Abstract
Background
Colorectal carcinoma is a common and often fatal disease in which methods of early detection and monitoring are essential. The present study was conducted for measuring serum levels of nucleosomes, carcinoembryonic antigen (CEA) and CA 19-9 in patients newly diagnosed with colorectal carcinoma and confirmed by clinicopathological study.
Method
Thirty subjects were included in the current study: six normal subjects as a control group with mean age (45.6 ± 7.9) and twenty four colorectal carcinoma patients with mean age (46.9 ± 15.6), which were classified pathologically according to the degree of malignant cell differentiation into well differentiated (group I), moderately differentiated (group II) and poorly differentiated (group III). Fasting venous blood samples were collected preoperative.
Results
The results revealed a significant increase in serum level of nucleosomes in patients with poorly differentiated tumors versus patients with well differentiated tumors (p = 0.041). The levels of CEA and CA19-9 showed no significant increase (p = 0.569 and 0.450, respectively).
Conclusion
In conclusion, serum level of nucleosomes provides a highly sensitive and specific apoptotic marker for colorectal carcinoma.
Keywords: colorectal carcinoma, nucleosomes, carcinoembryonic antigen (CEA) and CA 19-9, tumor marker
Background
Colorectal carcinoma is one of the leading causes of cancer-related death [1,2]. The 5-year survival rate for colon tumours in Europe ranges from 26% to 56% for men and from 29% to 59% for women. These differences in survival have been attributed to the stage and timing of diagnosis and, in some regions, to the quality of medical care [3]. Hence the need for early detection methods in colorectal cancer [4].
Tumor markers are used clinically for diagnosis, staging, and monitoring of the disease. They are proteins released from dying tumor cells or produced by neoplastic cells. There are two subcategories of these proteins, specific and non-specific. The specific proteins are expressed only in the tumor cells and are very useful for the detection and diagnosis of specific malignant tumors. Non-specific proteins or markers related to malignant cells are oncofetal or carcinogenic antigens, such as carcinoembryonic antigen (CEA), alphafetoprotein (AFP), prostate specific antigen (PSA), carbohydrate antigens CA15.3 and CA19-9. Recently nucleosomes, cytokeratine 18, and cyto-c in serum have been examined as markers for the evaluation of apoptotic death [5].
The basic unit of chromatin, known as a nucleosome, is composed of local wrapping of a short stretch of double stranded (ds) DNA (147 bp in length) around an octameric histone protein core of two molecules each of histones H2A, H2B, H3 and H4 (known as nucleosome core particle (NCP)), and the so-called linker DNA that varies between 10 and 100 bp and connects neighboring nucleosomes in a chain like pattern [6].
Apoptosis or programmed cell death has evolved in multicellular organisms to remodel tissue during development. It maintains tissue homeostasis (proliferation and apoptosis balance) by removing senescent cells and deleting cells with irreparable genetic damage. It is a highly regulated process with distinct morphologic and biochemical features [7,8].
The mechanism of apoptosis in tumors is unclear. However, it is known to be p53 and caspases dependent [9], a hallmark of cancer cells [10]. During apoptosis, caspases are activated leading to degradation of cell constituents [11]. High levels of circulating mono- and oligo-nucleosomal fragments are expected. In blood, circulating nucleic acids are not digested by endonucleases due to their close association with histone protein. High levels of circulating DNA fragments have been found in the blood of patients with most types of malignancy including colorectal, lung, gastrointestinal, breast, gynecological, renal, and nasopharyngeal cancers as well as lymphoma [12,13].
For colorectal cancer many biomarkers have been assessed but only a small number have been recommended for clinical use. The aim of the present study was to compare the circulating levels of nucleosomes, CEA and CA 19-9 as apoptotic markers of colorectal carcinoma and to determine which one is more specific and sensitive for clinical use.
CEA is a product of columnar and goblet cells in the normal colon cells as well as colonic cancer cells with a half life of 3-11 days. It is a glycoprotein with a molecular weight of 200 kDa; most of its carbohydrate content is composed of mannose, galactose, N-acetylglucosamine, fructose and sialic acid [14]. The serum levels of CEA may increase 4.5 to 8 months before the development of cancer symptoms. Therefore, CEA monitoring is the most cost-effective indicator for the disease [15].
CA 19-9 is an antigen originally isolated for the first time from human colorectal carcinoma, which is identified by monoclonal antibody designated 19-9. It was postulated that CA 19-9 could not be recommended for early diagnosis of colorectal carcinoma and its serial determination appears to provide little information to that of CEA in monitoring patients [16].
Methods
The present study was conducted in patients with colorectal cancer admitted to the Gastroenterology Department of EI-Minia University Hospital. Twenty four patients were included in the study (ten females and fourteen males) with ages ranging from 34 to 72 years (mean 46.9 ± 15.6). Six normal subjects were selected as a control group. All patients proved to have colorectal carcinoma by history, examination, investigations and biopsy. The blood samples were collected before surgical interference.
The circulating levels of nucleosomes were estimated by cell death detection ELISA (CDDE)plus supplied from Roche Diagnostic-Germany. CEA and CA 19-9 serum levels were determined by electrochemi-luminescence immunoassay (ECLISA) on Roche Elecsys 1010 immunoassay analyzer. The kits were used according to the manufacturer's instructions. The chemicals were supplied by Roche Diagnostics GmbH, D 68298, Mannheim, Germany. The patients were classified into three groups, nine patients with well differentiated tumor, nine patients with moderately differentiated tumor and six patients with poorly differentiated tumor according to The Modified Dukes classification of Astler and Coller [17].
Statistical methods
To test for normal distribution, frequency of data was plotted against normal distribution curve. Nonparametric statistical methods were used. Frequency, median, range and standard error of means were used to describe data. Kruskal-Wallis, nonparametric analysis of variance was used to test for variability between groups in quantitative variables while, Mann-Whitney u test was used to test for significance of difference in quantitative variables between each two groups. Nonparametric Kendall's correlation was used to test for linear relationship between different quantitative variables. Regression analysis was used for further analysis of the linear relationship between nucleosomes, CEA and CA 19-9. Receiver operating characteristic (ROC) curve analysis was done using MedCalc software for evolution of sensitivity and specificity of different markers. P value was considered significant if less than 0.05. These tests were run on an IBM compatible personal computer using the Statistical Package for Social Scientists (SPSS) for windows 7.5 (SPSS Inc., Chicago, IL, USA).
Results
Table (1) shows range, median and SE of the studied parameters in control versus patient groups (well, moderately and poorly differentiated tumors). There is significant increase in nucleosomes levels in all groups versus the control group (P < 0.001). CEA levels show a significant increase in all groups except the poorly differentiated tumor group versus the control group (P < 0.001 for the well differentiated tumor group, 0.037 for the moderately differentiated tumor group and 0.106 for the poorly differentiated tumor group). There is no significant increase in the levels of nucleosomes, also, no significant decrease in the levels of CEA with decreased grade of tumor differentiation. There is significant increase in the levels of nucleosomes in poor differentiated versus well differentiated tumors (p = 0.041).
Table 1.
Comparison between serum levels of nucleosomes, CEA and CA 19-9 in the three patient groups versus the control group and each group of patients versus the other groups
| Parameters Groups |
Nucleosomes (AU) |
CEA (ng/ml) |
CA 19-9 U/ml |
||
|---|---|---|---|---|---|
| Control group (n = 6) | Median | 282.6 | 2.3 | 20.9 | |
| S.E ± | 26.4 | 0.39 | 3.2 | ||
| Range | Min. | 181.5 | 0.8 | 7.8 | |
| Max. | 519.2 | 4.2 | 41.4 | ||
| Group I Patients (well differentiated tumor) (n = 9) | Median | 1081 | 6.6 | 32.86 | |
| S.E ± | 209.5 | 9.00 | 101.6 | ||
| Range | Min. | 712.4 | 1.29 | 8.4 | |
| Max. | 3382.5 | 1729 | 1895 | ||
| Group II Patients | Median | 1776.2 | 4.61 | 23.9 | |
| (moderate differentiated tumor) (n = 9) | S.E ± | 171.3 | 75.6 | 64.6 | |
| Range | Min. | 772.2 | 1.6 | 1.6 | |
| Max. | 3426.9 | 2400 | 2246 | ||
| Group III | Median | 1860 | 4.6 | 34 | |
| Patients (poor differentiated tumor) (n = 6) | S.E ± | 260.6 | 6.26 | 44.66 | |
| Range | Min. | 1149.7 | 1.72 | 3.16 | |
| Max. | 3482.5 | 52.6 | 386.2 | ||
| P control versus group I | <0.001 | <0.001 | 0961 | ||
| P control versus group II | <0.001 | 0.037 | 0.666 | ||
| P control versus group III | <0.001 | 0.106 | 0.816 | ||
| P group I versus group II | 0.086 | 0.464 | 0.116 | ||
| P group I versus group III | 0.041 | 0.569 | 0.450 | ||
| P group II versus group III | 0.566 | 0.861 | 0.627 | ||
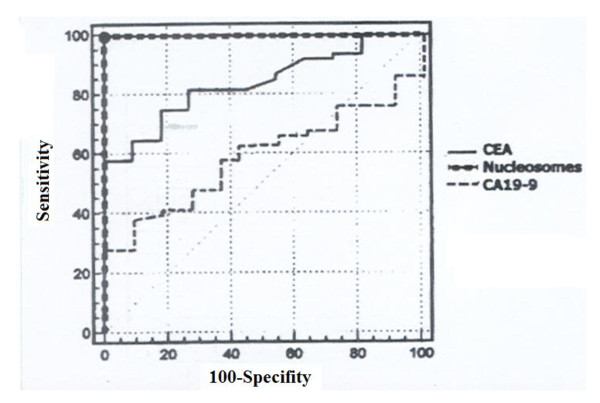
The overall positive rates obtained from ROC curve (Figure 1) of circulating CEA and CA 19-9 (using the cutoff values of 3.56 ng/ml and 28 U/ml, respectively) were 56.2% and 36.4% (Table 2).
Figure 1.
Receiver operating characteristic curve (ROC) of nucleosomes, CEA and CA 19-9 in colorectal carcinoma diagnosis.
Table 2.
Statistical evaluation of nucleosomes compared to CEA and CA 19-9 for colorectal carcinoma
| Parameter | Cutoff value | Sensitivity % | Specificity % | J index | Accuracy % | Kappa | PPV % | NPV % | ROC curve area |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Value | P | |||||||||
| CA 19-9 | 28 u/ml | 36.4 | 88.9 | 26 | 41 | 0.121 | 0.088 | 91.56 | 21.62 | 0.524 |
| CEA | 3.56 ng/ml | 56.2 | 100 | 55.8 | 62.2 | 0.290 | <0.001 | 100 | 30.40 | 0.808 |
| Nucleosomes | 421 | 100 | 100 | 100 | 100 | 1.00 | <0.001 | 100 | 100 | 1.0 |
Table (2) shows statistical evaluation of nucleosomes in comparison to CEA and CA 19-9 for colorectal carcinoma. By all statistical parameters, nucleosomes show the best values. As regards kappa it shows an excellent agreement between laboratory and actual diagnosis for nucleosomes while the values of CEA and CA 19 -9 show only weak agreement.
The curve shows the true positive rate (sensitivity) plotted versus false positive rate (100-specificity) for different cutoff points for nucleosomes, CEA and CA 19-9.
Discussion
Nuclear fragmentation as one of the morphologic features of apoptosis, results in a characteristic pattern of DNA complexed with histone proteins known as nucleosomes [18]. The measurement of these nucleosomes constitutes a feasible parameter for late stage of apoptosis [19]. Circulating nucleosomes can be quantified by real time PCR of the DNA. Moreover they can be estimated by stable immunologic assays that are particularly well suited for serial measurements.
CEA is a member of the immunoglobulin superfamily which was originally identified in human fetal colon and colorectal cancer. It is widely used as a tumor marker. However, little is known about its function except that it acts as a homotypic adhesion molecule that is implicated in cell aggregation [20]. It is over-expressed in numerous human cancers where it is present on the surface of cancer cells. It was reported that the over expression of CEA can protect cancer cells from apoptosis while a decrease in expression might lead to new approaches for management of cancer colon and other organs [21]. CEA is produced by more than 90% of colorectal cancers and contributes to the malignant characteristics of this type of cancer [22].
Although colorectal cancer screening is recommended for persons over 50 years and older its use is still low especially among older individuals [23].
An ideal tumor marker would be inexpensive screening that may help for early diagnosis in population at risk of cancer. Unfortunately currently available serological markers for colorectal carcinoma have not proven to be ideal [24].
The levels of serum nucleosomes show significant increase in patients as compared to control group (Table 1) with excellent discrimination between the two (area under curve equals 1 in table 2) that agrees with other published data [25]. There is an association of high nucleosomes levels with advanced stages of colorectal carcinoma. This may be due to a delayed clearance of nucleosomes from circulation where the bulky tumor tends to undergo peripheral apoptosis and central necrosis that in its turn would elicit local and systemic inflammatory responses and hence higher nucleosomes production [26].
The relationship between apoptosis and the degree of cell differentiation may play an important role in the susceptibility of the tumor cells to apoptosis [27]. An immature phenotype represents a block in the normal differentiation pathway [28]. Also, another study observed that the more the colon cells are stimulated to differentiate, the less likely they are to proliferate and hypothetically, the higher their rate of apoptosis [29].
After complete tumor resection of colorectal cancer, it was noticed that the level of nucleosomes increased in most patients rapidly reaching a maximum level during the first day. This was followed by a subsequent decrease, while the level was lower than in patients with postoperative or relapse therapy [30].
In the current study, the overall positive rates obtained from ROC curve (Figure 1) of circulating CEA and CA 19-9 (using the cutoff values of 3.56 ng/ml and 28 U/ml, respectively) were 56.2% and 36.4% (Table 2). These levels are higher than those reported by Zheng et al [24] who gave positive values of 29.2% and 25.2% for CEA and CA 19-9, their cutoff values of 5 ug/l and 31 Ku/l respectively. While the positive rates reported by Chan and Sell,[31], were 70% and 30% for CEA and CA 19-9, the cutoff values were of 3.5 ug/l and 37 Ku/l, respectively. CEA in the current study can provide a better discrimination between control subjects and patients than CA 19-9 (the areas under the ROC curve were 0.808 and 0.524 for CEA and CA 19-9, respectively).
Table (1) shows no significant difference in the levels of CA 19-9 in patients as compared to the control group while CEA shows a difference. Bhatnagar et al [32] noticed that in the well differentiated colorectal carcinoma there is more production of CEA/gram of total protein than in the poorly differentiated tumors in agreement with results obtained in the present study. On the other hand, it was observed that there is no significant correlation between levels of serum CA 19-9 and CEA [22] and the differentiation degree of the tumor.
Conclusion
In conclusion, our study confirms that the levels of nucleosomes provide highly specific and sensitive apoptotic marker for colorectal carcinoma which should be applied on a large scale of cancers with respect to clinicopathological variables. It can be used - for diagnosis, screening, prognosis, and in therapy monitoring.
List of abbreviations
CEA: Carcinoembryonic antigen; AFP: Alphafetoprotein; PSA: Prostate specific antigen; NCP: Nucleosome core particle; ECLISA: Electrochemi-luminescence immunoassay; ROC: Receiver operating characteristic.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors contributed equally to this work and read and approved the final manuscript.
Contributor Information
Jehad M Al-Shuneigat, Email: Dr.Jehad@mutah.edu.jo.
Samir S Mahgoub, Email: samir_mhgb@yahoo.com.
Fazlul Huq, Email: Fazlul.Huq@sydney.edu.au.
References
- Chan CC, Fan CW, Kuo YB, Chen YH, Chang PY, Chen KT, Hung RP, Chan EC. Multiple serological biomarkers for colorectal cancer detection. Int J Cancer. 2010;126:1683–1690. doi: 10.1002/ijc.24912. [DOI] [PubMed] [Google Scholar]
- Zhao-Hut H, Li-Hua L, Fan Y, Jin-Fu W. Detection of apparent methylation in fecal DNA as a molecular screening tool for colorectal cancer and precancerous lesions. World J Gastroenterol. 2007;14:950–954. doi: 10.3748/wjg.v13.i6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Esteva M, Cabeza E, Campillo C, Llobera J, Aguilo A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: A review. European journal of cancer. 2007;43:2467–2478. doi: 10.1016/j.ejca.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Ahlquist DA. Molecular Detection of Colorectal Neoplasia. Gastroenterology. 2010;138:2127–2139. doi: 10.1053/j.gastro.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Osaka A, Hasegawa H, Yamada Y, Yanagihara K, Hayashi T, Mine M, Aoyama M, Sawada T. A novel role of serum cytochrome c as a tumor marker in patients with operable cancer. J Cancer Res Clin Oncol. 2009;135:371–377. doi: 10.1007/s00432-008-0479-y. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Stieber P, Boden MH, Busch M, Pawel JV, Schalhom A, Nagel D, Seidel D. Circulating nucleosomes in serum. Ann N Y Acad Sci. 2001;945:93–102. doi: 10.1111/j.1749-6632.2001.tb03869.x. [DOI] [PubMed] [Google Scholar]
- Tan ML, Ooi JP, Ismail N, Moad AIH, Muhammad TST. Programmed Cell Death Pathways and Current Antitumor Targets. Pharmaceutical Research. 2009;26:1547–1560. doi: 10.1007/s11095-009-9895-1. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–774. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Aleman MJ, De Young MP, Tress M, Ketting P, Perry GW, Nara YR. Inhibition of single minded 2 gene expression mediates tumor - selective apoptosis and differentiation in human colon cancer cells. Proc Natl Acad Sci. 2005;102:12765–12770. doi: 10.1073/pnas.0505484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle-Bonnet M, Garrouste F, Baillat G, Andre F, Marvaldi J, Pommier G. Membrane rafts segregate pro-and anti-apoptotic insulin like growth factor- 1 receptor signaling in colon carcinoma cells stimulated by membranes of tumor necrosis factor superfamily. Am J Pathol. 2005;167:761–773. doi: 10.1016/S0002-9440(10)62049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D, Volkmann X, Anstatt M, Langer F, Manns MP, Schulze-Osthoff K, Bantel H. Serum biomarkers of cell death for monitoring therapy response of gastrointestinal carcinomas. European journal of cancer. 2010;4:1464–1473. doi: 10.1016/j.ejca.2010.01.037. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Stieber P. Apoptotic markers in cancer. Clinical Biochemistry. 2004;37:605–617. doi: 10.1016/j.clinbiochem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhuijze AEM, Van Lopik T, Smeenk RJT, Aarden LA. Time between onset of apoptosis and release of nucleosomes from apoptotic cells: putative implications for systemic lupus erythematosus. Ann Rheum Dis. 2003;62:10–14. doi: 10.1136/ard.62.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier J, Nicolson GL. The role of tumor cell adhesion as an important factor in formation of distant colorectal metastasis. Dis Colon Rectum. 2001;44:876–884. doi: 10.1007/BF02234713. [DOI] [PubMed] [Google Scholar]
- Flamen P, Hoekstra OS, Homans F, Van Cutsem E, Maes A, Stroobants S, Peeters M, Penninckx F, Filez L, Bleichrodt RP, Mortelmans L. Unexplained rising carcinoembryonic antigen (CEA) in the postoperative surveillance of colorectal cancer: the utility of positron emission tomography (PET) European Journal of Cancer. 2001;37:862–869. doi: 10.1016/S0959-8049(01)00049-1. [DOI] [PubMed] [Google Scholar]
- Duffy MJ, Van Dalen A, Haglund C, Hansson L, Klapdof R, Lamerz R, Nilsson O, Sturgron C, Toplocan O. Clinical utility of biochemical markers of CRC: European Group on Tumor marker (EGTM) guidelines. Eur J Cancer. 2003;39:718–727. doi: 10.1016/S0959-8049(02)00811-0. [DOI] [PubMed] [Google Scholar]
- Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Sur. 1954;139:846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PN, Smith AD. Biochemistry illustrated. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- Zeerleder S, Zwart B, Wuillemin WA, Aarden LA, Groeneveld ABJ, Caliezit C, Van Nieuwenhuijze AEM, Van Mierlo GJ, Eerenberg AJM, Lammle B, Hack CE. Elevated nucleosome levels in systemic inflammation and sepsis. Crit Care Med. 2003;31:1947–1951. doi: 10.1097/01.CCM.0000074719.40109.95. [DOI] [PubMed] [Google Scholar]
- Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as intercellular molecule. Cell. 1989;57:327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- Ueda K, Iwahashi M, Nakamori M. Improvement of carcinoembryonic antigen-specific prodrug gene therapy for experimental.cancer colon. Surgery. 2003;133:309–317. doi: 10.1067/msy.2003.73. [DOI] [PubMed] [Google Scholar]
- He Z, Shi C, Wen H, Li F, Wang B, Wang J. The potential of carcinoembryonic antigen, p53, Ki-67 and glutathione S-transferase-π as clinico-histopathological markers for colorectal cancer. Journal of Biomedical Research. 2010;24:51–57. doi: 10.1016/S1674-8301(10)60008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroukian SM, Xu F, Dor A, Woper GS. Colorectal cancer screening in the elderly population: disparities by dual Medicare - Medicaid enrollment status. Health Ser Res. 2006;41:2136–2154. doi: 10.1111/j.1475-6773.2006.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CX, Zhan WH, Zhao JZ, Zheng D, Wang DP, He YL, Zheng ZQ. The prognostic value of pre-operative serum levels of CEA, CA 19-9 and CA 72-4 in patients with colorectal cancer. World J Gastroenterol. 2001;7:431–434. doi: 10.3748/wjg.v7.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdenrieder S, Nagel D, Schalhorn A, Heinemann V, Wilkowski R, Von Pawel J, Raith H, Feldmann K, Kremer AE, Muller S, Geiger S, Hamann GF, Seidel D, Stieber P. Clinical Relevance of Circulating Nucleosomes in Cancer. Ann N Y Acad Sci. 2008;1137:180–189. doi: 10.1196/annals.1448.012. [DOI] [PubMed] [Google Scholar]
- Wigmore SJ, McMahon AJ, Sturgeon CM, Fearon CH. Acute-phase protein response, survival and tumor recurrence in patients with colorectal cancer. Br J Surg. 2001;88:255–260. doi: 10.1046/j.1365-2168.2001.01669.x. [DOI] [PubMed] [Google Scholar]
- Shoji Y, Saegusa M, Tanako Y, Ohbu M, Okayasu I. Correlation of apoptosis with tumor cell differentiation, progression and HPV infection in cervical carcinoma. J Clin Pathol. 1996;49:134–138. doi: 10.1136/jcp.49.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, Hickman JA. Differentiation: A suitable strategy for cancer chemotherapy. Anticancer drug Des. 1993;8:299–322. [PubMed] [Google Scholar]
- Jenab M, Thompson LU. Phytic acid in wheat bran affects colon morphology, cell differentiation and apoptosis. Carcinogenesis. 2000;21:1547–1552. doi: 10.1093/carcin/21.8.1547. [DOI] [PubMed] [Google Scholar]
- Andreas K, Stefan H, Petra S, Ralf W, Dorothea N, Dietrich S. Nucleosomes in colorectal cancer patients during radiochemotherapy. Tumor Biology. 2006;27:235–242. doi: 10.1159/000094694. [DOI] [PubMed] [Google Scholar]
- Chan DW, Sell S. In: Tietz Textbook of Clinical Chemistry. Burtis CA, Ashwood ER, editor. Philadelphia: W B Saunders Company; 1999. Tumor markers; pp. 722–724. [Google Scholar]
- Bhatnagar J, Tewari H, Bhatnagar M, Austin GE. Comparison of carcinoembryonic antigen in tissue and serum with grade and stage of colon cancer. Anticancer Res. 1999;19:2181–2187. [PubMed] [Google Scholar]