Abstract
Background
Gene therapy has attracted attention for its potential to treat several cardiovascular diseases. The use of adeno-associated viral (AAV) vectors to facilitate therapeutic gene transfer to suppress intimal hyperplasia is a promising concept. The objective of this study was to analyze the in vivo transduction of a novel recombinant AAV-2/9 vector with SM22α promoter, containing β-galactosidase gene (Lac Z) or green fluorescent protein (GFP) as reporter genes, to the medial layer smooth muscle cells (SMCs) of swine coronary and peripheral arteries.
Methods
The AAV2/9 vector containing SM22α (1×1013 pfu) were administered into carotid/femoral/coronary arteries of domestic swine using irrigating balloon catheter-based gene delivery. Following gene transfer, cryosections of arteries were processed for X-Gal and GFP analysis. Fluorescence microscopy and Western blotting were done to analyze the GFP expression in the SMCs.
Results
LacZ mRNA expression was visualized in the medial layer 7 days after vector administration. The GFP expression was detected at 7th day and lasted for at least 2 months showing the longer-lasting expression of the AAV2/9-vector. Control arteries did not show any expression of GFP or LacZ. There was no significant effect of AAV2/9 viral transduction on serum amylase, fibrinogen and serum CRP levels.
Conclusion
These finding support the use of AAV2/9 as a vector to effectively transduce a gene in SMCs of coronary and peripheral arteries without causing inflammation.
Keywords: Adeno-associated virus, Gene therapy, Intimal hyperplasia, Restenosis, Vascular smooth muscle cells, Vector
Introduction
Gene therapy is a potential strategy for the treatment of in-stent restenosis after angioplasty and vascular bypass graft occlusion for which no known effective therapy exists. The choice of an appropriate gene delivery system is the most crucial factor in the development of successful gene therapies. Adeno-associated viral (AAV) vectors have emerged as a versatile vehicle for gene delivery due to its efficient infection of dividing and non-dividing cells (1) in the presence of a helper virus, sustained maintenance of the viral genome (2) and a strong clinical safety profile. They are capable of holding various gene as well as promoter up to 4.7kb between their inverted terminal repeats (ITRs) (3). Another advantage of this vector system is that high titer viral stocks can be prepared (4).
The vast majority of past and current clinical trials employ AAV vectors based on serotype-2 (5, 6). The intracellular trafficking of AAV2 remains most well characterized among AAV serotypes (7). However, in vivo tropism of the AAV2 vectors in smooth muscle cells have only been reported in few studies(8, 9). The broad tissue tropism exhibited by the AAV2 vectors was a major drawback in terms of safety and specificity because non-target tissues may be transduced following vector application(10). Recent studies have shown that cross packaging of AAV2 vectors on to different serotypes have enhanced efficiency of transduction(11). Cross-packaged AAV2 genomes in AAV1, AAV3 and AAV4 capsids showed 900, 30 and 3-fold enhanced gene expression, respectively, in skeletal muscle compared to AAV2. AAV9 exhibits a similar profile with widely disseminated transduction as AAV2, albeit with much higher efficiency than AAV2. Hence, it may be plausible that the use of an AAV2 vector with AAV9 capsid cause stable transduction in cells. Another strategy adopted to increase specificity is the choice of a promoter that naturally drives expression of a particular gene to the tissue of interest (12). In this study, we designed the experiment to increase the specificity of the gene transfer to smooth muscle cells by using SM22α promoter.
The aim of our study was to evaluate the in vivo transduction efficiency of a recombinant AAV2 vector with SM22α promoter cross-packaged in AAV9 capsid (AAV 2/9) with LacZ/ GFP as reporter genes, in swine arterial smooth muscle cells (SMCs). The expression of viral proteins by transduced cells elicits immune response in animals. To determine the overall safety of this gene delivery vector we examined plasma fibrinogen and serum amylase and C-reactive protein (CRP) levels in the animals to verify the inflammatory response.
Materials and Methods
Construction and production of recombinant AAV Vector
The plasmids pZAC 2.1 eGFP3 and pAAV2.1 lacZ1 having the reporter genes, GFP (green fluorescent protein) and lacZ (β-galactosidase gene), with CMV promoter and ITRs were prepared at the Gene Therapy Resource Program (GTRP) of the NIH-NHLBI at the University of Pennsylvania Vector Core facility. Briefly, the vectors were amplified by transforming into the DH5α strain of E.coli through heat shock technique on LB agar plate with carbenicillin/ampicillin and inoculation in LB (Luria-Bertani) broth. Plasmids were isolated using the Maxi Prep kit (Qiagen, Valencia CA, USA). Double digestion was done with NheI and PstI restriction enzymes to remove the CMV promoter and to generate sites for ligation with SM22α promoter. Alkaline phosphatase treatment was done to prevent self ligation and the enzyme was heat-inactivated prior to the promoter ligation. Plasmid vector pGL2 having SM22α promoter was obtained from the Gene Therapy Resource Program (GTRP) of the NIH-NHLBI at the University of Pennsylvania Vector Core facility and was PCR amplified with primers having NheI and PstI restriction sites (Forward Primer: 5′-GCACCTAGCAGTCAAGACTAGTTCCCA-3′ Reverse Primer: 5′-TGGCTTCGATGAGAGGAAGGACGTCTA-3′). The product was further subjected to restriction digestion with the same restriction sites to generate sticky ends for ligation. Ligation was done with T4 DNA ligase. Vectors were propagated using the amplification procedure given above and plasmid DNA was isolated using the Maxi Prep kit (Qiagen, USA). Constructs were verified with restriction digestion. The cloned plasmids were sent to the Gene Therapy Resource Program (GTRP) of the NIH-NHLBI at the University of Pennsylvania Vector Core facility for the packaging. Briefly, packaging of the rAAV was done by a helper plasmid, having rep gene of AAV2 and cap gene of AAV9 to prepare AAV2/9. Helper plasmid was introduced by transfection of a third plasmid coding for adenoviral genes which is needed for AAV production or co-infection. Vector particles were then harvested from the HEK293 cells and purified by chromatography.
Animals
Domestic pigs (50-70 lbs) were purchased from the University of Nebraska-Lincoln swine facility in Mead, NE. Seven days and 2 months after the gene transfer the animals were euthanized with Beuthanasia-D (1mL/10 lb i.v.) and artery samples were collected. The experimental groups of pigs with the administration of AAV-GFP/LacZ included: (i) carotid artery, (ii) coronary artery, (iii) femoral artery, (iv) iliac artery for 7 days, and (v) carotid artery for 2 months. Each experimental group comprised of 4 pigs. The experimental protocol in this study was evaluated and approved by the Institutional Animal Care and Use Committee at Creighton University.
In vitro gene transfer in porcine coronary artery smooth muscle cells
Porcine coronary artery smooth muscle cells were seeded into 24-well plates at a density of 2 × 105 cells/cm2. After 24h, the cells were incubated at different concentrations of the GFP vector; 1 × 107, 1 × 109, 1 × 1011 and 1 × 1013 pfu. The cells were analyzed for gene transfer after 18h of incubation with the vector, using a multilabel reader (Enspire 2300, PerkinElmer, Turku, Finland) at excitation wavelength of 485nm and emission wavelength 520nm. The assay was done in four replicates. The gene transfer was also visualized using a fluorescence microscope (Olympus BX51, St. Louis, MO, USA).
In vivo gene transfer into arteries
For gene delivery, access to the femoral artery in the leg was created by an introducer needle. This was followed by the placement of a 6F sheath introducer to keep the artery open and control bleeding. A guiding catheter was introduced and pushed all the way to coronary arteries. Systemic heparin (100 U/Kg) was administered to maintain the blood flow and an additional 50 U/Kg, if the procedure exceeds 90 min. This was followed by insertion of a guide-wire through a guiding catheter and into the coronary artery. The ClearWay Vascular Irrigating PTFE balloon catheter (Atrium, Hudson, NH) with minute pores was then inserted at the back of the guidewire. The angioplasty catheter was gently pushed until the deflated balloon catheter is inside the coronary arteries. The balloon was then inflated to produce injury to the coronary artery SMCs. A 100μL solution of PBS alone (sham-control) or PBS containing AAV2/9-LacZ/eGFP (1 × 1013 pfu) vector was infused over a period of 30-60 seconds. For transfection in the carotid artery, the right external and common carotid arteries were accessed via femoral artery, and the endothelium of the common carotid artery was denuded with a 2-French Fogarty balloon catheter (Baxter Healthcare, USA). After balloon removal, the common carotid artery was flushed with phosphate-buffered saline (PBS), and about 3-cm segment was clamped followed by injection of 100μl solution of AAV-GFP/LacZ in this segment and left in the artery for 30 minutes. Similar procedures were used for injecting the vector in the femoral and iliac arteries. The catheters were removed and the carotid/femoral artery was sutured using 6-O proline sutures in a simple continuous pattern with buried knots at both ends to close the sub-cuticular layer. Intravenous fluids were continued until the IV catheter remained patent and swine was subsequently moved to the animal care facility.
Evaluation of in vivo gene transfer
Fluorescence microscopy for GFP expression analysis
The cryosections of the arteries obtained after 7 days of gene transfer were mounted with Vectashield containing DAPI (Vector Laboratories, Burlingame, CA, USA) and was analyzed for GFP expression using confocal microscope (Zeiss, LSM 510 Meta, Germany).
β-galactosidase reporter gene assay
Snap-frozen vessel segments were cut into 5μm sections using a cryostat and stained for β-galactosidase enzyme activity using β-galactosidase assay kit from Sigma (St. Louis, MO, USA). After staining, the β-galactosidase gene transfer was assessed by blue-stained cell nuclei in vessel cross-sections using bright field microscope (Olympus BX51, St. Louis, MO, USA).
Real time PCR
The endothelial and adventitial layers were carefully scraped off from the harvested vessel and the remaining medial layer was minced and treated with Trizol reagent (Sigma, St. Louis, USA) for isolation of total RNA. The yield of RNA was quantified using Genequant 1300 (GE Healthcare, USA). First-strand cDNA was synthesized using 1 μg total RNA with oligo dT (1μg), 5 X reaction buffer, MgCl2, dNTP mix and Improm II reverse transcriptase as per Improm II reverse transcription kit. Following the first strand synthesis, Real time PCR was done using 8μl cDNA, 10 μl SYBR green PCR master mix (Applied biosystems, Carlsbad, CA, USA) and forward and reverse primers (10picomol/ μl) (Integrated DNA Technologies, Coralville, IA, USA) using a 7500 Real Time PCR system (Applied biosystems, Carlsbad, CA, USA). The primer sequences used were GFP F-5′-CCTGAAGTTCATCTGCACCA-3′, R-5′-GTCCTCCTTGAAGTCGATGC-3′ and LacZ F5′-CCACGGCCACCGATATTATTTG-3′, R5′-CTCTCCGGCTGCGGTAGTTCA-3′. The PCR cycling conditions used were 5 min at 95 °C for initial denaturation, followed by 45 cycles of 45sec at 95 °C, 45 s at 55 °C and 45 sec at 72 °C. The specificity of the primers were analyzed by running a melting curve. The PCR data is expressed as the ratio of Ct value of GFP or LacZ relative to GAPDH. Statistical analysis of the qPCR results was performed using Single factor ANOVA.
Western blotting
From the medial layer of the arteries after 7days and 2 months of surgery protein was extracted using RIPA Buffer (Sigma-Aldrich, USA). The protein samples were run on an electrophoresis gel using SDS-PAGE and then transferred to a nitrocellulose membrane. After incubating the membranes with 5% milk in PBS (with 0.05% Tween-20) to block any nonspecific binding, they were incubated with primary antibodies for GFP (Abcam, Cambridge, MA, USA), at 1:500 dilution overnight, then with the secondary antibody conjugated to horseradish peroxidase for 1 h. The intensity of the protein expression was determined using the Bioimaging System (UVP, Upland, CA, USA). Densitometric analysis was done to relatively quantify the amount of protein. Statistical analysis of the results was performed using Single factor ANOVA.
Analysis for inflammatory reactions
The blood samples from pigs (n=8) was analyzed for plasma fibrinogen and serum CRP and α-amylase levels after 7 days of vector administration. The plasma fibrinogen was analyzed using STA-Fibrinogen kit in a Start4 Coagulation analyzer (Diagnostica Stago, Parsippany, NJ, USA). CRP levels were measured using ELISA kit (Innovative Research, Novi, MI, USA) according to the manufacturer’s protocol. Absorbance was measured using a microplate reader (Biorad Laboratories, Hercules, CA, USA) at 450nm. The serum α-amylase activity was quantified using the Quantichrom α-amylase assay kit (Bioassay Systems, Hayward, CA, USA) and absorbance was measured at 595nm according to the manufacturer’s protocol. Statistical analysis of the results was performed using Single factor ANOVA.
Results
AAV-mediated gene transfer leads to stable transgene expression in swine arteries
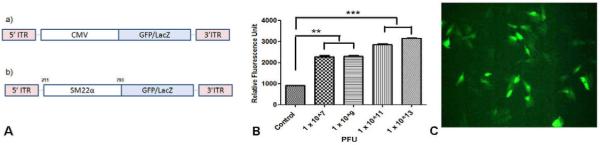
The recombinant vectors were created by inserting a transgene of interest GFP/LacZ flanked by the inverted terminal repeats (ITRs) of AAV2 into the capsid of another serotype AAV9. The CMV promoter of the vector was replaced by the SM22α promoter to increase the specificity of transfection (Fig. 1). The in vitro transfection efficiency was significantly higher at a vector concentration of 1 × 1013 pfu was used as shown by the flourimetry and fluorescence micrograph data (Fig. 1B&C). Hence, in vivo we delivered 1×1013 vector genomes of the AAV2/9 carrying the GFP/LacZ construct by Irrigating PTFE balloon catheter. The non-infected arteries from the same animal served as the control. This also helped to identify the specificity of the vector post gene delivery.
Figure 1.
A) Structure of cloned adeno-associated viral vector with (a) inverted terminal repeats (ITR) and CMV promoter and (b) CMV promoter was replaced by SM22α promoter to create the final construct. B) In vitro expression of GFP- Fluorimetry data showing expression of GFP at a vector different vector concentrations in porcine coronary artery SMCs (*** p <0.001, ** p <0.01). C) Fluorescence micrograph showing GFP expression in porcine coronary artery SMCs at vector concentration 1 × 1013 pfu.
Confocal micrographs revealed GFP expression in carotid artery 7days post gene transfer. The GFP expression was observed in the inner layers of the medial layer (Fig. 2). The relative qPCR data confirmed the presence of GFP. The GFP mRNA expression was detected at 7 days (Fig. 3) in all the arteries. There were similar GFP mRNA expression levels in all the arteries regardless of the type of the artery. The protein analysis using Western blot revealed GFP expression in carotid arteries at 7days and 60days after gene delivery (Fig. 4) showing the prolonged expression of the AAV2/9 vector. There was no expression of GFP in the control arteries. This data is promising since we expect the therapeutic gene to be expressed for longer period in order to suppress intimal hyperplasia.
Figure 2. GFP expression in vivo.
Confocal images showing GFP expression in AAV-GFP transduced carotid artery. (A) Blue nuclear staining of the cells by DAPI, (B) GFP expression shown in green, and (C) overlay image confirming the GFP is associated with the smooth muscle cells.
Figure 3. mRNA expression of Laz Z and GFP in arteries.
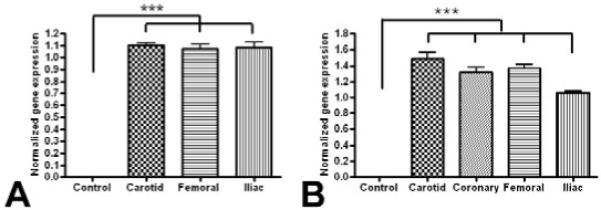
Real time PCR data showing mRNA expression of GFP (A) and Lac Z (B) in AAV-GFP/LacZ administered arteries normalized to GAPDH. The values are mean ± SD from 4 individual experiments. *** p <0.001
Figure 4. GFP protein expression.
GFP expression after 7days and 2months of AAV-GFP vector administration in carotid artery. The corresponding non-GFP injected carotid artery served as the control. The values are mean ± SD from 4 individual experiments. ***p < 0.001
β-galactosidase staining was visualized in the smooth muscle cells in the coronary, carotid and femoral arteries after 7days of gene delivery (Fig. 5). About 15-20 % of the cells were stained positively as blue-stained nuclei in the arteries. The tissue of the left femoral artery (control) did not show β-gal positive cells. The qPCR data showed expression of LacZ mRNA transcripts on the 7th day after gene transfer (Fig. 3). The level of LacZ mRNA expression was similar when different arteries were compared. There was no expression of GFP in heart, lung and spleen sections 2 months after the gene delivery confirming ing the specificity of the vector.
Figure 5. Lac Z expression.
Lac Z expression in the medial layer of coronary (A), carotid (B), femoral (C) and control femoral (D) artery after 7days of catheter-mediated AAV-LacZ gene transfer. LacZ expression in the medial layer smooth muscle cells is shown in blue color. This is a representative of 3 separate experiments.
The administration of AAV2/9 vector did not significantly affect the serum amylase and C-Reactive protein and plasma fibrinogen levels when compared to the control, suggesting no significant inflammatory reaction (Table 1).
Table I.
Fibrinogen, Serum amylase and CRP levels after 7 days of AAV gene delivery*
| Serum amylase levels (U/L) |
C-Reactive Protein (mg/dL) |
Fibrinogen (mg/dL) |
|
|---|---|---|---|
| Normal Range | 310 ± 90 | 0.66 ± 0.34 | 158 ± 7 |
| After vector delivery | 201 ± 54 | 0.12 ± 0.03 | 140 ± 4 |
The data is obtained 7 days after gene delivery and is expressed as mean ±SD N= 8). The statistical analysis was performed using Single factor ANOVA.
Discussion
In this study, using AAV2/9 vector with tissue-specific promoter, SM22α, and using a local delivery approach we were able to successfully transduce porcine coronary artery VSMCs. The potential toxicity due to the vector was insignificant and therefore, is a promising strategy for gene transfer of a therapeutic gene in the management of cardiovascular disorders.
The efficient delivery and long-term expression of therapeutic genes are necessary for genetic manipulation strategies in the cardiovascular system. A balloon injury method was adopted for introducing the vector to the medial SMCs to decrease the possibility of AAV transduction on unintended sites. Although systemic administration of AAV vectors was much easier it has resulted in significant hepatic tropism in previous studies (13). Another reason for the mechanical injury is that the barrier formed by the endothelium and internal elastic lamina can inhibit the effective diffusion of AAV vectors. In the ex vivo experiments by Maeda et al.(14), AAV-LacZ could not transduce medial VSMCs of aorta although Lac Z transduced cells were detected in the endothelial and adventitial layer. Lynch et al.(15) demonstrated significantly greater transduction in balloon injured carotids than uninjured arteries. In our approach, the injury resulted in the breakage of internal elastic lamina, which could have enabled the vector entry into the medial layer. The method maximized the local disposition of vector while minimizing systemic distribution, thus favoring productive vector-cell collisions.
The cross-packaging approach of AAV serotypes where the genome of one ITR serotype being packaged into a different serotype capsid has allowed broad tissue tropisms, with enhanced gene expression(16). Comparison of AAV-2 vector genomes cross-packaged into capsids of AAV-1 to -6 showed that AAV-1, -4, -5, and -6 capsids increased cardiac transduction efficiency by about 10-fold(17). AAV-9 vectors are recently reported to have similar mode of infection as AAV-2 and has shown to transduce a large number of cells with unique expression pattern (18). Hence, we used an AAV-9 capsid in an AAV-2 vector to increase the transduction of target cells.
In order to increase the specificity of transduction, smooth muscle cells-specific promoter element has been used. In a study, alkaline phosphatase (AP) recombinant protein was detected in neointimal (2.23%) and medial (0.56%) SMCs, but not in endothelial or adventitial cells, following introduction of adenoviral vectors having SM22α promoter with human placental AP gene into balloon-injured pig arteries (19). In contrast, adenoviral vectors with CMV promoter led to AP expression in intimal endothelial and SMC cells (39.14 %) and medial SMCs (2.84%) (19). Although the expression levels were lower compared to using the CMV promoter, the SM22α promoter caused specific transduction of SMCs which is safe and effective to target proliferating SMCs during restenosis. Furthermore, in contrast to the CMV promoter, SM22α-driven recombinant AP expression was not observed in organs outside the vasculature, including the liver or the lung of pigs following gene transfer, suggesting a favorable safety profile. This could be supported by the data in our study where LacZ and GFP positive cells were specifically detected only in the medial layer. This is promising as we intend to use such gene therapy approach to inhibit the proliferation of SMCs following interventional procedures. Ritcher et al.(8) in their approach showed that they were able to transduce VSMCs in the medial layer in vivo, without prior injury to the vessel with an apparent transduction efficiency of up to 58%. This might be due to the CMV promoter used in the vector which is more efficient but less specific compared to the SM22α promoter (20). Thus, our approach could be superior to the ones reported in the literature. Interestingly, we observed a higher percentage of GFP transfected cells after 7 days compared to LacZ. This might be because of the higher molecular weight of LacZ (116kDa) compared to that of eGFP (27 kDa). The higher molecular weight of LacZ might have resulted in slower transfection rate compared to eGFP.
The time for vector delivery in the arteries, especially coronary arteries, should be as minimal as possible to prevent ischemia and reperfusion injury. A high titer of viral vectors compensates for the time restrictions. The AAV viral vectors are feasible to be produced with titers of >1011 per ml. Hence, we introduced a vector titer value of 1 × 1013 pfu at the site of injury. The specificity of the gene delivery was evaluated by qPCR, confocal microscopy and Western blotting. The mRNA transcripts of GFP and LacZ could be observed 7 days after the gene delivery. Protein expression was detectable after 7 days and at least up to 2 months after the vector delivery. We observed an increase in both the mRNA and protein expression of GFP after 2 months of vector delivery when compared to the 7days. Since, in-stent restenosis is a process occurring over a period of time, we ideally wanted the therapeutic gene expression to occur over a relatively longer time period. The current method support the possibility of observing a gene expression even after 2 months of gene delivery, which could be promising while using a therapeutic gene.
Clinical safety and efficacy of any gene therapy depends on the immunological response that ensues following vector administration. Previous studies have shown that while AAV vectors transiently activate cells of the innate immune system, they have a limited capacity to trigger the expression of pro-inflammatory genes and induce inflammation in transduced tissues (21). The innate immune response caused by the AAV2 capsid is reported to be minimal (22). However, no adequate studies are reported on the AAV-9 capsid. The current study demonstrates that after 7days there was no significant increase in the levels of serum amylase, C-reactive protein and plasma fibrinogen, suggesting no significant immune response. This may also be due to the localized delivery of the vectors, where they are not trafficking through the spleen and the lymphoid organs to produce a significant immune reaction.
Thus, AAV2/9 vector could be efficient and promising in in vivo gene transfer with stable reporter gene expression in the SMCs of coronary and peripheral arteries. The transduction is specific and the tissues show long term gene expression. This modified vector may offer a reasonable alternative to other vectors for local arterial wall transduction, especially in treating intimal hyperplasia and restenosis where vascular SMCs are the principal target.
Acknowledgements
This work was supported by the LB692 State of Nebraska Tobacco Settlement Funds to Creighton University. We thank the Gene Therapy Resource Program (GTRP) of the NIH-NHLBI at the University of Pennsylvania Vector Core facility for providing the gene vectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Podsakoff G, Wong KK, Jr., Chatterjee S. Efficient gene transfer into nondividing cells by adeno-associated virus-based vectors. J Virol. 1994;68:5656–5666. doi: 10.1128/jvi.68.9.5656-5666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon I, Schaffer DV. Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm Res. 2008;25:489–499. doi: 10.1007/s11095-007-9431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecchini S, Negrete A, Kotin RM. Toward exascale production of recombinant adeno-associated virus for gene transfer applications. Gene Ther. 2008;15:823–830. doi: 10.1038/gt.2008.61. [DOI] [PubMed] [Google Scholar]
- 5.Ponnazhagan S, Mahendra G, Kumar S, Shaw DR, Stockard CR, et al. Adeno-associated virus 2-mediated antiangiogenic cancer gene therapy: long-term efficacy of a vector encoding angiostatin and endostatin over vectors encoding a single factor. Cancer Res. 2004;64:1781–1787. doi: 10.1158/0008-5472.can-03-1786. [DOI] [PubMed] [Google Scholar]
- 6.Tang JB, Cao Y, Zhu B, Xin KQ, Wang XT, et al. Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. an in vivo study. J Bone Joint Surg Am. 2008;90:1078–1089. doi: 10.2106/JBJS.F.01188. [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Richter M, Iwata A, Nyhuis J, Nitta Y, Miller AD, et al. Adeno-associated virus vector transduction of vascular smooth muscle cells in vivo. Physiol Genomics. 2000;2:117–127. doi: 10.1152/physiolgenomics.2000.2.3.117. [DOI] [PubMed] [Google Scholar]
- 9.Pajusola K, Gruchala M, Joch H, Luscher TF, Yla-Herttuala S, et al. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J Virol. 2002;76:11530–11540. doi: 10.1128/JVI.76.22.11530-11540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buning H, Perabo L, Coutelle O, Quadt-Humme S, Hallek M. Recent developments in adeno-associated virus vector technology. J Gene Med. 2008;10:717–733. doi: 10.1002/jgm.1205. [DOI] [PubMed] [Google Scholar]
- 11.Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, et al. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 12.Pacak CA, Sakai Y, Thattaliyath BD, Mah CS, Byrne BJ. Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet Vaccines Ther. 2009;7:3. doi: 10.1186/1479-0556-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koeberl DD, Alexander IE, Halbert CL, Russell DW, Miller AD. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda Y, Ikeda U, Ogasawara Y, Urabe M, Takizawa T, et al. Gene transfer into vascular cells using adeno-associated virus (AAV) vectors. Cardiovasc Res. 1997;35:514–521. doi: 10.1016/s0008-6363(97)00163-6. [DOI] [PubMed] [Google Scholar]
- 15.Lynch CM, Clowes MM, Osborne WR, Clowes AW, Miller AD. Long-term expression of human adenosine deaminase in vascular smooth muscle cells of rats: a model for gene therapy. Proc Natl Acad Sci U S A. 1992;89:1138–1142. doi: 10.1073/pnas.89.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller OJ, Leuchs B, Pleger ST, Grimm D, Franz WM, et al. Improved cardiac gene transfer by transcriptional and transductional targeting of adeno-associated viral vectors. Cardiovasc Res. 2006;70:70–78. doi: 10.1016/j.cardiores.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Howarth JL, Lee YB, Uney JB. Using viral vectors as gene transfer tools (Cell Biology and Toxicology Special Issue: ETCS-UK 1 day meeting on genetic manipulation of cells) Cell Biol Toxicol. 26:1–20. doi: 10.1007/s10565-009-9139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akyurek LM, Yang ZY, Aoki K, San H, Nabel GJ, et al. SM22alpha promoter targets gene expression to vascular smooth muscle cells in vitro and in vivo. Mol Med. 2000;6:983–991. [PMC free article] [PubMed] [Google Scholar]
- 20.Ribault S, Neuville P, Mechine-Neuville A, Auge F, Parlakian A, et al. Chimeric smooth muscle-specific enhancer/promoters: valuable tools for adenovirus-mediated cardiovascular gene therapy. Circ Res. 2001;88:468–475. doi: 10.1161/01.res.88.5.468. [DOI] [PubMed] [Google Scholar]
- 21.Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, et al. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]