Abstract
Noninfectious uveitis is a leading cause of blindness and is thought to involve autoimmune T cell responses to retinal proteins, e.g., retinal arrestin (S-Ag). There are no known biomarkers for the disease. Susceptibility is associated with HLA, but little is known about susceptible class II alleles or the potentially pathogenic epitopes that they present. Using a ‘humanized’ HLA-transgenic mouse model of S-Ag induced autoimmune uveitis, we identified several susceptible and resistant alleles of HLA-DR and –DQ genes and defined pathogenic epitopes of S-Ag presented by the susceptible alleles. The sequences of these epitopes overlap with some previously identified peptides of S-Ag (“M” and “N”), known to elicit memory responses in lymphocytes of uveitis patients. HLA-DR-restricted, S-Ag-specific CD4+ T cells could be detected in blood and draining lymph nodes of uveitic mice with HLA class II tetramers and transferred the disease to healthy mice. Importantly, tetramer-positive cells were detected in peripheral blood of a uveitis patient. These findings provide the first tangible evidence that an autoimmune response to retina is causally involved in pathogenesis of human uveitis, demonstrate the feasibility of identifying and isolating retinal antigen-specific T cells from uveitis patients and may facilitate their development as biomarkers for the disease.
Keywords: HLA class II, Uveitis, S-antigen, Arrestin, Autoimmune biomarker
Introduction
Uveitis is a major cause of blindness, and the primary manifestation in numerous ocular and systemic diseases. Strong HLA associations have been identified in various ethnic groups for a number of these diseases that include both HLA class I and class II alleles (1, 2)(Table I).
Table I.
HLA gene associations in autoimmune uveitic syndromes
| Disease | Ethnic group | HLA Genes (Relative risk) | References* |
|---|---|---|---|
| Birdshot Retinochoroidopathy | Caucasian | A29 (49.9 – 224.35) | (3–5) |
| Behcet's Disease | Caucasian, Oriental | B51 (4.0 – 18.2) | (6–8) |
| Anterior uveitis in Ankylosing Spondylitis | Caucasian | B27 (3.8 – 20.0) DR8 (34.3) |
(9–11) |
| Vogt-Koyanagi-Harada Disease | Oriental | DR4 (46.7 – 74.5), DQA3 (12.0), DQ4 (18.9 – 41.3), DP5 (3.8) |
(12, 13) |
| Sympathetic Ophthalmia | various | DR4 (5.6 – 13.7), DQA3 (3.9 – 12.8) |
(14, 15) |
| Intermediate Uveitis (unrelated to MS) | Caucasian | DR3 (Not reported) | (16) |
| Pars Planitis in Multiple Sclerosis | Caucasian | DR2 (3.2) DR51 (4.8) DR17 (14.3) |
(17) |
Peripheral blood mononuclear cells (PBMC) from uveitis patients respond to various ocular autoantigens in culture (18), with responses most often being directed against retinal arrestin (also known as Soluble antigen or S-Ag) and occasionally against interphotoreceptor retinoid binding protein (IRBP). A role for these responses in pathogenesis of uveitis is supported by a clinical study in which patients fed with S-Ag to induce oral tolerance had a positive clinical outcome (19). Responses to peptide fragments of human S-Ag have been studied using PBMC from uveitis patients (20), however, presence of in vitro proliferative or cytokine responses, while suggestive, is not sufficient to establish a causal relationship with disease. Furthermore, the patients had not been HLA-typed, so there is no information regarding restriction by particular HLA haplotypes.
Experimental Autoimmune Uveitis (EAU) is an animal model that mimics human uveitis (21). EAU can be induced in rodents by immunization of genetically susceptible strains with various retinal proteins (22). S-Ag as well as IRBP are pathogenic in Lewis rats, whereas IRBP is the stronger uveitogen in mice (23). Although the “classical” EAU model in mice and rats has helped to study the cellular and molecular mechanisms of uveitis, it cannot help to identify the antigenic epitopes that might be involved in human disease because rodent MHC molecules bind and present different antigenic epitopes as compared to humans. To address this, we developed a ‘humanized’ HLA-Tg model of EAU in mice that are transgenic for human class II molecules and deleted for mouse class II, and showed that, in contrast to their parental wild type strains, mice expressing HLA-DR3 (*0301) were highly susceptible to uveitis caused by S-Ag (24).
In the present study, we have not only identified DRB1*0402 and DQ8*0302 (DQA1*0301/DQB1*0302) as additional susceptible HLA class II alleles, but also demonstrated that some HLA alleles that confer higher risk for other autoimmune diseases are protective in uveitis, eg., HLA-DR2 alleles and the -DR4(0401) allele. Importantly, we have defined the uveitogenic S-Ag epitopes recognized by each of these HLA alleles using bioinformatic predictions and biological testing. It is of note that the HLA associated uveitogenic epitopes identified in our study overlap with the sequences of peptide M and peptide N of the bovine S-Ag that were previously reported to elicit recall responses in lymphocytes from uveitis patients (25, 26). HLA-DR3 tetramers loaded with a pathogenic epitope of S-Ag could detect antigen specific autoreactive T cells in draining lymph node cells of uveitic mice, and sorted tetramer-labeled cells could transfer EAU to unimmunized mice. Importantly, as proof of concept, the same tetramer could specifically detect antigen reactive T cells in the PBMCs from an HLA-DR3+ uveitis patient. These findings provide tangible evidence that autoimmune responses to retina restricted by human MHC class II are pathogenic and provide new insights into the biology of uveitis. Our data also suggest that epitope-specific HLA class II tetramer reagents could be developed as a biomarker for diagnosis and follow-up of human uveitis, and could potentially be useful in development of “personalized” immunotherapies adapted to particular MHC haplotypes.
Materials and Methods
Animals
HLA transgenic mice expressing alleles DRβ1*1501, -DRβ1*1502, -DRβ1*1503, -DRβ1*0301, -DRβ1*0401, -DRβ1*0402, -DQA1*0103/DQB1*0601 (DQ6), and DQA1*0301/DQB1*0302 (DQ8) were developed at Mayo Clinic, Rochester, Minnesota, as previously described (27–30). Transgenic lines were bred and maintained at the Mayo clinic or at National Institutes of Health under specific pathogen free conditions. The care and use of the animals complied with guidelines of the Institutional Animal Care and Use Committee and the Association for Research in Vision and Ophthalmology.
Patients
Research performed in this study was in compliance with guidelines of the National Institutes of Health Institutional Review Board and all procedures conformed to the tenets of the Declaration of Helsinki. Patients with well-defined clinical diagnosis of non-infectious uveitis enrolled into the National Eye Institute IRB protocol no. 03-EI-0122 were selected based on their HLA type. Informed consent was obtained from all patients for blood sampling. HLA typing was performed at the Department of Transfusion Medicine, National Institutes of Health using standard molecular typing techniques.
Antigens
Native S-Ag and IRBP were purified from bovine retinal tissue using published protocols (31, 32) with minor modifications (33). Twenty residue peptides overlapping by 10 residues and corresponding to the entire length of human S-Ag sequence (Suppl. Table I), as well as peptide ‘M’ (DTNLASSTIIKEGIDKTV), peptide ‘N’ (LLANNRERRGIALDGKIKHE), and truncated versions of peptide ‘N’ (N281–N290, Table II) were commercially synthesized by conventional solid phase technique using t-butyloxycarbonyl derivatives of the amino acids (aa) (Applied Biosystems, Foster City, CA) (34).
Table II.
Amino acid sequences of truncated versions of ‘Peptide N’ and the average score of EAU induced by these peptides in HLA-DR3 Tg mice.
| Peptide ID residues | Amino acid sequence | Average EAU | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N - hSA 287–306 | L | L | A | N | N | R | E | R | R | G | I | A | L | D | G | K | I | K | H | E | 1.14 ± 1.06 |
| N281 - hSA 289–306 | A | N | N | R | E | R | R | G | I | A | L | D | G | K | I | K | H | E | 0.48 ± 0.27 | ||
| N282 - hSA 291–306 | N | R | E | R | R | G | I | A | L | D | G | K | I | K | H | E | 1.38 ± 1.64 | ||||
| N283 - hSA 293–306 | E | R | R | G | I | A | L | D | G | K | I | K | H | E | 0.83 ± 1.16 | ||||||
| N284 - hSA 295–306 | R | G | I | A | L | D | G | K | I | K | H | E | 0.62 ± 0.88 | ||||||||
| N285 - hSA 297–306 | I | A | L | D | G | K | I | K | H | E | 0.09 ± 0.19 | ||||||||||
| N286 - hSA 291–300 | N | R | E | R | R | G | I | A | L | D | ND | ||||||||||
| N287 - hSA 290–301 | N | N | R | E | R | R | G | I | A | L | D | G | 0.0 ± 0.0 | ||||||||
| N288 - hSA 289–302 | A | N | N | R | E | R | R | G | I | A | L | D | G | K | 0.0 ± 0.0 | ||||||
| N289 - hSA 288–303 | L | A | N | N | R | E | R | R | G | I | A | L | D | G | K | I | 0.02 ± 0.07 | ||||
| N290 - hSA 287–304 | L | L | A | N | N | R | E | R | R | G | I | A | L | D | G | K | I | K | 0.89 ± 1.03 | ||
Induction of EAU and disease scoring
Mice were immunized subcutaneously with 150 µg of the specified antigens emulsified 1:1 v/v with Complete Freund’s Adjuvant (Sigma-Aldrich) containing 2.5 mg/ml of M. tuberculosis H37RA (Sigma-Aldrich) in a total volume of 200 µl, divided among both thighs and base of tail, and were given 0.4 µg pertussis toxin (#P7208, Sigma-Aldrich, USA) intraperitoneally (i/p) in 100 µl RPMI + 1% mouse serum. The progression of disease was monitored by fundus examination and eyes were collected for histopathological confirmation at the end of the experiment (day 30–32). Severity of EAU was scored on a scale of 0–4 based on the degree of inflammation in the posterior uvea and the damage to the photoreceptor layer, using previously described criteria (21).
Quantitation of antigen specific lymphocyte proliferation and cytokine release
Cells from spleen and lymph nodes (LN) draining the site of immunization (iliac and inguinal) were collected from immunized animals 32–34 days after immunization and cultured with the immunizing antigens as described (23). Briefly, 5 × 105 cells/well were cultured in 200µl of RPMI-1640 medium (Gibco, Paisley, UK), supplemented with 1% normal mouse serum, mercaptoethanol, antibiotics, glutamine, and non-essential amino acids with native S-Ag (20µg/ml or as specified) or with synthetic peptides (25µg/ml) for a total of 72 hours and pulsed with 3H-thymidine during the last 16–18 hours of incubation. Isotope uptake was determined by liquid scintillation counting. Cytokine production was measured in 48 hrs culture supernatants (1 × 106 cells/well in a total of 200µl HL-1 medium, Lonza Inc, Walkersville, MD), by multiplex ELISA using the Search Light Technology (Aushon Biosystems, Billerica, MA).
Protein sequence alignment and epitope prediction
The S-Ag (retinal S-Arrestin) protein sequences from human (NP_000532), bovine (NP_851343) and murine (NP_033144) species were aligned by global multiple sequence alignment method using ‘MultAlin’ (35) and by ClustalW2 (36). T cell epitopes of the human S-Ag sequence for the HLA-DRB1*0301 (DR17), -DRB1*0402, and –DQ8 alleles were predicted using the online epitope prediction tools PROPRED (37) and RANKPEP (38).
Expansion and adoptive transfer of T cells
HLA transgenic mice were immunized with S-Ag in CFA as described above for EAU. After 10 days a booster dose of 150µg of S-Ag emulsion was given in incomplete Freund’s adjuvant (Sigma Aldrich) (100µl on the nape and 50µl on either forelimbs). Ten to 12 days later peripheral blood and draining LN were collected. Single cell suspensions of lymphocytes were made from draining LN and from peripheral blood, the latter after lysing red blood cells using ACK lysis buffer (Lonza). LN cells were purified using CD4+ T cell isolation kit on an autoMACS™ Pro (Miltenyi Biotec Inc. CA). Cells (4 – 5 million/2ml/well) were stimulated for 2 days in 24-well Linbro plates (Hampton Research, Aliso Viejo, CA) with 20 µg/ml full length peptide N (LLANNRERRGIALDGKIKHE) or peptide N282 (NRERRGIALDGKIKHE) in DMEM (Lonza) supplemented with 100U/ml Penicillin, 100µg/ml Streptomycin, and 100U/ml IL-2 (‘Proleukin’ Novartis Pharmaceuticals, East Hanover, NJ) 10% fetal calf serum (FCS) (Lonza), 10% rat spleen conditioned medium (SCM, supernatant from Con-A stimulated rat splenocytes), 10ng/ml IL-12 (Peprotech Inc, Rocky Hill, NJ), and 10µg/ml anti-IL-4 (clone 11B.11, Biological Research Branch, NCI, Frederick, MD). Five million irradiated (3000 rads) naïve syngeneic splenocytes were added per well as antigen presenting cells. Cultures were incubated at 37°C and 10% CO2. Following two-day antigenic stimulation cells were split using medium with 10% SCM without peptide to maintain a concentration of approximately 4–5 million cells/well, and expanded for 7–8 days with partial medium changes every other day. Additional rounds of stimulation were performed in the same way. At the end of fourth cycle, cells were stained with peptide-loaded HLA-DR3 tetramer-PE, anti-TCRβ-FITC and anti-CD4-APC antibodies as described below. Tetramer and TCR double-positive cells from the CD4+ population were sorted using a BD FACS Aria (BD Bioscience, CA) into DMEM medium containing 20% FCS and antibiotics. The purity of sorted cells was at least 70% tetramer-positive cells out of the CD4+ lymphocyte population. Sorted cells were restimulated for two days with peptide N282 (NRERRGIALDGKIKHE) and 5 million cells (after removing irradiated splenocytes and dead cells by Ficoll purification) were injected intraperitoneally into recipient HLA-DR3 transgenic mice. Animals were evaluated by fundus examination. Eyes were collected for histopathology on day 15 after transfer.
HLA-DRB1*(0301) Tetramer staining of HLA-DR3 transgenic mouse cells
The generation of soluble HLA-DRA1*0101 /B1*0301 molecules, and the procedure for loading the peptides have been described (39). Peptide N282 of S-Ag that was uveitogenic in HLA-DR3(0301) strain (Table II), was used to generate HLA-DR3 tetramers (Benaroya Research Institute at Virginia Mason, Seattle, WA). Cells were stained with phycoerythrin (PE)-labeled tetramers (0.5µg of / 0.5 × 106 cells in 50µl HL-1 medium [Lonza]), in presence of 1µg Fc block (BD Bioscience, NJ) for one hour at 37°C, followed by surface staining for 20 minutes on ice with anti-TCRβ-fluorescein isothiocyanate (FITC) and anti-CD4-allophycocyanin (APC) (BD Bioscience, NJ). To exclude dead cells, 5µl of 7-AAD (BD Bioscience, NJ) was added to each sample 10 minutes before analysis. Seventy five thousand events in the live lymphocyte gate were collected using a FACS Calibur flow cytometer and CellQuest II software (Becton Dickinson, Mountain view, CA) and were further analyzed for tetramer+, TCRβ+ CD4+ T cells using FlowJo 8.8.6 software (Treestar, Inc., CA).
Detection of tetramer-positive T cells in patient PBMC
PBMCs were isolated from peripheral blood of DR3+ uveitis patients using Histopaque-1077 (Sigma-Aldrich Inc. USA), washed and resuspended in RPMI medium (Lonza) containing 100U/ml Penicillin, 100µg/ml Streptomycin and 10% heat inactivated human AB serum (Sigma-Aldrich Inc. USA). Cells were cultured in a 24-well Linbro plate (Hampton Research, Aliso Viejo, CA) at 4 million cells/2ml/well in the presence of peptide, N282 (50µg/ml), 10µg/ml Anti-human CD28 (Cat. No. 302913, BioLegend, CA), and 1µg/ml Anti-human CD49d (Cat. No. 304309, BioLegend, CA) at 37°C and 5% CO2 for seven days. Cells from confluent wells were split using fresh culture medium. After 7 days, cells were collected, washed and re-stimulated with the peptide and co-stimulants as above. After day 10, culture medium was refreshed with medium supplemented with 10 IU/ml of recombinant human IL-2 (‘Proleukin’ Novartis Pharmaceuticals corp. NJ) every other day. The cells were stained with peptide N282 loaded DR3 tetramers as described above after 17 days in culture. Staining for T cell markers was done with anti-CD3 (FITC) and anti-CD4 (APC) (BD Bioscience, NJ).
Results
Susceptibility of HLA Class II transgenic mice to Uveitis
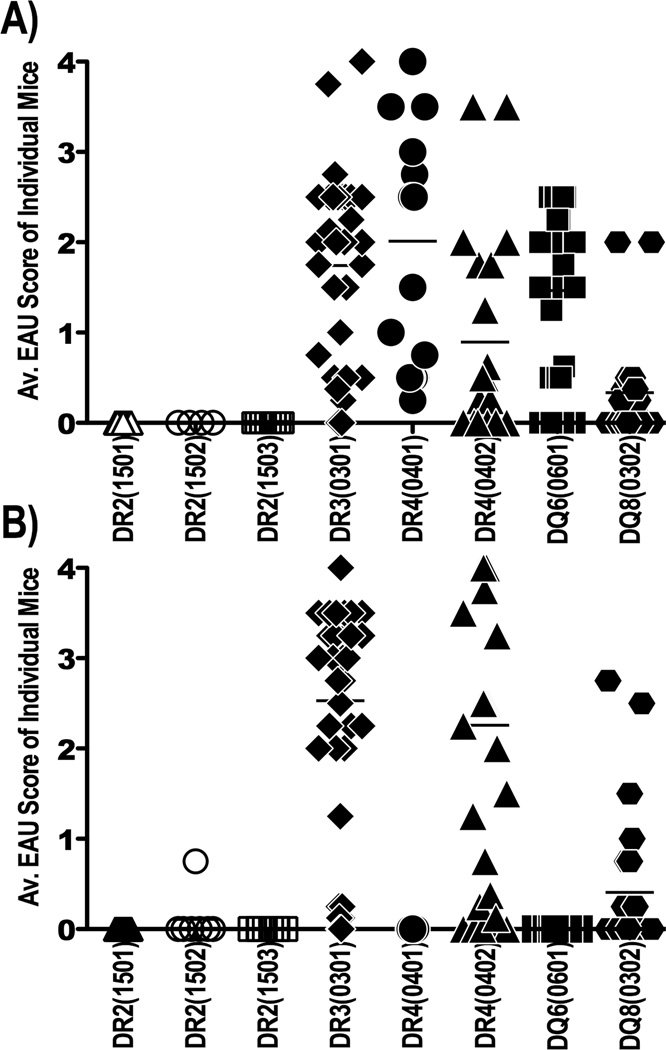
HLA class II transgenic (Tg) mice deleted for murine MHC class II molecules and expressing different HLA-DR or –DQ alleles were immunized with the uveitogenic retinal proteins IRBP and S-Ag. Among the eight HLA-Tg strains tested for susceptibility, most developed EAU after immunization with IRBP at varying degrees of severity (Figure 1A). However, susceptibility to S-Ag-induced uveitis was restricted to HLA-DR3(0301), HLA-DR4(0402) and HLA-DQ8 strains, with the HLA-DQ8 strain being the least susceptible of the three (Figure 1B). Interestingly, a minor difference in the amino acid sequence (3 residues) between the two allelic forms of HLA-DR4 was associated with a major difference in their susceptibility to S-Ag induced EAU. In contrast to HLA-DR4(0402), HLA-DR4(0401) Tg mice were resistant to EAU induced with S-Ag, although both were equally susceptible to EAU induced with IRBP (Figure 1). None of the three HLA-DR2 (DRB1*1501, *1502, and *1503) alleles conferred susceptibility to either IRBP or S-Ag. Because uveitis patients tend to have immunological responses to S-Ag rather than to IRBP (20), further studies concentrated on S-Ag induced responses.
Figure 1. Susceptibility to IRBP vs. S-Ag induced EAU in various HLA class II Tg mice.
The indicated strains of HLA transgenic mice were immunized with a uveitogenic protocol of either IRBP or S-Ag, as described in Methods. (A) IRBP induced EAU; (B) S-Ag induced EAU. The data are combined from five independent experiments. Shown is severity of EAU assessed by histopathological analysis of eyes collected 24 – 28 days after immunization. Each point represents one mouse (average score of both eyes). Horizontal one denotes the mean score of the group.
S-Antigen specific proliferation and cytokine profile
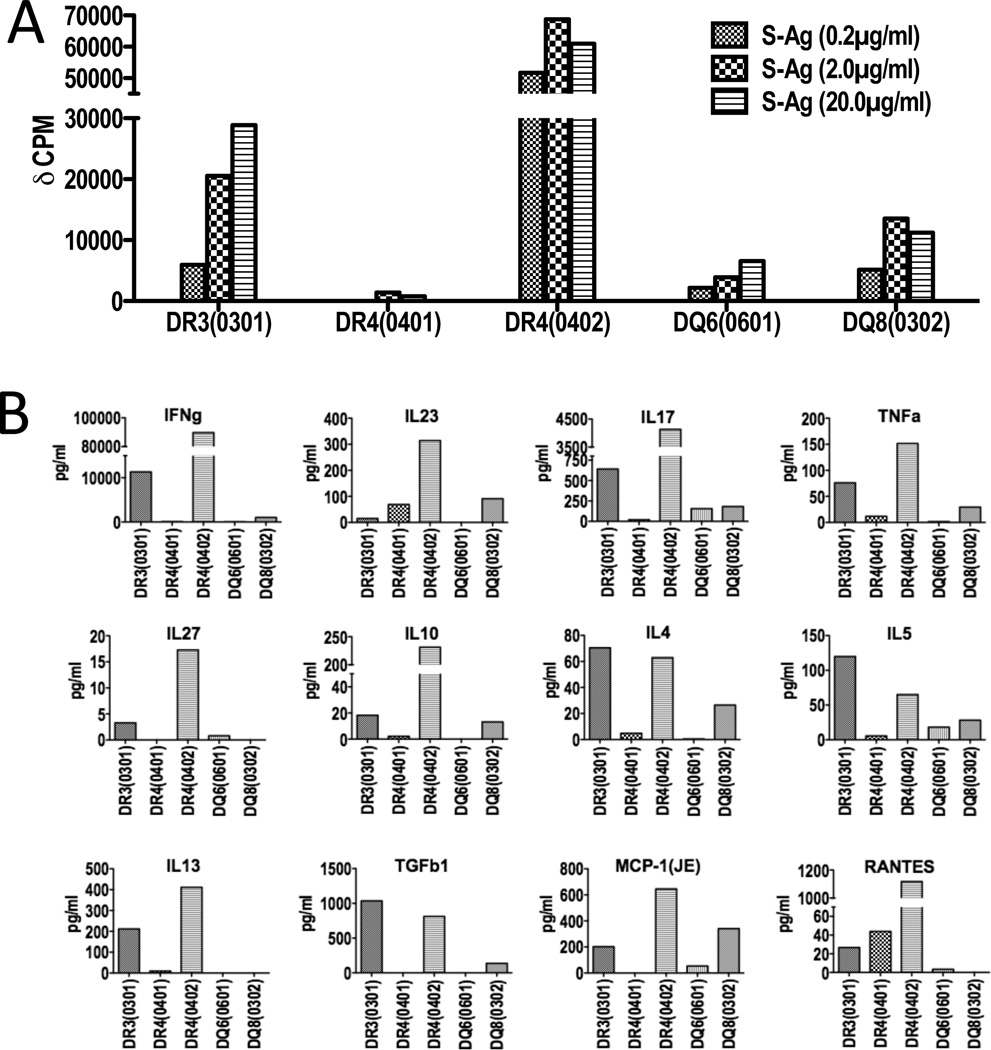
Draining LN cells of HLA-DR3, HLA-DR4(0402), and HLA-DQ8 transgenic mice immunized with a uveitogenic regimen of S-Ag proliferated in-vitro in response to S-Ag in a dose dependant manner (Figure 2A). Notably, antigen-specific proliferative responses were considerably higher in the susceptible strains, namely HLA-DR3 and HLA-DR4(0402), than in resistant strains such as HLA-DR4(0401) and HLA-DQ6(0601).
Figure 2. S-Ag specific T cell responses of immunized HLA class II Tg mice.
EAU susceptible and resistant HLA class II transgenic strains were immunized with a uveitogenic regimen of S-Ag. Proliferative and cytokine responses of cells from lymph nodes draining the site of immunization were examined. (A) Draining lymph node cells collected 30 days after immunization were cultured with serial dilutions of S-Ag. Average background H3 uptake by the cells in the absence of antigen was approximately 4000 cpm and is subtracted (representative data from one of two experiments). (B) Cytokine production was measured by multiplex ELISA, in cell culture supernatants collected 48 hrs after in-vitro antigenic stimulation. Antigen specific cytokine production is plotted after subtraction of the background levels in the absence of antigen (representative data from one of three experiments).
Th1 and Th17 cytokines have been reported to play a pathogenic role in various EAU models (40, 41) and both types of responses have been reported in human uveitis (42–44). We, therefore, evaluated the S-Ag specific cytokine production by LN cells from susceptible and resistant strains of HLA Tg mice with S-Ag-induced uveitis. As with proliferation, susceptible strains produced higher levels of both Th1 and Th17 type cytokines as compared to the resistant strains (Figure 2B). LN cells from HLA-DR4(0402) Tg mice also produced higher amounts of IL-27 and as well as the inflammatory chemokines MCP-1 and RANTES.
Epitopes of human S-Ag restricted by HLA-DR3, -DR4, and -DQ8 alleles
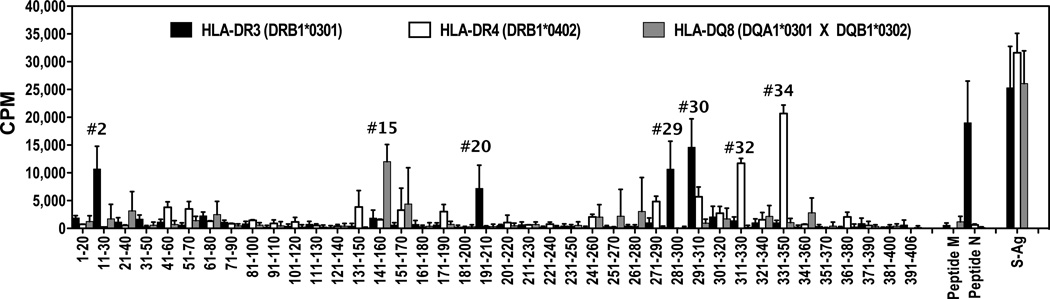
To identify S-Ag epitopes presented by susceptible HLA class II alleles, we used epitope prediction tools (PROPRED and RANKPEP) to identify the regions in the human S-Ag protein molecule that could potentially bind to HLA-DR3(DRB1*0301), –DR4(DRB1*0402), and -DQ8(DQA1*0301 × DQB1*0302) alleles with higher affinity. The results from these predictions are shown in Supplementary Table II. It is notable that multiple registers from a given region of the S-Ag protein were predicted for all the three alleles.
The immunogenicity of various regions of S-Ag in the context of uveitis-susceptible HLA class II alleles was examined using a standard peptide-mapping strategy based on immunizing mice with the native S-Ag protein and recalling responses in culture with overlapping 20 residue peptides representing the entire sequence of human S-Ag (Supplementary Table I). Because of the unavailability of native human S-Ag, bovine S-Ag was used for immunization. We recognize that despite the very high interspecies homology, we may inevitably have missed some epitopes that are not shared between the bovine and human proteins. However, since the target for uveitis in the HLA transgenic mice is the murine protein, uveitogenic epitopes detected in this model are likely to represent sequences that are conserved among species.
The response profile of the susceptible HLA class II alleles, -DR3(0301), -DR4(0402) and -DQ8 to different regions of human S-Ag protein sequence are shown in Figure 3. Dominant epitopes recognized by each of these alleles were distinct. The two dominant epitopes of HLA-DR3 spanned overlapping residues 281–300 and 291–310, whereas dominant epitopes of HLA-DR4(0402) were two distinct non-overlapping regions between residues 311–330 and 331–350. Notably, these regions corresponded to the predicted epitopes for -DR3(0301), -DR4(0402), and -DQ8 alleles shown in Supplementary Table II.
Figure 3. Immunodominant epitope profiles of human S-Ag protein for the susceptible HLA class II alleles.
Spleen cells from S-Ag immunized mice were used in an in-vitro lymphocyte proliferation assay in the presence of 25µM of each of 20 amino acid long overlapping synthetic 20mer peptides spanning the human S-Ag protein sequence, peptide M, peptide N, and whole S-Ag protein (20µg/ml). [3H] Thymidine uptake by cells was plotted after subtracting the background counts in cells cultured in the absence of antigen (average background counts ≤ 5000 cpm). Data shown is the average of five individual mice for each strain.
Although DR4(0401) differs from the DR4(0402) only by three residues, mice carrying this allele were resistant to S-Ag induced EAU. We therefore examined epitope recognition in these two strains of HLA transgenic mice. Results of a peptide scan performed as above showed that resistant DR4(0401) mice recognized a distinct immunodominant peptide than did the susceptible DR4(0402) mice, spanning residues 301–320 (Supplementary Figure 1).
Uveitogenicity of the immunodominant epitopes
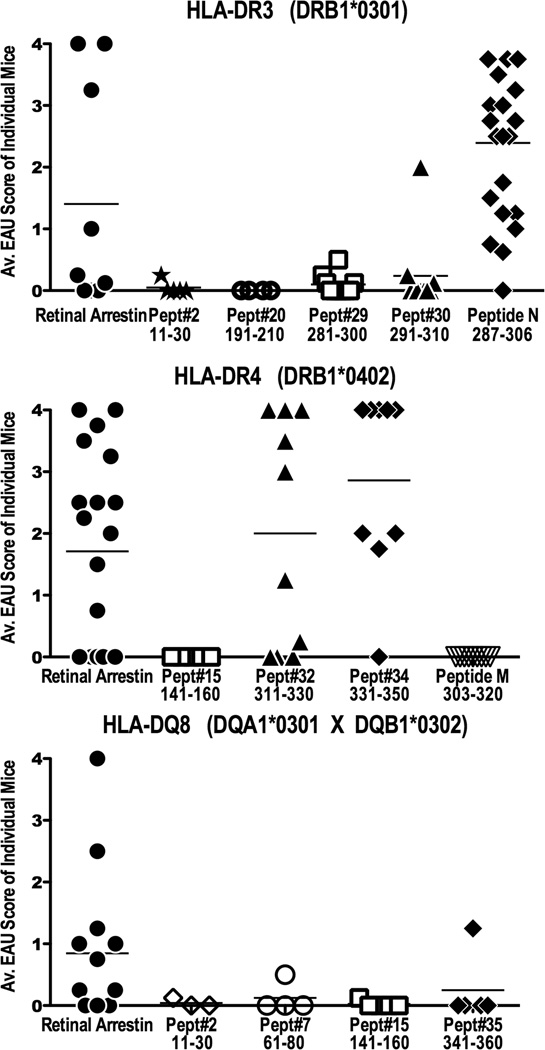
To determine the uveitogenic potential of the immunodominant peptides identified for each of the HLA class II alleles, we immunized each of the susceptible strains of HLA-Tg mice with their corresponding immunodominant peptides to induce EAU. Because several of these immunodominant peptide sequences overlap with the previously defined ‘peptide M’ and ‘peptide N’ that elicit responses in humans as well as in other species (45), the original peptide M and peptide N sequences (based on bovine S-Ag), were also used. Histological scores of the severity of EAU lesions induced with the various peptides are shown in Figure 4.
Figure 4. Uveitogenicity of immunodominant epitopes of human S-Ag in HLA Tg mice.
(A) HLA-DR3(0301), (B) HLA-DR4(0402) and (C) HLA-DQ8 transgenic mice were immunized with 150µg of the respective immunodominant human S-Ag peptides identified in Figure 3, peptide M, peptide N or whole S-Ag protein, and were given 0.4µg of PTX i/p. Eyes were evaluated for EAU by histopathological examination 28–30 days post immunization. Each point represents one mouse (average score of both eyes). Disease scores are combined from six independent experiments for peptide N in DR3(0301) strain, two experiments for peptide#32 and #34 for DR4(0402) strain. Peptides that failed to elicit disease were not studied further.
Although in HLA-DR3 Tg mice peptide #29 (residues 281–300) and #30 (residues 291–310) were highly immunogenic (Figure 3), these peptides elicited at best only marginal EAU at the doses used in this study (Figure 4). Notably, the 20 amino acid ‘peptide N’ (residues 287–306, LLANNRERRGIALDGKIKHE), which is fully conserved between murine, bovine and human S-Ag (Supplementary Figure 2), induced very severe uveitis in HLA-DR3 Tg mice. Shorter peptides were synthesized to identify the minimal core region of ‘peptide N’ for the HLA-DR3(0301) allele (Table II). The results showed that there could be multiple HLA-DR3(0301) registers, between amino acid positions 287–306 which could induce uveitis in the context of this allele (Table II), which may underlie the high pathogenicity of this peptide.
In HLA-DR4(0402) Tg mice two adjacent but non-overlapping peptides, #32 (aa 311–330) and #34 (aa 331–350) induced severe disease (Figure 4). The amino acid sequences of one of these peptides, #32 (ASSTIIKEGIDRTVLGILVS) overlapped with the sequence of ‘peptide M’ which spans residues aa 303–320 of the bovine S-Ag (DTNLASSTIIKEGIDKTV), elicits responses in PBMC of some uveitis patients (26) and is pathogenic in Lewis rats (45). However, peptide M did not induce uveitis in HLA-DR4(0402) Tg mice (Figure 4). The human ‘peptide M’ equivalent (aa 307–325, DTNLASSTIIKEGIDRTVL) also did not elicit disease in this strain (data not shown).
Specific binding of S-Ag peptide-loaded HLA-DR3 tetramer to primary T lymphocytes of DR3 Tg mice with uveitis
MHC class II tetramers loaded with specific epitopes have been used as biomarkers to detect and to study antigen specific autoreactive CD4+ T cells present in peripheral blood and in tissues of patients (46–48). Identification of the uveitogenic epitopes of S-Ag specific to different HLA class II alleles could have direct application to the study of Ag specific T cells in uveitis.
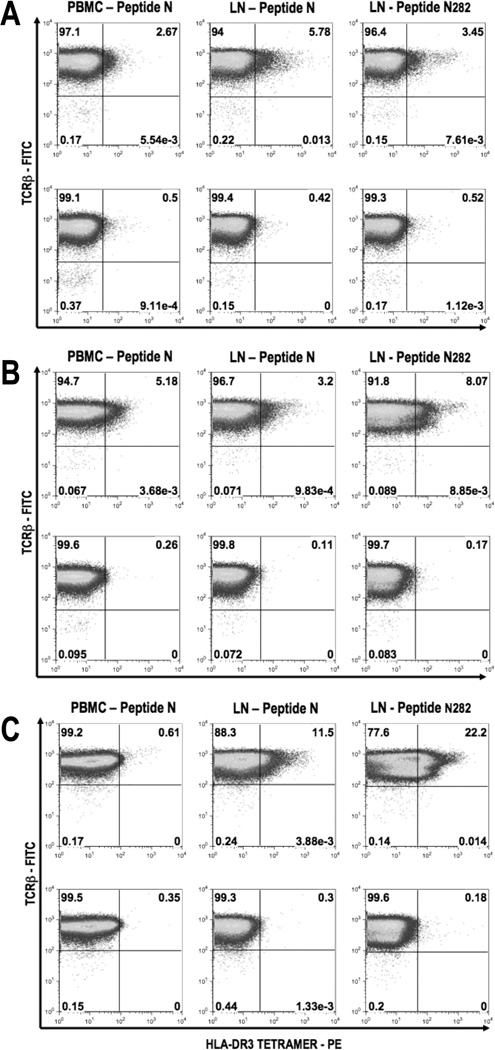
To determine whether S-Ag-specific CD4 T cells could be detected using MHC class II tetramers in blood and lymphoid tissues of mice primed to native S-Ag (to mimic the clinical situation) we used HLA-DR3 tetramers loaded with peptide N282 (NRERRGIALDGKIKHE, Table II). To increase the frequency of epitope specific T cells for detection, the cells were first expanded in culture with peptide N or its shorter version N282 for one or more stimulation cycles. Several fold higher frequencies of S-Ag-specific CD4+ T cells were detected in the PBMC and draining lymph node cells already after a single stimulation cycle (Figure 5A), and the proportion of tetramer-positive cells continued to increase with further stimulations, demonstrating specificity of the response (Figure 5B and 5C).
Figure 5. Peptide loaded HLA-DR3 tetramer detect epitope-specific CD4+ T cells from peripheral blood and draining lymph nodes of immunized HLA-DR3(0301) Tg mice.
HLA-DR3 Tg mice were immunized with S-Ag twice as described in Methods. Cells were isolated from either peripheral blood or draining lymph nodes 12 days after the second immunization. PBMC were in-vitro stimulated with peptide N and draining LN cells were stimulated with peptide N or peptide N282 for 48 hours. Cells were expanded in IL-2 containing medium for 7 days and stained using HLA-DR3 tetramer (PE) loaded with peptide N282 as shown in the upper half, or with unloaded HLA-DR3 tetramer (PE) as shown in the lower half of each panel, at 37°C for one hour followed by anti-CD4 (APC) and anti-TCRβ (FITC). Cells were also stained with 7AAD to exclude the dead cells from analysis. Percentage of tetramer+ TCRβ+ cells are shown after gating CD4+ 7-AAD− cells after the first round (A), second round (B), and third round (C) of stimulation and expansion. Data shown is from one representative experiment of two.
S-Ag specific HLA-DR3 tetramer+ T cells are uveitogenic in HLA-DR3 Tg mice
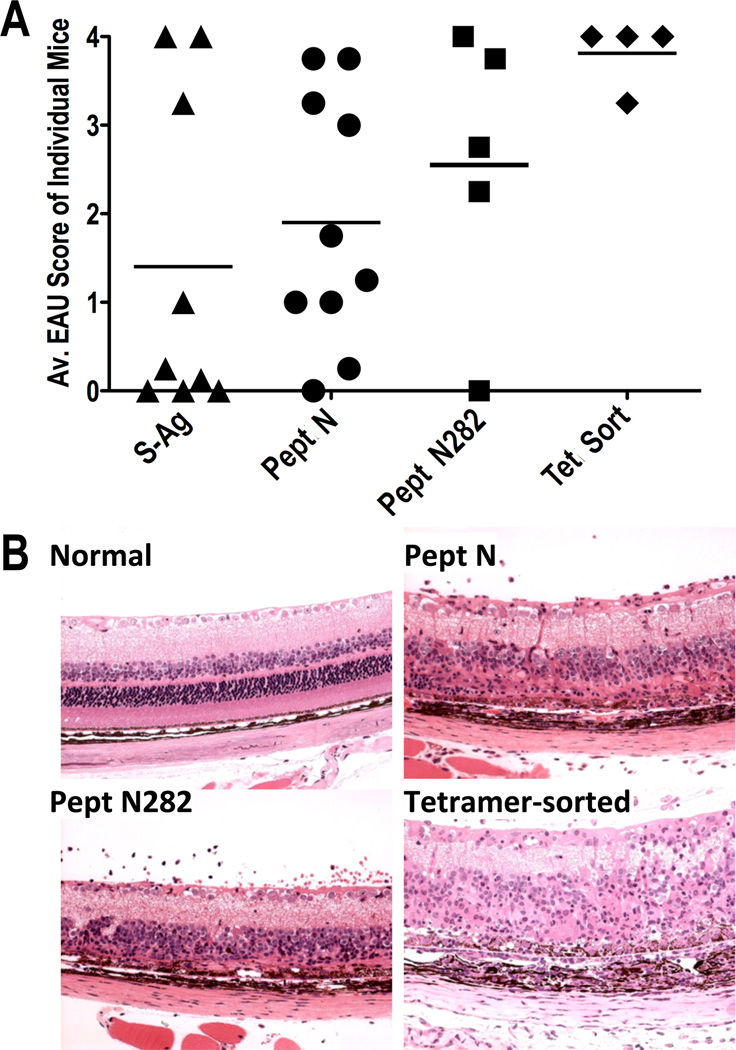
To examine whether the tetramer-positive T cells that bound the N282 -loaded HLA-DR3 tetramers represent uveitogenic effector T cells, we infused five million sorted CD4+ TCRβ+, HLA-DR3 tetramer+ T cells after 4 cycles of expansion into naïve HLA-DR3 Tg recipient mice. Severe inflammation with cellular infiltrate was detected in the eyes of recipient mice by fundus examination as early as five days after cell transfer. Severity of inflammation and retinal damage induced by tetramer positive cells was considerably higher than EAU induced by active immunization of HLA-DR3 Tg mice with whole S-Ag protein or peptide N, or its truncated version N282 (Figure 6A). Histopathological analysis of eyes collected 15 days after adoptive transfer showed cellular infiltrate in the posterior segments of the eyes and a partial or complete destruction of the photoreceptor cells (Figure 6B).
Figure 6. T cells positive for HLA-DR3 tetramer loaded with S-Ag epitope are pathogenic in HLA-DR3 Tg mice.
HLA-DR3 tetramer loaded with the peptide N282 was used to sort CD4+ gated TCRβ+Tetramer+ cells from cultures expanded in-vitro with the same peptide. Sorted cells were stimulated with the same peptide and expanded in-vitro to obtain sufficient numbers of tetramer positive T cells for adoptive transfer into recipient HLA-DR3 Tg mice. After expansion for seven days cells were re-stimulated with the peptide 48 hours before adoptively transferring 5 million live cells/mouse intraperitoneally. Eyes from the recipient mice were collected for histopathological evaluation 15 days after adoptive transfer of cells. (A) Average disease scores of both eyes in four individual mice that received tetramer sorted cells, and (B) histopathological lesions in the eyes of one representative mouse, in comparison to the severity of disease in mice actively immunized with peptide N or peptide N282 or normal eye are shown.
HLA class II tetramers detect S-Ag-responsive cells in uveitis
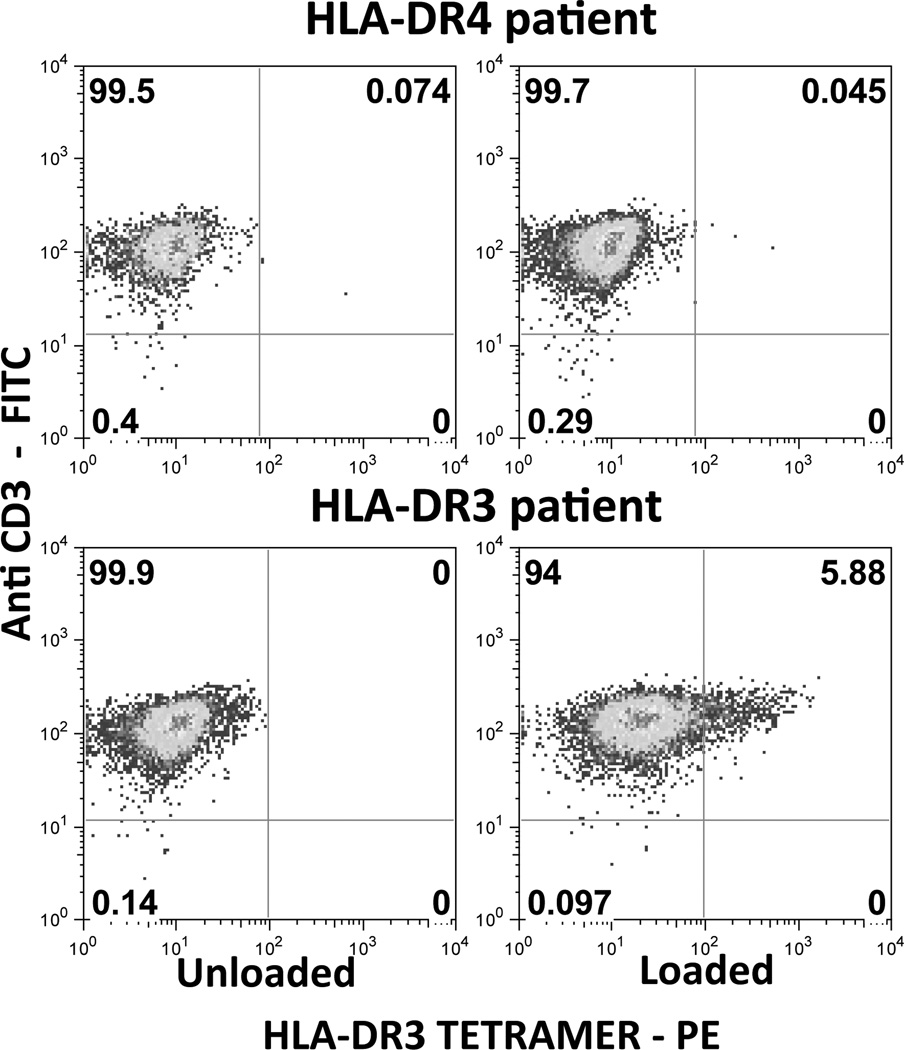
As proof of concept that antigen-reactive cells could be detected in the circulation of S-Ag-responsive uveitis patients, HLA-DR3 tetramer staining was next tested using patient samples. HLA-DR3 tetramers loaded with peptide N282 could successfully detect a population of CD4+ T cells specific to this peptide in an HLA-DR3-positive patient (Figure 7) after selective expansion of antigen-specific cells in culture. Close to 6% of the live CD4+ cells were CD3 and tetramer double-positive cells in this patient. PBMCs from an HLA-DRB1*0401 positive patient not expressing the HLA-DR3 allele did not stain for this specific epitope under similar culture and staining conditions.
Figure 7. HLA class II tetramers detect S-Ag-responsive cells in PBMC of uveitis patient.
PBMC from HLA-DR3 or from HLA-DR4 uveitis patients were stimulated with peptide N282 for 7 days as described in Methods. Cells were re-stimulated on day 7 and expanded in IL-2 containing medium. On day17 of culture cells were stained with HLA-DR3 tetramer (PE) loaded with N282 peptide (NRERRGIALDGKIKHE) or with unloaded HLA-DR3 tetramer (PE) followed by anti-CD4 (APC) and anti-CD3 (FITC). Dead cells were excluded from analysis (7AAD). Percentages of tetramer+ CD3+ cells are shown after gating on CD4+ 7-AAD− cells.
Discussion
In the present study we analyze the immunodominant and pathogenic epitopes of human arrestin (S-Ag), an autoantigen recognized by lymphocytes of uveitis patients, which is believed to have a role in driving progression of uveitis. Using available HLA Tg mouse strains, we identify permissive and non-permissive HLA class II alleles, and characterize the immunodominant and uveitogenic epitopes that they recognize. Importantly, we find that the pathogenic regions that elicit EAU in HLA class II Tg mice overlap or are identical with previously identified sequences known as peptide M and peptide N, which elicit lymphocyte responses in experimental animals and in human uveitis patients.
Although in human uveitis the initial triggers are unknown, some types of uveitis show a clear class II association. Furthermore, effector responses in the EAU model and proliferative responses to S-Ag in patients are primarily CD4 restricted, as expected for responses to a soluble protein. We therefore used class II Tg mice for the present studies. The choice of strains was dictated by published MHC class II associations and by the availability of HLA class II Tg strains. It is interesting that minor differences in the amino-acid sequence between two related allelic forms of HLA-DR4 (29) drastically changed the susceptibility to S-Ag induced EAU. This is reminiscent of observations in type 1 diabetes, for which DR0401 and DR0405 confer increased risk, while DR0403 is protective (49) The immunodominant epitopes of S-Ag presented by DR4(0401) and DR4(0402) alleles are adjacent, but do not overlap with each other. Unlike in the arthritis model (29), HLA-DR4(0401) is a non-permissive allele for S-Ag induced uveitis. However the strain itself is not inherently incapable of developing uveitis, as the disease can be induced with IRBP, and is even more severe than in DR4(0402) mice (see Figure 1A). In contrast, HLA-DR3(0301) allele is permissive to both IRBP and S-Ag induced disease (Figure 1). Association of various forms of uveitis and HLA genes in different populations is not well explored and it is possible that the importance of HLA-DR3 may have been underestimated. As mentioned in Table I, intermediate uveitis (not related to Multiple Sclerosis) had been reported to be associated with HLA-DR3. There are many reports about the association of HLA-DR3 in ocular Sarcoidosis (50, 51). HLA-DR3 is a predisposing gene in autoimmune thyroiditis (52), type 1 diabetes (53), type 1 autoimmune hepatitis (54) etc. Since there is clustering of multiple autoimmune diseases in families, we speculate that HLA-DR3 can be an important gene for uveitis susceptibility as well. HLA-DR2 has been reported to be associated with pars planitis (17, 55, 56) or uveitis associated with MS (Table I). Even though all three alleles of HLA-DR2 examined here were resistant to IRBP as well as to S-Ag induced uveitis (Figure 1), it is possible that other allelic forms of HLA-DR2 could be susceptible. As well, S-Ag and IRBP resistant DR2 alleles could be permissible to uveitis induced with other retinal proteins, such as RPE-65, rhodopsin, recoverin, phosducin etc. The finding that both DR4 and DQ8 alleles in the Tg mice are permissive to S-Ag induced EAU, may perhaps help to explain the relatively higher risk reported for individuals with HLA-DR4DQ8 haplotype to have increased susceptibility to and severity of uveitis (57).
Our results demonstrate that epitope predictions made using various algorithms can accurately pinpoint the immunogenic epitopes of S-Ag in the context of particular HLA class II alleles (Supplementary Table II). However, current bioinformatic tools cannot predict whether the immunogenic epitopes are also pathogenic. This question can only be approached experimentally in a ‘humanized’ uveitis model like the one reported here. Since immunodominant sequences of the human S-Ag presented by HLA-DR3 overlapped with peptide N, and sequences presented by HLA-DR4(0402) overlapped with peptide M, which are both known to elicit immune responses from PBMC of uveitis patients (25, 26), both peptides were examined for ability to induce disease in the respective strains. The peptide N sequence, which is completely conserved among the mouse, human and bovine species (Supplementary Figure 2A), induced severe uveitis in HLA-DR3 Tg mice (Figure 4). However, neither the original peptide M (based on the bovine S-Ag sequence, Figure 4) nor its human equivalent (data not shown), elicited disease in the HLA-DR4(0402) Tg mice.
It should be noted that due to the nature of the experimental system and its logistics, three kinds of S-Ag are players in this study. Because we are interested in human peptides binding to human HLA molecules, the overlapping synthetic 20 amino acid peptides were derived from the human S-Ag sequence. Autoimmunization resulting in uveitis occurs to the whole protein, therefore, we immunized mice with native S-Ag, which had to be bovine, as native human S-Ag cannot be obtained in sufficient quantities. Finally, the target Ag in the uveitic eye is the mouse protein. This experimental design will necessarily tend to select for evolutionarily conserved epitopes that remain immunologically cross-reactive among these species, i.e. approximately 85% of the sequence (Supplementary Figure 2B). Thus, it is possible that some epitopes that are not conserved among species may have been missed.
In conventional mice, EAU development appears not to be restricted to a particular type of effector response. We recently reported that EAU in C57BL/6 and B10.RIII mice can develop in the context of either a Th1 (IFN-γ) or a Th17 (IL-17) effector response (58, 59), and others have demonstrated that in immunodeficient hosts a Th2 response can also be permissive to EAU development (60). In uveitis patients, increased levels of both IFN-γ and IL-17 have been reported (42–44). These observations are directly supported by the present data, showing that HLA-DR3 and HLA-DR4(0402) Tg mice develop S-Ag induced EAU with equal severity and that both Th1 and Th17 cytokines are part of the immunological response profiles (Figure 2B).
Multiple genes contribute to the susceptibility to human uveitis, but by far the strongest associations have been reported with HLA (2). Previous studies documented responses in a proportion of the patients to S-Ag and some of its peptides, but detection of the responding cells and their quantitation as a biomarker of disease has not been possible. We demonstrate here for the first time the specificity of uveitogenic epitopes in the context of susceptible HLA allele using an MHC class II tetramer reagent (Figure 5). The observed frequency of positive T cells in the peripheral blood and primary lymph node cells of uveitic mice is comparable to what has been reported earlier in similar systems (61, 62). Our results show that it is possible to select, identify and characterize rare populations of HLA allele-specific uveitogenic CD4+ T cells for diagnosis and study. Adoptive transfer of uveitis in HLA transgenic mice can be also achieved with T cells restricted by IRBP, to which these mice are also susceptible as a uveitis-inducing antigen by active immunization. Here, we specifically concentrated on N282 tetramer positive T cells, because we wished to show relevance of this T cell population to human disease, where responses to S-Ag are more frequently encountered than to IRBP.
Importantly, our data provide proof of concept that detection of such cells is possible in human uveitis. Out of nine DR3 patients tested, all already on immunosuppressive therapy, one showed presence of autoreactive T cells specific to S-Ag epitope detectable with HLA-DR3 tetramer loaded with peptide N282. Absence of tetramer positive cells in the HLA-DR4 uveitis patient, whose cells also proliferated to S-Ag, reveals the specificity of this reagent. The frequency of tetramer-positive cells is comparable to that of patients with arthritis and type 1 diabetes to their respective disease-associated antigens (47, 48). This tetramer-positive patient was in an active inflammatory stage of the disease at the time when blood was obtained for testing. Notably, after 20 days of immunosuppressive therapy we could no longer detect tetramer-positive cells in this patient. The other eight patients were already under prolonged immunosuppressive therapy at the time of blood collection and their proliferative responses not only to S-Ag, but also to tetanus toxoid, purified protein derivative of tuberculin and to phytohemagglutinin were several-fold lower than those of healthy donors (data not shown). Thus, presence of tetramer-positive cells appears associated with disease activity. While clearly much additional work needs to be done with patients during different clinical stages of uveitis and expressing different HLA allele/epitope combinations, our data provide a proof of principle that this technology has the potential to be developed into the first disease biomarker for clinical diagnosis and follow up as well as for isolation and study of uveitis-relevant T cells in the S-Ag responsive patient population.
In conclusion, we have identified alleles of HLA class II genes that confer susceptibility or resistance to uveitis-associated antigens in “humanized” mice and have identified pathogenic epitopes that they bind and present in the context of human MHC class II molecules. We further provide proof of concept that these findings may lead to development of the first disease biomarker(s) for uveitis. Knowledge of the risk associated HLA alleles and of the pathogenic epitopes that they present promises to contribute towards the improved understanding, diagnosis and treatment of uveitis.
Supplementary Material
Acknowledgements
Authors are grateful for the helpful advice of Dr. Gerald Nepom (Benaroya Research Institute at Virginia Mason, WA). We thank Ms. Angelia M Viley and Mr. Arrash Yazdani (Lab. Immunology, NEI) and Mr. Rafael Villasmil (NEI Flow Cytometry Core) for excellent technical help. The authors thank Ms. Julie Hanson and Ms. Michele Smart (Immunogenetics mouse colony, Mayo clinic, MN) for generous supply of genotyped HLA Tg mice.
This work was supported by the intramural research grant of National Eye Institute, NIH. This project was also supported in part with federal funds from the National Institutes of Allergy and Infectious diseases (NIAID) & National Institute of Dental and Craniofacial Research (NIDCR) under Grant Number K22AI07812 & R21DE018339 to JJM. JHMcD was supported by NIH grants EY014864, EY006225 and an unrestricted departmental grant from Research to Prevent Blindness.
Abbreviations
- EAU
Experimental Autoimmune Uveitis
- S-Ag
S-antigen
- IRBP
Interphotoreceptor Retinoid Binding Protein
- Tg
transgenic
Footnotes
Conflict of interest disclosure: The authors declare no competing financial interests.
References
- 1.Levinson RD. Immunogenetics of ocular inflammatory disease. Tissue Antigens. 2007;69:105–112. doi: 10.1111/j.1399-0039.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 2.Martin TM, Kurz DE, Rosenbaum JT. Genetics of uveitis. Ophthalmol. Clin. North Am. 2003;16:555–565. doi: 10.1016/s0896-1549(03)00071-3. [DOI] [PubMed] [Google Scholar]
- 3.Baarsma GS, Priem HA, Kijlstra A. Association of birdshot retinochoroidopathy and HLA-A29 antigen. Curr. Eye. Res. 1990;9 Suppl:63–68. doi: 10.3109/02713689008999422. [DOI] [PubMed] [Google Scholar]
- 4.LeHoang P, Ozdemir N, Benhamou A, Tabary T, Edelson C, Betuel H, Semiglia R, Cohen JH. HLA-A29.2 subtype associated with birdshot retinochoroidopathy. Am. J. Ophthalmol. 1992;113:33–35. doi: 10.1016/s0002-9394(14)75749-6. [DOI] [PubMed] [Google Scholar]
- 5.Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am. J. Ophthalmol. 1982;94:147–158. doi: 10.1016/0002-9394(82)90069-1. [DOI] [PubMed] [Google Scholar]
- 6.Arber N, Klein T, Meiner Z, Pras E, Weinberger A. Close association of HLA-B51 and B52 in Israeli patients with Behcet's syndrome. Ann. Rheum. Disease. 1991;50:351–353. doi: 10.1136/ard.50.6.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilmartin DJ, Finch A, Acheson RW. Primary association of HLA-B51 with Behcet's disease in Ireland. Br. J. Ophthalmol. 1997;81:649–653. doi: 10.1136/bjo.81.8.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuki N, Inoko H, Tanaka H, Kera J, Tsuiji K, Ohno S. Human leukocyte antigen serologic and DNA typing of Behcet's disease and its primary association with B51. Invest. Ophthalmol. Vis. Sci. 1992;33:3332–3340. [PubMed] [Google Scholar]
- 9.Brewerton DA. The genetics of acute anterior uveitis. Trans. Ophthalmol. Soc. U. K. 1985;104(Pt 3):248–249. [PubMed] [Google Scholar]
- 10.Jaakkola E, Herzberg I, Laiho K, Barnardo MC, Pointon JJ, Kauppi M, Kaarela K, Tuomilehto-Wolf E, Tuomilehto J, Wordsworth BP, Brown MA. Finnish HLA studies confirm the increased risk conferred by HLA-B27 homozygosity in ankylosing spondylitis. Ann. Rheum. Disease. 2006;65:775–780. doi: 10.1136/ard.2005.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Numaga J, Islam MS, Mitsui H, Maeda H. Anterior uveitis with ankylosing spondylitis and HLA. Nippon. Ganka. Gakkai Zasshi. 1996;100:292–295. [PubMed] [Google Scholar]
- 12.Kim MH, Seong MC, Kwak NH, Yoo JS, Huh W, Kim TG, Han H. Association of HLA with Vogt-Koyanagi-Harada syndrome in Koreans. Am. J. Ophthalmol. 2000;129:173–177. doi: 10.1016/s0002-9394(99)00434-1. [DOI] [PubMed] [Google Scholar]
- 13.Shindo Y, Inoko H, Yamamoto T, Ohno S. HLA-DRB1 typing of Vogt-Koyanagi-Harada's disease by PCR-RFLP and the strong association with DRB1*0405 and DRB1*0410. Br. J. Ophthalmol. 1994;78:223–226. doi: 10.1136/bjo.78.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilmartin DJ, Wilson D, Liversidge J, Dick AD, Bruce J, Acheson RW, Urbaniak SJ, Forrester JV. Immunogenetics and clinical phenotype of sympathetic ophthalmia in British and Irish patients. Br. J. Ophthalmol. 2001;85:281–286. doi: 10.1136/bjo.85.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shindo Y, Ohno S, Usui M, Ideta H, Harada K, Masuda H, Inoko H. Immunogenetic study of sympathetic ophthalmia. Tissue Antigens. 1997;49:111–115. doi: 10.1111/j.1399-0039.1997.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 16.Cuccia Belvedere M, Martinetti M, de Paoli F, Mazzacane D, Redaelli C, Tafi A, Morone G. Genetic heterogeneity in uveitis. Dis. Markers. 1986;4:243–246. [PubMed] [Google Scholar]
- 17.Oruc S, Duffy BF, Mohanakumar T, Kaplan HJ. The association of HLA class II with pars planitis. Am. J. Ophthalmol. 2001;131:657–659. doi: 10.1016/s0002-9394(00)00863-1. [DOI] [PubMed] [Google Scholar]
- 18.de Smet MD, Yamamoto JH, Mochizuki M, Gery I, Singh VK, Shinohara T, Wiggert B, Chader GJ, Nussenblatt RB. Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Am. J. Ophthalmol. 1990;110:135–142. doi: 10.1016/s0002-9394(14)76981-8. [DOI] [PubMed] [Google Scholar]
- 19.Nussenblatt RB, Gery I, Weiner HL, Ferris FL, Shiloach J, Remaley N, Perry C, Caspi RR, Hafler DA, Foster CS, Whitcup SM. Treatment of uveitis by oral administration of retinal antigens: results of a phase I/II randomized masked trial. Am. J. Ophthalmol. 1997;123:583–592. doi: 10.1016/s0002-9394(14)71070-0. [DOI] [PubMed] [Google Scholar]
- 20.de Smet MD, Bitar G, Mainigi S, Nussenblatt RB. Human S-antigen determinant recognition in uveitis. Invest. Ophthalmol. Vis. Sci. 2001;42:3233–3238. [PubMed] [Google Scholar]
- 21.Caspi r. Experimental autoimmune uveoretinitis in the rat and mouse. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Curr. Prot. Immunol. Hoboken, New Jersey: John Wiley and Sons Inc; 1997. p. 15.16.11. [Google Scholar]
- 22.de Smet MD, Chan CC. Regulation of ocular inflammation--what experimental and human studies have taught us. Prog. Retin. Eye. Res. 2001;20:761–797. doi: 10.1016/s1350-9462(01)00011-8. [DOI] [PubMed] [Google Scholar]
- 23.Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z, Nussenblatt RB. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol. 1988;140:1490–1495. [PubMed] [Google Scholar]
- 24.Pennesi G, Mattapallil MJ, Sun SH, Avichezer D, Silver PB, Karabekian Z, David CS, Hargrave PA, McDowell JH, Smith WC, Wiggert B, Donoso LA, Chan CC, Caspi RR. A humanized model of experimental autoimmune uveitis in HLA class II transgenic mice. J. Clin. Invest. 2003;111:1171–1180. doi: 10.1172/JCI15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto JH, Minami M, Inaba G, Masuda K, Mochizuki M. Cellular autoimmunity to retinal specific antigens in patients with Behcet's disease. Br. J. Ophthalmol. 1993;77:584–589. doi: 10.1136/bjo.77.9.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirose S, Donoso LA, Shinohara T, Palestine AG, Nussenblatt RB, Gery I. Lymphocyte responses to peptide M and to retinal S-antigen in uveitis patients. Jpn. J. Ophthalmol. 1990;34:298–305. [PubMed] [Google Scholar]
- 27.Kong YC, Lomo LC, Motte RW, Giraldo AA, Baisch J, Strauss G, Hammerling GJ, David CS. HLA-DRB1 polymorphism determines susceptibility to autoimmune thyroiditis in transgenic mice: definitive association with HLA-DRB1*0301 (DR3) gene. J. Exp. Med. 1996;184:1167–1172. doi: 10.1084/jem.184.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabozny GH, Baisch JM, Cheng S, Cosgrove D, Griffiths MM, Luthra HS, David CS. HLA-DQ8 transgenic mice are highly susceptible to collagen-induced arthritis: a novel model for human polyarthritis. J. Exp. Med. 1996;183:27–37. doi: 10.1084/jem.183.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taneja V, Taneja N, Behrens M, Pan S, Trejo T, Griffiths M, Luthra H, David CS. HLA-DRB1*0402 (DW10) transgene protects collagen-induced arthritis-susceptible H2Aq and DRB1*0401 (DW4) transgenic mice from arthritis. J. Immunol. 2003;171:4431–4438. doi: 10.4049/jimmunol.171.8.4431. [DOI] [PubMed] [Google Scholar]
- 30.Nooh MM, El-Gengehi N, Kansal R, David CS, Kotb M. HLA transgenic mice provide evidence for a direct and dominant role of HLA class II variation in modulating the severity of streptococcal sepsis. J. Immunol. 2007;178:3076–3083. doi: 10.4049/jimmunol.178.5.3076. [DOI] [PubMed] [Google Scholar]
- 31.Buczylko J, Palczewski K. Purification of arrestin from bovine retinas. In: Hargrave P, editor. Photoreceptor cells. San Diego: Academic Press; 1993. pp. 226–236. [Google Scholar]
- 32.Redmond TM, Wiggert B, Robey FA, Nguyen NY, Lewis MS, Lee L, Chader GJ. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry. 1985;24:787–793. doi: 10.1021/bi00324a038. [DOI] [PubMed] [Google Scholar]
- 33.Puig J, Arendt A, Tomson FL, Abdulaeva G, Miller R, Hargrave PA, McDowell JH. Synthetic phosphopeptide from rhodopsin sequence induces retinal arrestin binding to photoactivated unphosphorylated rhodopsin. FEBS Lett. 1995;362:185–188. doi: 10.1016/0014-5793(95)00225-x. [DOI] [PubMed] [Google Scholar]
- 34.Donoso LA, Merryman CF, Shinohara T, Dietzschold B, Wistow G, Craft C, Morley W, Henry RT. S-antigen: identification of the MAbA9-C6 monoclonal antibody binding site and the uveitopathogenic sites. Curr. Eye. Res. 1986;5:995–1004. doi: 10.3109/02713688608995181. [DOI] [PubMed] [Google Scholar]
- 35.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 37.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–1237. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 38.Reche PA, Glutting JP, Zhang H, Reinherz EL. Enhancement to the RANKPEP resource for the prediction of peptide binding to MHC molecules using profiles. Immunogenetics. 2004;56:405–419. doi: 10.1007/s00251-004-0709-7. [DOI] [PubMed] [Google Scholar]
- 39.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J. Clin. Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res. 2008 doi: 10.1007/s12026-008-8031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luger D, Caspi RR. New perspectives on effector mechanisms in uveitis. Semin. Immunopathol. 2008;30:135–143. doi: 10.1007/s00281-008-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chi W, Zhu X, Yang P, Liu X, Lin X, Zhou H, Huang X, Kijlstra A. Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Invest. Ophthalmol. Vis. Sci. 2008;49:3058–3064. doi: 10.1167/iovs.07-1390. [DOI] [PubMed] [Google Scholar]
- 43.Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, Chen L, Zhou H, Huang X, Kijlstra A. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J. Allergy Clin. Immunol. 2007;119:1218–1224. doi: 10.1016/j.jaci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Hamzaoui K, Hamzaoui A, Guemira F, Bessioud M, Hamza M, Ayed K. Cytokine profile in Behcet's disease patients. Relationship with disease activity. Scand. J. Rheumatol. 2002;31:205–210. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 45.Donoso LA, Merryman CF, Sery TW, Shinohara T, Dietzschold B, Smith A, Kalsow CM. S-antigen: characterization of a pathogenic epitope which mediates experimental autoimmune uveitis and pinealitis in Lewis rats. Curr. Eye. Res. 1987;6:1151–1159. doi: 10.3109/02713688709034888. [DOI] [PubMed] [Google Scholar]
- 46.Nepom GT. Tetramer Analysis of Human Autoreactive CD4-Positive T Cells. Adv. Immunol. 2005;88:51–71. doi: 10.1016/S0065-2776(05)88002-2. [DOI] [PubMed] [Google Scholar]
- 47.Kotzin BL, Falta MT, Crawford F, Rosloniec EF, Bill J, Marrack P, Kappler J. Use of soluble peptide-DR4 tetramers to detect synovial T cells specific for cartilage antigens in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. U S A. 2000;97:291–296. doi: 10.1073/pnas.97.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reijonen H, Novak EJ, Kochik S, Heninger A, Liu AW, Kwok WW, Nepom GT. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 2002;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 49.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P f. t. T. D. G. Consortium. HLA DR-DQ Haplotypes and Genotypes and Type 1 Diabetes Risk; Analysis of the Type 1 Diabetes Genetics Consortium Families. Diabetes. 2008;57:1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grunewald J, Janson CH, Eklund A, Ohrn M, Olerup O, Persson U, Wigzell H. Restricted V alpha 2.3 gene usage by CD4+ T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients correlates with HLA-DR3. Eur. J. Immunol. 1992;22:129–135. doi: 10.1002/eji.1830220120. [DOI] [PubMed] [Google Scholar]
- 51.Hedfors E, Lindstrom F. HLA-B8/DR3 in sarcoidosis. Correlation to acute onset disease with arthritis. Tissue Antigens. 1983;22:200–203. doi: 10.1111/j.1399-0039.1983.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J. Autoimmun. 2008;30:58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin. Chem. 57:176–185. doi: 10.1373/clinchem.2010.148221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin. Liver Dis. 2002;6:635–647. doi: 10.1016/s1089-3261(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 55.Raja SC, Jabs DA, Dunn JP, Fekrat S, Machan CH, Marsh MJ, Bressler NM. Pars planitis: clinical features and class II HLA associations. Ophthalmology. 1999;106:594–599. doi: 10.1016/S0161-6420(99)90122-7. [DOI] [PubMed] [Google Scholar]
- 56.Tang WM, Pulido JS, Eckels DD, Han DP, Mieler WF, Pierce K. The association of HLA-DR15 and intermediate uveitis. Am. J. Ophthalmol. 1997;123:70–75. doi: 10.1016/s0002-9394(14)70994-8. [DOI] [PubMed] [Google Scholar]
- 57.Levinson RD, See RF, Rajalingam R, Reed EF, Park MS, Rao NA, Holland GN. HLA-DRB1 and -DQB1 alleles in mestizo patients with Vogt-Koyanagi-Harada's disease in Southern California. Hum. Immunol. 2004;65:1477–1482. doi: 10.1016/j.humimm.2004.07.236. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimura T, Sonoda KH, Miyazaki Y, Iwakura Y, Ishibashi T, Yoshimura A, Yoshida H. Differential roles for IFN-gamma and IL-17 in experimental autoimmune uveoretinitis. Int. Immunol. 2008;20:209–214. doi: 10.1093/intimm/dxm135. [DOI] [PubMed] [Google Scholar]
- 59.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SJ, Zhang M, Vistica BP, Chan CC, Shen DF, Wawrousek EF, Gery I. Induction of ocular inflammation by T-helper lymphocytes type 2. Invest. Ophthalmol. Vis. Sci. 2002;43:758–765. [PubMed] [Google Scholar]
- 61.Tian C, Bagley J, Cretin N, Seth N, Wucherpfennig KW, Iacomini J. Prevention of type 1 diabetes by gene therapy. J. Clin. Invest. 2004;114:969–978. doi: 10.1172/JCI22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bischof F, Hofmann M, Schumacher TN, Vyth-Dreese FA, Weissert R, Schild H, Kruisbeek AM, Melms A. Analysis of autoreactive CD4 T cells in experimental autoimmune encephalomyelitis after primary and secondary challenge using MHC class II tetramers. J. Immunol. 2004;172:2878–2884. doi: 10.4049/jimmunol.172.5.2878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.