Abstract
The intranasal trigeminal system is a third chemical sense in addition to olfaction and gustation. As opposed to smell and taste, we still lack knowledge on the relationship between receptor binding and perception for the trigeminal system. We therefore investigated the sensitivity of the intranasal trigeminal system towards agonists of the trigeminal receptors TRPM8 and TRPA1 by assessing subjects’ ability to identify which nostril has been stimulated in a monorhinal stimulation design. We summed the number of correct identifications resulting in a lateralization score. Stimuli were menthol (activating TRPM8 receptors), eucalyptol (TRPM8), mustard oil (TRPA1) and two mixtures thereof (menthol/eucalyptol and menthol/mustard oil). In addition, we examined the relationship between intensity and lateralization scores and investigated whether intensity evaluation and lateralization scores of the mixtures show additive effects.
All stimuli were correctly lateralized significantly above chance. Across subjects the lateralization scores for single compounds activating the same receptor showed a stronger correlation than stimuli activating different receptors. Although single compounds were isointense, the mixture of menthol and eucalyptol (activating only TRPM8) was perceived as weaker and was lateralized less accurately than the mixture of menthol and mustard oil (activating both TRPM8 and TRPA1) suggesting suppression effects in the former mixture.
In conclusion, sensitivity of different subpopulations of trigeminal sensory neurons seems to be related, but only to a certain degree. The large coherence in sensitivity between various intranasal trigeminal stimuli suggests that measuring sensitivity to one single trigeminal chemical stimulus may be sufficient to generally assess the trigeminal system’s chemosensitivity. Further, for stimuli activating the same receptor a mixture suppression effect appears to occur similar to that observed in the other chemosensory systems.
Keywords: irritation, chemosensory, lateralization, smell, intensity, monorhinal
Introduction
In addition to smell and taste, the trigeminal nerve of the somatosensory system constitutes the third of the chemical senses, which allow for monitoring the chemical environment of the exterior and interior worlds. Trigeminal stimulation leads to sensations such as burning, cooling, and tingling, even in the absence of an olfactory percept (Laska et al., 1997). Most odorous substances at high enough concentrations activate the trigeminal nerve in addition to the olfactory nerve (Doty et al., 1978), with some odorous substances being strong trigeminal stimuli, such as allyl-thioisocyanate (mustard oil) (Brand and Jacquot, 2002), nicotine (Thuerauf et al., 1999), or menthol (Parikh et al., 2009).
An interesting feature in humans that sets the trigeminal system apart from its olfactory counterpart is its ability to physically identify the site of stimulation (von Skramlik, 1924, Frasnelli et al., 2008). When an odorous stimulus is presented to one nostril only (monorhinal stimulation) we can only identify the stimulated nostril, if the odor also activates the trigeminal system (Kobal et al., 1989, Hummel et al., 2003, Frasnelli et al., 2009, Kleemann et al., 2009). This is not possible with pure odorants, i.e. stimuli which activate the olfactory sense exclusively (Kobal et al., 1989, Frasnelli et al., 2011). By assessing how well subjects can identify the stimulated nostril - a test called “odor lateralization” which results in a “lateralization score” (Hummel et al., 2003, Boyle et al., 2006) - one can measure the degree to which a stimulus activates the human trigeminal system (Frasnelli et al., 2011). The underlying assumption is that lateralization scores reflect how sensitive subjects are towards trigeminal stimuli per se. In other words, it is assumed that subjects who are good at lateralizing one trigeminal stimulus are also good at lateralizing independent of the kind of trigeminal stimulus. We aimed to assess this assumption by testing our initial hypothesis: (I.) lateralization scores for different trigeminal stimuli are correlated.
For a long time, it was assumed that the trigeminal system was activated via nonspecific interactions between chemical compounds and free endings of the trigeminal nerve (Cain and Murphy, 1980, Radil and Wysocki, 1998). However, over the last fifteen years different trigeminal receptors have been identified; trigeminal chemosensation is therefore based on the interaction of a ligand with a receptor, similar to olfaction and gustation. For example, TRPV1 responds to capsaicin (the pungent ingredient of hot chili peppers) leading to a burning sensation (Caterina et al., 1997). Other known trigeminal receptors include TRPM8 which is activated by substances such as menthol and mediates sensations of cooling and freshness (McKemy et al., 2002, Behrendt et al., 2004), and TRPA1 that is activated by compounds such as mustard oil and elicits a stinging and burning sensation (Story et al., 2003, Jordt et al., 2004). Interestingly, while TRPA1 and TRPV1 are often co-expressed on the same sensory neuron (Story et al., 2003, Salas et al., 2009), TRPA1 and TRPM8 are exclusively expressed in different populations of sensory neurons (Story et al., 2003). It is, however, unclear whether the relative independence of these distinct TRPA1- and TRPM8-expressing trigeminal receptor neurons has functional implications. In hypothesis I we postulated that lateralization scores for different trigeminal stimuli are correlated; here we extend this, by formulating a second hypothesis, i.e., (II.) lateralization scores for two agonists of the same trigeminal receptor show a higher degree of correlation than lateralization scores for agonists of two different receptors, who exhibit a lower, but still significant degree of correlation.
As stated above, activation of the trigeminal system is based on the interaction of a stimulus with a receptor, similarly to olfaction and gustation. However, the analogy between the chemical senses may extend beyond this. One important question is how the sensory system reacts to stimulus mixtures. In the olfactory system, simultaneous presentation of two compounds in a 50:50 mixture generally evokes the same intensity as each single compound at 100% concentration. This is true even if both compounds are perceptually relatively different (Olsson and Cain, 2000, Boyle et al., 2009). In the gustatory system, however, a different picture emerges: binary taste mixtures consisting of two stimuli, which each evoke the same taste quality (e.g., sweet), may show effects of enhancement or suppression. Similarly, binary mixtures of different quality stimuli (e.g., sweet and sour) can, depending on the taste qualities, show enhancement, suppression or no effect (for an overview, see Keast and Breslin, 2003). We planned to explore the effect of mixing trigeminal stimuli on both perceived intensity and lateralization scores. Specifically, we provided subjects with a mixture consisting of agonists of one trigeminal receptor (menthol and eucalyptol; both TRPM8 agonists) and a mixture of agonists of two different trigeminal receptors (menthol; TRPM8 agonist; and mustard oil; TRPA1 agonist). We hypothesized that (III.) a heterogeneous mixture is perceived as having a different intensity and can consequently be lateralized more poorly or better than a mixture stimulating one type of receptor.
In summary, we aimed to test three hypotheses in this study: (I.) lateralization scores for different trigeminal stimuli are correlated; (II.) lateralization scores for two agonists of the same trigeminal receptor show a higher correlation than lateralization scores for two agonists of different receptors; (III.) a mixture of stimuli binding to different trigeminal receptors is perceived as differently intense and can consequently better or worse be lateralized than a mixture of stimuli binding to the same trigeminal receptor.
Material and Methods
A total of 20 subjects (10 women; mean age of 25 years (SD 4.5, range 19–35)) participated in the study. Individuals with a reduced sense of smell (as assessed with the Sniffin’ Sticks 16-item identification test (Hummel et al., 2007)) or suffering from diseases and conditions that may be accompanied by an altered olfactory or trigeminal sensitivity (e.g., a cold, allergic rhinitis, epilepsy, traumatic brain injury, etc) were not included in the study. All subjects provided written informed consent and all aspects of the study were approved in advance by the University of Pennsylvania’s Institutional Review Board.
We assessed subjects’ ability to lateralize three single compounds, i.e., (1) a 17.85% w/v L-menthol solution (menthol; CAS 2216-51-5: Fisher Scientific, Acros Organics; dissolved in 1,2-propanediol: CAS 57-55-9: Fisher Scientific, Acros Organics), (2) a 60% v/v eucalyptol solution (eucalyptol: CAS 470-82-6: Sigma-Aldrich, Aldrich Chemical; in PEG), and (3) a 0.075% v/v allyl isothiocyanate solution (mustard oil; CAS 57-06-7: Sigma Aldrich; in 1,2-propanediol). These specific concentrations were used since a pilot study using a separate cohort demonstrated equal intensity judgments and lateralization scores between stimuli. This cohort consisted of six experienced subjects. They rated different concentrations of the single compounds with regards to intensity on a visual analog scale. We then chose that concentration of each stimulus, which was rated as an average intense stimulus (between 60 and 70 on a scale of 100). In addition to these three single compounds, subjects’ ability to lateralize two mixtures, i.e., (4) a mixture of menthol and eucalyptol (mixture consisting of 50% of (1) and (2); both binding to the same receptor TRPM8; menthol/eucalyptol) and (5) a mixture of menthol and mustard oil (consisting of 50% of (1) and (3); binding to receptors TRPM8 and TRPA1, respectively. Thus, subjects were tested with five different stimuli: menthol, eucalyptol, mustard oil, menthol/eucalyptol, and menthol/mustard oil. Testing was performed on two different days to limit fatigue. On day one, subjects performed the lateralization test for three stimuli, on day two they were tested with the remaining two stimuli. The five stimuli were presented in a pseudo-randomized and counterbalanced order.
We assessed the ability to lateralize the stimuli by presenting the target odorant to either nostril in an amber glass bottle (total volume 280mL) filled with 20ml of the respective solution; at the same time an identical bottle filled with 20mL of odorless solvent (1,2-propanediol) was presented to the contralateral nostril. The bottles were fitted with nostril-shaped Teflon nose pieces to allow for a tight connection to the nostril as well as ventilation holes to allow a natural sniff (a more detailed method description can be found in (Wysocki et al., 2003)). When prompted, the blindfolded subjects grabbed the bottles with the nose pieces and moved them close to their nostrils so that the top of the nose pieces sealed the nostrils. They took one sniff, allowing themselves to sample the air from the head space of the left and right bottle simultaneously.
For each stimulus, a total of 40 presentations were applied to the blindfolded subjects at a timed intertrial interval of 40s resulting in 26 minutes of testing time per condition. Stimulation of the left or right nostril followed a pseudo-randomized sequence, thus each nostril was stimulated 20 times. After each trial, subjects made a two-alternative (left/right), forced-choice judgement on the position of the stimulus by raising the corresponding hand. Task performance was assessed by counting the number of correct lateralizations (von Skramlik, 1924, von Békésy, 1964, Kobal et al., 1989, Hummel et al., 2003, Wysocki et al., 2003, Frasnelli et al., 2008) resulting in a score for each stimulus. Tests of different stimuli were separated by a 10-minute break.
At the end of each testing block, subjects rated the intensity and pleasantness of the stimulus on a 10 cm visual analog scale with “not intense at all” (or “very unpleasant”) and “extremely intense” (or “very pleasant”) as anchor points. In addition, they chose three descriptors from a list of seventeen identical to the ones used by (Laska et al., 1997), and ranked the selected descriptors regarding suitability. The descriptors were: stinging, burning, painful, sharp, astringent, furry, warm, scratchy, tickling, prickly, sneeze, cool, fresh, sweet, salty, bitter, and sour (Laska et al., 1997).
Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago IL). In order to test hypothesis I we calculated Pearson’s correlation coefficient between the scores obtained for the three single compounds. Hypothesis II was tested in two different ways. First, we examined if the correlation coefficient between scores of menthol and eucalyptol (agonists of the same receptor) was higher than between mustard oil and eucalyptol as well as between mustard oil and menthol. Second, if there is a high agreement between the scores for two compounds, the difference between two scores should be relatively similar across subjects and therefore have a low variance, as compared to high variance when there is little similarity. We therefore computed the differences between the subjects’ scores for the single compounds (deltaME: menthol - eucalyptol; deltaMA: menthol - mustard oil; deltaEA: eucalyptol - mustard oil). In order to test if deltaEA has a lower variance than the other two, as postulated by hypothesis II, we compared variances by calculating Pitman’s t for the three pairings deltaME-deltaMA, deltaME-deltaAE, and deltaMA-deltaAE (Pitman, 1939). A significant result for this test indicates that the two correlated samples have different variances. In order to test hypothesis III, we computed the following statistics. To establish qualitative differences between the single compounds and the mixtures, the frequency of the descriptors was examined by weighting the best suited descriptor with 3 points, the second best suited with 2 points, and the third best suited with one point. Based on these weights, a total score was calculated for each stimulus and descriptor. Since another qualitative aspect of perception is pleasantness, we computed a repeated measures ANOVA with stimulus (five levels: menthol, eucalyptol, mustard oil, menthol/eucalyptol, and menthol/mustard oil) as a within-subject factor for pleasantness ratings. Finally, we computed repeated measures ANOVAs with stimulus (five levels, as above) as a within-subject factor separately on intensity and lateralization scores. Unless we had an a priori hypothesis, Holm-Bonferroni correction of alpha values was employed to correct for multiple statistical comparisons in post hoc testing. P-values < 0.05 were considered significant.
Results
All stimuli clearly activated the trigeminal system as demonstrated by the ease subjects had in lateralizing them. Performance scores were significantly above expected chance values for all individual stimuli (all p<0.001). See table 1 for average scores.
Table 1.
Mean lateralization scores as well as intensity and pleasantness ratings for 5 odors/odor mixtures (A = Allyl isothiocyanate, E = Eucalyptol, M = Menthol, MA = Mixture of M and A, ME = Mixture of M and E).
| Odor | Lateralization score |
Intensity ratings |
Pleasantness ratings |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | post hoc |
Mean | SD | post hoc |
Mean | SD | post hoc |
|
| A | 32.4 | 8.8 | a,b | 67.3 | 4.4 | a,b | 35.7 | 4.0 | a |
| E | 32.0 | 6.8 | a,b | 63.7 | 3.5 | a,b | 62.7 | 3.7 | c |
| M | 34.8 | 5.5 | a,b | 62.1 | 3.9 | a,b | 58.5 | 3.4 | b,c |
| MA | 35.4 | 6.0 | a* | 71.8 | 3.5 | a | 42.7 | 4.9 | a,b |
| ME | 33.6 | 6.5 | b* | 54.6 | 4.1 | b | 62.4 | 3.8 | c |
Significant differences in post hoc comparisons between stimuli are indicated by different letters in the post hoc columns (p < 0.05, *: one sided t-test).
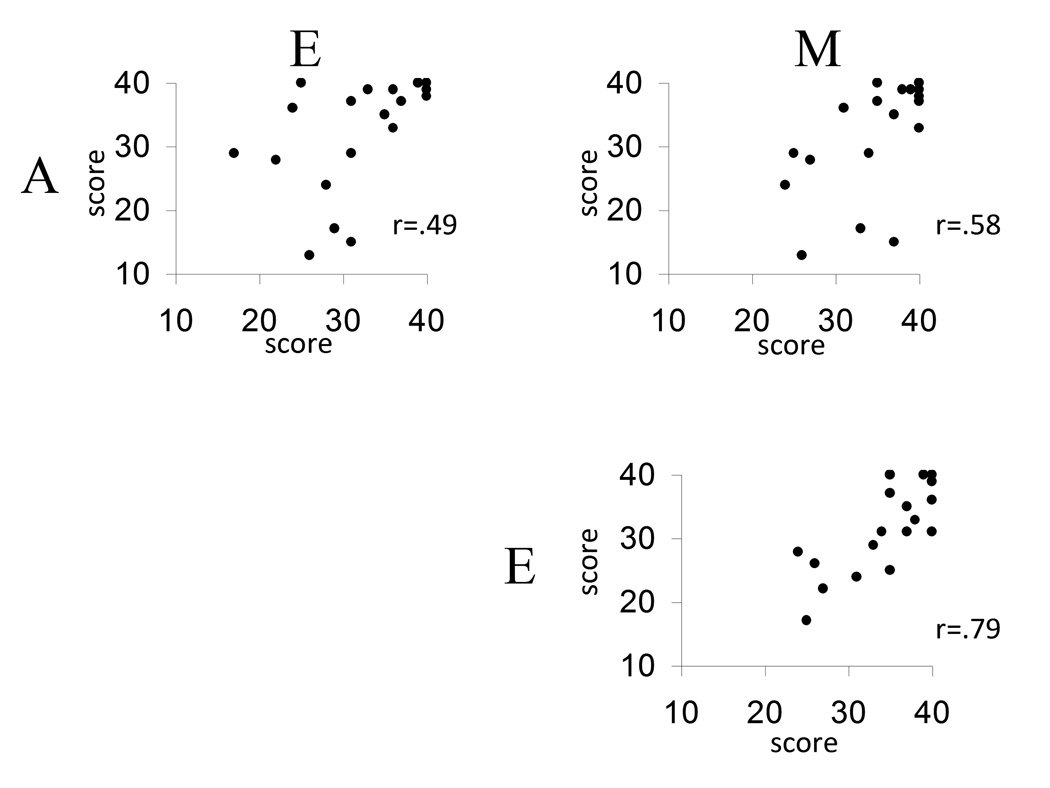
There was a general correspondence in performance between scores for all three single compounds (Figure 1). The scores for menthol and eucalyptol showed the highest correlation (r20=0.79; p<0.001), whereas lower, but still significant, correlations were observed between scores for menthol and mustard oil (r20=0.58; p=0.007) as well as eucalyptol and mustard oil (r20=0.49; p=0.028)
Figure 1.
Correlations between lateralization scores (number of correct responses of 40) for 3 single substances (A: mustard oil; E: eucalyptol; M: menthol) in 20 subjects.
The variance of deltaME (17.4) was considerably lower than the variances of deltaMA (51.2) and deltaAE (64.8), indicating a high congruency between scores of menthol and eucalyptol. Indeed, Pitman’s t was highly significant for deltaME-deltaMA (t=7.1; p<0.001) and deltaME-deltaAE (t=9.9; p<0.001); whereas no statistically significant difference was observed for deltaMA-deltaAE (t=0.3; p=ns). This indicates that there was a significantly higher correlation between the scores for menthol and eucalyptol than between menthol and mustard oil, or between eucalyptol and mustard oil.
With regards to the descriptors, subjects most often characterized mustard oil as stinging (on average 1.2 points [p]), followed by burning (1.1 p) and sharp (1.0 p). Eucalyptol and menthol were described as predominantly cool (2.4p and 2.3 p, respectively), fresh (both 1.4p) and tickling (0.5p and 0.7p, respectively). Similarly, the mixture menthol/eucalyptol was described as predominantly cool (2.6p), fresh (1.6p), but also astringent (0.5p), whereas menthol/mustard oil shared descriptors with mustard oil (burning: 1.2p; sharp: 0.8p) as well as with menthol and eucalyptol (cool: 1.0 p). See Figure 2 for an overview.
Figure 2.

Average descriptor score for 3 single substances (A: mustard oil; E: eucalyptol; M: menthol) and 2 mixtures (MA: menthol/mustard oil; ME: menthol/eucalyptol) in 20 subjects. Subjects’ best suited descriptor was weighted with 3 points, the second best suited with 2 points, and the third best suited one with one point.
There was a significant difference between the stimuli in ratings of their perceived pleasantness (F[4,76] = 13.0, p<0.001). Whenever subjects were presented with a stimulus, which contained mustard oil, they rated it as significantly less pleasant than all stimuli without mustard oil (all p<0.008), with the exception of menthol vs. menthol/mustard oil, which did not differ significantly in respect of their pleasantness ratings (Figure 3).
We observed a significant main effect of stimulus on intensity ratings (F[4,76] = 3.1; p=0.036; Greenhouse-Geisser corrected). There was no difference in the intensity ratings for the single compounds; they were thus perceived as isointense. The mixture menthol/mustard oil was however perceived as significantly stronger than menthol/eucalyptol (p=0.015); there was no difference between any other of the intensity ratings.
The factor Stimulus failed to show a significant main effect on lateralization scores after Greenhouse-Geisser correction (F(4,76)=2.73; p=0.07). However, since the results on intensity ratings predict subjects to reach higher scores for the mixture menthol/mustard oil than to the mixture menthol/eucalyptol we performed a one sided t-test. Subjects reached significantly higher scores for menthol/mustard oil than for menthol/eucalyptol (p=0.029).
We additionally investigated possible presentation order effects (e.g., effects of adaptation/habituation, learning, or fatigue), by comparing the summed score of the first 20 trials with those of the last 20 trials for each stimulus. For none of the included stimuli, was there a significant difference between the two scores. Furthermore, in order to investigate sex related effects on the scores, we calculated repeated measures ANOVAs, separately for lateralization scores, intensity ratings, and pleasantness ratings, with stimulus as within subject variables and sex as between subject variables. There was no significant effect of the factor sex for any variable or interaction.
Discussion
We report three major results: first, lateralization scores for different trigeminal stimuli were generally correlated. Despite this general correlation, we observed, as a second finding, the clear dissociation of lateralization scores between agonists of different trigeminal receptors. Thirdly, we observed mixture effects, in that a mixture consisting of agonists of different trigeminal receptors was perceived as more intense and therefore was lateralized better than the mixture of two agonists that act at the same receptor.
The first result of this study is that lateralization scores of isointense stimuli of different trigeminal receptors are correlated between substances across subjects. Subjects who perform well when lateralizing one trigeminal stimulus also scored high for another one as demonstrated by the significant correlations between stimuli. One can compare these findings to studies on olfactory function, which is much better investigated, but for which results seem less straightforward. Cain and Gent reported correlation coefficients between detection threshold scores obtained for four different odorants which were comparable to the ones obtained in the present study (ranging from r=0.66 to r=0.86). However, in order to achieve these high correlation coefficients detection threshold scores were averaged over four repetitions for each odorant; single assessments revealed considerably lower coefficients in the range of r=0.3 (Cain and Gent, 1991). In fact, when working with threshold data from single assessments, detection thresholds for phenyl ethyl alcohol and n-butanol (Zernecke et al., 2010) as well as androstadienone and phenyl ethyl alcohol (Lundstrom et al., 2003) were not at all correlated. Another study, however, reported detection thresholds for phenyl ethyl alcohol and n-butanol to be significantly correlated (Croy et al., 2009). In summary, sensitivity measures for different trigeminal stimuli exhibit a high correlation whereas in olfaction this seems to not necessarily be the case. This suggests that it is sufficient to test one compound (e.g., using the lateralization paradigm) in order to assess the trigeminal system’s chemosensitivity as a whole.
In addition to this general responsiveness of the trigeminal chemosensory system, we also observed a dissociation between measures for agonists of different subsets of trigeminal neurons. Specifically, lateralization scores for the two TRPM8 agonists were more closely correlated than each of them with the score for the TRPA1 agonist. This suggests that the lateralization score is a behavioral measure which reflects the receptor to which the compound binds. The sensitivities of both fiber subsets seem to be distinct; further studies should address functional differences between the trigeminal sensory neuron subsets in more detail. Our findings provide support in humans for the notion of that TRPA1 and TRPM8 receptors are predominantly expressed in different populations of sensory neurons (Story et al., 2003). Bandell et al. (2004) reported mustard oil to exclusively activate mouse TRPA1 but not mouse TRPM8 receptors. In the same report, the authors show strong support for the mutually exclusively expression of TRPA1 and TRPM8. Along the same line, TRPA1 and TRPM8 receptors were found to be expressed by separate subpopulations of dorsal roots ganglion neurons in rats (Kobayashi et al., 2005). In addition, no functional response to menthol (a TRPM8 agonist) and cinnamaldehyde (a TRPA1 agonist) was observed in the same cells of mouse dorsal root ganglion (Hjerling-Leffler et al., 2007). Some recent findings may put the assumption of agonist selectivity and/or specificity into question. For example, half of the menthol sensitive cells in the rat trigeminal ganglion also responded to cinnamaldehyde, and vice versa (Klein et al., 2011). Similarly, there is evidence that menthol may also activate mouse TRPA1 receptors (Karashima et al., 2007). In support of less than perfect agonist selectivity is a human psychophysical experiment, in which menthol and cinnamaldehyde exhibited mutual cross-desensitization (Klein et al., 2011); however this experiment cannot distinguish at which level - receptor, neuron or higher central nervous system - the desensitization takes place.
As a third finding, we observed a difference in the perception of a mixture of stimuli activating the same receptor compared to a mixture of stimuli activating two different receptors. When presented with menthol and eucalyptol, our subjects rated the same-receptor mixture as less intense when compared to the mixture of menthol and mustard oil. This was accompanied by lower lateralization scores for the former mixture than for the latter one. The single compounds were not different from the mixtures, with regard to the lateralization score and intensity. The two mixtures however were (a) perceived significantly different from each other and (b) lateralized to a different degree. Mixture-specific effects have been described for the other chemosensory systems as well. In gustation, there is a well-known mixture suppression effect. When two suprathreshold taste stimuli are mixed together, the intensity of the resulting mixture is less than the sum of the single components’ intensity (Keast and Breslin, 2003). When the bitter tastant quinine is mixed with sweet tasting sucrose, bitterness is decreased with increasing amounts of sucrose (Veldhuizen et al., 2006). Similar rules apply for the olfactory system. Two equally strong odors of similar chemical composition, mixed together, will be perceived as more intense than the single components, but less intense than the sum of the intensities of the single components (Cain et al., 1995). Our study cannot be directly compared to those studies since our stimuli clearly evoked sensations in two distinct sensory systems, i.e., the trigeminal system and the olfactory system, with potential interactions between the two chemosensory systems (Cain and Murphy, 1980, Livermore et al., 1992, Frasnelli et al., 2007).
Unfortunately, usual methods to exclude olfactory co-stimulation could not be applied in the present study. For almost all odorants, the concentration of the odorant at the olfactory threshold is lower compared to the trigeminal threshold. Therefore the use of concentrations below the olfactory threshold would not have evoked a trigeminal sensation. Similarly, masking with other odors was not possible as it is difficult to assure that the olfactory, but not the trigeminal portion of a stimulus is masked at a given concentration. In addition, although most odorants activate the trigeminal system, we only know few volatile stimuli that (relatively) specifically activate trigeminal receptors. A viable option would be to recruit anosmic subjects. However, this induces other confounding factors as it has been shown that anosmia also affects the trigeminal system (Hummel et al., 2003, Frasnelli et al., 2007); hence results from anosmic subjects can not be generalized to healthy subjects. We do however think that the fact that our stimuli had an olfactory component had - if any at all - only a minor impact on the main findings of the study. In order to investigate this more closely, it may be an interesting suggestion for future studies to investigate the effect of additional olfactory stimulation when lateralizing a trigeminal stimulant; a study that could be done using the odorless gas carbon dioxide.
When menthol and eucalyptol were mixed, the resulting mixture was nominally less intense than the two components. When rating the mixed mustard oil and menthol, the mixture was perceived as nominally stronger than the two individual components. Since similar effects were observed for lateralization performance, a measure of trigeminal activation (Kobal et al., 1989), we attribute these effects to the trigeminal system rather than the olfactory system. Thus, this suggests that mixture suppression can also be observed for trigeminal stimuli sharing a common receptor. Conversely, for stimuli that are agonists of different receptors, additive effects appear to take place. One study indicates a possibility that an interaction between the TRPA1 agonist and the TRPM8 agonists may occur in the periphery. In in-vitro experiments Macpherson and colleagues showed that the TRPM8 agonist menthol and the TRPA1 agonist cinnamaldehyde inhibited responses to TRPA1 and TRPM8 agonists, respectively (Macpherson et al., 2006). However, we did not observe an inhibition, but rather additive effects in the menthol/mustard oil mixture, suggesting that if cross inhibition occurs in the periphery, it appears to be overcome by activation of multiple subtypes or modalities of somatosensory nerve endings.
Unpleasant odors and tastants are known to dominate over pleasant ones, when they are mixed together (Lawless, 1977). We report here similar effects for the trigeminal system in that the unpleasant stimulus mustard oil dominated the percept when mixed with the more pleasant menthol. The mixture menthol/mustard oil was significantly less pleasant than menthol alone, but not significantly different from mustard oil. The pleasantness of mixtures of trigeminal stimuli thus seems to be similar to the mixture effects observed for smell and taste (Lawless, 1977), thus indicating a mechanism independent of the individual chemical sense. However, it is likely that the pleasantness of our stimuli was affected by an olfactory sensation.
In conclusion, our data suggest that measuring sensitivity to one single trigeminal chemical stimulus using lateralization may be sufficient to generally assess the trigeminal system’s chemosensitivity. Higher sensitivity to an agonist of one trigeminal receptor predicts better performance to another agonist of the same receptor, and to an agonist of another receptor. Mixing trigeminal stimuli showed mixture suppression when stimuli activating the same receptor were used, whereas a mixture of stimuli activating different receptors exhibited additive effects.
Acknowledgements
JF is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR). JA was partly supported by a fellowship within the postdoctoral program of the German Academic Exchange Service (DAAD D/08/40252). JNL was partly supported by the National Institute on Deafness and other Communication Disorders (NIDCD R03DC009869).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JA, Djordjevic J, Olsson MJ, Lundstrom JN, Jones-Gotman M. The Human Brain Distinguishes between Single Odorants and Binary Mixtures. Cereb Cortex. 2009;19:66–71. doi: 10.1093/cercor/bhn058. [DOI] [PubMed] [Google Scholar]
- Boyle JA, Lundstrom JN, Knecht M, Jones-Gotman M, Schaal B, Hummel T. On the trigeminal percept of androstenone and its implications on the rate of specific anosmia. J Neurobiol. 2006;66:1501–1510. doi: 10.1002/neu.20294. [DOI] [PubMed] [Google Scholar]
- Brand G, Jacquot L. Sensitization and desensitization to allyl isothiocyanate (mustard oil) in the nasal cavity. Chem Senses. 2002;27:593–598. doi: 10.1093/chemse/27.7.593. [DOI] [PubMed] [Google Scholar]
- Cain WS, Gent JF. Olfactory sensitivity: reliability, generality, and association with aging. J Exp Psychol. 1991;17:382–391. doi: 10.1037//0096-1523.17.2.382. [DOI] [PubMed] [Google Scholar]
- Cain WS, Murphy CL. Interaction between chemoreceptive modalities of odour and irritation. Nature. 1980;284:255–257. doi: 10.1038/284255a0. [DOI] [PubMed] [Google Scholar]
- Cain WS, Schiet FT, Olsson MJ, deWijk RA. Comparison of models of odor interaction. Chemical Senses. 1995;20:625–637. doi: 10.1093/chemse/20.6.625. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Croy I, Lange K, Krone F, Negoias S, Seo HS, Hummel T. Comparison between Odor Thresholds for Phenyl Ethyl Alcohol and Butanol. Chemical Senses. 2009;34:523–527. doi: 10.1093/chemse/bjp029. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WPE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20:175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Charbonneau G, Collignon O, Lepore F. Odor localization and sniffing. Chem Senses. 2009;34:139–144. doi: 10.1093/chemse/bjn068. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Hummel T, Berg J, Huang G, Doty RL. Intranasal Localizability of Odorants: Influence of Stimulus Volume. Chem Senses. 2011 doi: 10.1093/chemse/bjr001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasnelli J, Schuster B, Hummel T. Interactions between olfaction and the trigeminal system: what can be learned from olfactory loss. Cereb Cortex. 2007;17:2268–2275. doi: 10.1093/cercor/bhl135. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Ungermann M, Hummel T. Ortho- and retronasal presentation of olfactory stimuli modulates odor percepts. Chemosens Percept. 2008;1:9–15. [Google Scholar]
- Hjerling-Leffler J, Al Qatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. Journal of Neuroscience. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Futschik T, Frasnelli J, Huttenbrink KB. Effects of olfactory function, age, and gender on trigeminally mediated sensations: a study based on the lateralization of chemosensory stimuli. Toxicol Lett. 2003;140–141:273–280. doi: 10.1016/s0378-4274(03)00078-x. [DOI] [PubMed] [Google Scholar]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the "Sniffin' Sticks" including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast SJR, Breslin PAS. An overview of binary taste-taste interactions. Food Qual Prefer. 2003;14:111–124. [Google Scholar]
- Kleemann AM, Albrecht J, Schopf V, Haegler K, Kopietz R, Hempel JM, Linn J, Flanagin VL, Fesl G, Wiesmann M. Trigeminal perception is necessary to localize odors. Physiol Behav. 2009;97:401–405. doi: 10.1016/j.physbeh.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Klein AH, Carstens MI, Zanotto KL, Sawyer CM, Ivanov M, Cheung S, Carstens E. Self- and Cross-desensitization of Oral Irritation by Menthol and Cinnamaldehyde (CA) via Peripheral Interactions at Trigeminal Sensory Neurons. Chem Senses. 2011;36:199–208. doi: 10.1093/chemse/bjq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal G, Van Toller S, Hummel T. Is there directional smelling? Experientia. 1989;45:130–132. doi: 10.1007/BF01954845. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with A delta/C-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Laska M, Distel H, Hudson R. Trigeminal perception of odorant quality in congenitally anosmic subjects. Chem Senses. 1997;22:447–456. doi: 10.1093/chemse/22.4.447. [DOI] [PubMed] [Google Scholar]
- Lawless HT. The pleasantness of mixtures in taste and olfaction. Sens Processes. 1977;1:227–237. [PubMed] [Google Scholar]
- Livermore A, Hummel T, Kobal G. Chemosensory event-related potentials in the investigation of interactions between the olfactory and the somatosensory (trigeminal) systems. Electroencephalogr Clin Neurophysiol. 1992;83:201–210. doi: 10.1016/0013-4694(92)90145-8. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN, Hummel T, Olsson MJ. Individual differences in sensitivity to the odor of 4,16-androstadien-3-one. Chem Senses. 2003;28:643–650. doi: 10.1093/chemse/bjg057. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Olsson MJ, Cain WS. Psychometrics of odor quality discrimination: Method for threshold determination. Chemical Senses. 2000;25:493–499. doi: 10.1093/chemse/25.5.493. [DOI] [PubMed] [Google Scholar]
- Parikh V, Lee-Lim AP, Halpern BP. Retronasal and oral-cavity-only identification of air-phase trigeminal stimuli. Chemosens Percept. 2009;2:9–24. [Google Scholar]
- Pitman EJG. A note on normal correlation. Biometrika. 1939;31:9–12. [Google Scholar]
- Radil T, Wysocki CJ. Spatiotemporal masking in pure olfaction. Ann N Y Acad Sci. 1998;855:641–644. doi: 10.1111/j.1749-6632.1998.tb10638.x. [DOI] [PubMed] [Google Scholar]
- Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, Mclntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Thuerauf N, Kaegler M, Dietz R, Barocka A, Kobal G. Dose-dependent stereoselective activation of the trigeminal sensory system by nicotine in man. Psychopharmacology (Berl) 1999;142:236–243. doi: 10.1007/s002130050885. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, van Rooden AP, Kroeze JH. Dissociating pleasantness and intensity with quinine sulfate/sucrose mixtures in taste. Chem Senses. 2006;31:649–653. doi: 10.1093/chemse/bjl005. [DOI] [PubMed] [Google Scholar]
- von Békésy G. Olfactory analogue to directional hearing. J Appl Physiol. 1964;19:369–373. doi: 10.1152/jappl.1964.19.3.369. [DOI] [PubMed] [Google Scholar]
- von Skramlik E. Über die Lokalisation der Empfindungen bei den niederen Sinnen. Z Sinnesphysiol. 1924;56:69. [Google Scholar]
- Wysocki CJ, Cowart BJ, Radil T. Nasal trigeminal chemosensitivity across the adult life span. Percept Psychophys. 2003;65:115–122. doi: 10.3758/bf03194788. [DOI] [PubMed] [Google Scholar]
- Zernecke R, Vollmer B, Albrecht J, Kleemann AM, Haegler K, Linn J, Fesl G, Bruckmann H, Wiesmann M. Comparison of two different odorants in an olfactory detection threshold test of the Sniffin Sticks. Rhinology. 2010;48:368–373. doi: 10.4193/Rhino09.212. [DOI] [PubMed] [Google Scholar]