Abstract
Background
Nearly-universal cardiomyopathy in Duchenne muscular dystrophy (DMD) contributes to heart failure and death. As DMD patients show myocardial fibrosis well before functional impairment, we postulated that earlier treatment using drugs with anti-fibrotic effect may be beneficial.
Methods and Results
Three groups of 10 utrn+/−;mdx or “het” mice with skeletal myopathy and cardiomyopathy that closely mimics clinical DMD were studied. One het group received spironolactone and lisinopril starting at 8 weeks-of-life (het-treated-8), a second received the same starting at 4 weeks-of-life (het-treated-4), and the third het group was untreated. At 20 weeks, all mice had normal EFs though circumferential strain rate was abnormal (−0.21±0.08) in untreated hets. This improved to −0.40±0.07 in het-treated-8 mice (p=0.003), and further improved to −0.56±0.10 in het-treated-4 mice (p=0.014 for het-treated-4 vs. het-treated-8). Treated mice showed less cardiomyocyte damage, with a 44% reduction in intra-cardiomyocyte serum IgG localization in het-treated-8 mice (p<0.0001), and further 53% reduction in het-treated-4 mice (p=0.0003 vs. het-treated-8); matrix metalloproteinases were similarly reduced. Cardiac, limb and diaphragm function by ex vivo muscle testing remained at 80% of normal with early treatment compared to a decline to 40% of normal skeletal muscle function without treatment.
Conclusions
These findings offer clinically-available medications with proven anti-fibrotic effect as a new therapeutic strategy in DMD. Early initiation greatly attenuated myocardial disease and, for the first time with these drugs, improved skeletal myopathy. Thus, early initiation of such agents warrants further clinical evaluation to maintain ambulatory, respiratory and cardiac function for DMD and related myopathies.
Keywords: cardiomyopathy, muscles, aldosterone antagonist
Inherited myopathies produce progressive immobility due to limb muscle degeneration, respiratory failure due to diaphragm involvement and cardiomyopathy due to myocardial disease1. The most common form is Duchenne muscular dystrophy (DMD), an X-linked disorder that leads to absence of the sarcolemmal protein dystrophin and impairs ambulation beginning in children age 3–7 years. DMD patients universally develop cardiomyopathy by the third decade of life, and present guidelines advocate periodic screening with echocardiography2, 3. Current guidelines dictate that drugs like angiotensin converting enzyme (ACE) inhibitors may be started once there is evident left ventricular (LV) systolic dysfunction, particularly reduced ejection fraction (LVEF)4. Use of drugs like aldosterone antagonists that have anti-fibrotic effects are only advocated for treatment of patients with advanced heart failure4. Recent evidence supports initiation earlier in the progression of cardiac disease albeit still only in symptomatic patients with evident LV dysfunction5.
Our present study was motivated by increasing evidence suggesting that myocardial disease is developing in DMD patients well before LVEF becomes abnormal6. Observing that the early histopathological changes that occur in both skeletal and cardiac muscle invariably leads to fibrosis, we postulated that drugs with an anti-fibrotic effect may be most beneficial if started earlier in the disease course. Therefore, we implemented a prospective, blinded study to test the hypothesis that early vs. late treatment with clinically-available ACE inhibitors and aldosterone antagonists provides significantly greater myocardial protection in a mouse model of DMD. In addition, because of: 1) the reported “anti-fibrotic” properties of spironolactone and lisinopril5, 7–9, 2) the absence of any published investigations of these drugs on skeletal muscles in any muscular dystrophy model, and 3) the presence of large amounts of fibrosis in het mice, we decided to include analysis of skeletal muscle effects in addition to cardiac effects of the drug treatment in our studies.
Methods
All protocols were approved by the OSU institutional animal review board. For this study we used a mouse model deficient for dystrophin that is also haploinsufficent for its partially compensating homolog utrophin, utrn+/−;mdx “het” mice, because we have previously shown that their skeletal muscle fibrosis is more severe than that of mdx mice10, making it a more accurate phenotypic model of DMD. Het mice were bred and genotyped as previously described10. Groups of 10 of these mice, housed 2–3 to a cage, were given water bottles containing 66 mg/l lisinopril (Sigma L 6292) + 250 mg/l spironolactone (Sigma S3378) (dissolved in 0.1% Ethanol) in reverse osmosis (RO) water starting at 4 or 8 weeks-of-age, or provided RO water only. Medicated water bottles were replaced 3 times per week. Mice were weighed and volume of water consumed was recorded to calculate the average drug dosages for the first several weeks of treatment, which were found to be reported effective dosages for a mouse of 10 mg/kg/day lisinopril and 37.5 mg/kg/day spironolactone.
Using medicated water bottles containing lisinopril and spironolactone (drug treatment), we treated one group of het mice starting at 4 weeks-of-age (het-treated-4, n=10), before onset of apparent cardiac damage and at an early stage of skeletal muscle damage, and a second group of het mice starting at 8 weeks-of-age (het-treated-8, n=10) when initial functional and histological signs of cardiomyopathy are apparent11, 12. We compared these groups to an untreated group (n=10) of het mice (het-untreated) at 20 weeks-of-age.
One day prior to 20 weeks-of-age, injected mice were weighed and underwent CMR on a vertical bore 11.7-Tesla, 30-mm bore magnetic resonance imaging system (Bruker Biospin, Ettlingen, Germany) under gas anesthesia with electrocardiographic leads and temperature control maintaining body core temperature at 37°C. Fast low-angle shot gradient recalled echo cine imaging was acquired in contiguous short axis planes covering the left ventricle. From short axis cines, endocardial borders at end-diastole and end-systole were automatically delineated using dedicated software for CMR analysis (Segment, Medviso AB; Lund, Sweden), allowing computation of LVEF from end-diastolic and end-systolic volumes: LVEF=(EDV-ESV)/EDV, %. Myocardial strain and strain rate were computed from apical, mid and basal short axis cines using vector-based feature tracking software (Vector Velocity Imaging, Siemens, Mountain View, California)13.
On the day following in vivo CMR examination, ECGs were recorded and the mice were then euthanized. In vitro cardiac muscle function was assessed as previously described in detail for a related mouse model12. Briefly, small, linear multicellular preparations were dissected and electrically stimulated to contract under near-physiological conditions. In addition to baseline function, the main three mechanisms (length-dependent activation, frequency-dependent activation, and β-adrenergic stimulation) that regulate in vivo myocardial force development were assessed. Isolated strips of diaphragm muscle were assessed on their force generating ability, while maximal tetanic strength of the extensor digitorum longus (EDL) muscle as well as its susceptibility to repetitive eccentric stress were assessed as previously described14, 15.
The remainder of the heart tissue and skeletal muscles including diaphragm and quadriceps were embedded in Optimal Cutting Temperature medium and frozen on liquid-nitrogen cooled isopentane for subsequent histological analyses. 8 μm cryosections were stained by hematoxylin and eosin by standard methods or stained for intracellular IgG. IgG immunostaining was performed using a CY3-conjugated anti-mouse IgG antibody (1:100) (Jackson Immunoresearch Laboratories 115-165-146) as previously described11 with or without co-staining with anti-Collagen I or anti-ERTR7 antibodies (Abcam ab7778 and ab51824, respectively). The percentage of IgG stained pixels in composites of photomicrographs that represented the majority of heart or quadriceps sections from each mouse were quantified using Image J. For in situ zymography, heart cryosections were fixed and incubated for 9 hours at 37 °C with a solution of gelatin conjugated to Oregon Green 488 (Invitrogen Molecular Probes) then washed with 10 mM EDTA to stop activity. Summary values are presented as mean ± standard error. One-way ANOVA followed by post-hoc test using Bonferroni adjustment, where applicable, was used to determine statistically significant differences.
Results
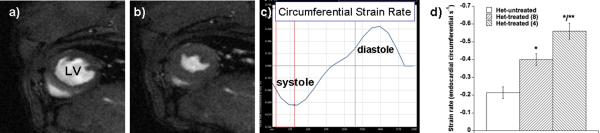
CMR showed normal LVEF in all three groups. Strain analysis, however, showed measurable differences in both systolic and diastolic function (Figure 1); these were more significant in basal LV segments compared to the apex, similar to DMD cardiomyopathy in humans that initially demonstrate abnormalities in the base vs. the apex of the LV16. Mean systolic circumferential strain rate measured in a short-axis cine view at the base of the heart was −0.21±0.08 in untreated het mice, improved to −0.40±0.07 in het mice whose treatment was initiated at 8 weeks of life, and improved even more to −0.56±0.10 in het mice whose treatment was initiated at 4 weeks of life (p=0.003 for het-untreated vs. het-treated-8, p<0.0001 for het-untreated vs. het-treated-4, and p=0.014 for het-treated-4 vs. het-treated-8). Diastolic strain measurement showed similar improvements: 0.24±0.09 in untreated hets, 0.42±0.10 in hets whose treatment began at 8 weeks, and 0.59±0.08 in hets whose treatment began at 4 weeks (p=0.007 for het-untreated vs. het-treated-8, p<0.0001 for het-untreated vs. het-treated-4, and p=0.012 for het-treated-4 vs. het-treated-8).
Figure 1.
In vivo cine CMR in the het mouse showed normal global systolic function in all mice, treated and untreated (data not shown). A–B) Comparison of an end-diastolic frame (a) to an end-systolic frame (b) indicates systolic thickening in all regions, resulting in a normal LV ejection fraction. C) Measurement of myocardial deformation using vector velocity image segmentation allows measurement of LV circumferential strain rate, a sensitive measure of subtle abnormalities in both systolic and diastolic function. D) Despite normal LVEF, untreated het mice had abnormal circumferential strain rate that was significantly improved with treatment initiation at 8 weeks with lisinopril and spironolactone (p=0.003 compared to het-untreated); this improvement was even more marked if treatment was started at 4 weeks of life (p=0.014 comparing het-treated-8 to het-treated-4; p<0.0001 het-untreated vs. het-treated-4).
Electrocardiographic recordings obtained in conscious, unrestrained mice showed no significant difference in heart rate among C57BL/10 controls, het-untreated, het-treated-8 and het-treated-4 mice (p=0.19), nor were there significant differences across groups in QT interval (p=0.31).
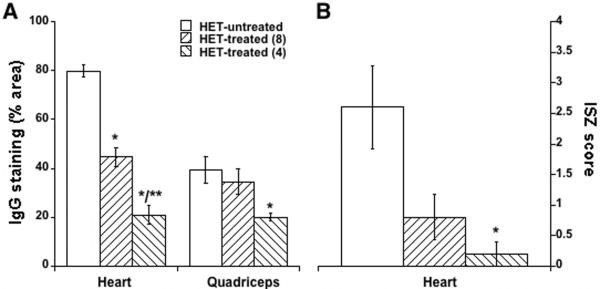
In vitro cardiac muscle force and response to isoproterenol also showed a clear trend towards improvement in the het-treated-4 group (Figure 2A–F) at 20 weeks-of-age compared to untreated het mice. The profound cardiac damage present in untreated het mice was almost completely prevented by the drug treatment in both groups of treated mice (Figure 3A). Degenerating cardiomyocytes, as detected by intracellular serum IgG localization were prevalent throughout the left ventricle of untreated het mice (Figure 3A). These were reduced 44% in het-treated-8 mice (p<0.0001 vs. het-untreated), and showed a further 53% reduction from these levels in the het-treated-4 group (p<0.0001 vs. het-untreated; p=0.0003 vs. het-treated-8) (Figure 4). Drug treatment also prevented matrix metalloproteinase (MMP) gelatinase activity, a key indicator of ventricular remodeling (Figure 3) and the infiltration of activated fibroblasts that secrete both MMPs and collagen (Figure 5).
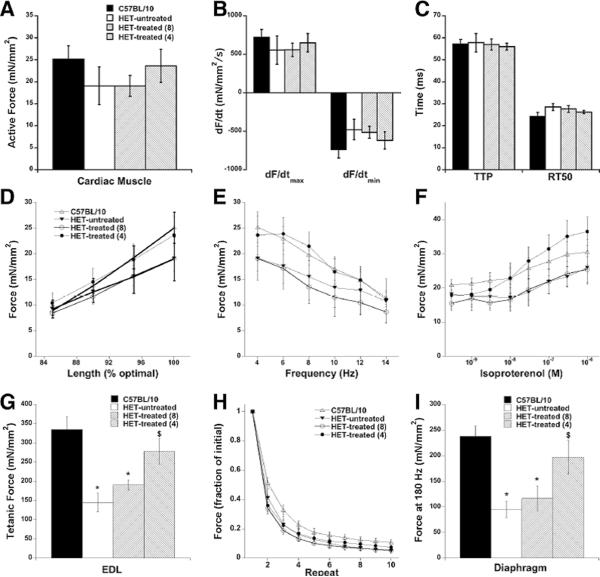
Figure 2.
In vitro muscle physiology showed that compared to untreated het mice, trabeculae isolated from het-treated-4 mice trended to improve active developed force at 4 Hz, 37 °C (A), improve maximal rates of contraction and relaxation (B), and faster timing kinetics (C). Cardiac regulatory systems regarding length-dependency (D), frequency-dependency (E), and β-adrenergic responsiveness were assessed similarly as previously reported12 and trended to be normalized to levels in control mice. In EDL muscles, specific force (150 Hz tetanus for 500 ms at 30 °C) was significantly improved upon treatment (G), and resistance to eccentric contractions was also improved (H). In isolated diaphragm muscle (180 Hz tetanus for 500 ms at 37 °C), specific force development was also significantly improved. n=6–9 group, * P<0.05 vs. C57BL/10 control, $ P<0.05 vs. het-untreated animals.
Figure 3.
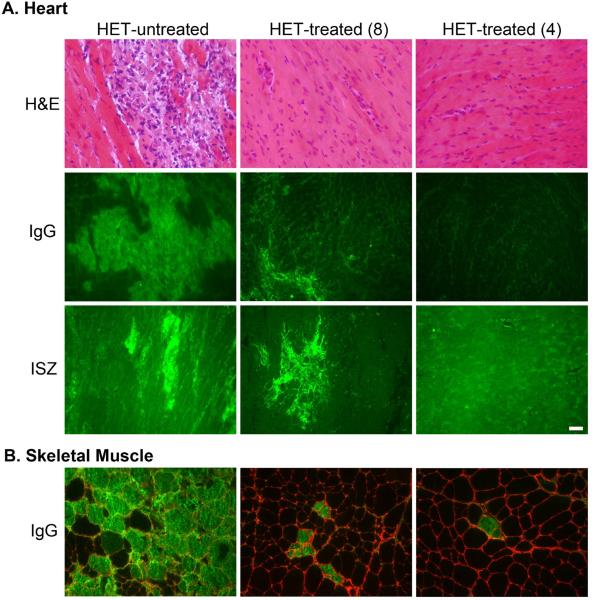
Drug treatment improves histological parameters of heart and skeletal muscles. A) Hematoxylin and Eosin (H&E)-stained left ventricular sections show the cardiac damage prevalent throughout het-untreated hearts that is almost completely prevented in both treatment groups. Intracellular localization of mouse IgG (green) indicates damaged myocardium that is significantly attenuated in het-treated-8 and even further improved in het-treated-4 hearts. Gelatinase in situ zymography (ISZ) shows the combined activity of matrix metalloproteinases 2 and 9 (bright green), indicative of ventricular remodeling, that is also attenuated in the het-treated-8 hearts, and almost entirely prevented in the het-treated-4 hearts. B) IgG localization (green) in quadriceps skeletal muscle sections indicates a profound and significant reduction of ongoing myofiber damage in the het-treated-4 group, with intermediate effects in the het-treated-8 group compared to untreated hets. Localization of Collagen I (red) in the matrix surrounding individual muscle fibers is shown to demonstrate the intracellular localization of the IgG staining. Bar = 50 μm.
Figure 4.
A) The average percentage (± SE) of section area stained for mouse IgG for heart (left) and quadriceps skeletal muscles from het-untreated and treated groups shows significant reductions in ongoing muscle damage. B) Blinded visual scoring of gelatinase activity from in situ zymography supports reductions of MMP remodeling in treated groups. * P<0.03 vs. untreated, ** P<0.05 vs. 8-week. Panel A, n=7–10/group. Panel B, n=5/group.
Figure 5.
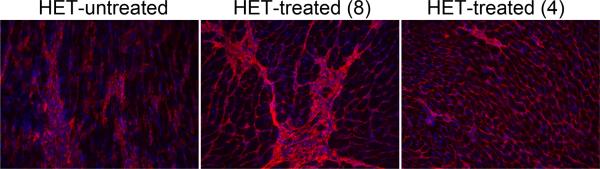
ERTR7 immunolocalization (red) in heart sections shows that the mononuclear cell infiltrate (nuclei stained blue) in damaged regions of het-untreated cardiac muscle is mostly composed of fibroblasts. The less frequent areas of damage in the het-treated-8 group still contain fibroblasts, suggesting that the drug treatment is not acting directly on fibroblast proliferation or migration. Since very few areas of cardiac damage are present in het-treated-4 hearts, clusters of activiated fibroblasts are not typically present.
Surprisingly, het mice started on drug treatment at 4 weeks-of-age showed a dramatic improvement in both diaphragm and EDL muscle function to 80% of isogenic normal (C57BL/10) control muscle force compared to 40% of normal force in untreated het mice, (Figure 2G, EDL: p=0.0006 C57BL/10 vs. het-untreated; p=0.0067 C57BL/10 vs. het-treated-8; p=0.013 het-untreated vs. het-treated-4. Fig 2I, Diaphragm: p=0.0017 C57BL/10 vs. het-untreated; p=0.0086 C57BL/10 vs. het-treated-8; p=0.044 het-untreated vs. het-treated-4).
Limb muscles demonstrated a similar reduction of ongoing muscle degeneration as shown in the heart (Figure 3B), with a 2-fold reduction in the het-treated-4 group (p=0.025 het-untreated vs. het-treated-4) (Figure 4). However, gross limb muscle histopathology was similar in all groups. Despite the profound functional improvement in diaphragm conferred by early drug treatment initiation in het-treated-4 muscles, the diaphragm showed no obvious improvement in any histopathological parameter assessed (not shown).
Discussion
We show for the first time in a mouse model of DMD a remarkable protective effect on both cardiac as well as skeletal muscle of very early initiation of treatment with an aldosterone antagonist plus an ACE inhibitor. Treatment was initiated well before evident cardiac dysfunction, and even untreated animals at 20 weeks of life still showed preserved EF despite extensive myocardial fibrosis and injury. Despite genetics that would otherwise dictate inevitable cardiomyopathy and skeletal muscle disease, early treatment with spironolactone in combination with lisinopril provided near-normalization of muscle function and considerable prevention of tissue changes.
Gross histopathology of limb muscle was similar in all groups despite less muscle degeneration by IgG staining and MMP activity; this may be explained by ongoing damage in dystrophic skeletal muscle at 4 weeks-of-age when such damage is not yet evident in cardiac muscle. Also, despite dramatic improvement in diaphragm function, there was no appreciable histopathological improvement in diaphragm. This result is likely due to the well-documented crisis period of damage that occurs in dystrophin-deficient mouse diaphragm between 3.5 and 4 weeks-of-age17, prior to the initiation of treatment in either group, and supports that treatment prior to damage likely results in the most improvement in all parameters.
Numerous pharmacological treatment approaches have been investigated to attenuate the devastating sequelae of cardiomyopathy and skeletal myopathy in DMD, though none prior to this work have shown such remarkable efficacy for both myocardial and skeletal muscle disease. A recent critical review of the literature listed therapeutics with sufficient evidence for clinical use, and endorsed the use of glucocorticoids that limit decline in muscle strength and function18. However, chronic steroid therapy brings a wide range of attendant complications19. Further, glucocorticoids have shown inconsistent effects on myocardial disease ranging from modest protection20 and reduced fibrosis21 to accelerated progression22 or even increased fibrosis23. Standard heart failure medications such as ACE inhibitors and beta-blockers are advocated once LVEF is abnormal by echocardiography, though evidence suggests that changes in myocardial structure and function are well underway even with normal LVEF using more sensitive CMR-based strain and fibrosis imaging techniques6.
Mechanistic insights from this work include evidence supporting that the drugs' benefit are likely not mediated via inhibition of fibroblast proliferation and migration. As precise molecular pathways are better defined through ongoing preclinical studies, these results behoove initiation of clinical trials to evaluate the potentially significant therapeutic benefits across a broad range of genetic and acquired cardiomyopathies. Any myocardial disease marked by fibrosis, from hypertensive heart disease to diabetic cardiomyopathy, warrants evaluation of earlier use of aldosterone antagonism that what is presently advocated. Similarly, our findings of these drugs' effect on attenuating damage and maintaining strength of skeletal muscle suggest potential benefit for conditions such as sarcopenia and cachexia where therapeutic options are presently limited.
The threshold doses for lisinopril and spironolactone were not established in this study, but were based on therapeutic doses commonly used in other mouse studies and relate to the allometric scaling needed to account for different pharmacokinetics between rodents and humans. Clinical trials testing the efficacy of these drugs in DMD patients will likely begin at the comparable per-kg body weight doses typically used clinically. Because both lisinopril and spironolactone were administered, we cannot determine from these data if the benefits demonstrated were due to the combination of drugs or spironolactone alone. Ongoing studies with administration of each drug individually should address this question.
In conclusion, combining aldosterone antagonism with ACE inhibition at an extremely early stage of genetically-determined myocardial and skeletal muscle disease potentially offers maximal inhibition of tissue injury and fibrosis with superior outcomes. Our work indicates that such a combined approach yields 1) improvements in both skeletal and cardiac muscle histology unlike prior investigations that showed improvements in one tissue but not in another and 2) preservation of function to an extent that far exceeds that of any prior pharmacological therapy regimen studied in mouse models of DMD24–26.
Duchenne muscular dystrophy (DMD) is a universally fatal disorder, with cardiomyopathy and skeletal myopathy leading to progressive immobility, respiratory impairment, heart failure and death. Current treatment options are limited to glucocorticoids that produce inconsistent effects on myocardial disease. Guidelines for management of cardiomyopathy advocate medications such as angiotensin converting enzyme (ACE) inhibitors and beta-blockers only when left ventricular ejection fraction drops below normal. We tested the hypothesis that early treatment using the ACE inhibitor lisinopril in combination with the aldosterone antagonist spironolactone with proven anti-fibrotic effect might prevent muscle damage and preserve muscle function. We found that these drugs dramatically improved function of cardiac muscle, leg muscle and diaphragm in utrn+/−;mdx or “het” mice whose skeletal myopathy and cardiomyopathy closely mimics clinical DMD. Furthermore, histology demonstrated striking reduction in cardiomyocyte damage. These results offer clinically-available medications as a new therapeutic strategy in DMD. For the first time, these historically cardioprotective drugs were shown to protect skeletal muscle as well. These preclinical results suggest that ACE inhibition plus aldosterone antagonism may deliver improved ambulation, respiratory function and cardiac status for patients with DMD. Further investigations are also warranted to tests these drugs' efficacy in attenuating the skeletal myopathy that accompanies a variety of myocardial disorders.
Acknowledgements
The authors are grateful to the Davis Heart and Lung Research Institute Small Animal Imaging Core Lab for their services. The authors also thank Dr. John Kissel for his support.
Funding Sources: BallouSkies (S.V.R., J.A.R-F.), The Beckman Foundation (N.C., R.G.), NIH R01HL095563 and R01HL102450 (S.V.R.), AHA EIA0740040N (P.M.L.J.) and T32HL098039 (D.D.)
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verhaert D, Richards K, Rafael-Fortney JA, Raman SV. Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations. Circ Cardiovasc Imaging. 2011;4:67–76. doi: 10.1161/CIRCIMAGING.110.960740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.English KM, Gibbs JL. Cardiac monitoring and treatment for children and adolescents with neuromuscular disorders. Dev Med Child Neurol. 2006;48:231–235. doi: 10.1017/S0012162206000491. [DOI] [PubMed] [Google Scholar]
- 3.Bushby K, Muntoni F, Bourke JP. 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th–9th June 2002, Naarden, the Netherlands. Neuromuscul Disord. 2003;13:166–172. doi: 10.1016/s0960-8966(02)00213-4. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2010;364:11–21. [Google Scholar]
- 6.Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, Benson DW, Gottliebson WM. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol. 2009;53:1204–1210. doi: 10.1016/j.jacc.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 8.Sanderson JE. New treatments for myocardial fibrosis. Cardiovasc Drugs Ther. 2002;16:181–182. doi: 10.1023/a:1020636002954. [DOI] [PubMed] [Google Scholar]
- 9.Chan AK, Sanderson JE, Wang T, Lam W, Yip G, Wang M, Lam YY, Zhang Y, Yeung L, Wu EB, Chan WW, Wong JT, So N, Yu CM. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007;50:591–596. doi: 10.1016/j.jacc.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Rafael-Fortney JA, Huang P, Zhao XS, Cheng G, Zhou X, Kaminski HJ, Liu L, Ransohoff RM. Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J Neurol Sci. 2008;264:106–111. doi: 10.1016/j.jns.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hainsey TA, Senapati S, Kuhn DE, Rafael JA. Cardiomyopathic features associated with muscular dystrophy are independent of dystrophin absence in cardiovasculature. Neuromuscul Disord. 2003;13:294–302. doi: 10.1016/s0960-8966(02)00286-9. [DOI] [PubMed] [Google Scholar]
- 12.Janssen PML, Hiranandani N, Mays TA, Rafael-Fortney JA. Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2005;289:H2373–2378. doi: 10.1152/ajpheart.00448.2005. [DOI] [PubMed] [Google Scholar]
- 13.Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P, Al-Khalidi HR, Chung ES, Benson DW, Mazur W. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3:144–151. doi: 10.1016/j.jcmg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Peterson JMKW, Canan BD, Ricca DJ, Kaspar BK, Delfín DA, DiRienzo K, Clemens PR, Robbins PD, Baldwin AS, Flood P, Kaumaya P, Frietas M, Kornegay JN, Mendell JR, Rafael-Fortney JA, Guttridge DC, Janssen PML. Peptide-based inhibition of NF-κB rescues diaphragm muscle contractile dysfunction in a murine model of Duchenne muscular dystrophy. Molecular Medicine. 2011 Jan 20; doi: 10.2119/molmed.2010.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodino-Klapac LR, Janssen PM, Montgomery CL, Coley BD, Chicoine LG, Clark KR, Mendell JR. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, Pack N, Dibella E, Gottliebson WM. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging. 2009;25:57–63. doi: 10.1007/s10554-008-9352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 18.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 19.Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008:CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18:365–370. doi: 10.1016/j.nmd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Marques MJ, Oggiam DS, Barbin IC, Ferretti R, Santo Neto H. Long-term therapy with deflazacort decreases myocardial fibrosis in mdx mice. Muscle Nerve. 2009;40:466–468. doi: 10.1002/mus.21341. [DOI] [PubMed] [Google Scholar]
- 22.Bauer R, Straub V, Blain A, Bushby K, MacGowan GA. Contrasting effects of steroids and angiotensin-converting-enzyme inhibitors in a mouse model of dystrophin-deficient cardiomyopathy. Eur J Heart Fail. 2009;11:463–471. doi: 10.1093/eurjhf/hfp028. [DOI] [PubMed] [Google Scholar]
- 23.Guerron AD, Rawat R, Sali A, Spurney CF, Pistilli E, Cha HJ, Pandey GS, Gernapudi R, Francia D, Farajian V, Escolar DM, Bossi L, Becker M, Zerr P, de la Porte S, Gordish-Dressman H, Partridge T, Hoffman EP, Nagaraju K. Functional and molecular effects of arginine butyrate and prednisone on muscle and heart in the mdx mouse model of Duchenne Muscular Dystrophy. PLoS One. 2010;5:e11220. doi: 10.1371/journal.pone.0011220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartel JV, Granchelli JA, Hudecki MS, Pollina CM, Gosselin LE. Impact of prednisone on TGF-beta1 and collagen in diaphragm muscle from mdx mice. Muscle Nerve. 2001;24:428–432. doi: 10.1002/1097-4598(200103)24:3<428::aid-mus1018>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen LH, Blain A, Greally E, Laval SH, Blamire AM, Davison BJ, Brinkmeier H, MacGowan GA, Schroder HD, Bushby K, Straub V, Lochmuller H. Long-term blocking of calcium channels in mdx mice results in differential effects on heart and skeletal muscle. Am J Pathol. 2011;178:273–283. doi: 10.1016/j.ajpath.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreetta F, Bernasconi P, Baggi F, Ferro P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R, Confalonieri P. Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy. J Neuroimmunol. 2006;175:77–86. doi: 10.1016/j.jneuroim.2006.03.005. [DOI] [PubMed] [Google Scholar]