Abstract
Background
Wilms tumor (WT) is thought to arise in children of black African ancestry with greater frequency than whites. To clarify the biological basis for race disparities in WT, we first verified that black children residing in Tennessee have an increased incidence of WT, and second, established molecular profiles in WT that are specific to race.
Materials and Methods
To assess race disparities in WT epidemiology, the Tennessee Cancer Registry (TCR) was queried for all in-state patients less than 20 years of age and registered between 1999–2008. To explore race disparities in WT biology, six black and four white WT specimens acquired in Tennessee were analyzed using imaging mass spectrometry (IMS).
Results
TCR data show that black children are overrepresented among WT patients (29%) relative to all other childhood cancers (18.5%; p=0.01). WT ranked the 5th most common cancer diagnosis among blacks, but 9th among whites. The diagnosis of WT occurred 79% more frequently among blacks (n=28) than whites (n=69; p=0.01), and proportionally more blacks tended to present with distant disease. Although overall survival from WT was not statistically different between blacks (92.9%) and whites (94.0%), black males showed the lowest survival (85%; p=0.21). IMS analysis identified peptide spectra from both WT blastema and stroma that independently classify specimens according to race with greater than 80% accuracy.
Conclusions
In Tennessee, black children appear more susceptible than whites to develop WT. Race-specific molecular profiles can be determined that may help to clarify pathways of Wilms tumorigenesis and the biological basis for race disparities in WT incidence and biology.
Keywords: Wilms tumor, race disparities in cancer, imaging mass spectrometry
INTRODUCTION
Wilms tumor (WT) is the most common malignancy arising in the kidney of children, yet its incidence varies between race groups around the world (1–3). Epidemiologic studies suggest that race, more than geography or nationality, is a greater risk factor for the development of WT (2). Specifically, North American children of black African ancestry have an increased incidence of WT (11 cases per million children less than 15 years of age) compared to whites (8 cases per million), whereas children of Asian ancestry show the lowest incidence (3 cases per million) (2, 4). Furthermore, black children born in sub-Saharan Africa show particularly high incidence rates of WT and experience dismal outcomes (5–7). Although 4-year survival from WT exceeds 90% in most developed countries, African-American children, when compared to white Americans, continue to experience lower late survival from WT despite similar enrollment in treatment protocols (1, 8).
The seminal paper describing race disparities in WT documented a nearly two-fold increase in its incidence among blacks residing in the Greater Delaware Valley (9). Importantly from that study, those congenital anomalies that associate with the development of WT (aniridia, genito-urinary malformations, mental retardation, and disorders of sexual differentiation, WT1; and the overgrowth syndromes, hemihypertrophy and Beckwith-Wiedemann, WT2) were shown to occur with greater frequency in blacks. These anomalies when present in patients who subsequently develop WT have now been associated with loss of heterozygosity (LOH) at WT1 (11p13) and loss of imprinting (LOI) at WT2 (11p15.5). However, no specific analysis of racial disparity in the mutational status of either gene in WT patients has been reported to date. Moreover, no analysis at any molecular level has been reported attempting to clarify observed race disparities in WT.
The biology and behavior of WT may vary between patients, and several tumor factors have been identified that correlate with advanced stages of disease and outcome, including CITED1, telomerase, IGFR-I, topoisomerase II, and CRABP2 (10–15). Microarray studies have shown further that a number of genes active in nephrogenesis, yet normally downregulated in the adult kidney, are overrepresented in WT specimens (11, 16). Activating mutations of β-catenin and WTX also have been described in 15% to 30% of WT (17–19). Finally, LOH at 1p, 7p and 16q have been identified in a spectrum of WT and portend worse outcome (20–22). However, an analysis of how these complex molecular circumstances implicated in WT development and disease progression contribute to racially disparate incidences and outcomes has not been reported.
To clarify the disparate incidence and behavior of WT between blacks and whites, a formal biological analysis is needed. First, we verified that race disparities in incidence exist in the larger population comprising our institutional tissue repository. Second, we performed imaging mass spectrometry on a sample of our WT tissues to test the hypothesis that WT show a unique molecular profile specific to race and histologic compartment.
MATERIALS AND METHODS
Tennessee Cancer Registry
In order to validate our strategy to explore the biological basis for race disparities in WT using specimens archived in our institutional tissue repository, we sought first to confirm that Tennessee children indeed show a racially disparate behavior in WT. To evaluate race disparities in WT within the larger population of children contributing tissues to our repository, we queried the Tennessee Cancer Registry (TCR) for all cancer cases affecting residents less than 20 years of age and registered between 1999–2008. Measured variables included cancer diagnosis, race, gender, stage of disease at presentation, days to first cancer treatment, length of follow up in days, and vital status. Notably, the TCR defines a cancer diagnosis according to the International Classification of Childhood Cancer (Third Edition: ICCC-3) and defines stage of disease according to the Surveillance Epidemiology and End Results (SEER) summary for stage of disease.
Imaging Mass Spectrometry
Imaging Mass Spectometry (IMS) was used to analyze the proteome of a subset of our repository WT specimens. To control for sampling error across these often large specimens, two tissue blocks from the same WT specimen, but remote from one another, were selected after review of the hematoxylin and eosin (H & E) slides. Blocks were chosen to include regions of histologic interest, enriched with blastema and tumor stroma, and showing negligible tumor necrosis. One section from each of two blocks of the last 6 black and 4 white patients treated for WT at our Tennessee institution were submitted for IMS. These WT patients were matched for primary resection (i.e. no neoadjuvant therapy), favorable histology, and stage of disease.
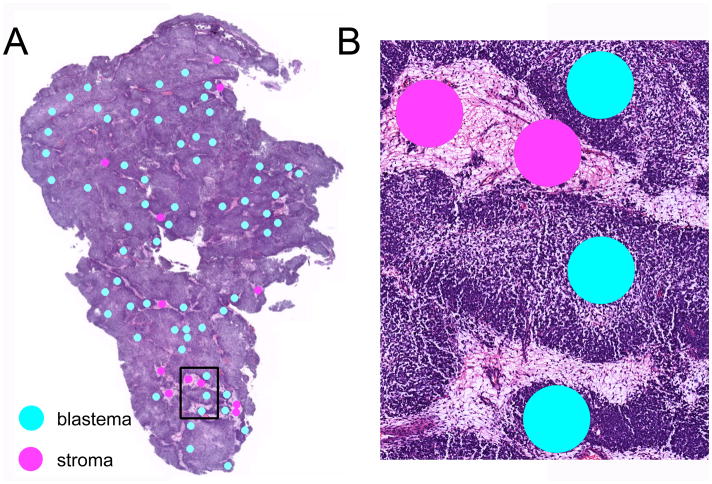
IMS was used as previously described (23). Briefly, two serial sections from each specimen block were cut at 6 μm thickness, and one section of each pair was stained with hematoxylin and eosin (H & E). Photomicrographs of these 20 H & E slides were marked for 300 μm diameter histologic regions of histologic interest within blastemal and stromal compartments (Figure 1: blastema, teal; stroma, pink). Tissue sections for mass spectrometry analysis were deparaffinized and subjected to heat-induced antigen retrieval. Digital images of the stained sections were matched to the corresponding unstained serial sections using Photoshop (Adobe, San Jose, CA). Coordinates of the histological annotations were determined and transferred to an acoustic robotic microspotter for deposition of trypsin and α-cyano-4-hydroxycinnamic acid matrix (10 mg/ml in 50:49.9:0.1 acetonitrile:water:trifluoroacetic acid). Spectra were collected using a Bruker UltrafleXtreme mass spectrometer equipped with a SmartBeam™ laser (Bruker Daltonics, Billerica, MA). Each spectrum was the sum of 1600 laser shots collected throughout the entire area of each matrix spot. The data were preprocessed (baseline subtraction, noise level estimation, and alignment to common peaks), and classifiers were determined using a support vector machine algorithm.
Figure 1.
Hematoxylin and eosin staining of a representative Wilms tumor specimen. (A) Cellular compartments of blastema (teal) and tumor stroma (pink) are marked for IMS analysis. (B) Higher power image of marked histologic regions within rectangle in (A).
To demonstrate feasibility in the sequencing of peptides that are unique to either race group, fragmentation (MS/MS) spectra were generated from statistically relevant peaks obtained directly from the tissue section. MS/MS spectra were submitted to Mascot software (Matrix Science, Boston, MA) for database searching to determine the amino acid sequence and parent protein identity of the peptides.
Statistical Analysis
Statistical analysis of TCR data was performed using SAS statistical package (SAS, Raleigh, NC). Specific tests are reported with results. Statistical significance was set at p<0.05. For IMS data, statistical analyses between race groups were accomplished by feeding data into ClinPro Tools statistical analysis software package (Bruker Daltonics). Peak picking was performed manually to assure proper peak boundaries and selection of only the monoisotopic peak. Unsupervised clustering, principal component analyses, and receiver operating characteristics (ROC) were performed to classify peptide spectra according to race. Peptides with an area under the curve from the ROC plots of greater than 0.8 were considered significant. To determine accuracy of protein sequencing data, an ion’s score is −10*Log(P), where P is the probability that the observed match is a random event. For the peptide discussed below, individual ion scores greater than 37 indicate peptides with significant homology, whereas individual ion scores greater than 47 indicate identity or extensive homology (p<0.05).
RESULTS
Demographics of the Tennessee Cancer Registry Cohort
After exclusion of other race groups, 2,515 cancer patients (black, n=475; white, n=2,040) less than 20 years of age were registered in the TCR as in-state residents for the study period, and 97 had WT (black, n=28; white, n=69). A rank order of ICCC-3 site/histology codes was generated and showed that WT was the 5th most common cancer arising in black children, but was only 9th for whites (Table 1). With the exception of Site Group XI, all other site/histology codes ranked similarly between the two race groups.
Table 1.
| International Classification of Childhood Cancer, 3rd edition | Race | Race | ||
|---|---|---|---|---|
| Black | Rank | White | Rank | |
| Cancer Type | n | n | ||
| Site Group I: Leukemias, Myeloproliferative & Myelodysplastic Diseases | 109 | 1 | 491 | 1 |
| Lymphoid Leukemias | 70 | 351 | ||
| Acute Myeloid Leukemias | 29 | 80 | ||
| Other | 10 | 60 | ||
| Site Group II: Lymphomas & Reticuloendothelial Neoplasms | 65 | 3 | 260 | 3 |
| Hodgkins Lymphomas | 32 | 146 | ||
| Non-Hodgkins Lymphoma (Except Burkitt’s) | 31 | 73 | ||
| Burkitt’s Lymphoma | * | 25 | ||
| Other | * | 16 | ||
| Site Group III: CNS & Miscellaneous Intracranial & Intraspinal Neoplasms | 80 | 2 | 337 | 2 |
| Astrocytomas | 37 | 173 | ||
| Intracranial & Intraspinal Embryonal Tumors | 16 | 72 | ||
| Other | 27 | 92 | ||
| Site Group IV: Neuroblastoma & Other Peripheral Nerve Cell Tumors | 22 | 7 | 97 | 8 |
| Neuroblastoma & Ganglioneuroblastoma | 22 | 97 | ||
| Site Group V: Retinoblastoma | 9 | 9 | 35 | 10 |
| Retinoblastoma | 9 | 35 | ||
| Site Group VI: Renal Tumors | 34 | 5 | 80 | 9 |
| Wilms Tumor | 28 | 69 | ||
| Other | 6 | 11 | ||
| Site Group VII: Hepatic Tumors | 1 | 11 | 19 | 11 |
| Hepatoblastoma | * | 19 | ||
| Site Group VIII: Malignant Bone Tumors | 22 | 7 | 101 | 7 |
| Ewing’s Sarcoma & Related Sarcomas of Bone | 0 | 36 | ||
| Osteosarcomas | 21 | 57 | ||
| Other | * | 8 | ||
| Site Group IX: Soft Tissue & Other Extraosseous Sarcomas | 39 | 4 | 135 | 5 |
| Rhabdomyosarcomas | 16 | 66 | ||
| Other | 23 | 69 | ||
| Site Group X: Germ Cell Tumors, Trophoblastic Tumors & Neoplasms of Gonads | 24 | 6 | 104 | 6 |
| Malignant Gonadal Germ Cell Tumors | 16 | 81 | ||
| Other Germ Cell Tumors | 8 | 23 | ||
| Site Group XI: Other Malignant Epithelial Neoplasms & Malignant Melanomas | 19 | 10 | 179 | 4 |
| Other | 19 | 179 | ||
| Total cases reported in this table | 433 | 1838 | ||
| Total childhood cancer cases with behavior code 3 (invasive) | 444 | 1863 |
To assess disparity in WT incidence between race groups, TCR data showed that blacks were overrepresented among WT patients (29%) relative to all other cancer types (18.5%; Mantel-Haenszel χ2 p=0.01; Table 2). The diagnosis of WT was 79% more likely to occur among black cancer patients than whites (Odds ratio 1.79, 95% CI 1.14–2.81; p=0.01). This ratio represents the increased odds for black cancer patients less than 20 years of age to be diagnosed with WT relative to all other forms of cancer and, importantly, does not reflect risk to the general population. Gender did not affect this increased incidence in WT among Tennessee blacks, as the white male to black male incidence ratio was 0.70, and the white female to black female ratio was 0.74.
Table 2.
| 1999–2008 | African-American 16% of TN census |
Caucasian 82% of TN census |
Totals |
|---|---|---|---|
| Wilms’ tumor (%) | 28 (28.9) | 69 (71.1) | 97 |
| Other cancers (%) | 447 (18.5) | 1,971 (81.5) | 2,418 |
| Total cancers (%) | 475 (18.9) | 2,040 (81.1) | 2,515 |
| Wilms’/Total cancers | 5.9% | 3.4% | 3.9% |
p= 0.0104
Stage of Wilms Tumor at Presentation
To assess disparity in the stage of WT at presentation, stage of disease data showed that more black children (25%) diagnosed with WT tended to present with distant disease than did whites (15%; p=0.26, Mantel-Haenszel χ2). Among children presenting with distant disease for all cancer types, black patients were diagnosed more frequently with WT (3.5%) than whites (1.4%; Fisher’s Exact Test, p=0.047; Table 3). Importantly, and consistent with previous reports, the median age at presentation of WT tended to be older for blacks (4.1±0.9 years) than whites (3.2±0.4 years; Kruskal-Wallis, p=0.06).
Table 3.
| Race | Wilms’ Tumor Distant Disease | Other Tumor Distant Disease | Total | % |
|---|---|---|---|---|
| Black | 7 | 194 | 201 | 3.5 |
| White | 10 | 723 | 733 | 1.4 |
| Total | 17 | 917 | 934 | 1.8 |
P = 0.047
Survival
To evaluate disparate outcomes between these race groups, overall survival from WT was not statistically different between blacks (92.9%) and whites (94.0%) residing and treated in Tennessee, although black males showed the lowest survival (85%) for race-gender subgroups (p=0.21, Fisher’s Exact Test).
Time from WT Diagnosis to Initiation of Therapy
To determine whether a disparity in initiation of cancer therapy for WT between race groups existed in Tennessee, we compared days from diagnosis to first therapeutic intervention for WT patients. Blacks were treated within a mean of 1.4±0.6 days and whites within 3.0±1.4 days (ANOVA; p=0.46). Median length of recorded follow-up was not statistically different between race groups either: blacks were followed to 245 days (interquartile range, 200–547 days) and whites to 195 days (IQR, 87–352 days; Wilcoxon Rank Sum, p<0.08). Taken together, no difference in time from diagnosis to initial treatment was found in this cohort of Tennessee WT patients.
IMS of Tennessee WT Specimens
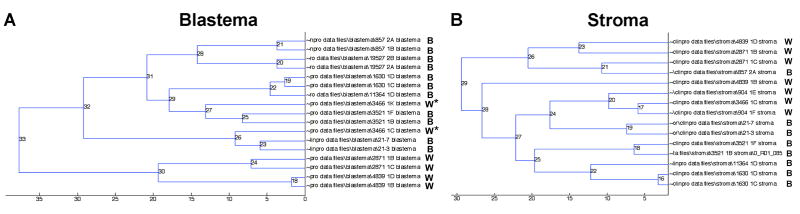
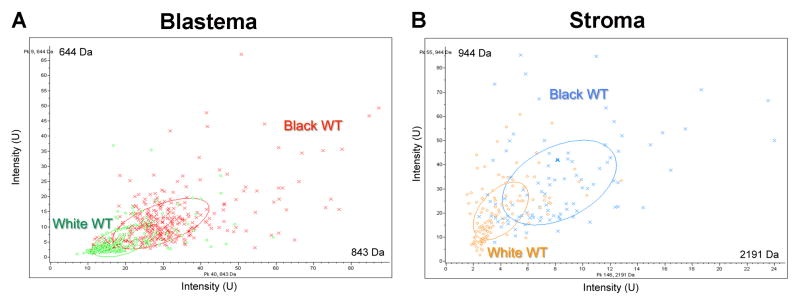
To begin to clarify the biological basis for these race disparities in Tennessee WT patients, we performed independent IMS analyses of blastemal and stromal histologic compartments in WT specimens derived from black and white patients. We chose to analyze separately these two distinct histologic compartments to control for differences in protein composition of tissues segregating solely according to racial ancestry. Unsupervised clustering of peptide spectra from either cellular compartment was able to classify tumors according to race with greater than 80% accuracy (Figure 2: (A) blastema; (B) stroma). Sections from separate tissue blocks in the same patient clustered adjacent to one another with the exception of one patient (Figure 2). Importantly, these peptide spectra from either histologic compartment were distinct from one another, suggesting race and cancer cell specificity of the WT proteome. Principal component analysis was used to identify peptide peaks within blastemal and stromal compartments that could discriminate WT specimens according to race (Figure 3: (A) blastema; (B) stroma).
Figure 2.
Unsupervised clustering of all peptide spectra from (A) blastema and (B) stroma is able to classify Wilms tumor specimens according to race (B = black and W = white patient tumor specimens). The asterisks (*) in (A) indicate the only patient whose tumor specimens did not cluster immediately next to one another.
Figure 3.
Example of a principal component analysis of peptide peaks within (A) blastema and (B) stroma that discriminate Wilms tumor specimens according to race.
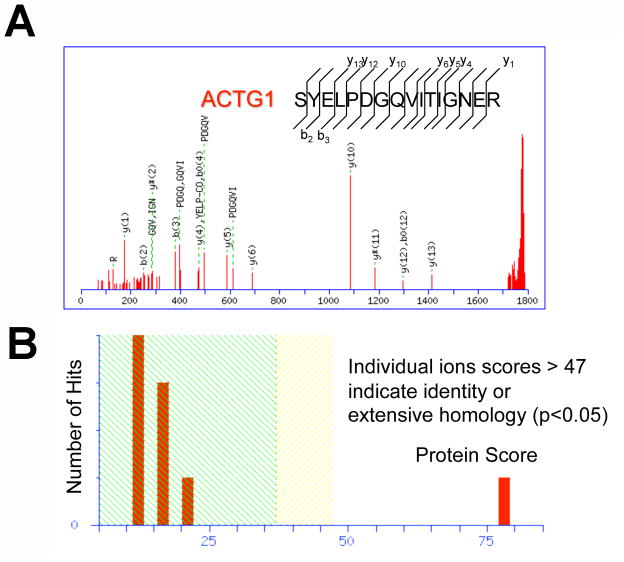
Peptide identification and sequencing detected proteins that appear differentially expressed in WT blastema between race groups. For example, ACTG1, a protein active in cell motility, was uniquely detected in blastema of tissues from white patients with extensive homology (Figure 4: (A) sequencing data of ACTG1; (B) individual ions scores > 47 indicate identity, p<0.05).
Figure 4.
Peptide identification and sequencing detects proteins that appear differentially expressed in Wilms tumor according to race. (A) sequencing of ACTG1. (B) individual ions scores > 47 indicate identity, p<0.05).
DISCUSSION
These TCR data suggest that WT is more of a health concern for blacks than whites residing in Tennessee. Importantly, these TCR data support previous studies that document an increased incidence of WT to develop in children of black African ancestry, regardless of residence in geographically disparate regions of the world, and implicate a racial predisposition to develop WT. Coupled with the mass spectrometry data generated from two distinct cellular compartments within racially diverse specimens, it appears that a race-specific biology may be at play in both the tumorigenesis and pathogenesis of WT.
This study provides two important contributions to the understanding of race disparities in both WT epidemiology and biology. First, regarding epidemiology, this study represents the initial description of how WT incidence and behavior may differ between race groups from Tennessee, which serves as a surrogate for the greater South Region. To monitor population cancer trends in the United States, the National Cancer Institute established the Surveillance, Epidemiology and End Results (SEER) Program. The SEER registry accounts for approximately 26% of the US population, but does not include our population in Tennessee. Furthermore blacks comprise only 7.3% of SEER registered patients. The South Region, as designated by the United States Census Bureau, is comprised of 17 states, where collectively 56% of the African-American population resides. However, only three states in this South Region, Georgia, Kentucky and Louisiana, contribute data to the SEER registry. Therefore, SEER data may be insufficient to evaluate race disparities in a rare cancer such as WT and may blur geographical variations in, and region-specific environmental modifiers of, its incidence. This limitation of the SEER database provides additional rationale to explore variation in cancer epidemiology between race groups registered in a state cancer database.
Although our study does not have the power to show statistically significant differences in survival from WT, the race-gender subgroup in the TCR having the lowest survival was black male. Larger reports have shown that blacks lag whites in late survival from WT, between 2 and 4% (1, 8). In our cohort, we did not identify any disparities between race groups in the initiation of WT therapy or in early follow up, although compliance with late follow up could not be assessed. Notably, a report from the National Wilms Tumor Study Group showed that blacks are at significant risk to become lost to follow up, which certainly could confound any outcome data (1). Nevertheless, the TCR does capture all deaths of its registrants. A study to investigate race disparities in compliance with late survivorship clinic evaluation and in chronic health issues of survivors is warranted. Factors that could influence these disparate outcomes from WT include divergent access to routine preventive medicine and a delayed age at presentation. Although not statistically significant observations, black children in our relatively small TCR cohort of WT patients showed both a more advanced age and stage of disease at presentation, which could reflect delays or inequalities in access to and provision of routine health care.
A query of a state cancer registry can be meritorious to assess regional trends and race disparities in malignant diseases, yet this analysis of TCR data has several limitations. Specifically, the TCR does not record all features known to influence WT behavior, which could differ according to race and therefore account for the disparate behavior observed between study groups. As a result, we could not assess disparities in the frequency of favorable or unfavorable histology, in Children’s Oncology Group (COG) pathologic staging, and in disease-relapse, considerations that are standard to any WT study. As well, factors that are widely considered prognostic determinants of WT behavior, such as mutations of 1p, 7p, 16q WT1, WT2, and WTX, are not measureable currently through the TCR. Finally, the influence of environmental modifiers and of societal barriers to preventive medicine for generations of underrepresented minorities was not discernible.
The second unique aspect of this study is underscored by the attempt to clarify the biological basis for race disparities in a childhood cancer documented within the same population. To explore the biologic underpinnings of these race disparities in WT epidemiology, we chose to perform a proteomic analysis of WT between race groups. Matrix Assisted Laser Desorption Ionization Time of Flight (MALDI-TOF) Imaging Mass Spectrometry (IMS) has been shown recently to be an innovative, high-throughput means to analyze the proteome of formalin fixed, paraffin embedded tissues, the most common means of specimen archiving (23). For these reasons, we postulated that MALDI-TOF-IMS could help tease apart unique patterns of protein expression that vary according to race and may reflect biologic differences in the development and behavior of WT along racial lines. Importantly, to control for histologic differences between specimens having cellular heterogeneity, as is typical of WT, IMS permits the proteomic analysis of specific cellular compartments within a tumor (24). For example, comparisons can be made between cell-specific WT compartments across multiple specimens (e.g. blastema versus blastema; epithelia versus epithelia; stroma versus stroma), so that variability in histologic subtypes of individual specimens does not confound peptide spectral differences between grouped samples. As evident in the current study, the capacity of peptide spectra from two distinct histologic compartments to classify independently WT specimens according to race ensures that the observed patterns of protein expression are specific to the cancer cell type and are not surrogate markers of other biologically unique aspects of race. This ability of MALDI-TOF-IMS peptide spectra to classify WT specimens according to race represents an early peek into the biological basis for the current and previous epidemiologic observations discussed above. Taken together, WT appears to display specific peptide profiles that could account for its variability in incidence and behavior observed between race groups.
Although encouraging, the preliminary nature of our molecular studies also limits interpretation of this report. We are currently validating these initial observations with increased numbers of racially disparate WT specimens both from Vanderbilt and the COG. Importantly, to evaluate the biological basis for race disparities in adverse WT behavior, half of these COG specimens derive from patients who subsequently experienced disease-relapse.
As the first report to describe race disparities in WT from the South Region, our study supports previous work that race, more than geographic region, predisposes to the development of WT, further suggesting a heritable and disparate biology between race groups. The mass spectrometry data provide initial evidence that a race-specific biology may indeed underlie observed disparities in WT development and behavior. Through our more detailed and ongoing molecular analysis, perhaps we can begin to identify peptide spectra that correlate with, or specific proteins that contribute to, the increased incidence of WT among children of black African ancestry, as reflected by the TCR data and the larger body of literature. Such molecular knowledge will help to discover race-specific targets of future, more personalized WT therapies, which can be further tailored to the risk for specific race groups to experience adverse behavior and disease-relapse, as also predicted by tumor-specific molecular fingerprints. Our exploration of the biological basis for race disparities in a rare childhood cancer could serve as a paradigm for the investigation of other racially disparate cancers in both children and adults. Additional studies are warranted to clarify the effect of genetic and epigenetic variability on race disparities in WT.
Acknowledgments
This work was supported in part by the Vanderbilt CTSA grant, UL1 RR024975, from the National Center for Research Resources of the National Institutes of Health, by the National Cancer Institute Grant, 5T32CA106183-06A1 (JA and AJM), and by NCI Grant 4R00CA135695-03 (HNL). Mass spectrometry work was supported in part by Vanderbilt Ingram Cancer Center Core Support Grant P30-CA68485 and the Department of Defense Grant DOD W81XWH-05-1-0179. The TCR is supported in part by funds received from the Centers for Disease Control & Prevention’s (CDC), National Program of Cancer Registries, Contract # DP07-703 DP00822-04. The opinions expressed herein are those of the authors and may not represent the views of the CDC. The authors would like to thank Dr. James A. O’Neill, Jr. for his critical review of the manuscript and Jamie L. Allen for help with sample preparation. All experiments were conducted in accordance with the ethical standards, and with prior approval from the Vanderbilt University Institutional Review Board (IRB study numbers 020888 and 100734).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breslow N, Olshan A, Beckwith JB, Moksness J, Feigl P, Green D. Ethnic variation in the incidence, diagnosis, prognosis, and follow-up of children with Wilms’ tumor. J Natl Cancer Inst. 1994;86:49–51. doi: 10.1093/jnci/86.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Breslow N, Olshan A, Beckwith JB, Green DM. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993;21:172–181. doi: 10.1002/mpo.2950210305. [DOI] [PubMed] [Google Scholar]
- 3.Kramer S, Meadows AT, Jarrett P, Evans AE. Incidence of childhood cancer: experience of a decade in a population-based registry. J Natl Cancer Inst. 1983;70:49–55. [PubMed] [Google Scholar]
- 4.Stiller CA, Parkin DM. International variations in the incidence of childhood renal tumours. Br J Cancer. 1990;62:1026–1030. doi: 10.1038/bjc.1990.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilde JC, Lameris W, van Hasselt EH, Molyneux EM, Heij HA, Borgstein EG. Challenges and outcome of Wilms’ tumour management in a resource-constrained setting. Afr J Paediatr Surg. 7:159–162. doi: 10.4103/0189-6725.70416. [DOI] [PubMed] [Google Scholar]
- 6.Abdallah FK, Macharia WM. Clinical presentation and treatment outcome in children with nephroblastoma in Kenya. East Afr Med J. 2001;78:S43–47. [PubMed] [Google Scholar]
- 7.Uba AF, Chirdan LB. Childhood Wilms’ tumour: prognostic factors in North Central Nigeria. West Afr J Med. 2007;26:222–225. doi: 10.4314/wajm.v26i3.28314. [DOI] [PubMed] [Google Scholar]
- 8.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113:2575–2596. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer S, Meadows AT, Jarrett P. Racial variation in incidence of Wilms’ tumor: relationship to congenital anomalies. Med Pediatr Oncol. 1984;12:401–405. doi: 10.1002/mpo.2950120609. [DOI] [PubMed] [Google Scholar]
- 10.Tretiakova M, Turkyilmaz M, Grushko T, Kocherginsky M, Rubin C, Teh B, Yang XJ. Topoisomerase IIalpha in Wilms’ tumour: gene alterations and immunoexpression. J Clin Pathol. 2006;59:1272–1277. doi: 10.1136/jcp.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovvorn HN, Westrup J, Opperman S, Boyle S, Shi G, Anderson J, Perlman EJ, Perantoni AO, Wills M, de Caestecker M. CITED1 expression in Wilms’ tumor and embryonic kidney. Neoplasia. 2007;9:589–600. doi: 10.1593/neo.07358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dome JS, Bockhold CA, Li SM, Baker SD, Green DM, Perlman EJ, Hill DA, Breslow NE. High telomerase RNA expression level is an adverse prognostic factor for favorable-histology Wilms’ tumor. J Clin Oncol. 2005;23:9138–9145. doi: 10.1200/JCO.2005.00.562. [DOI] [PubMed] [Google Scholar]
- 13.Natrajan R, Reis-Filho JS, Little SE, Messahel B, Brundler MA, Dome JS, Grundy PE, Vujanic GM, Pritchard-Jones K, Jones C. Blastemal expression of type I insulin-like growth factor receptor in Wilms’ tumors is driven by increased copy number and correlates with relapse. Cancer Res. 2006;66:11148–11155. doi: 10.1158/0008-5472.CAN-06-1931. [DOI] [PubMed] [Google Scholar]
- 14.Natrajan R, Williams RD, Hing SN, Mackay A, Reis-Filho JS, Fenwick K, Iravani M, Valgeirsson H, Grigoriadis A, Langford CF, Dovey O, Gregory SG, Weber BL, Ashworth A, Grundy PE, Pritchard-Jones K, Jones C. Array CGH profiling of favourable histology Wilms tumours reveals novel gains and losses associated with relapse. J Pathol. 2006;210:49–58. doi: 10.1002/path.2021. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Yang XJ, Lavery TT, Furge KA, Williams BO, Tretiakova M, Montag A, Vogelzang NJ, Re GG, Garvin AJ, Soderhall S, Kagawa S, Hazel-Martin D, Nordenskjold A, Teh BT. Gene expression profiling of favorable histology Wilms tumors and its correlation with clinical features. Cancer Res. 2002;62:6598–6605. [PubMed] [Google Scholar]
- 16.Li CM, Guo M, Borczuk A, Powell CA, Wei M, Thaker HM, Friedman R, Klein U, Tycko B. Gene expression in Wilms’ tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am J Pathol. 2002;160:2181–2190. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiti S, Alam R, Amos CI, Huff V. Frequent association of beta-catenin and WT1 mutations in Wilms tumors. Cancer Res. 2000;60:6288–6292. [PubMed] [Google Scholar]
- 18.Rivera MN, Kim WJ, Wells J, Driscoll DR, Brannigan BW, Han M, Kim JC, Feinberg AP, Gerald WL, Vargas SO, Chin L, Iafrate AJ, Bell DW, Haber DA. An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science. 2007;315:642–645. doi: 10.1126/science.1137509. [DOI] [PubMed] [Google Scholar]
- 19.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 20.Grundy PE, Breslow NE, Li S, Perlman E, Beckwith JB, Ritchey ML, Shamberger RC, Haase GM, D’Angio GJ, Donaldson M, Coppes MJ, Malogolowkin M, Shearer P, Thomas PR, Macklis R, Tomlinson G, Huff V, Green DM. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 21.Grundy RG, Pritchard J, Scambler P, Cowell JK. Loss of heterozygosity on chromosome 16 in sporadic Wilms’ tumour. Br J Cancer. 1998;78:1181–1187. doi: 10.1038/bjc.1998.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy RG, Pritchard J, Scambler P, Cowell JK. Loss of heterozygosity for the short arm of chromosome 7 in sporadic Wilms tumour. Oncogene. 1998;17:395–400. doi: 10.1038/sj.onc.1201927. [DOI] [PubMed] [Google Scholar]
- 23.Groseclose MR, Massion PP, Chaurand P, Caprioli RM. High-throughput proteomic analysis of formalin-fixed paraffin-embedded tissue microarrays using MALDI imaging mass spectrometry. Proteomics. 2008;8:3715–3724. doi: 10.1002/pmic.200800495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornett DS, Mobley JA, Dias EC, Andersson M, Arteaga CL, Sanders ME, Caprioli RM. A novel histology-directed strategy for MALDI-MS tissue profiling that improves throughput and cellular specificity in human breast cancer. Mol Cell Proteomics. 2006;5:1975–1983. doi: 10.1074/mcp.M600119-MCP200. [DOI] [PubMed] [Google Scholar]