with NaOH. The DRGs were then digested with Liberase TH (0.19 U/ml; Roche Diagnostics Corp., Indianapolis, IN) for 20 min, and for another 20 min with Liberase TL (0.25 U/ml; Roche Diagnostics) and papain (30 U/ml, Worthington Biochemical, Lakewood, NJ) in CSS containing 0.5 mM EDTA at 37°C. After enzymatic digestion, the cells were dissociated by gentle trituration in cultured medium containing 1 mg/ml bovine serum albumin and 1 mg/ml trypsin inhibitor (Boehringer Mannheim, Germany), and placed on poly-D-lysine/laminin coated glass coverslips (BioCoat, BD Biosciences, MA). The culture medium contained equal amounts of DMEM and F12 (Gibco, Grand Island, MD) with 10% FCS (HyClone Laboratories, Logan, UT) and 1% penicillin and streptomycin (Invitrogen). The cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2 and used within 24 hours.
Preparation of IgG immune complex
IgG-IC was prepared by using the normal mouse IgG (Santa Cruz Biotechnology, CA) as antigen and the affinity-purified rat anti-mouse IgG (Jackson ImmunoResearch, PA) as antibody, which has been pretreated to avoid non-specific self-binding with rat IgG. Unlike the studies by Andoh and Kuraishi (2004) that used IgG purified from the serum of ragweed pollen-sensitized mice, we used commercially available antibody and antigen so that the concentration could be better controlled in the experiments. To avoid the possible toxic and non-specific effects of sodium azide on DRG neurons, the storage buffer of all the IgGs (containing sodium azide) was changed to HEPES buffer using Zeba™ spin desalting columns (Thermo Scientific, Rockford, IL) before application.
IgG-IC were formed by incubating 10 μg/ml antigen and antibody at the ratio of 1:1 for 1 h at 25°C, and then diluting to the concentrations of 0.001, 0.01, 0.1, 1, and 10 μg/ml. In control experiments, the individual components of IC ( i.e. rat anti-mouse IgG, normal mouse IgG), or the mixture of the normal mouse IgG and normal rat IgG (Santa Cruz Biotechnology, CA), all at the concentration of 0.1 μg/ml, were applied. To assess the function of the Fc portion of IgG, the intact rat anti-mouse IgG in the IC preparations was replaced with the affinity-purified F(ab/)2 fragment rat anti-mouse IgG (Jackson ImmunoResearch Laboratories, PA).
Calcium imaging
The cultured rat DRG neurons were loaded with Fura 2-acetoxymethyl ester (2 μM, Invitrogen) in the dark for 45 min at 37°C. After loading, DRG neurons were washed twice in HEPES buffer to remove extracellular dye, and placed in a recording chamber continuously perfused with HEPES buffer at a flow rate of 1.5 ml/min at room temperature. The HEPES buffer contained (in mM): 145 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 glucose and 10 HEPES (adjusted to pH 7.4 with NaOH). For some experiments, Ca2+-free bath solution was applied, which was the normal bath solution (HEPES buffer) modified by the removal of 2 mM CaCl2, the addition of 0.1 mM EGTA and an increase in the concentration of MgCl2 (4 mM) (Lu et al., 2006).
Ratiometric calcium imaging was performed at room temperature (20-22°C) using an upright Olympus BX-51WI microscope equipped with a ratiometric imaging system (Indec Biosystems, CA). The calcium signals by 340 nm and 380 nm excitation (switched by the monochromator, Polychrome V, TILL Photonics, NY) were recorded at 2-s intervals using a cooled CCD camera (Sensicam, PCO, Germany) controlled by a computer with Image Workbench 5.2 software (Indec Biosystems, CA). The ratio of 340nm/380nm fluorescence intensity (R340/380) within a certain region of interest after background subtraction was used as a relative measure of intracellular calcium concentration ([Ca2+]i) (Grynkiewicz et al., 1985). Calibration with external standards (Calcium Calibration Buffer Kit, Invitrogen) showed that R340/380 increased linearly with [Ca2+]i up to about 1 μM and R340/380 of 0.7–1.25 corresponded to basal [Ca2+]i of 90–180 nM. Therefore, only small-diameter neurons (≤ 30 μm) with R340/380 at the range of 0.7–1.25 were included in this study.
Neurons were considered capsaicin sensitive (CAP+) if a 10-s application of 1 μM capsaicin evoked an increase in R340/380 that was equal or greater than 20% above baseline. The viability of the neurons was confirmed by the increase in [Ca2+]i produced by a 5-s application of 50 mM K+ at the end of each experiment. The proportion of DRG neurons responsive to IC was calculated as the number of IC-responsive neurons divided by all the neurons tested (c.f. Qu et al., 2010a; Qu et al., 2010b). All agents were dissolved in HEPES buffer and applied locally to the neuronal cell bodies through a micropipette (with a tip diameter of 100 μm) and a 8-channel pressure-controlled drug application system (AutoMate Scientific, Berkely, CA) (Ma et al., 2006). The interval between drug applications was at least 3 min. CPA (cyclopiazonic acid) was purchased from Ascent Scientific (Princeton, NJ). Anti-CD64 (FcRI) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless indicated.
Electrophysiological recording
Whole-cell patch-clamp recordings were performed on dissociated DRG neurons at room temperature using a Multiclamp 700A amplifier with Pclamp 9 software (Molecular Device, Sunnyvale, CA) as described (Sun et al., 2006; Zheng et al., 2007). Patch pipettes were pulled from borosilicate glass capillaries (Sutter Instrument; 1.2 mm outer diameter, 0.69 mm inner diameter; Novato, CA) using a horizontal puller (Model P97, Sutter Instrument, Novato, CA). The resistance of the patch pipettes was 3–4 MΩ when filled with an internal solution consisting of (in mM): K+-gluconate 120, KCl 20, CaCl2·2H2O 1, MgCl2·6H2O 2, EGTA 11, HEPES-K+ 10, MgATP 2, adjusted to a pH of 7.2 with Tris-base and having an osmolarity of 290-300 mOsm (Zheng et al., 2007). The series resistance was routinely compensated at 60-80%. Resting membrane potential (RMP) was recorded for each neuron under the current clamp mode after stabilization (within 4 min). A neuron was included if the RMP was more negative than −40 mV and the spike overshoot was > 15 mV. Action potentials (APs) were evoked by a series of depolarizing current steps, each 500 ms duration, in increments of 50 pA up to 2 nA. The current threshold (rheobase) was defined as the minimal depolarizing current required to evoke an AP. The number of APs evoked by a suprathreshold stimulus was estimated by injecting a 500-ms depolarizing current of a magnitude at twice the rheobase. Input resistance was obtained from the slope of a steady-state current-voltage plot in response to a series of hyperpolarizing currents steps from -200 to -50 pA. Only small-diameter neurons (≤ 30 μm) were recorded. Capsaicin (1 μM) was applied for 10 s at the end of whole-cell recordings.
Statistical analysis
Data values are presented as means with standard errors (mean ± SEM). Statistical analyses were performed using the SPSS software (version 17.0, IBM Crop., Somers, NY). A Student's t-test was used to test the statistical significance of a difference between mean responses for two groups. Statistical comparisons of differences among three or more groups were made with a one-way analysis of variance (ANOVAs) followed by Scheffe's post hoc test. Chi-Square tests were used to compare the incidence of neuronal responses. The criterion for statistical significance was a value of p < 0.05.
Results
Expression of FcγRI on rat DRG neurons
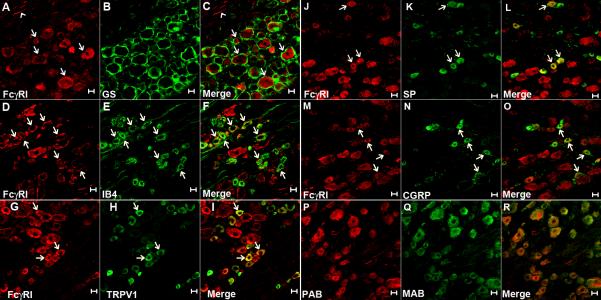
Immunoreactivity (IR) for FcγRI (Fig. 1A) was observed in 37.4% (176 of 471) of DRG neurons (including the somata and axons) across the neuronal size range examined, but not in cells that were identified as satellite glia by their positive IR for GS (Fig. 1B,C). The percentages of DRG neurons that showed FcγRI-IR within each size category were: 19.1% (56/293) of small-, 54.1% (66/122) of medium- and 96.4% (54/56) of large-sized neurons. No IR was detected for FcγRIIB or FcγIII (data not shown), consistent with a previous study in mouse DRG neurons (Andoh and Kuraishi, 2004). In addition, some FcγRI-immunopositive DRG neurons with various sizes were also immunopositive for the nociceptive neuronal markers IB4 (detected in 52.0% of neurons with FcγRI-IR, Fig. 1D-F), TRPV1 (37.0%, Fig. 1G-I), substance P (13.1%, Fig. 1J-L) and CGRP (25.9%, Fig. 1M-O). It is notable that the majority (73.3%) of FcγRI-immunopositive small-sized DRG neurons expressed TRPV1. To confirm the specificity of the primary antibody for FcγRI, control experiments were performed using the normal goat IgG, or adding the blocking peptide for the primary antibody. Both control groups revealed negative results (not shown). The staining pattern remained the same when the primary antibody for FcγRI was compared with a monoclonal FcγRI antibody from a different vendor (Fig. 1P-R).
Figure 1.
Expression of FcγRI on rat DRG neurons. Immunoreactivity for FcγRI (red) was found in both the somata (arrows) and axons (arrowhead) of neurons (A), but not in the satellite glial cells that were immunopositive for GS (green, in B). The merged image (C) indicates the lack of co-localization between FcγRI and GS. FcγRI (D, G, J and M) was found co-expressed with IB4 (E), TRPV1 (H), SP (K) and CGRP (N) in some DRG neurons (arrows in merged image F, I, L and O). The staining pattern remained the same when the primary antibody for FcγRI (PAB, in P) and a monoclonal FcγRI antibody from a different vendor (MAB, in Q) were used for double-labeling the same section (merged image in R). Scale bar: 20 μm.
IC-induced [Ca2+]i increase via neuronal FcγRI in dissociated DRG neurons
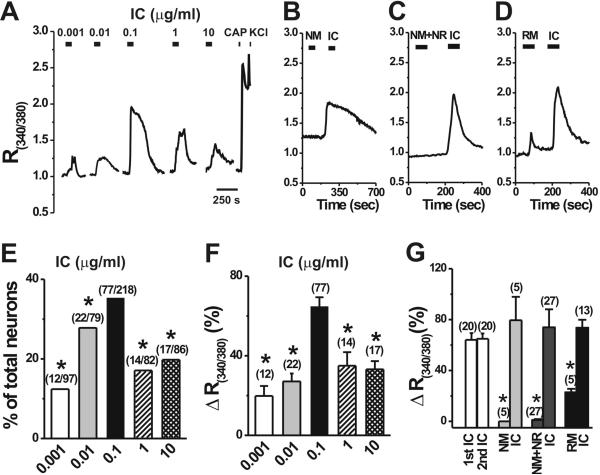
In order to test the potential role of neuronal FcγRI in the activation of nociceptive sensory afferents, we focused our functional studies on small-diameter dissociated DRG neurons which are mostly C-type primary afferents (Harper and Lawson, 1985) and are more likely to mediate nociception and pain (Kumazawa, 1996) than the larger A-type afferents. We first investigated the effects of different concentrations of IC formed by rat anti-mouse IgG (antibody) and normal mouse IgG (antigen), on the [Ca2+]i in the small-diameter dissociated DRG neurons (Fig. 2A,E,F). A bath application of IC at different concentrations caused a significant [Ca2+]i increase in rat DRG neurons [F(4, 136 ) = 10.93 , p < 0.001]. The proportion of neurons responding to the IC of 0.1 μg/ml with an increase in [Ca2+]i was the largest (35.3%) among these five concentrations (Fig. 2E). Moreover, the IC-induced [Ca2+]i increase was significantly greater for 0.1 μg/ml than for other concentrations (Fig. 2F). The [Ca2+]i returned slowly to baseline at least 3 min after washout of IC in most neurons tested. When IC (0.1 μg/ml) was applied twice (at an interval of 10 min), the magnitude of the second R340/380 increase was 64.9 ± 4.2%, which was not significantly different from the 64.1 ± 5.3% of the first (Fig. 2G; p > 0.05; n = 20), suggesting that no desensitization occurs during repetitive application of the IC. Therefore, the concentration of 0.1 μg/ml was chosen as the optimal dose of IC and used throughout the study. IC-induced calcium responses were observed in both capsaicin-sensitive and capsaicin-insensitive neurons. Among the capsaicin-sensitive DRG neurons, 36.7% (57 out of 155) showed a [Ca2+]i rise in response to 0.1 μg/ml IC (Fig. 2A), indicating the co-expression of FcγRI and TRPV1 on the same DRG neuron. Twenty of the 63 capsaicin-insensitive neurons (31.7%) responded to IC. In addition, among the IC-responsive neurons 74.0% (57 out of 77) was capsaicin-sensitive, similar to the proportion (73.3%) of FcγRI-immunopositive small-sized DRG neurons that co-expressed TRPV1 as observed in the above immunostaining studies.
Figure 2.
IC evoked [Ca2+]i increases in dissociated DRG neurons. Representative traces of changes in [Ca2+]i (R(340/380)) induced by IC at different doses (A), normal mouse IgG (NM, 0.1 μg/ml, B), the mixture of NM and normal rat IgG (NR, 0.1 μg/ml, C) or rat-anti-mouse IgG (RM, 0.1 μg/ml, D) were demonstrated on the upper row, and summary graphs were given below (E-G). The dose of 0.1 μg/ml IC evoked the most robust response (A), as measured by the percentage of responsive neurons (E) or changes (Δ) in R(340/380) among the responsive neurons (F). Application of capsaicin (CAP; 1 μM; 10-s) and KCl (50 mM; 5-s) induced Ca2+ transients, respectively (A). The control experiments in BD were performed on different groups of DRG neurons, and IC was subsequently applied to the same neuron following the application of NM, RM or NR+NM. No obvious changes in [Ca2+]i were detected in response to NM (B) or the mixture of NM and NR (C). Application of RM alone evoked a mild [Ca2+]i increase in some neurons (D). G: Sequential application of IC evoked similar increases in [Ca2+]i, but calcium responses to the control solutions were always significantly lower than those evoked by a subsequent application of IC. Number of neurons tested was given in the parentheses. *p < 0.05 versus 0.1 μg/ml IC, Chi-square test (E); *p < 0.05 versus 0.1 μg/ml IC, Scheffe's post hoc test following one-way ANOVA (F); *p < 0.001 versus 0.1 μg/ml IC, student's t-test (G).
In control experiments, bath application of the normal mouse IgG alone (Fig. 2B) or the mixture of normal mouse IgG and normal rat IgG (Fig. 2C), all at 0.1 μg/ml failed to induce any significant changes in the basal [Ca2+]i whereas a subsequent application of IC produced a large rise in [Ca2+]i in the same neuron (Fig. 2B,C,G). Although the rat-anti-mouse IgG alone increased R340/380 by 22.8 ± 4.5% in 5 of 37 (13.5%) neurons tested, the increase in R340/380 was much more robust in response to a subsequent application of IC (Fig. 2D,G). Furthermore, a significantly larger percentage of neurons (13 out of 37) responded to IC than to rat-anti-mouse IgG alone (37.8% vs.13.5%, p < 0.001, Chi-square test).
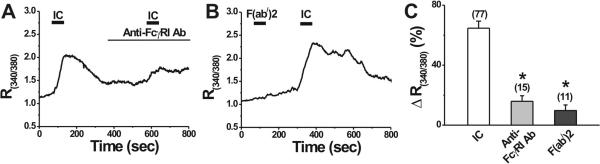
To examine whether FcγRI was involved in the IC-induced [Ca2+]i increase, DRG neurons were pretreated with the primary antibody against FcγRI (0.2 μg/ml, buffer exchanged in HEPES solution) for 3-5 min. In the presence of FcγRI antibody, the rise in [Ca2+]i evoked by IC was significantly less than that in the control medium without FcγRI antibody (Fig. 3A, C). In addition, after the Fc portion in the IC was removed, i.e. by replacing the intact rat-anti-mouse IgG with the F(ab/)2 fragment of rat-anti-mouse IgG, the [Ca2+]i rise was almost abolished (Fig. 3B, C), whereas the response to intact IC, subsequently applied, could still be evoked (Fig. 3B). These results suggest that the interaction between the Fc portion of IgG and neuronal FcγRI is essential for the IC-induced increase in [Ca2+]i.
Figure 3.
The neuronal FcγRI and the IgG Fc region are required for IC-induced increase in [Ca2+]i. A, Representative trace showing that the [Ca2+]i responses (changes in R340/380) to IC was significantly reduced after pretreatment with anti-FcγRI antibody. B, Application of the mixture of NM and the F(ab/)2 fragment of RM failed to induce a change in the basal [Ca2+]i, but the following application of intact IC evoked a robust increase in [Ca2+]i. C, Mean percentage change in R340/380. *p < 0.001 versus IC, one-way ANOVA followed by Scheffe's post hoc test.
Mechanisms of IC-induced [Ca2+]i increase in DRG neurons
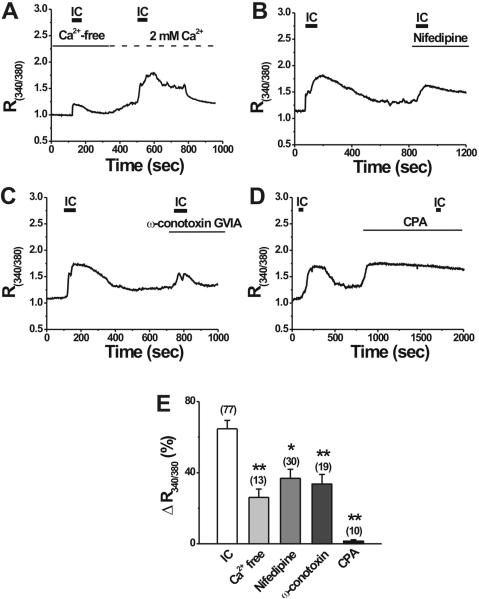
To investigate whether IC-induced [Ca2+]i increase was due to calcium influx from extracellular space, extracellular calcium in bath perfusion was removed and 0.1 mM EGTA was added. In Ca2+-free extracellular solution, IC did not change basal [Ca2+]i in 30 out of 43 DRG neurons, indicating that Ca2+-permeable ion channels on the cell membrane might contribute to the IC-induced [Ca2+]i increase. However, in the remaining 13 DRG neurons, IC still increased [Ca2+]i in Ca2+-free extracellular solution, but significantly smaller than the changes of [Ca2+]i seen in normal extracellular solution containing 2 mM Ca2+ (Fig. 4A, E). This result suggests that IC may also stimulate calcium release presumably from intracellular stores in a subpopulation of DRG neurons.
Figure 4.
Mechanisms of IC-induced [Ca2+]i increase in DRG neurons. A-D, Representative traces of the effects of 0.1 μg/ml IC on [Ca2+]i in Ca2+-free extracellular solution (buffered by 0.1 EGTA), L- (nifedipine; 5 μM) and N- (ω-conotoxin GVIA; 1 μM) type Ca2+ channel blockers and a sarco/endoplasmic reticulum calcium ATPase inhibitor, cyclopiazonic acid (CPA; 5 μM). E, Summary of IC effects on [Ca2+]i under various conditions. Removal of extracellular calcium and blockade of L- and N- type Ca2+ channels by nifedipine (5 μM) and ω-conotoxin GVIA (1 μM) significantly reduced IC-induced rise in [Ca2+]i. No IC-induced [Ca2+]i increase was observed after depletion of intracellular stores by CPA (5 μM). Numbers in the parentheses indicate the number of DRG neurons tested. *p < 0.05, **p <0.001 versus 0.1 μg/ml IC, one-way ANOVA followed by Scheffe's post hoc test.
To test whether L- or N-type Ca2+ channels on the membrane of DRG neurons contributed to IC-induced Ca2+ influx, we examined the effects of nifedipine, a selective blocker of L-type Ca2+ channels, and ω-conotoxin GVIA, a selective N-type Ca2+ channel blocker on IC-induced [Ca2+]i increase, respectively. In the presence of nifedipine (5 μM), IC increased R340/380 by 36.8 ± 5.1% (Fig. 4B, E; n = 30), which was significantly less than the 64.7 ± 4.7% (n = 77) in control medium without nifedipine. In addition, pretreatment with 1 μṂ ω-conotoxin GVIA for 20-30 s significantly attenuated IC-induced [Ca2+]i increase (Fig. 4C, E; n = 19). These results suggest that IC induces [Ca2+]i increase partly by Ca2+ influx through L- or N-type Ca2+ channels in DRG neurons.
To further explore the contribution of intracellular stores to IC-induced Ca2+ release, DRG neurons were pretreated with CPA (5 μM), the sarco/endoplasmic reticulum calcium ATPase (SERCA) blocker (Lu et al., 2006). Bath application of CPA alone for 10-15 min caused a rise in resting [Ca2+]i, which decreased slowly to some extent after initial addition of CPA, but did not return the baseline levels before CPA application (Fig. 4D), consistent with previous reports (Lu et al., 2006). The inhibition of SERCA by CPA almost abolished the IC-induced [Ca2+]i increase in all tested DRG neurons (Fig. 4D, E; n = 10). However, high K+ still evoked Ca2+ transients in the presence of CPA (data not shown), as previously reported (Lu et al., 2006). This result suggests that intracellular stores are a main source of IC-induced calcium release.
FcγRI activation increased the excitability of DRG neurons
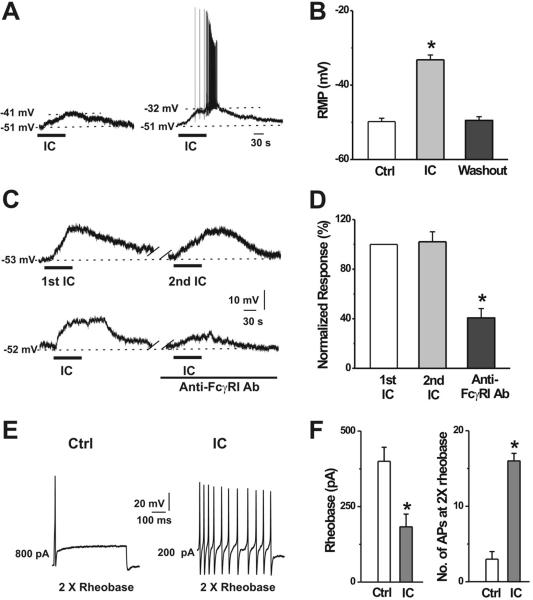
In whole-cell current clamp recordings, bath application of 0.1 μg/ml IC depolarized the resting membrane potential (RMP) by 16.6 ± 1.3 mV in rat DRG neurons (n = 29), which were identified as responsive to IC by calcium imaging firstly (Fig. 5A, B). Furthermore, in 14 of these 29 activated neurons, the depolarization was accompanied by action potential (AP) discharges (Fig. 5A). The RMP returned to the baseline within 3 minutes after washout. When ICs were applied repetitively at an interval of 6 to 7 min, the membrane potential depolarization induced by the second IC challenge was 17.2 ± 1.8 mV (n = 13), similar to that induced by the first (18.4 ± 1.6 mV; n =13; Fig. 5C, D). In the presence of FcγRI antibody (0.2 μg/ml), IC depolarized the RMP by only 6.8 ± 1.2 mV, which is significantly less than the amplitude of depolarization (17.2 ± 1.8 mV; n = 13) in control medium without anti-FcγRI antibody (Fig. 5C, D). Similar to the calcium imaging experiments, neither application of normal mouse IgG (n = 6) or rat anti-mouse IgG (n = 9) alone, nor the mixture of normal mouse IgG and normal rat IgG (n = 10) significantly affected the RMP of DRG neurons (data not shown). Among the IC-responsive neurons, 69.0% (20 out of 29) exhibited a membrane depolarization in response to capsaicin (1 μM) applied for 10-s at the end of recordings.
Figure 5.
IC increased the excitability of DRG neurons. A, Typical current-clamp recordings of 0.1 μg/ml IC-induced membrane potential depolarization (left) and AP discharges (right) in small-diameter DRG neurons. B, Mean RMP before (Ctrl) and during IC application (IC), and after washout (n = 29). C, Representative recordings of repetitive IC application and in the presence of FcγRI antibody. D, The mean magnitude of depolarization did not change with a subsequent application of IC at an interval of 6-7 min (n = 13), but was significantly reduced in the presence of FcγRI antibody (n = 4). *p < 0.001 versus 2nd IC, one-way ANOVA followed by Scheffe's post hoc test. E, Typical traces of APs evoked by a 500-ms depolarizing current pulse at 2X rheobase before and during IC application. F, IC significantly decreased the mean rheobase and increased the number of APs evoked by a depolarizing current at 2X rheobase (n = 6). *p < 0.01 versus control, paired t-test.
In addition, bath application of IC significantly decreased the rheobase (Fig. 5F), and increased (by 7.7-fold) the number of APs evoked by a depolarizing current pulse at 2X rheobase (Fig. 5E, F). The input resistance was significantly reduced from 446.5 ± 27.9 MΩ to 213.2 ± 24.8 MΩ (n = 6; p < 0.001, paired t-test) during exposure to IC , suggesting an increase in the opening of resting ion channels.
Discussion
In this study, we demonstrated that the high-affinity IgG receptor FcγRI is expressed on a subpopulation of rat DRG neurons including those co-expressing nociceptive neuronal markers. Activation of FcγRI by IgG-IC caused an increase in [Ca2+]i, induced a depolarization of RMP and sometimes evoked action potential discharges in dissociated small-diameter DRG neurons. To our knowledge, this is the first study directly demonstrating the co-expression pattern of FcγRI with other nociceptive neuronal markers in the DRG and the enhanced excitability of DRG neurons following FcγRI activation.
Expression of FcγRI in the DRG
FcγRI is typically expressed on immune cells to regulate immunity (Nimmerjahn and Ravetch, 2008). Recent studies revealed the presence of FcγRI on both DRG (Andoh and Kuraishi, 2004) and superior cervical ganglion neurons of mice (van der Kleij et al., 2010). The present study showed that FcγRI, but not FcγRII or FcγRIII, is expressed on a subpopulation of rat DRG neurons, consistent with previous reports (Andoh and Kuraishi, 2004). In addition, we found that FcγRI was present on both the somata and axons of DRG neurons, suggesting that IC might activate the FcγRI on the DRG neuronal somata and/or the axons including nerve terminals in the peripheral tissue. Furthermore, we provided direct evidence for the first time that FcγRI is co-expressed with nociceptive neuronal markers IB4, TRPV1, CGRP, and substance P in rat DRG neurons. The coexpression of FcγRI and substance P might account for the FcγRI-mediated substance P release from DRG neurons (Andoh and Kuraishi, 2004). In contrast with prior findings (Andoh and Kuraishi, 2004) there was no immunostaining for FcγRI in satellite glia. This discrepancy between the two studies is likely due to our use of GS as the marker for satellite glial cells (Hanani, 2005; Zhang et al., 2009), while in the study by Andoh and Kuraishi (2004), the lack of Protein Gene Product 9.5 (PGP9.5) was used as a criterion for satellite glial cells. Since PGP9.5 as a general neuronal marker is absent in both glial cells and other non-neuronal cells such as immune cells, the PGP9.5-negative cells that showed immunostaining for FcγRI might actually be immune cells such as macrophages.
Although FcγRI was expressed on DRG neurons across all size categories, our functional studies were focused on small-diameter DRG neurons. Whether medium- and large-diameter neurons expressing FcγRI can be excited by IgG-IC is unknown. The cellular mechanisms of FcγRI activation in medium- and large-diameter neurons might be different from those in small-diameter neurons due to the distinct ion channel configurations and calcium-regulating mechanisms in different subpopulations of DRG neurons (Lu et al., 2006). Regardless, the possible IC-induced activation of medium- and large-diameter neurons may contribute to paresthesias, allodynia and hyperalgesia (Decosterd et al., 2002; Ma and Woolf, 1996; Woolf and Doubell, 1994) in immune-related diseases. The expression of FcγRI in the axons might suggest a potential role of neuronal FcγRI in axonal degeneration and regeneration following nerve injury (Stirling and Stys, 2010).
Activation of FcγRI by IC induces [Ca2+]i increase
In addition to the expression pattern of FcγRI in the DRG, we also assessed whether FcγRI expressed in DRG neurons is functional. We showed that bath application of IC triggered an increase in [Ca2+]i, as previously reported (Andoh and Kuraishi, 2004; van de Winkel et al., 1990). Moreover, replacement of the intact IgG with F(ab/)2 fragments lacking the Fc portion or pretreatment with FcγRI antibody prevented the IC-induced [Ca2+]i increase, suggesting an essential role of the interaction between Fc portion of IgG and neuronal FcγRI in IC-induced calcium response. In addition, individual components of IC or the mixture of normal rat IgG and normal mouse IgG failed to trigger [Ca2+]i increase, indicating that only the intact IC might have the conformation capable of activating FcγRI on primary sensory neurons.
Surprisingly, the IC at the concentration of 0.1 μg/ml displayed stronger effects on [Ca2+]i than the two higher concentrations (1 and 10 μg/ml) of IC. A previous study also demonstrated that the activation of FcγRI by the Fc fragment of IgG (IgG Fc) at the higher dose induced smaller changes in CGRP release and cAMP levels in DRG neurons, as compared to the lower dose of IgGFc (Harada et al., 2010). One possible reason is that the higher concentration of IC mixtures contain a relative larger amount of monometric IgG that competitively blocks the effects of FcγRI on the neuronal surface (Nimmerjahn and Ravetch, 2006). Additionally, the IC may form large aggregates at higher concentrations, which might make them less accessible to the FcγRI on the neuronal surface. Finally, exposure to the higher concentration of IC might cause the FcγRI desensitization. These hypotheses remain to be tested.
A lower percentage of small diameter neurons were immunostained for FcγRI than exhibited calcium responses or membrane depolarization to IgG-IC. One possible reason is that the immunofluorescent staining method was not sensitive enough to detect all the neurons that express functional FcγRI in the DRG. Another possibility is that the expression and function of FcγRI may be different in the intact vs. dissociated DRG neurons, the latter deprived of satellite cells and axons.
Mechanisms of FcγRI-mediated [Ca2+]i increase
The [Ca2+]i increase could be mediated by two main candidate calcium sources: (1) extracellular Ca2+ influx through plasmalemmal ion channels, or (2) calcium release from the endoplasmic reticulum (ER). Our findings indicate that both mechanisms are involved in FcγRI-mediated [Ca2+]i increase.
In this study, we observed that the removal of extracellular calcium ions suppressed the FcγRI-induced [Ca2+]i increase, suggesting the contribution of calcium entry from extracellular space. Furthermore, the blockade of L- or N-type voltage-gated calcium channels (VGCCs) attenuated FcγRI-induced [Ca2+]i, indicating a role of these VGCCs in calcium entry. Several types of high voltage-activated calcium channels are activated by strong membrane depolarization, particularly during AP discharges (Tsien et al., 1988). Indeed, we found that the IC induced a membrane depolarization and evoked AP discharges in neurons that initially exhibited an increase in [Ca2+]i. These results suggest that FcγRI activation depolarizes the RMP of DRG neurons, which results in the activation of L- and N- type VGCCs and a subsequent influx of extracellular calcium through these VGCCs. However, our study also demonstrated that IC still induced intracellular calcium increase after the removal of extracellular calcium, suggesting that calcium influx from extracellular space only partially contributes to the IC-induced calcium increase. Calcium entry through VGCCs may further induce calcium release from internal stores (CICR) (Gover et al., 2009; Lu et al., 2006). In addition, other Ca2+-permeable mechanisms such as store-operated calcium entry and certain TRP channels (Gees et al., 2010; Lu et al., 2006; Philipp et al., 1998) may also be involved in IC-induced calcium influx.
Additionally, calcium release from internal stores may also contribute to the FcγRI-mediated [Ca2+]i increase in DRG neurons. After extracellular calcium was removed, IC still induced a small but significant [Ca2+]i increase in 30% of DRG neurons. In addition, depletion of intracellular calcium stores via the blockade of SERCA with CPA abolished the FcγRI-mediated [Ca2+]i increase. Previous studies have shown that the activation of FcγRI in certain immune cells results in tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motif and subsequent activation of spleen tyrosine kinase, and activates a number of intracellular signal pathways (Nimmerjahn and Ravetch, 2007) including the phospholipase C (PLC) -inositol 1,4,5-trisphosphate (IP3) pathway which triggers the Ca2+ release from intracellular stores in macrophages (Myers and Swanson, 2002) and monocytic cell lines (Liao et al., 1992; van de Winkel et al., 1990). Whether this signaling pathway is involved in FcγRI-mediated [Ca2+]i increase in DRG neurons remains to be determined.
FcγRI increases the excitability of DRG neurons
The present study provides novel evidence that FcγRI activation by IgG-IC increases the excitability of DRG neurons. IgG-IC-induced activation of neuronal FcγRI (1) depolarized the membrane potential and trigged action potential discharges; (2) reduced the rheobase of DRG neurons; and (3) increased the number of action potentials generated by a depolarizing current pulse at 2X rheobase. However, the mechanism of the increased excitability of DRG neurons remains to be determined. In macrophages and monocytes, the activation of FcγRI triggers a Ca2+-dependent, nonselective cation channel which is mainly permeable to Na+ and induces membrane depolarization (Floto et al., 1997; Nelson et al., 1985; Young et al., 1983). It is possible that IC activates a nonselective cation channel in DRG neurons that contributes to the decrease in input resistance, lower rheobase, and membrane potential depolarization. Additionally, DRG neurons express multiple voltage-gated Na+ and K+ ion channels that may also contribute to the IC-induced increase in neuronal excitability. These mechanisms remain to be investigated.
It is worth noting that since both the calcium imaging and electrophysiological experiments were performed at room temperature, there may be differences in the excitatory effects of FcγRI activation at body temperature in vivo.
Implications for pain
Several findings in our study suggest a potential role of neuronal FcγRI in pain sensation and the development of chronic pain. First, FcγRI is coexpressed with nociceptive markers IB4, TRPV1, CGRP and substance P in a subpopulation of DRG neurons. Second, the activation of FcγRI evoked [Ca2+]i increases in the nociceptive DRG neurons may trigger a series of intracellular signaling pathways leading to the release of certain neurotransmitters (Cheng and Ji, 2008) such as substance P (Andoh and Kuraishi, 2004), CGRP (Harada et al., 2010) or pro-inflammatory cytokines. These pain mediators are able to excite DRG neurons via their own receptors expressed on DRG neurons in a paracrine or autocrine manner (Tang et al., 2007; van Rossum et al., 1997). Finally, the activation of FcγRI increases membrane excitability and can even evokes action potential discharges in nociceptive DRG neurons. Excitation of primary nociceptive neurons is known to cause pain sensation, and a sustained increase in excitability leading to peripheral and central sensitization could contribute to the development and maintenance of chronic pain (Cheng and Ji, 2008).
This novel mechanism may play a role in the widely existing painful symptoms in autoimmune demyelinating diseases (Moulin, 1998). Under these conditions, autoantibodies bind to self-antigens such as myelin components that are readily present in the lesions, and may directly activate nociceptive neurons via neuronal FcγRI. Another possible pain mechanism is that under allergic or infectious conditions, IC may directly activate the neuronal FcγRI in the peripheral nerve terminals of nociceptive sensory neurons, producing pain and other unpleasant sensations such as itch (Andoh and Kuraishi, 2004; Ma et al., 2009; Oaklander, 2008; Wittkowski et al., 2007). In addition, IC may trigger the release of pro-inflammatory cytokines from macrophages and other immune cells via the activation of Fc receptors (Nimmerjahn and Ravetch, 2008), inducing peripheral and central sensitization (DeLeo and Yezierski, 2001; Thacker et al., 2007). These mechanisms may work together to contribute to the development and maintenance of chronic pain.
Acknowledgements
This work was supported by NINDS grant NS065091 (to CM) and NS014624 (to RHL), and the Research Startup Grant (to CM) from Department of Anesthesiology, Yale University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
References
- Andoh T, Kuraishi Y. Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J. 2004;18:182–184. doi: 10.1096/fj.02-1169fje. [DOI] [PubMed] [Google Scholar]
- Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Allchorne A, Woolf CJ. Progressive tactile hypersensitivity after a peripheral nerve crush: non-noxious mechanical stimulus-induced neuropathic pain. Pain. 2002;100:155–162. doi: 10.1016/s0304-3959(02)00275-0. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Choi JS, Macala LJ, Tyrrell L, Black JA, Cummins TR, Waxman SG. Transfection of rat or mouse neurons by biolistics or electroporation. Nat Protoc. 2009;4:1118–1126. doi: 10.1038/nprot.2009.90. [DOI] [PubMed] [Google Scholar]
- Ferguson AC, Salinas FA. Elevated IgG immune complexes in children with atopic eczema. J Allergy Clin Immunol. 1984;74:678–682. doi: 10.1016/0091-6749(84)90229-x. [DOI] [PubMed] [Google Scholar]
- Floto RA, Somasundaram B, Allen JM, Mahaut-Smith MP. Fcgamma receptor I activation triggers a novel Ca2+-activated current selective for monovalent cations in the human monocytic cell line, U937. J Biol Chem. 1997;272:4753–4758. doi: 10.1074/jbc.272.8.4753. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel A, Baranowski A, Maurer K, Ghiai A, McCabe C, Ambler G. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med. 2010;152:152–158. doi: 10.7326/0003-4819-152-3-201002020-00006. [DOI] [PubMed] [Google Scholar]
- Gover TD, Moreira TH, Weinreich D. Role of calcium in regulating primary sensory neuronal excitability. Handb Exp Pharmacol. 2009:563–587. doi: 10.1007/978-3-540-79090-7_16. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haanpaa M, Dastidar P, Weinberg A, Levin M, Miettinen A, Lapinlampi A, Laippala P, Nurmikko T. CSF and MRI findings in patients with acute herpes zoster. Neurology. 1998;51:1405–1411. doi: 10.1212/wnl.51.5.1405. [DOI] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K. Stimulation of Fc gammaRI on primary sensory neurons increases insulin-like growth factor-I production, thereby reducing reperfusion-induced renal injury in mice. J Immunol. 2010;185:1303–1310. doi: 10.4049/jimmunol.0902051. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humle Jorgensen S, Sorensen PS. Intravenous immunoglobulin treatment of multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;233:61–65. doi: 10.1016/j.jns.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, van Lent PL, Hofhuis FM, van Ojik HH, Sedlik C, da Silveira SA, Gerber J, de Jong YF, Roozendaal R, Aarden LA, van den Berg WB, Saito T, Mosser D, Amigorena S, Izui S, van Ommen GJ, van Vugt M, van de Winkel JG, Verbeek JS. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- Kenner M, Menon U, Elliott DG. Multiple sclerosis as a painful disease. Int Rev Neurobiol. 2007;79:303–321. doi: 10.1016/S0074-7742(07)79013-X. [DOI] [PubMed] [Google Scholar]
- Kumazawa T. The polymodal receptor: bio-warning and defense system. Prog Brain Res. 1996;113:3–18. doi: 10.1016/s0079-6123(08)61078-x. [DOI] [PubMed] [Google Scholar]
- Liao F, Shin HS, Rhee SG. Tyrosine phosphorylation of phospholipase C-gamma 1 induced by cross-linking of the high-affinity or low-affinity Fc receptor for IgG in U937 cells. Proc Natl Acad Sci U S A. 1992;89:3659–3663. doi: 10.1073/pnas.89.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol. 2006;577:169–190. doi: 10.1113/jphysiol.2006.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Greenquist KW, Lamotte RH. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol. 2006;95:2098–2107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Enhanced excitability of dissociated primary sensory neurons after chronic compression of the dorsal root ganglion in the rat. Pain. 2005;113:106–112. doi: 10.1016/j.pain.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ma C, Zhang P, Sikand P, LaMotte RH, Gu Q. Antigen-specific immune mechanisms of chronic pain. Society for Neuroscience Abstract Program No. 459.5; Chicago, IL: 2009. [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- Moulin DE. Pain in central and peripheral demyelinating disorders. Neurol Clin. 1998;16:889–898. doi: 10.1016/s0733-8619(05)70103-1. [DOI] [PubMed] [Google Scholar]
- Moulin DE, Hagen N, Feasby TE, Amireh R, Hahn A. Pain in Guillain-Barre syndrome. Neurology. 1997;48:328–331. doi: 10.1212/wnl.48.2.328. [DOI] [PubMed] [Google Scholar]
- Myers JT, Swanson JA. Calcium spikes in activated macrophages during Fcgamma receptor-mediated phagocytosis. J Leukoc Biol. 2002;72:677–684. [PubMed] [Google Scholar]
- Nelson DJ, Jacobs ER, Tang JM, Zeller JM, Bone RC. Immunoglobulin G-induced single ionic channels in human alveolar macrophage membranes. J Clin Invest. 1985;76:500–507. doi: 10.1172/JCI111999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9:S10–18. doi: 10.1016/j.jpain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Okun E, Mattson MP, Arumugam TV. Involvement of Fc receptors in disorders of the central nervous system. Neuromolecular Med. 2010;12:164–178. doi: 10.1007/s12017-009-8099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalie A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LG, Cunha TM, Vieira SM, Lemos HP, Verri WA, Jr., Cunha FQ, Ferreira SH. IL-17 mediates articular hypernociception in antigen-induced arthritis in mice. Pain. 2010;148:247–256. doi: 10.1016/j.pain.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Qu L, Zhang P, LaMotte RH, MA C. A Novel Antigen-Specific Immune Mechanism of Pain.. 13th World Congress on Pain Presentation No. PM170.; Montreal, Quebec, Canada. 2010a. [Google Scholar]
- Qu L, Zhang P, MA C. Mechanisms of neuronal Fc-gamma receptor I-mediated activation of the rat dorsal root ganglion neurons. Society for Neuroscience Abstract Program No. 586.2; San Diego, CA: 2010b. [Google Scholar]
- Stinissen P, Raus J, Zhang J. Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies. Crit Rev Immunol. 1997;17:33–75. doi: 10.1615/critrevimmunol.v17.i1.20. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Stys PK. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med. 2010 doi: 10.1016/j.molmed.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Tang HB, Li YS, Arihiro K, Nakata Y. Activation of the neurokinin-1 receptor by substance P triggers the release of substance P from cultured adult rat dorsal root ganglion neurons. Mol Pain. 2007;3:42. doi: 10.1186/1744-8069-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Valks R, Conde-Salazar L. Painful dermatitis of the fingertip. Am J Contact Dermat. 2003;14:219–220. [PubMed] [Google Scholar]
- van de Winkel JG, Tax WJ, Jacobs CW, Huizinga TW, Willems PH. Cross-linking of both types of IgG Fc receptors, Fc gamma RI and Fc gamma RII, enhances intracellular free Ca2+ in the monocytic cell line U937. Scand J Immunol. 1990;31:315–325. doi: 10.1111/j.1365-3083.1990.tb02774.x. [DOI] [PubMed] [Google Scholar]
- van der Kleij H, Charles N, Karimi K, Mao YK, Foster J, Janssen L, Chang Yang P, Kunze W, Rivera J, Bienenstock J. Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. J Allergy Clin Immunol. 2010;125:757–760. doi: 10.1016/j.jaci.2009.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr., Cunha TM, Parada CA, Poole S, Liew FY, Ferreira SH, Cunha FQ. Antigen-induced inflammatory mechanical hypernociception in mice is mediated by IL-18. Brain Behav Immun. 2007;21:535–543. doi: 10.1016/j.bbi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr., Guerrero AT, Fukada SY, Valerio DA, Cunha TM, Xu D, Ferreira SH, Liew FY, Cunha FQ. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci U S A. 2008;105:2723–2728. doi: 10.1073/pnas.0712116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkowski A, Richards HL, Griffiths CE, Main CJ. Illness perception in individuals with atopic dermatitis. Psychol Health Med. 2007;12:433–444. doi: 10.1080/13548500601073928. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Doubell TP. The pathophysiology of chronic pain--increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Yao H, Donnelly DF, Ma C, LaMotte RH. Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J Neurosci. 2003;23:2069–2074. doi: 10.1523/JNEUROSCI.23-06-02069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Unkeless JC, Kaback HR, Cohn ZA. Macrophage membrane potential changes associated with gamma 2b/gamma 1 Fc receptor-ligand binding. Proc Natl Acad Sci U S A. 1983;80:1357–1361. doi: 10.1073/pnas.80.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki N, Yoshino H, Sato S, Shinozawa K, Miyatake T. Severe acute axonal form of Guillain-Barre syndrome associated with IgG anti-GD1a antibodies. Muscle Nerve. 1992;15:899–903. doi: 10.1002/mus.880150806. [DOI] [PubMed] [Google Scholar]
- Zandman-Goddard G, Blank M, Shoenfeld Y. Intravenous immunoglobulins in systemic lupus erythematosus: from the bench to the bedside. Lupus. 2009;18:884–888. doi: 10.1177/0961203309106921. [DOI] [PubMed] [Google Scholar]
- Zhang H, Mei X, Zhang P, Ma C, White FA, Donnelly DF, Lamotte RH. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia. 2009;57:1588–1599. doi: 10.1002/glia.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JH, Walters ET, Song XJ. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J Neurophysiol. 2007;97:15–25. doi: 10.1152/jn.00559.2006. [DOI] [PubMed] [Google Scholar]