Abstract
Background
The kappa opioid receptor (KOR) system contributes to the prodepressive and aversive consequences of stress, and is implicated in the facilitation of conditioned fear and anxiety in rodents. Here we sought to identify neural circuits that mediate KOR system effects on fear and anxiety in rats.
Methods
We assessed whether fear conditioning induces plasticity in KOR or dynorphin (the endogenous KOR ligand) mRNA expression in the basolateral (BLA) and central (CeA) nuclei of the amygdala, hippocampus (HIP), or striatum (STR). We then assessed whether microinfusions of the KOR antagonist JDTic (0–10.0 μg/side) into the BLA or CeA affect the expression of conditioned fear or anxiety. Finally, we examined whether fear extinction induces plasticity in KOR mRNA expression that relates to the quality of fear extinction.
Results
Fear conditioning upregulated KOR mRNA in the BLA by 65%, and downregulated it in the STR by 22%, without affecting KOR levels in the CeA or HIP, or dynorphin levels in any region. KOR antagonism in either the BLA or CeA decreased conditioned fear in the fear-potentiated startle paradigm, whereas KOR antagonism in the BLA but not the CeA produced anxiolytic-like effects in the elevated plus maze. Effective fear extinction was associated with a 67% reduction in KOR mRNA in the BLA.
Conclusions
These findings suggest that fear conditioning and extinction dynamically regulate KOR expression in the BLA, and provide evidence that the BLA and CeA are important neural substrates mediating the anxiolytic-like effects of KOR antagonists in models of fear and anxiety.
Keywords: fear-potentiated startle, elevated plus maze, quantitative PCR, conditioned fear, anxiety, JDTic
INTRODUCTION
The kappa opioid receptor (KOR) system is widely implicated in mediating the prodepressive emotional and behavioral consequences of stress. KOR activation produces dysphoria in humans (1, 2) and prodepressive-like behavioral signs in rodents, including dysphoria (3, 4), anhedonia (5–7), and passive coping strategies (8–13). In contrast, KOR antagonism or ablation of the genes encoding KORs or their endogenous ligand dynorphin have antidepressant-like effects (9–14). We reported that systemic administration of KOR antagonists also produces anxiolytic-like effects in models of conditioned fear and anxiety in rats (15). Recent work has confirmed these findings (16, 17) and demonstrated an anxiolytic-like phenotype in independent lines of prodynorphin (PDyn, the dynorphin precursor) knockout mice that is reproduced in wild-type mice treated with a KOR antagonist (18, 19). Thus the KOR system appears to be an important mediator of both prodepressive and anxious mood states. Given the high comorbidity of clinical depression and anxiety disorders (20, 21), and their association with stress (20, 22, 23), these findings suggest that dysregulation of KOR signaling within neural circuits involved in mood and motivation contributes to the etiology of depression and anxiety disorders.
Studies characterizing the role of KOR systems in depressive behavior have focused mainly on the mesocorticolimbic dopamine system and hippocampus (HIP) (5, 7, 11, 12, 24–26). However, little is known about the neural circuits that mediate KOR system effects on fear and anxiety. KORs and dynorphin are expressed throughout brain areas involved in fear and anxiety, including in the basolateral (BLA) and central (CeA) nuclei of the amygdala and the HIP, in humans and rodents (27–30). The amygdala is critically involved in conditioned fear and anxiety-related behaviors, and many stress hormones and neuropeptides modulate fear learning and memory via effects in this region (31–39). Although it is not known if KOR signaling in the amygdala modulates fear learning and memory, evidence suggests that plasticity in KOR system gene expression in limbic brain regions contributes to the enduring prodepressive consequences of stress (11–13, 25, 26, 40). Because fear memory is associated with altered gene expression in a broad neural circuitry that includes the amygdala and HIP (41–46), we hypothesized that fear conditioning might induce plasticity in KOR system gene expression in brain regions involved in fear and anxiety, and that KOR signaling in these regions might facilitate conditioned fear and anxiety.
Here we used qPCR to examine whether fear learning induces plasticity in KOR or PDyn mRNA expression in the BLA, CeA, HIP, and striatum (STR). We then examined the behavioral consequences of disrupting KOR signaling in the amygdala using local microinfusions of the KOR antagonist JDTic into the BLA or CeA prior to testing conditioned fear and anxiety. Finally, we examined whether fear extinction training alters KOR mRNA expression in the BLA in a manner that relates to the quality of extinction.
MATERIALS AND METHODS
Rats
A total of 198 male Sprague-Dawley rats (325–375g; Charles River Laboratories, Raleigh, NC) were used. Rats were singly housed following surgery and maintained on a 12 hr light-dark cycle with unrestricted access to food and water. Protocols were approved by McLean Hospital’s Institutional Animal Care Committee and consistent with National Institutes of Health policies.
Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (qPCR)
Thirty-eight rats were used to determine if fear conditioning affects KOR or PDyn mRNA expression in the BLA, CeA, and HIP; we also assessed gene expression in a portion of the striatum (STR) dorsal to the amygdala in anticipation of using this region as a dorsal control site in drug microinfusion studies. An additional 20 rats were used to determine if fear extinction affects KOR mRNA expression in the BLA. We assessed levels of glutamic acid decarboxylase 65 and 67 kDa (GAD65, GAD67) and PDyn in the BLA and CeA as an index of dissection accuracy. GAD65 levels are decreased in the BLA 24 hr after fear conditioning (47, 48), and thus GAD65 expression was used to confirm that our training parameters induced molecular correlates of conditioned fear. On four consecutive days, rats were briefly handled and then placed for 30 min into unilluminated fear-potentiated startle (FPS) chambers (60 dB background noise) for acclimation. The next day, rats received a training session consisting of 10 light-shock presentations (Light+Shock; 3.7 sec light conditioned stimulus (CS) co-terminating with a 0.5 sec, 0.6 mA foot-shock unconditioned stimulus (US); ~3 min inter-trial interval [ITI]). A separate group received 10 light presentations (Light Alone). Eighteen hours after training, 20 rats (10 Light+Shock, 10 Light Alone) were killed by decapitation for qPCR analyses, and 24 hr after training, 18 rats were tested for FPS to confirm that our training parameters induced conditioned fear. The remaining 20 rats were used to assess the molecular effects of fear extinction. Methods for tissue collection and qPCR are provided in the Supplement.
Drugs
JDTic was synthesized at Research Triangle Institute (Research Triangle Park, NC) and dissolved in artificial cerebrospinal fluid (Harvard Apparatus, Holliston, MA); drug doses are based on the salt form of JDTic.
Surgery and Microinfusions
To determine the effect of KOR antagonism in the amygdala on fear and anxiety, rats received surgery to implant bilateral cannulas in the BLA (n=56) or CeA (n=54), as previously described (49). Cannulas (23-gauge; Plastics One, Roanoke, VA) were directed 1.5 mm above the BLA (relative to bregma in mm: anteroposterior (AP) −2.8, mediolateral (ML) ±5.1, ventral to dura (DV) −7.7) or CeA (AP −2.6, ML ±4.5, DV −7.3). Stainless steel obturators and infusion stylets (30-gauge) extended 1.5 mm beyond the cannula. A dorsal control group had bilateral cannulas implanted in the STR, 2.5 mm above the BLA (AP −2.8, ML ±5.1, DV −5.2) or 1.5 mm above the CeA (AP −2.6, ML ±4.5, DV −5.8) infusion sites. Behavioral data for sites dorsal to the BLA or CeA did not differ and thus were combined into a single group.
Microinfusions were administered as previously described (49). Rats received infusions of JDTic (3–10 μg/side; 5.4–18.0 nmol) in 0.5 μL/side over 5 min, 24 hr prior to behavioral testing. JDTic is a highly selective KOR antagonist (50) that has a slow onset of antagonism (effects peak ~24 hr after systemic administration) that is maintained for at least 7 days (51, 52).
Fear-Potentiated Startle (FPS)
Seventy-five rats were used to test the effect of KOR antagonism in the amygdala on FPS (BLA, n=38; CeA, n=24; STR, n=13). A description of the FPS apparatus (Med Associates, St. Albans VT) is provided in the Supplement. Before surgery, rats received a habituation session consisting of 100 startle stimuli (50-msec, 100-dB, 30-sec inter-stimulus interval [ISI]) to determine baseline startle magnitudes. Following recovery from surgery, rats received two habituation sessions separated by 48 hr to acclimate them to the holding chambers and startle stimuli. Two and five days later, rats received conditioning sessions consisting of 10 light-shock pairings (3.7-sec light co-terminating with a 0.5-sec, 0.6-mA foot-shock, ~3-min ITI). Three days after conditioning, rats received a pre-test consisting of 15 habituating startle stimuli and 20 startle stimuli in which half were preceded by the light CS, to form experimental groups with similar levels of fear. Rats received microinfusions of JDTic or vehicle 20 min after the pre-test and received a full-length FPS test 24 hr later consisting of 15 habituating startle stimuli and 60 startle stimuli in which half were preceded by the CS. The operational measure of fear was the difference in startle in the presence and absence of the CS (percentage of FPS=([startle in the presence of the light–startle in the dark]/startle in the dark) × 100).
Fear Extinction
To determine if fear extinction alters KOR mRNA expression in the BLA, 20 rats were subjected to fear conditioning as described above. One day later, rats were tested for acquisition of FPS, and the following day were given extinction training consisting of 60 presentations of the CS (3.7 sec light, 30 sec ISI) alone. The next day, rats were retested for FPS to assess the magnitude of fear extinction. Rats with the lowest (“good” extinction, n=4) or highest (“poor” extinction, n=4) levels of FPS were inferred to have effectively or ineffectively extinguished conditioned fear, respectively, and were killed immediately after testing by decapitation and gene expression was analyzed using qPCR (see Supplement).
Data for each brain region were analyzed separately using one-way (treatment) ANOVAs, and significant effects were analyzed using Dunnett’s post hoc tests. Two-way (treatment × time, group × time) ANOVAs were used to analyze the time course of FPS, followed by Bonferroni post hoc tests. Studies comparing two treatment groups were analyzed using Student’s t tests.
Elevated Plus Maze (EPM)
Sixty-five rats were used to test the effect of KOR antagonism in the amygdala on anxiety in the EPM (BLA, n=18; CeA, n=30; STR, n=17). Methods for EPM and histology are presented in the Supplement.
RESULTS
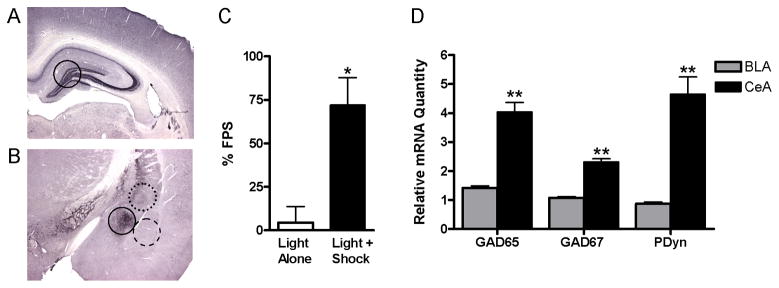
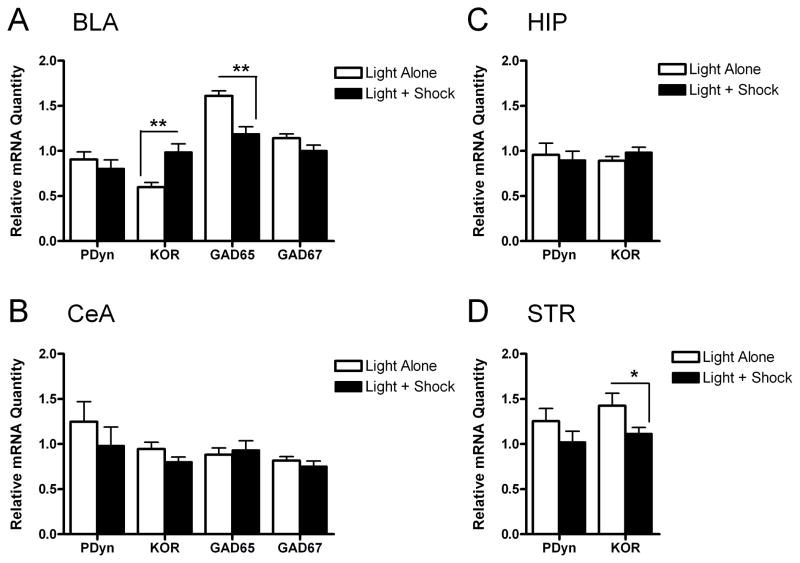
Tissue punches were obtained from the BLA, CeA, HIP, and STR, which have varying degrees of dynorphin expression (27, 29) (Fig. 1A,B). Rats that received Light+Shock training displayed marked conditioned fear, as indicated by a 72% increase in startle in the presence of the light (Fig. 1C). Rats exposed to Light Alone did not show altered startle in the presence of the light and had significantly lower FPS (4%) than rats receiving Light+Shock training (t16=2.87, P<0.05) (Fig. 1C), demonstrating conditioned fear only in rats that received paired training. We confirmed the accuracy of our dissections by comparing levels of GAD65, GAD67, and PDyn in the BLA and CeA. The CeA contains high levels of dynorphin (27) and a much higher density of GABAergic neurons (53, 54) than the BLA, which is composed primarily of glutamatergic neurons and contains little dynorphin (27, 55, 56). CeA dissections had significantly higher levels of GAD65 (U=0.0, n1=n2=10, P<0.01), GAD67 (U=0.5, n1=n2=10, P<0.01), and PDyn mRNA (U=0.0, n1=n2=10, P<0.01) than BLA dissections (Fig. 1D). Histological analyses also confirmed the accuracy of our dissections (not shown). Melt curves and gel electrophoresis confirmed the specificity of each primer pair used to amplify KOR, PDyn, and control gene mRNA (not shown). Light+Shock training increased KOR mRNA by 65% (U=7.0, n1=n2=10, P<0.01) and decreased GAD65 mRNA by 26% (U=10.5, n1=n2=10, P<0.01) in the BLA, without affecting PDyn or GAD67 mRNA (Fig 2A), consistent with prior studies (47, 48). Light+Shock training also decreased KOR mRNA in the STR by 22% (U=20.0, n1=n2=10, P<0.05), without affecting PDyn mRNA (Fig. 2D). There was no effect of Light+Shock training on KOR or PDyn mRNA in the CeA (Fig. 2B) or HIP (Fig. 2C).
Figure 1. Control data for quantitative PCR analysis of gene expression.
(A and B) Micrographs of coronal rat brain sections showing prodynorphin immunoreactivity (methods in the Supplement) and the approximate location and size of bilateral tissue punches (1mm3) taken from (A) the hippocampus (solid-line) and (B) the central nucleus of the amygdala (CeA, solid-line), basolateral nucleus of the amygdala (BLA, dashed-line), and striatum (STR, dotted-line). (C) Rats that received Light+Shock training (n=12) showed increased fear-potentiated startle (FPS) compared to those that received Light Alone training (n=6) (mean ± S.E.M.; Student’s t test). (D) Levels of glutamic acid decarboxylase 65 and 67 kDa (GAD65, GAD67) and prodynorphin (PDyn) were higher in tissue punches taken from the CeA compared to the BLA (n=20; mean ± S.E.M.; Mann-Whitney tests). *P<0.05 vs. Light Alone (C), **P<0.01 vs. BLA tissue punches (D).
Figure 2. Fear conditioning increases the relative quantity of kappa opioid receptor (KOR) mRNA in the basolateral nucleus of the amygdala (BLA).
(A) Fear conditioning increased KOR mRNA and decreased glutamic acid decarboxylase 65 kDa (GAD65) mRNA in the BLA, without affecting prodynorphin (PDyn) or glutamic acid decarboxylase 67 kDa (GAD67) mRNA expression (n=10/group; mean ± S.E.M.; Mann-Whitney tests). Fear conditioning did not affect mRNA quantities in the central nucleus of the amygdala (CeA) (B) or in the hippocampus (HIP) (C), but decreased KOR mRNA in the striatum (STR), without affecting PDyn mRNA (D). *P<0.05, **P<0.01 vs. Light Alone control.
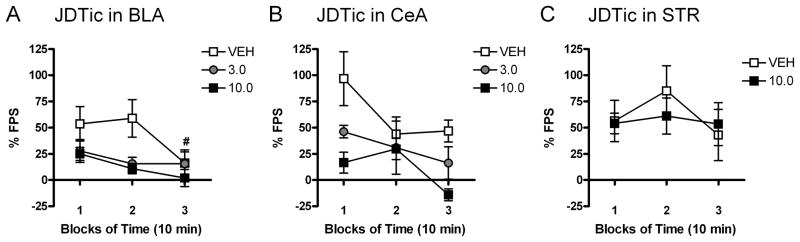
We microinfused the KOR antagonist JDTic into the BLA, CeA, or STR to test the hypothesis that KOR signaling within these regions contributes to conditioned fear. Representative images of cannula tracks and microinfusion placements for these regions are provided in Figure S1 (see Supplement). Time course analyses indicated that JDTic effects were most apparent within the first 20 min of the 30-min test session (Fig. 3). The magnitude of FPS tended to decrease during the 30-min test session, and was significantly reduced at the 30-min time point (block 3) in rats that received microinfusions of vehicle into the BLA (t9=2.82, P<0.05) (Fig. 3A; Student’s t test versus block 2), consistent with within-session extinction (57). Reductions in FPS were not detected in vehicle treated rats in the CeA (B) and STR (C) groups, which likely reflects higher levels of initial fear in these groups. Comparison of FPS in rats that received vehicle into the BLA, CeA, or STR (Fig. 3A–C) suggested that conditioning produced different levels of fear depending on the location of the cannulas, although these effects were not statistically significant. Rats with cannulas in the BLA showed ~54% FPS in first 10-min block of testing (Fig. 3A), while rats with cannulas in the CeA showed ~97% FPS (Fig. 3B), suggesting that cannulation of the BLA decreased the efficacy of training. The within-session extinction profile for STR rats treated with vehicle (Fig. 3C), suggests an inverted U-shaped function (i.e., FPS magnitudes tended to increase in block 2); considering that high levels of fear can elicit a freezing response that competes with expression of potentiated startle (58), these data suggest that our training parameters established the highest levels of fear in this group.
Figure 3. Kappa opioid receptor (KOR) antagonism in the basolateral (BLA) and central (CeA) nuclei of the amygdala affects the time course of fear-potentiated startle (FPS).
Microinfusions of the KOR antagonist JDTic or vehicle were administered 24 hr before FPS testing. The effects of JDTic in the BLA (A), CeA (B), and striatum (STR) (C) on FPS were most apparent within the first 20 min (blocks 1–2) of the 30-min test session (n=5–12/group; mean ± S.E.M.). FPS was significantly reduced at the 30-min time point (block 3) in rats that received microinfusions of vehicle into the BLA (A) (Student’s t tests, # denotes significant difference from block 2, P<0.05). Significant reductions in FPS were not detected in vehicle treated rats in the CeA (B) and STR (C) groups, which may be due to increased levels of fear in these groups. The magnitude of FPS in block 1 tended to be higher in vehicle treated rats with cannulas in CeA (B) than in the BLA (A), and the extinction profile for vehicle treated rats with cannulas in the STR (C) suggests an inverted U-shaped function relating FPS to fear that is characteristic of high fear levels (58).
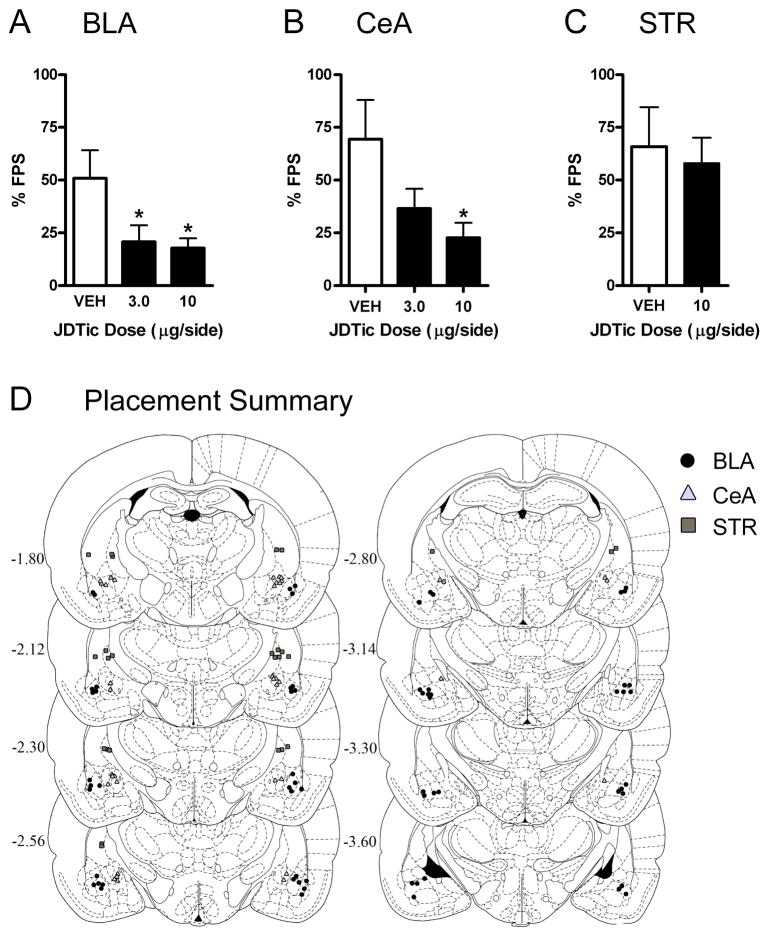
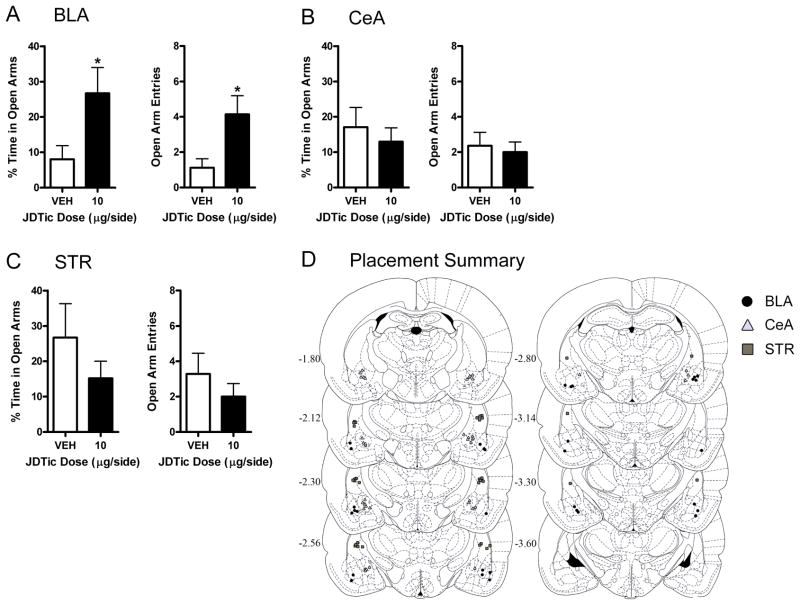
In subsequent analyses we examined KOR antagonist effects on FPS during the first 20 min of testing, which was associated with the most reliable levels of fear. Microinfusion of JDTic into the BLA affected the expression of the conditioned response (F2,30=4.15, P<0.05), without affecting baseline startle (not shown): FPS was decreased in rats after treatment with 3.0 (P<0.05, Dunnett’s tests) or 10 (P<0.05) μg/side (Fig. 4A). Microinfusion of JDTic into the CeA also affected FPS (F2,16=4.05, P<0.05) without affecting baseline startle (not shown): FPS was decreased in rats after treatment with 10 μg/side (P<0.05) (Fig. 4B). Microinfusion of JDTic into a dorsal control site in the STR did not affect FPS (Fig. 4C) or baseline startle (not shown). Histological analyses were used to identify rats with bilateral cannula placements in the BLA (n=33; 10–12/group), CeA (n=19, 5–8/group), or STR (n=13, 6–7/group) (Fig. 4D). Rats with misplaced cannulas (n=10) were excluded from the analyses.
Figure 4. Kappa opioid receptor (KOR) antagonism in the basolateral (BLA) and central (CeA) nuclei of the amygdala decreases fear-potentiated startle (FPS).
Microinfusions of the KOR antagonist JDTic or vehicle were administered 24 hr prior to FPS testing. JDTic in the BLA (A) or CeA (B) decreased FPS (mean ± S.E.M.; Dunnett’s tests), without affecting baseline startle (not shown). (C) JDTic in the striatum (STR) did not affect FPS (Student’s t test). (D) Summary of cannula tip placements in the BLA (black circles; n=10–12/group), CeA (gray triangles; n=5–8/group), or STR (gray squares; n=6–7/group). *P<0.05 vs. vehicle. Images in D published in The Rat Brain in Stereotaxic Coordinates, 3rd ed. (93) and reprinted with permission, Copyright Elsevier (1996).
We also microinfused JDTic into the BLA, CeA, or STR to examine effects on unconditioned anxiety in the EPM. Microinfusion of JDTic into the BLA affected behavior in the EPM: JDTic (10 μg/side) increased the percentage of time rats spent in the open arms (t14=2.40, P<0.05) and the number of entries rats made into the open arms (t14 = 2.78, P < 0.05) (Fig. 5A), without affecting closed arm entries or maze crosses (not shown). In contrast, microinfusion of JDTic into the CeA (Fig. 5B) or STR (Fig. 5C) did not affect the percentage of time rats spent in the open arms or open arm entries, or closed arm entries or maze crosses (not shown). Comparison of open arm exploration in rats that received microinfusions of vehicle into the BLA, CeA, or STR (Fig. 5A–C) suggested that basal levels of anxiety depended on the location of the cannulas, although these effects were not statistically significant. Histological analyses were used to identify rats with bilateral cannula placements in the BLA (n=16, 7–9/group), CeA (n=20, 10/group), or STR (n=16, 7–9/group) (Fig. 5D). Rats that had misplaced cannulas (n=10) or fell from the maze (n=3) were excluded.
Figure 5. Kappa opioid receptor (KOR) antagonism in the basolateral (BLA) and central (CeA) nuclei of the amygdala increases open arm exploration in the elevated plus maze (EPM).
Microinfusions of the KOR antagonist JDTic or vehicle were administered 24 hr prior to EPM testing. (A) JDTic in the BLA increased the percentage of time rats spent in the open arms and the number of open arm entries (mean ± S.E.M.; Student’s t tests). JDTic in the CeA (B) or striatum (STR) (C) did not affect the percentage of time rats spent in the open arms or the number of open arm entries. None of the drug treatments affected closed arm entries or maze crosses (not shown). (D) Summary of cannula tip placements in the BLA (black circles; n=7–9/group), CeA (gray triangles; n=10/group), or STR (gray squares; n=7–9/group). *P<0.05, **P<0.01 vs. vehicle. Images in D published in The Rat Brain in Stereotaxic Coordinates, 3rd ed. (93) and reprinted with permission, Copyright Elsevier (1996).
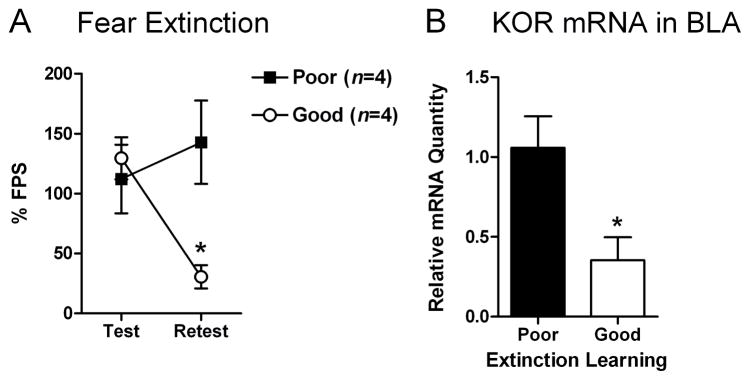
Since KOR mRNA expression is upregulated by fear conditioning, we examined if KOR mRNA expression would be downregulated by extinction training. Rats with good versus poor extinction learning had significantly different levels of FPS during the retest (group × time interaction: F1,6=6.87, P<0.05): good extinction learners had lower levels of FPS during the retest than poor extinction learners (P<0.05) (Fig. 6A). Rats with good extinction learning had 67% less KOR mRNA in the BLA than those with poor extinction learning (t6=2.88, P<0.05) (Fig. 6B).
Figure 6. Effective fear extinction decreases the relative quantity of kappa opioid receptor (KOR) mRNA in the basolateral nucleus of the amygdala (BLA).
Rats were subjected to fear conditioning (10 light-shock pairings) and tested 24 hr later for fear-potentiated startle (FPS). One day later, rats were given extinction training (60 presentations of the light alone) and the next day they were retested for FPS. Rats with the lowest (“good” extinction; n=4) or highest (“poor” extinction; n=4) levels of FPS were killed immediately after testing by decapitation and gene expression in the BLA was analyzed using qPCR. (A) Rats that had good extinction learning had significantly less FPS during the retest than rats with poor extinction learning (mean ± S.E.M.). (B) Rats that had good extinction learning had 67% less KOR mRNA in the BLA than those that had poor extinction learning. *P<0.05.
DISCUSSION
Systemic administration of KOR antagonists produces anxiolytic-like effects in models of fear and anxiety (15). Here we identify the BLA as a site of increased KOR mRNA expression following fear learning and decreased KOR mRNA expression following effective fear extinction. We also identify the BLA and CeA as regions that mediate the anxiolytic-like effects of KOR antagonists. These findings provide evidence that fear conditioning and extinction dynamically regulate KOR expression in the BLA—a region critically involved in fear learning—and that KOR signaling in the BLA and CeA plays an important role in the expression of conditioned fear. Our observation that KOR antagonism in the BLA decreases anxiety in the EPM is consistent with evidence that KOR signaling in the BLA mediates the anxiogenic effects of corticotropin releasing factor (CRF) and stress (19, 59).
We used qPCR to determine if fear conditioning induces plasticity in KOR or PDyn gene expression in fear circuits. We examined gene expression 18 hr after training based on evidence that PDyn and GAD65 mRNA expression are stably altered following stress (25) or fear conditioning (47, 48), respectively; thus changes in gene expression could reflect molecular correlates of fear acquisition or consolidation. Fear was not quantified in rats used for the qPCR analyses to avoid detecting changes in gene expression induced by the behavioral procedures themselves. However, we obtained indirect evidence that our training parameters induced conditioned fear: separate rats that received the same light-shock training showed significant FPS, and these training parameters produced molecular correlates of memory consolidation (decreased GAD65 mRNA in the BLA (47, 48)) in rats used for qPCR analyses. The observation that fear conditioning upregulates KOR mRNA expression in the BLA is consistent with work establishing that this region is a critical site of plasticity during fear learning (33, 43, 60, 61) and that KOR signaling in the BLA contributes to the anxiogenic effects of stress (19, 59). Reductions in KOR mRNA in the STR were unexpected, since this region is not usually considered an essential component of fear circuitry. However, the STR is involved in forms of aversive learning in humans (62, 63) and rodents (64–67). In addition, our dissections included portions of the amygdalostriatal transition area, which shows high levels of plasticity during fear learning and may contribute to fear-related behaviors (68, 69). These analyses suggest that upregulation of KOR mRNA expression in the BLA and downregulation in the STR contribute to the development or expression of conditioned fear.
Additional studies are needed to confirm that alterations in KOR mRNA levels are accompanied by corresponding alterations in protein levels; this work is made difficult by the current unavailability of highly selective KOR antibodies. The high affinity of dynorphin for KORs (70) suggests that even small changes in KOR protein expression could have physiologically-relevant effects. Moreover, our microinfusion studies and fear extinction studies provide compelling evidence that KOR signaling in the BLA facilitates conditioned fear and anxiety, and that effective fear extinction involves a downregulation of KOR signaling in the BLA. Because both stress and associative learning can regulate gene expression (42, 43, 71), our findings provide rationale for future work to examine how these processes influence KOR expression.
The absence of altered PDyn expression was unexpected considering prior reports that stress increases PDyn expression in limbic brain regions (11, 25, 72), and that fear conditioning produces neuroadaptations associated with stress (26). Our training parameters were selected to induce moderate fear levels compatible with FPS (58) and may not have been optimal for affecting PDyn expression; alternatively, PDyn mRNA regulation might have a different time course or be highly localized to subregions within these nuclei (37). Future studies assessing the spatiotemporal distribution of mRNA and protein expression may provide insight into how plasticity in KOR systems contributes to conditioned fear.
KOR antagonism in the BLA and CeA markedly reduced FPS, and KOR antagonism in the BLA increased open arm exploration in the EPM. These anxiolytic-like effects were site-specific: KOR antagonism in the STR was without effect in both paradigms and KOR antagonism in the CeA was without effect in the EPM. The similarity between the dose-effect functions for intra-BLA and -CeA JDTic in the FPS paradigm suggests local drug effects that are not the result of diffusion to neighboring regions (73, 74). However, it is likely that our drug microinfusions affected KORs within the intercalated cell masses (ITCs), which lie interposed between the BLA and CeA and receive moderate dynorphinergic innervation (27). While the ITCs are difficult to target selectively because of their small size and diffuse distribution, they have a critical role in gating amygdala activity (75–78). Nevertheless, the anxiolytic-like effects of intra-amygdala KOR antagonism are consistent with prior evidence that systemic administration of KOR antagonists reduces conditioned fear and anxiety (15, 16, 18). Importantly, intra-amygdala JDTic did not affect baseline startle—a measure of sensory function—in the FPS paradigm or locomotor activity in the EPM, providing evidence that KOR antagonism selectively affects fear and anxiety-related behaviors. Because fear conditioning decreases KOR mRNA expression in the STR, we might not have been able to detect further behavioral effects of KOR antagonism in the STR in the FPS paradigm. However, the lack of effect of KOR antagonism in the STR in the EPM suggests that KOR signaling in this region does not contribute to basal anxiety levels.
We also examined if the quality of fear extinction would relate to KOR mRNA levels in the BLA. Prior studies have shown that extinction learning is associated with reversal of neuroadaptations that accompany fear learning (48, 79, 80). The reduction in KOR mRNA expression in the BLA of rats that were good (compared to poor) extinction learners is consistent with these findings, and suggests that KOR levels in the BLA regulate the magnitude of conditioned fear. Considering that KOR signaling is an important mediator of both the rapid and cumulative effects of stress (81), our findings raise the possibility that prior and ongoing stress may produce long-term changes in amygdala function through alterations in KOR expression and signaling. Moreover, because KOR mRNA expression differed between good and poor extinction learners, understanding the molecular mechanisms that regulate KOR expression and signaling in the amygdala might provide insight into novel treatments for clinical anxiety disorders that involve impaired fear extinction, such as posttraumatic stress disorder (57).
In prior studies of systemic KOR antagonist effects on FPS, we were not able to distinguish between KOR antagonist effects on distinct phases of fear learning and memory due to the long-lasting effects of these drugs (15). Here, microinfusions of JDTic were administered three days after training—well after the acquisition and consolidation of fear memory (82)—and 24 hr prior to testing, providing evidence that KOR antagonism disrupted the expression of conditioned fear. These effects could be mediated by blockade of the acute actions of dynorphin at KORs during testing; recent work has shown that other stressors that induce anxiogenic-like responses in mice trigger dynorphin-mediated KOR activation in the BLA (19). Considering that KOR antagonists have a slow onset and prolonged duration of antagonism (52), KOR antagonism may have produced neuroadaptations that reversed or counteracted the development or maintenance of conditioned fear. Consistent with this hypothesis, PDyn knockout mice and wild-type mice treated with KOR antagonist for 48 hr show decreased serum corticosterone levels and altered CRF and neuropeptide Y expression in the amygdala and hypothalamus (18), suggesting that KOR antagonism induces neuroadaptations that could indirectly affect conditioned fear. Alternatively, KOR antagonism after the pre-test may have interfered with the reconsolidation of fear memory (83, 84), a process that depends on intact intracellular signaling and protein synthesis (85, 86). Although KOR antagonism alters intracellular signaling (50, 87, 88), disrupting KOR signaling does not produce associative learning deficits in other paradigms that involve repeated training sessions and therefore multiple rounds of memory formation, retrieval, and reconsolidation (4, 10, 40, 89). Thus KOR antagonism may reduce the aversive quality of fear-associated cues through acute effects on KOR signaling or neuroadaptations. Similar mechanisms may underlie the anxiolytic-like effects of systemic and intra-BLA KOR antagonism in the EPM, which occur when KOR antagonists are administered 1–7 days prior to testing (15, 19). Delineation of the mechanism(s) of KOR system effects on fear and anxiety, and replication of these results with a structurally dissimilar KOR antagonist, is a high priority for future research and will be facilitated by the development of KOR antagonists with improved pharmacokinetics.
The effects of KOR signaling on neuronal activity in the amygdala are not well understood, although there is some evidence that KOR activation inhibits excitatory synaptic transmission in the BLA (90) and preferentially inhibits local interneurons in the CeA (91, 92). Future studies are needed to determine the mechanism(s) of KOR-mediated effects within the amygdala, and to examine the role of KORs outside the amygdala in fear and anxiety. For example, recent work suggests that KOR activation in the nucleus accumbens produces anhedonia without affecting the magnitude of fear (26). Nevertheless, the present studies provide evidence that fear conditioning and extinction dynamically regulate KOR mRNA expression in the BLA, and that KOR antagonism in the amygdala can produce anxiolytic-like effects. These findings suggest that treatments which decrease KOR function in the amygdala may have utility as therapeutics for anxiety disorders.
Supplementary Material
Acknowledgments
This research was funded by grants from the National Institutes of Health (F31MH078473, to ATK; F32DA026250, to JWM; R01DA009045, to FIC; R01MH074000, to CK; and R01MH063266, to WAC).
Footnotes
FINANCIAL DISCLOSURES
Dr. Knoll, Dr. Muschamp, Ms. Daws, Dr. Ferguson, Dr. Dietz, Dr. Meloni, Dr. Nestler, and Dr. Konradi report no biomedical financial interests or potential conflicts of interest. Dr. Carlezon discloses that he and McLean Hospital are co-owners of a US patent claiming the use of kappa-opioid receptor antagonists in the treatment of depression. Dr. Carroll discloses that he and the Research Triangle Institute are co-owners of US patents claiming the composition of JDTic.
Supplementary material cited in this article is available online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 2.Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9:187–198. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- 4.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 6.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 7.Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 12.Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- 16.Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolanos Guzman CA. Kappa-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology. 2009;34:1339–1350. doi: 10.1038/npp.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr GV, Lucki I. Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology (Berl) 2010;210:295–302. doi: 10.1007/s00213-010-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 22.Axelson DA, Birmaher B. Relation between anxiety and depressive disorders in childhood and adolescence. Depress Anxiety. 2001;14:67–78. doi: 10.1002/da.1048. [DOI] [PubMed] [Google Scholar]
- 23.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 24.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muschamp JW, Van’t Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, et al. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.5973-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- 28.Hurd YL. Differential messenger RNA expression of prodynorphin and proenkephalin in the human brain. Neuroscience. 1996;72:767–783. doi: 10.1016/0306-4522(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 29.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 30.Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- 31.Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci U S A. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28:12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang W, Wilson SP, Wilson MA. Overexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepines. Neuropsychopharmacology. 2000;22:77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 36.Nair HP, Gutman AR, Davis M, Young LJ. Central oxytocin, vasopressin, and corticotropin-releasing factor receptor densities in the basal forebrain predict isolation potentiated startle in rats. J Neurosci. 2005;25:11479–11488. doi: 10.1523/JNEUROSCI.2524-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrovich GD, Scicli AP, Thompson RF, Swanson LW. Associative fear conditioning of enkephalin mRNA levels in central amygdalar neurons. Behav Neurosci. 2000;114:681–686. doi: 10.1037//0735-7044.114.4.681. [DOI] [PubMed] [Google Scholar]
- 38.Rotzinger S, Vaccarino FJ. Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. J Psychiatry Neurosci. 2003;28:171–181. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z, Yang Y, Walker DL, Davis M. Effects of substance P in the amygdala, ventromedial hypothalamus, and periaqueductal gray on fear-potentiated startle. Neuropsychopharmacology. 2009;34:331–340. doi: 10.1038/npp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 41.Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mei B, Li C, Dong S, Jiang CH, Wang H, Hu Y. Distinct gene expression profiles in hippocampus and amygdala after fear conditioning. Brain Res Bull. 2005;67:1–12. doi: 10.1016/j.brainresbull.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav Neurosci. 1999;113:276–282. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- 45.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pape HC, Stork O. Genes and mechanisms in the amygdala involved in the formation of fear memory. Ann N Y Acad Sci. 2003;985:92–105. doi: 10.1111/j.1749-6632.2003.tb07074.x. [DOI] [PubMed] [Google Scholar]
- 49.Chartoff EH, Barhight MF, Mague SD, Sawyer AM, Carlezon WA., Jr Anatomically dissociable effects of dopamine D1 receptor agonists on reward and relief of withdrawal in morphine-dependent rats. Psychopharmacology (Berl) 2009;204:227–239. doi: 10.1007/s00213-008-1454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 51.Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 52.Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, et al. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 53.Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- 54.McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- 55.Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- 56.McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- 57.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 58.Walker DL, Cassella JV, Lee Y, De Lima TC, Davis M. Opposing roles of the amygdala and dorsolateral periaqueductal gray in fear-potentiated startle. Neurosci Biobehav Rev. 1997;21:743–753. doi: 10.1016/s0149-7634(96)00061-9. [DOI] [PubMed] [Google Scholar]
- 59.Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 60.Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 61.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 62.Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc Lond B Biol Sci. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira TL, Shammah-Lagnado SJ, Bueno OF, Moreira KM, Fornari RV, Oliveira MG. The indirect amygdala-dorsal striatum pathway mediates conditioned freezing: insights on emotional memory networks. Neuroscience. 2008;153:84–94. doi: 10.1016/j.neuroscience.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Viaud MD, White NM. Dissociation of visual and olfactory conditioning in the neostriatum of rats. Behav Brain Res. 1989;32:31–42. doi: 10.1016/s0166-4328(89)80069-5. [DOI] [PubMed] [Google Scholar]
- 66.White NM, Salinas JA. Mnemonic functions of dorsal striatum and hippocampus in aversive conditioning. Behav Brain Res. 2003;142:99–107. doi: 10.1016/s0166-4328(02)00402-3. [DOI] [PubMed] [Google Scholar]
- 67.White NM, Viaud M. Localized intracaudate dopamine D2 receptor activation during the post-training period improves memory for visual or olfactory conditioned emotional responses in rats. Behav Neural Biol. 1991;55:255–269. doi: 10.1016/0163-1047(91)90609-t. [DOI] [PubMed] [Google Scholar]
- 68.Jolkkonen E, Pikkarainen M, Kemppainen S, Pitkanen A. Interconnectivity between the amygdaloid complex and the amygdalostriatal transition area: a PHA-L study in rat. J Comp Neurol. 2001;431:39–58. doi: 10.1002/1096-9861(20010226)431:1<39::aid-cne1054>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 69.Wang C, Kang-Park MH, Wilson WA, Moore SD. Properties of the pathways from the lateral amygdal nucleus to basolateral nucleus and amygdalostriatal transition area. J Neurophysiol. 2002;87:2593–2601. doi: 10.1152/jn.2002.87.5.2593. [DOI] [PubMed] [Google Scholar]
- 70.Chavkin C. Dynorphins are endogenous opioid peptides released from granule cells to act neurohumorly and inhibit excitatory neurotransmission in the hippocampus. Prog Brain Res. 2000;125:363–367. doi: 10.1016/S0079-6123(00)25025-5. [DOI] [PubMed] [Google Scholar]
- 71.Herringa RJ, Nanda SA, Hsu DT, Roseboom PH, Kalin NH. The effects of acute stress on the regulation of central and basolateral amygdala CRF-binding protein gene expression. Brain Res Mol Brain Res. 2004;131:17–25. doi: 10.1016/j.molbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Przewlocki R, Lason W, Hollt V, Silberring J, Herz A. The influence of chronic stress on multiple opioid peptide systems in the rat: pronounced effects upon dynorphin in spinal cord. Brain Res. 1987;413:213–219. doi: 10.1016/0006-8993(87)91012-2. [DOI] [PubMed] [Google Scholar]
- 73.Nicholson C, Chen KC, Hrabetova S, Tao L. Diffusion of molecules in brain extracellular space: theory and experiment. Prog Brain Res. 2000;125:129–154. doi: 10.1016/S0079-6123(00)25007-3. [DOI] [PubMed] [Google Scholar]
- 74.Stukel JM, Parks J, Caplan MR, Tillery SI. Temporal and spatial control of neural effects following intracerebral microinfusion. J Drug Target. 2008;16:198–205. doi: 10.1080/10611860801886695. [DOI] [PubMed] [Google Scholar]
- 75.Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. Neuroscientist. 2007;13:532–545. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Paré D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2009 doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 83.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 84.Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- 85.Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- 86.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 87.Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carr KD, Kutchukhidze N, Park TH. Differential effects of mu and kappa opioid antagonists on Fos-like immunoreactivity in extended amygdala. Brain Res. 1999;822:34–42. doi: 10.1016/s0006-8993(99)01088-4. [DOI] [PubMed] [Google Scholar]
- 89.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huge V, Rammes G, Beyer A, Zieglgansberger W, Azad SC. Activation of kappa opioid receptors decreases synaptic transmission and inhibits long-term potentiation in the basolateral amygdala of the mouse. Eur J Pain. 2009;13:124–129. doi: 10.1016/j.ejpain.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 91.Chieng BC, Christie MJ, Osborne PB. Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol. 2006;497:910–927. doi: 10.1002/cne.21025. [DOI] [PubMed] [Google Scholar]
- 92.Zhu W, Pan ZZ. Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience. 2004;127:871–879. doi: 10.1016/j.neuroscience.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 93.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. San Diego, CA: Academic Press; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.