Abstract
AMPA receptors are glutamate receptors that are tetramers of various combinations of GluR1-4 subunits. AMPA receptors containing GluR1, 3 and 4 are Ca2+ permeable, however, AMPA receptors containing even a single subunit of GluR2 are Ca2+ impermeable. Most AMPA receptors are Ca2+ impermeable due to the presence of GluR2. GluR2 confers special properties on AMPA receptors through the presence of arginine at the pore apex; other subunits (GluR1, 3, 4) contain glutamine at the pore apex and allow Ca2+ influx. Normally, an RNA editing step changes DNA-encoded glutamine to arginine, introduces arginine in the GluR2 pore apex. GluR2 RNA editing is carried out by an RNA-dependent adenosine deaminase (ADAR2). Loss of GluR2 editing leads to the formation of highly excitotoxic AMPA channels (Mahajan and Ziff, 2007) and is shown to contribute to loss of motor neurons in Amyotrophic Lateral Sclerosis (ALS). Relatively higher levels of Ca2+ permeable AMPA receptors are found in motor neurons and this has been correlated with lower GluR2 mRNA levels. However, the reason for loss of GluR2 editing is not known. Here we show that exposure of neurons to excitotoxic levels of glutamate leads to specific cleavage of ADAR2 that leads to generation of unedited GluR2. We demonstrate that cleaved ADAR2 leads to decrease or loss of GluR2 editing, which will further result in high Ca2+ influx and excitotoxic neuronal death.
Keywords: ADAR2, excitotoxicity, RNA editing, Amyotrophic Lateral Sclerosis (ALS), AMPA receptors, calpain activation
Introduction
Glutamate is the major neurotransmitter in the central nervous system (CNS) that mediates fast excitatory synaptic transmission and is involved in learning and memory. Glutamate excess due to ischemic injury or underlying disease conditions of the CNS causes neurodegeneration due to excitotoxicity (Lau and Tymianski, 2010). In particular, elevated extracellular levels of glutamate in Amyotrophic Lateral Sclerosis (ALS) patients may arise from abnormal functioning of astrocytic glutamate transporters (Foran and Trotti, 2009). Excess glutamate can potentially activate three types of ionotropic glutamate receptors, namely NMDA receptors, AMPA receptors and kainate receptors.
The activation of NMDA receptors and their role in glutamate-mediated excitotoxicity is well established (Lau and Tymianski, 2010). NMDA receptors are blocked by voltage and Mg2+-dependent mechanism under physiological conditions. They are not activated by the binding of glutamate alone, and require both membrane depolarization and glutamate binding for activation. Ischemic conditions, however, fulfill both prerequisites for NMDA receptor activation, thereby leading to uncontrolled, pathological NMDA receptor activation.
AMPA receptors are composed of various combinations of GluR1-4 subunits and are either Ca2+ permeable (containing exclusively GluR1, GluR3 or GluR4) or Ca2+ impermeable, containing GluR2 and other GluR subunits (GluR1, 3 and 4) (Hollmann and Heinemann, 1994). AMPA receptors are largely Ca2+ impermeable due to the presence of the GluR2 subunit, which prevents Ca2+ permeability and only allows Na+ influx (Lu et al., 2009). AMPA receptors are activated simply upon ligand binding and often their activation precedes NMDA receptor activation. GluR2 incorporation changes the properties of AMPA receptors due to the presence of arginine in GluR2 at the pore apex of the channel. Posttranscriptionally the codon for arginine is introduced in the GluR2 RNA by an RNA editing enzyme, adenosine deaminase dependent on RNA 2 (ADAR2) (Melcher et al., 1996a, Melcher et al., 1996b). Interestingly, due to it’s editing, GluR2 plays a crucial role in moderating synaptic transmission. Under physiological conditions upon high frequency stimulation, the Ca2+ influx through Ca2+ permeable AMPA receptors triggers a change in the composition of AMPA receptors by activating the incorporation of GluR2 into the synaptic surface AMPA receptor pool, thereby decreasing the Ca2+ influx and preventing excitotoxicity through Ca2+ permeable AMPA receptors (Gardner et al., 2005). Under physiological conditions GluR2 editing is almost 100% complete throughout life and in the embryonic stages of mice (Brusa et al., 1995). However, Kawahara et al (Kawahara et al., 2004) have shown that GluR2 editing is lowered or absent in motor neurons of ALS patients, but not in Purkinje cells of ALS patients or Purkinje cells or motor neurons of control subjects. GluR2, which is in its edited form has a protective effect on the neuron, may become very toxic upon failure of editing as was suggested in the case of sporadic ALS patients. Unedited GluR2, when incorporated into AMPA receptors, creates channels that are even more toxic than Ca2+ permeable AMPA receptors lacking GluR2, due to their fast synaptic trafficking nature and high Ca2+ permeability (Mahajan and Ziff, 2007). Hideyama et al (Hideyama et al., 2010) further confirmed that lowered editing of GluR2 in a conditional knock out of ADAR2 in mouse leads to motor neuron disease. Mice with a general knock out of ADAR2, in contrast, succumb to seizures (Higuchi et al., 2000). Loss of editing of GluR2 has been observed in ALS patients, but how the editing is lowered is not known. It is not known whether the ADAR2 enzyme is absent or is rendered non-functional. If ADAR2 loses function, it is not established how and what could be the cause of the functional loss of ADAR2. The results of this report clearly demonstrate that one mechanism for lowering editing of GluR2 is by activity-dependent degradation of ADAR2. We show that exposure of neurons to high levels of glutamate causes ADAR2 enzyme cleavage and decrease in GluR2 editing via the activation of the NMDA receptor and the subsequent activation of calpain. Notably, this is a first report that demonstrates that long term damaging effects of excess glutamate on AMPA receptor function occur through the activation of a new form of NMDA receptor-dependent excitotoxicity.
Experimental Procedures
Primary neuronal cultures
Neurons obtained from E 19 Sprague Dawley rat embryos were processed and maintained as described (Osten et al., 1998). For hippocampal neurons in 6 well plates, the neurons were plated at a density of 150,000 per well on cover slips precoated with poly-L-Lysine and maintained for 2 weeks. For cortical neuronal cultures, used in western blotting, cortical neurons were plated on 10 cm dishes for 10 days.
Constructs
The ADAR2b cDNA obtained from Dr. Ron Emeson was sub cloned into Xba I and Mlu I sites of a modified (attenuated) Sindbis virus vector (Mahajan and Ziff, 2007). A Flag-tag was introduced at the C terminus of full length ADAR2.
Treatment of neuronal cultures
Cortical neuronal cultures at 10 d.i.v. for western blots were treated either untreated or treated with 100 µM glutamate (Tocris) for 30 min or 1 hr in the kinetics experiment. AMPA (Tocris) was used at 20 µM or 100 µM, NMDA (Tocris) was also used at 20 µM or 100 µM as specified in the experiments. NMDA receptor blocker, APV (100 µM) (Tocris) and AMPA receptor blocker, CNQX (20 µM) (Tocris) were added 2 min prior to addition of the agonist (NMDA, AMPA). All other inhibitors such as N-Omega-Nitro-L-Arginine (L-NAME, 100 µM), nifedipine (20 µM) calpain inhibitor 1 (50 µM) (Calbiochem), calpain inhibitor X ( 10 µM) (Calbiochem), MG132 (20 µM) (Calbiochem) , epoxomicin ( 20 µM) (Peptide International), caspase 3 inhibitor (1 µM), caspase inhibitor 3 (15 µM) (Santa Cruz Biotech. Inc) and zVAD-FMK (50 µM) (Promega) were added 5 min prior to the treatment with glutamate. In all experiments except the kinetics assay, glutamate treatment and treatment with other inhibitors was done for 1 hr.
Antibodies
Anti-N-terminus antibodies were made to the sequence 48-STSRKRPLEEGSNGC-61 of ADAR2b to detect ADAR2b in neurons using immunofluorescence. Anti-ADAR2 antibody used to detect endogenous ADAR2 in the western blot is a gift from Ron Emeson.
Nuclear extract
Crude nuclear extracts were prepared by the protocol of Schreiber et al (Schreiber et al., 1989). Briefly, approximately 3 million cells were washed with 1X PBS collected in PBS, and centrifuged to collect the cell pellet. The cells were resuspended in 400 µL of buffer (10 mM HEPES pH7.9, 10mM KCL, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF) by gently pipetting up and down. The cells were left on ice for 15 minutes, 25 µL of 25% NP-40 was added and the suspension was vortexed vigorously for 10 sec. The homogenate was centrifuged and the pellet containing crude nuclei was collected and resuspended in buffer (20 mM HEPES pH 7.9, 0.4 M NaCl, I mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF). The tube was vigorously rocked for 15 min at 4o C and centrifuged to obtain the supernatant of the crude nuclear extract. In our experiments 3 million cells were used per sample for detecting exogenous expression of ADAR2 and 6 million cells were used to detect endogenous ADAR2 expression.
Western blot
Exogenous ADAR2 expression was detected using anti Flag antibodies followed by anti-mouse secondary antibody conjugated to horseradish peroxidase. Endogenous ADAR2 was detected by anti-ADAR2 antibody (gift of Ron Emeson) followed by anti-sheep secondary antibody conjugated to horseradish peroxidase. Horseradish peroxidase was detected by ECL using Pierce reagents.
Immunofluorescence
Endogenous ADAR2: Hippocampal neurons were fixed in 4% for 10 min paraformaldehyde and permeabilized using 0.2% Triton-X100 for 5 min. The neurons were incubated with anti N-ter ADAR2 and anti MAP-2 antibodies followed by FITC conjugated anti rabbit and rhodamine conjugated anti mouse secondary antibodies. The images were captured using confocal microscopy using a Nikon PCM 2000 confocal microscope and simple 32 software (Compix). Exogenous ADAR2: Hippocampal neurons were infected with ADAR2b-expressing Sindbis virus. Neurons were fixed in 4 % paraformaldehyde for 10 min followed by permeabilization by 0.2% Triton-X 100 for 5 min. ADAR2b was detected using anti Flag antibody and, neurons were detected using anti MAP2 antibody followed by secondary antibodies FITC conjugated anti mouse and rhodamine conjugated anti-goat. The images were captured using confocal microscopy.
Total RNA extraction and reverse transcription
Cortical neurons (150,000)/well in 6 well plates at 10 d.i.v. were either untreated or treated with 100µM glutamate for 1 hr. Media in the samples was replaced with conditioned media without glutamate at 1hr. Samples were collected in RNAsol (Biorad) and processed for total RNA extraction at various time intervals. Total RNA was resuspended in 20 µl DEPC treated water and digested with DNase 1 (Fermentas) for 1 hr in 30 µl volume and 2 µl of RNA was used for reverse transcription using Maxima Reverse Transcriptase (Fermentas) and oligodT (20mer) in a 20 µl volume at 50° C for 1 hr. The reactions were terminated by heating the samples at 85°C for 5 min.
Editing assay
The editing assay was performed using the method described by Kawahara et al (Kawahara et al., 2003). Briefly, first PCR was performed using 2 µl of above cDNA samples using the primers; GluR2F1-5';-TCTGGTTTTCCTTGGGTGCC-3' and GluR2R1-5'-AGATCCTCAGCACTTTCG-3' and for the nested PCR the primers used were GluR2F2-5'-GGTTTTCCTTGGGTGCCTTTAT-3’ and GluR2RR2-5'-ATCCTCAGCACTTTCGATGG-3'. The PCR reactions were performed using Dream Taq polymerase from Fermentas. The PCR product obtained with the nested PCR was 182 bp. The edited samples upon BbvI digestion would produce 116bp and 66bp fragments and the unedited GluR2 would produce 81bp, 66bp and 35bp fragments. The digested nested PCR fragments were separated on a 4% agarose gel. MAP-2 primers were used to amplify cDNA to serve as an internal control using primers, MAP-2F-5’-CCAAGGAGTCTGATTGCAGGA-3’ and MAP2-R-5’-CCTCAACCACAGCTCAAATGC-3’. The MAP-2 product is 198bp.
Results
1. Structure and expression
1.1 Exogenous expression of ADAR2 in hippocampal neurons
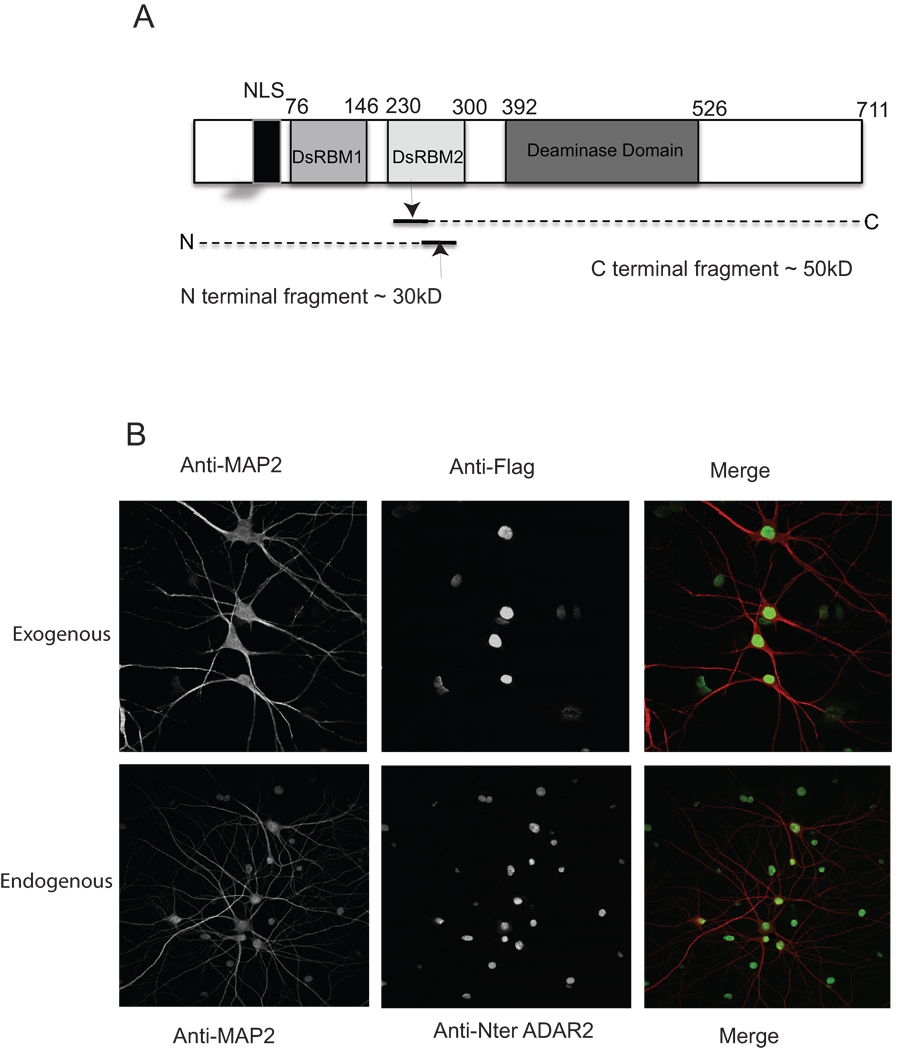
To study the regulation of ADAR2 under excitotoxic conditions induced by glutamate in neurons, the cellular localization of virally expressed ADAR2 was examined and compared with endogenous ADAR2. Complementary DNA of ADAR2b was expressed in 14 div hippocampal neurons from E18 pups using a Sindbis vector. An attenuated form of the Sindbis virus expression vector was used to reduce toxicity resulting from the viral protein expression load as described earlier (Mahajan and Ziff, 2007). Virally expressed ADAR2 in this experiment was the full-length protein that contained an NLS at the N terminus, two double stranded RNA binding domains, DRBM1 and DRBM2, and a C terminal deaminase domain and a Flag-tag at the C terminus (Figure 1A). Hippocampal neurons were infected with Flag-tagged ADAR2 expressing Sindbis virus for 17 hrs. Uninfected neurons were used to compare the endogenous and virally expressed ADAR2. As expected, the exogenously expressed ADAR2 (Figure 1B upper panel) showed nuclear and nucleolar localization similar to endogenous ADAR2 (Figure 1B lower panel).
Figure 1. Structure of ADAR2, virally expressed ADAR2 shows nuclear localization like the endogenous ADAR2.
A. Structure of ADAR2.
B. Virally expressing hippocampal neurons were fixed with 4% paraformaldehyde and immunostained with anti-flag antibody to detect ADAR2 expression and with anti-MAP-2 antibody (upper panel). Endogenous ADAR2 expression was detected by immunostaining the neurons with anti-Nter ADAR2 antibody and anti-MAP2 (lower panel). Secondary antibody staining was carried out using FITC and rhodamine conjugated secondary antibodies, followed by confocal microscopy.
2. ADAR2 is cleaved upon glutamate exposure
2.1 Kinetics of glutamate induced ADAR2 cleavage
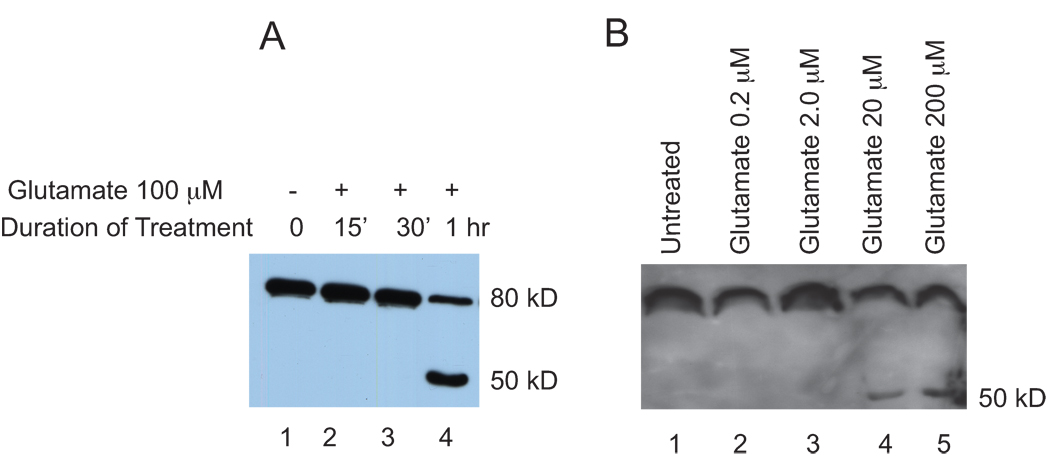
Flag-ADAR2 in cortical neurons also showed similar nuclear localization upon viral expression (data not shown). The kinetics of the effects of glutamate stimulation in cortical neurons was studied. Cortical neurons in 10 cm dishes at 10 d.i.v were infected with ADAR2 containing Sindbis virus. Seventeen hr post infection, the neurons were either left untreated or treated with 100 µM glutamate for 15 min, 30 minutes or 1 hr. Crude nuclear extracts were prepared and analyzed by western blotting using anti-flag antibodies (Figure 2A). Cleavage of ADAR2 as revealed by bands migrating ahead of the full-length protein, was seen at 1hr of glutamate treatment (lane 4) but not in untreated neurons or in neurons treated for 15’ or 30’ (lanes 1, 2 and 3). The cleavage product detected was a ~50 kD C terminal ADAR2 fragment. The N terminal product, which lacks a Flag-tag and was not detected in this experiment, is predicted to be approximately 30kD. The approximate cleavage site is shown in Figure 1A. However, we cannot exclude the possibility that additional cleavages occur in the N-terminal fragment.
Figure 2. Kinetics and dosage dependent curve of ADAR2 cleavage upon glutamate treatment.
A. Cortical neurons 10 d.i.v expressing Flag-ADAR2 were treated with 100µM glutamate for time points 0 min, 15 min, 30 min and 1hr. Nuclear extracts were subjected to western blotting with anti-Flag antibody. B. Cortical neurons expressing Flag-ADAR2 were either not treated (lane 1) treated with glutamate at 0.2µM (lane 2), 2µM (lane 3), 20µM (lane 4) and 200µM (lane5).
2.2 Glutamate dose dependent cleavage of ADAR2
We next determined if glutamate-induced cleavage of ADAR2 was dose-dependent. ADAR2 was expressed in cortical neurons 10 d.i.v by infection for seventeen hrs, and neurons were either left untreated or were treated with glutamate at concentrations of 0.2 µM, 2.0 µM, 20 µM and 200 µM. One hour post-treatment, crude nuclear extracts were prepared and analyzed by Western blotting using anti-Flag antibody (Figure 2B). A glutamate dose-dependent increase in ADAR2 cleavage was observed at 20 µM (lane 4) and 200 µM (lane 5), however, no cleavage was observed in the untreated neurons or in neurons treated with 0.2 µM (lane 2) or 2.0 µM (lane 3) glutamate.
3. Glutamate induced ADAR2 cleavage depends upon NMDA receptor activation
3.1 A NMDA receptor antagonist blocks glutamate induced ADAR2 cleavage
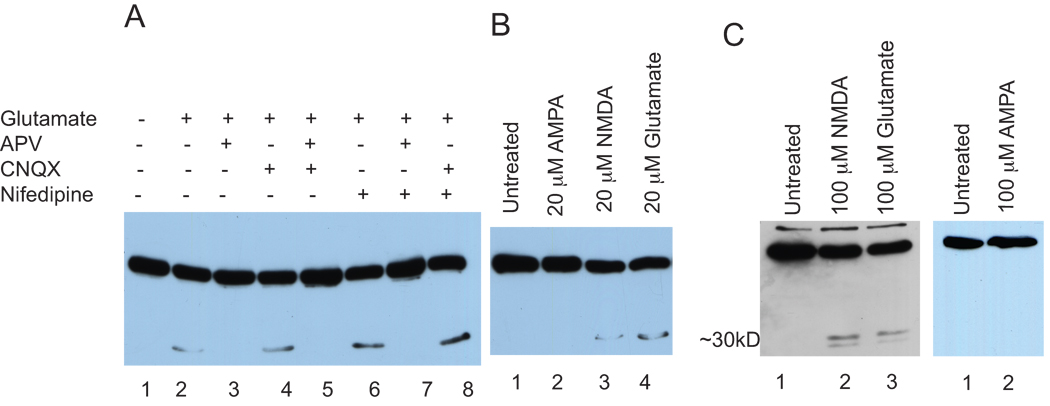
Glutamate activates both NMDA and AMPA receptors. To determine the type of receptor involved in glutamate dependent ADAR2 cleavage, we analyzed glutamate induced ADAR2 cleavage under conditions when specific receptors were blocked. We applied the receptor blockers APV (NMDA receptor antagonist, 100 µM), CNQX (AMPA receptor antagonist, 20µM) and nifedipine (L-type Ca2+ channel blocker, 20 µM) to virally expressing ADAR2 cortical neurons together with glutamate. Nuclear extracts were prepared and analyzed by SDS-PAGE and western blotting using anti-Flag antibody (Figure 3A). An unstimulated control did not show cleavage of ADAR2 (lane 1). Glutamate (100 µM for 1hr), as expected, induced cleavage (lane 2), and the glutamate-induced cleavage was blocked by APV (lane 3) but not by CNQX (lane 4) or nifedipine (lane 6). A combination of APV and CNQX, or APV and nifedipine treatments resulted in the blockage of ADAR2 cleavage (lanes 5 and 7), however, combined treatment with CNQX and nifedipine did not block glutamate dependent cleavage (lane 8). These results demonstrate that glutamate induced ADAR2 cleavage requires the activation of the NMDA receptor but not the AMPA receptor or L-type Ca2+ channels.
Figure 3. Both endogenous and exogenous ADAR2 cleavage occurs through the activation of NMDA receptors.
A. Cortical neurons expressing Flag-ADAR2 were either untreated (lane 1) or treated with glutamate (lane 2), and the glutamate and APV (lane 3) glutamate and CNQX (lane 4) glutamate with APV and CNQX (lane 5), glutamate and nifedipine (lane 6), glutamate with APV and nifedipine (lane 7) and glutamate with CNQX and nifedipine (lane8).
B. Cortical neurons expressing Flag-ADAR2 were either not treated or treated with AMPA, NMDA or glutamate. Nuclear extracts were analyzed using western blotting using anti-Flag antibody. Lane 1 untreated, lane 2 AMPA 20)µM, lane 3 NMDA 20µM and lane 4 glutamate 20µM for 1 hr.
C. Cortical neurons were either not stimulated or stimulated with NMDA or glutamate. Nuclear extracts were prepared and analyzed using SDS-PAGE followed by western blotting. Endogenous ADAR2 was detected using anti-ADAR2 antibody. Untreated (lane), NMDA (lane 2), glutamate (lane 3).
D. Cortical neurons expressing Flag-ADAR2 were either not treated or treated with 100µM AMPA for I hr. Nuclear extracts were prepared and analyzed using SDS-PAGE followed by western blotting using anti-Flag antibody. Untreated (lane1), 100µM AMPA (lane 2).
3.2 ADAR2 cleavage is induced by glutamate and NMDA but not by AMPA
To confirm that the activation of NMDA receptors was involved in glutamate induced ADAR2 cleavage, we analyzed the cleavage of ADAR2 following stimulation with selective agonists, NMDA and AMPA. Cortical neurons expressing ADAR2 were either not treated or treated with AMPA (20 µM), NMDA (20 µM), or glutamate (20 µM). Nuclear extracts were analyzed using SDS-PAGE and western blotting using anti-Flag antibody. The results in Figure 3B showed that ADAR2 cleavage was induced by both NMDA (lane 3) and glutamate (lane 4) but not by AMPA (lane 2) and no cleavage was seen in the untreated sample (lane 1). To confirm that a high concentration of AMPA does not induce cleavage, we treated cortical neurons expressing Flag-ADAR2 with 100µM AMPA for 1hr. No cleavage was observed in either an untreated control sample shown in Figure 3D (lane 1) or in a 100µM AMPA treated sample (lane2). This data confirmed that the cleavage of ADAR2 was induced by the activation of NMDA receptors and not through AMPA receptors.
4. Endogenous ADAR2 cleavage by glutamate
To determine whether ADAR2 cleavage was not an artifact of over expression of ADAR2, we analyzed the effect of glutamate on endogenous ADAR2 in cortical neurons. Cortical neurons were either not stimulated or stimulated with NMDA or glutamate. Nuclear extracts were prepared and analyzed using SDS-PAGE followed by western blotting. Endogenous ADAR2 was detected using anti-ADAR2 antibody (Figure 3C) (gift from Ron Emeson). Stimulation by NMDA (100 µM) (lane 2) or glutamate (100 µM) (lane 3) induced ADAR2 cleavage. This result demonstrated that cleavage of exogenously expressed ADAR2 induced by glutamate was recapitulated by the cleavage of endogenous ADAR2, and suggests that ADAR2 cleavage induced by glutamate was not an artifact of over expression of ADAR2.
5. A neuronal nitric oxide synthase inhibitor does not block ADAR2 cleavage induced by glutamate
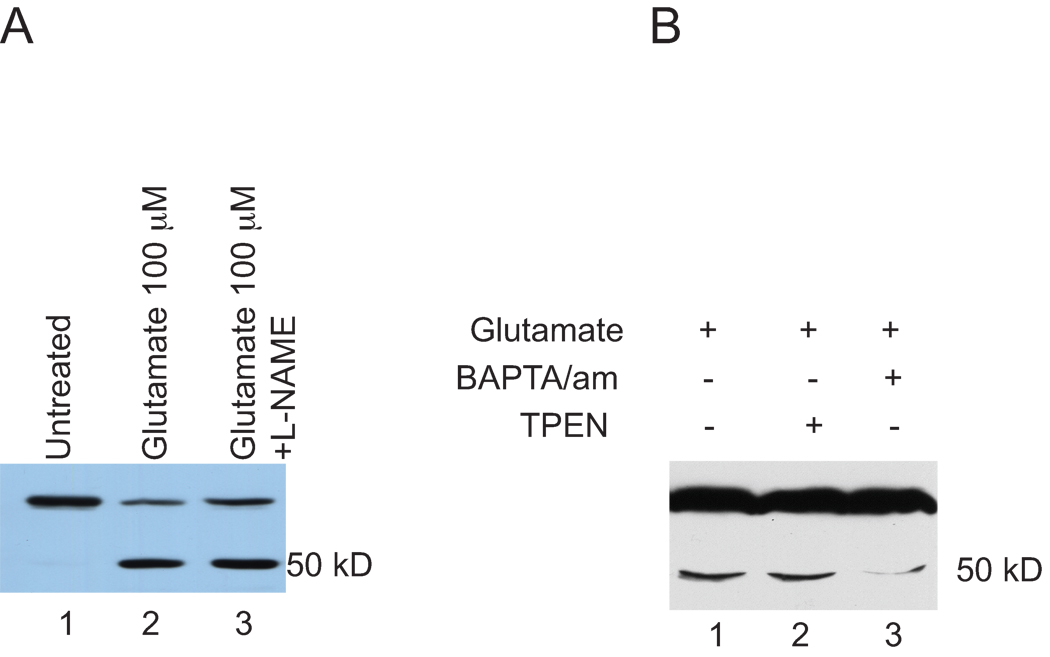
NMDA receptor activation is coupled to the activation of neuronal NOS (nNOS) (Bredt and Snyder, 1992). Since our results thus far demonstrated that the activation of NMDA receptors was involved in glutamate dependent ADAR2 cleavage, we investigated the involvement of nNOS in the induction of ADAR2 cleavage by glutamate. Cortical neuron cultures virally expressing ADAR2 were either not stimulated or were stimulated with glutamate or glutamate and the nNOS inhibitor, N-Omega-Nitro-L-Arginine (L-NAME, 100 |M). Nuclear extracts were prepared from these samples and analyzed using SDS-PAGE and western blotting using anti-Flag antibodies (Figure 4A). Untreated samples did not show ADAR2 cleavage (lane 1). Glutamate stimulation induced cleavage (lane 2), and L-NAME did not block glutamate induced ADAR2 cleavage (lane 3). This result demonstrated that ADAR2 cleavage induced by the activation of NMDA receptors was independent of nNOS activation.
Figure 4. Glutamate induced ADAR2 cleavage is blocked by Ca2+ chelator but not by Zn2+ chelator.
A. Virally expressing ADAR2 cortical neuron cultures with either not stimulated or stimulated with glutamate or glutamate and L-NAME. Nuclear extracts were prepared from these samples and analyzed using SDS-PAGE and western blotting using anti-Flag antibodies. Untreated samples did not show ADAR2 cleavage (lane 1), glutamate stimulation induced cleavage (lane 2), neuronal nitric oxide inhibitor, L-NAME, did not block glutamate induced ADAR2 cleavage (lane 3).
B. Cortical neurons expressing exogenous ADAR2 were either not treated, or treated with BAPTA/am, an extracellular Ca2+ chelator, prior to glutamate treatment, treated with TPEN, a transition metal chelator, and glutamate (Figure 4B). Glutamate treatment as expected induced ADAR2 cleavage (lane 1), treatment with TPEN did not blocked ADAR2 cleavage induced by glutamate (lane 2) but treatment with BAPTA/am blocks glutamate induced ADAR2 cleavage efficiently (lane 3).
6. Glutamate induced ADAR2 cleavage is dependent on Ca2+ influx
NMDA receptor activation leads to Ca2+ influx followed by synaptic cascades initiated by a rise in local Ca2+ concentration (Nicholls et al., 1999). However, Ca2+ influx-independent NMDA receptor activation has been shown to play an important role in neuronal death (Kato and Murota, 2005). ADAR2 cleavage may lead to inactivation of ADAR2 followed by loss or decrease in GluR2 editing, which may in turn trigger neuronal death (Mahajan and Ziff, 2007). We studied the involvement of Ca2+ influx in ADAR2 cleavage. Cortical neurons expressing exogenous ADAR2 were either not treated, or treated with BAPTA/am, an extracellular Ca2+ chelator, prior to glutamate treatment, or were treated with TPEN, a transition metal chelator, plus glutamate (Figure 4B). Glutamate treatment induced ADAR2 cleavage (lane 1) as expected, and treatment with TPEN did not block ADAR2 cleavage induced by glutamate (lane 2) but treatment with BAPTA/am blocked glutamate induced ADAR2 cleavage efficiently (lane 3). This result demonstrated that Ca2+ influx was necessary for ADAR2 cleavage, however, a transition metal chelator did not have any effect on glutamate induced ADAR2 cleavage.
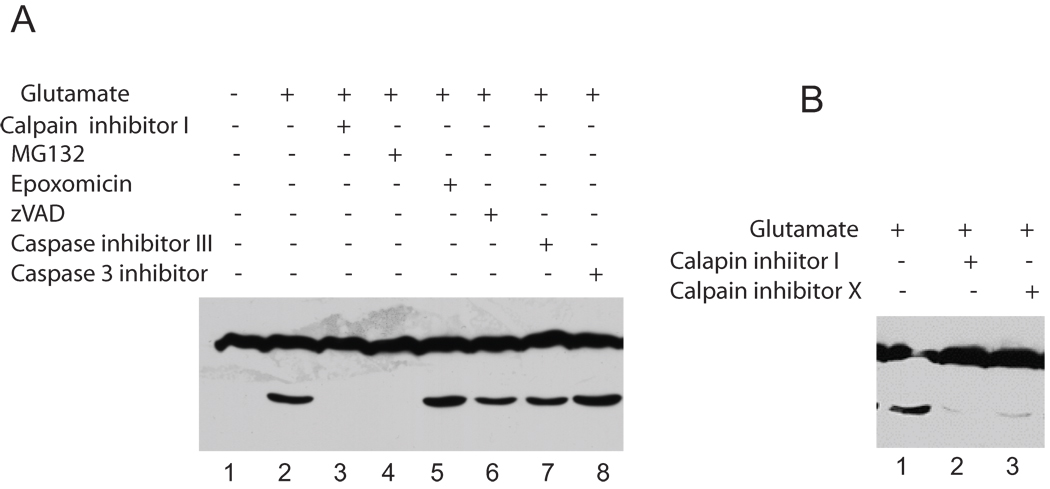
7. Glutamate induced ADAR2 cleavage is calpain dependent
Our results suggested that the ADAR2 cleavage was Ca2+-dependent and thus may be carried out by any of the cascades that are initiated by an increase in local Ca2+ concentration. We investigated the cleavage pathway further to determine the protease that may be involved in ADAR2 cleavage. A series of protease inhibitors was applied to the neurons including proteosome inhibitor, caspase inhibitors and calpain inhibitors. Specifically, cortical neurons expressing exogenous ADAR2 were either not treated or were treated with glutamate (20 µM), or glutamate and plus the proteosome inhibitors, MG132 (20 µM) or epoxomicin (20 µM), or with glutamate and the caspase inhibitors, zVAD (50 µM) or Caspase inhibitor 3 (15 µM) or caspase 3 inhibitor (1 µM), or with glutamate and calpain inhibitor I (50 µM) (Figure 5A). Untreated neurons did not show ADAR2 cleavage (lane 1). Glutamate treatment resulted in ADAR2 cleavage (lane 2), and the proteosome/calpain inhibitor MG132 blocked ADAR2 cleavage (lane 4). However, another proteosome inhibitor, epoxomicin, did not block ADAR2 cleavage, and none of the caspase inhibitors, zVAD, caspase inhibitor III or caspase 3 inhibitor blocked the cleavage (lane 6, 7 and 8). Interestingly, calpain inhibitor I blocked ADAR2 cleavage (lane 3). These results demonstrated that calpain inhibitor I completely blocked ADAR2 cleavage. MG132 has been shown to inhibit both the proteosome and calpain (Figueiredo-Pereira et al., 1994) (Lee and Goldberg, 1998). Our studies suggest that the MG132 mediated block of calpain activity is the basis for the block of ADAR2 cleavage because the inhibition of ADAR2 cleavage is replicated by Calpain inhibitor I but not by another proteosome inhibitor, epoxomicin.
Figure 5. Glutamate induced ADAR2 cleavage is blocked by calpain inhibitor.
A. Cortical neurons expressing exogenous ADAR2 were either not treated or were treated with glutamate, or glutamate and plus the proteosome inhibitors such as MG132 or epoxomicin, or with glutamate and the caspase inhibitors zVAD or caspase inhibitor 3 or caspase 3 inhibitor, or with glutamate and calpain inhibitor (Figure 5A). Untreated neurons did not show ADAR2 cleavage (lane 1). Glutamate treatment resulted in ADAR2 cleavage (lane 2), and the proteosome/calpain inhibitor MG132 blocked ADAR2 cleavage (lane 4). However, another proteosome inhibitor, epoxomicin, did not block ADAR2 cleavage, and none of the caspase inhibitors, zVAD, caspase inhibitor III or caspase 3 inhibitor blocked the cleavage (lane 6, 7 and 8). Interestingly, calpain inhibitor I blocked ADAR2 cleavage (lane 3).
B. Cortical neurons expressing ADAR2 were treated with glutamate as described above using the two-calpain inhibitors. Calpain inhibitor I (lane 2) and calpain inhibitor X (lane 3) both blocked glutamate induced ADAR2 cleavage.
To confirm the result that calpain inhibitor I completely blocks ADAR2 cleavage, we used a second Calpain inhibitor, Calpain inhibitor X (Figure 5B). Cortical neurons were treated with glutamate as described above using the two calpain inhibitors. Calpain inhibitor I (50 µM) (lane 2) and calpain inhibitor X (10 µM) (lane 3). Both blocked glutamate induced ADAR2 cleavage. This result thus confirmed the involvement of calpain in mediating ADAR2 cleavage upon glutamate stimulation.
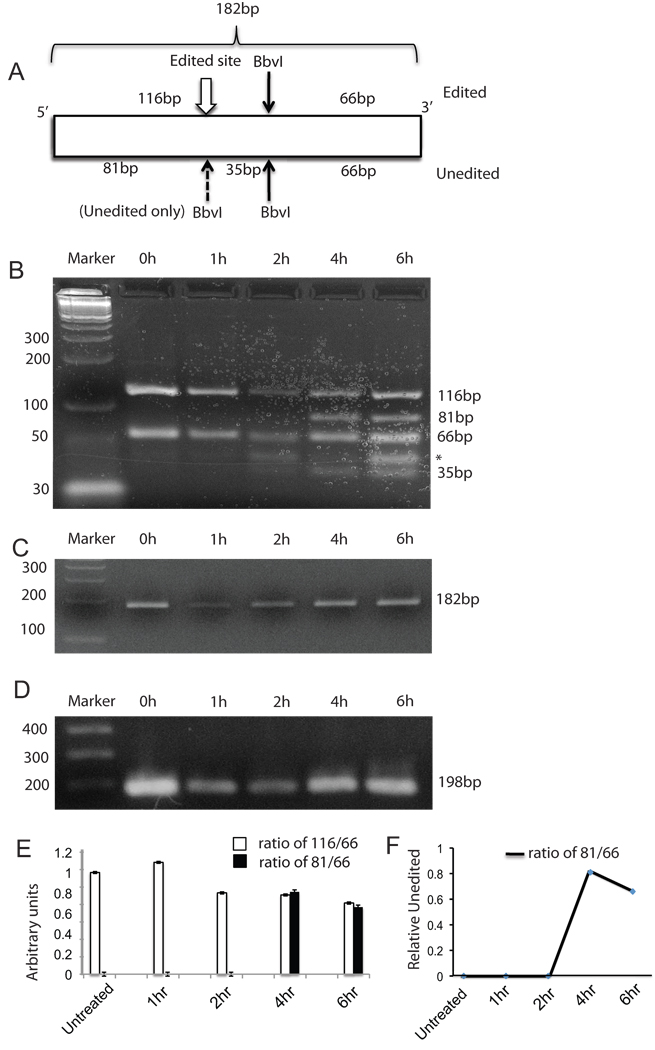
8. ADAR2 cleavage reduces GluR2 RNA editing
We analyzed editing levels by the method of Kawahara et al (Kawahara et al., 2003), in which Q/R RNA editing site sequences were amplified by means of PCR primers complementary to intronic and exonic sequences that surround the editing site. RNA editing is assayed by changes in the BbVI restriction pattern. Total RNA was extracted from either untreated cortical neurons or cortical neurons treated with 100µM glutamate for 1hr, 2hr, 4 hr and 6 hr post glutamate treatment. The RNA was used for cDNA synthesis followed by 2 sets of PCR and samples were digested with BbvI to assess editing by ADAD2 as described in the Experimental Procedures section. The fragment sizes obtained with BbvI digestion are depicted in Figure 6A. The results in Figure 6B show nested PCR products digested with BbvI. The sample at 0h, 1hr and 2hr show complete editing represented by two fragments of sizes 116bp and 66bp, however, at 4h and 6h two new fragments 81bp and 35bp are observed that are generated from the unedited GluR2 RNA as depicted in Figure 6A. A non-specific band (marked by an asterisk) appears immediately above the 35bp band but observed even in the untreated sample and therefore is irrelevant to glutamate treatment. The undigested nested PCR product is shown in Figure 6C. MAP-2 cDNA was amplified using the samples to serve as an internal control (Figure 6D). The undigested nested PCR products shown in Figure 6C and the MAP-2 PCR products shown in 6D fluctuate between the samples, which and may indicate fluctuations due to mRNA stability following glutamate treatment. The bands were quantified from three independent experiments and the ratios of intensities of 166bp/66bp and 81bp/66bp are plotted in Figure 6E. Relative levels of unedited GluR2 RNA at 4h and 6h are shown in Figure 6F. The results show a significant loss of editing of GluR2 RNA at 4h and 6h. This result confirms that excitotoxic treatment with glutamate that causes cleavage of ADAR2 also results in loss of GluR2 editing.
Figure 6. Glutamate induced ADAR2 cleavage leads to loss of GluR2 editing.
A. Cartoon showing the size of the nested PCR product (182bp) and the sizes of BbvI digestion fragments of nested PCR for edited GluR2 (116bp and 66bp) and for unedited GluR2 (81bp, 66bp and 35bp).
B. Total RNA from cortical neurons was used for the editing assay. Marker, 0h, 1hr and 2 hr samples showed two fragments of sizes 116bp and 66bp after BbvI digestion, however, 4 hr and 6hr show an 81bp fragment in addition to 116bp and 66bp fragments generated due to the presence of both edited and unedited GluR2 RNA.
C. Products of nested PCR (182bp) are shown before BbvI digestion (undigested). Marker, 0 h, 1 hr, 2 hr, 4 hr and 6 hr.
D. PCR products for MAP-2 cDNA were used as an internal control (198bp). Marker, 0 h, 1 hr, 2 hr, 4 hr and 6 hr.
E. Quantification and ratio of intensities of 116bp/66 and 81bp/66. The bands were quantifies using Image J and the ratio of the band intensities is plotted. This graph represents data from three independent experiments.
F. The ratios of intensities of 81/66 are plotted to show relative levels of unedited GluR2 RNA.
Discussion
Excessive activation of NMDA receptors by glutamate can lead to excitotoxicity, however, the involvement of AMPA receptors has been recently shown to occur as well, particularly in neurodegenerative diseases like ALS (Grosskreutz et al., 2010) and ischemia (Arundine and Tymianski, 2003, Kwak and Weiss, 2006, Forder and Tymianski, 2009). Glutamate excitotoxicity is caused by the unregulated elevation of levels of glutamate that occurs under conditions of ischemia or in ALS. Elevated levels of glutamate were observed in the cerebrospinal fluid (CSF) of 40% of ALS patients and high levels of glutamate were correlated with the spinal onset of ALS (Spreux-Varoquaux et al., 2002). Abnormal glutamate metabolism has been shown in ALS patients (Plaitakis and Caroscio, 1987, Rothstein et al., 1990). In both sporadic and familial ALS, glutamate reuptake by the glutamate astroglial transporter, GLT1, was decreased or absent, in both motor cortex and spinal cord (Rothstein et al., 1995, Fray et al., 1998, Sasaki and Iwata, 2000).
Sustained increased levels of glutamate at synapses would lead to the activation of AMPA receptors that in turn depolarize the neuron through Na+ entry, and activate NMDA receptors through coincident membrane depolarization and glutamate binding (Hollmann and Heinemann, 1994). The majority of AMPA receptors are Na+ permeable and Ca2+ impermeable due to the presence of GluR2 subunit, which controls Ca2+/Zn2+ permeability of the heteromeric AMPA receptor channel. Insertion of single subunit of GluR2 ensures Ca2+ impermeability of the AMPA receptor, and channels lacking GluR2 subunits are Ca2+ permeable (Seeburg et al., 1998). Ca2+ permeable AMPA receptors play an important role in AMPA receptor mediated excitotoxicity (Gorter et al., 1997, Pellegrini-Giampietro et al., 1997, Weiss and Sensi, 2000, Cull-Candy et al., 2006, Kwak and Weiss, 2006, Liu and Zukin, 2007, Buckingham et al., 2008). Under normal conditions, most of the AMPA receptors are Ca2+ impermeable, however, in motor neurons the level of Ca2+ permeable AMPA receptors is higher due to relatively lower levels of GluR2 mRNA (Carriedo et al., 1996, Van Den Bosch et al., 2002). Also the Ca2+ buffering capacity of motor neurons is limited (Van Den Bosch et al., 2002), leading to a greater potential for glutamate and Ca2+ dependent toxicity.
Another factor that contributes to glutamate-induced toxicity is the lower levels or absence of GluR2 editing. Kawahara et al (Kawahara et al., 2004) (Aizawa et al., 2010) have demonstrated decreased editing of GluR2, specifically in motor neurons of ALS patients. Decreased editing of GluR2 can dramatically alter the conductance properties and trafficking properties of unedited GluR2 containing AMPA receptors (Mahajan and Ziff, 2007). Unedited GluR2 is highly toxic due to increased Ca2+ influx, and unedited GluR2 traffics to the plasma membrane efficiently, thereby contributing further to the toxicity of unedited GluR2 containing AMPA receptors (Mahajan and Ziff, 2007). Furthermore, expression of GluR2 with an aspargine at the pore apex, which controls Ca2+ permeability, has been shown to contribute to motor neuron toxicity and late onset motor neuron disease, suggesting that the increased Ca2+ permeability through altered GluR2 containing AMPA receptors contributes to motor neuron toxicity in ALS (Kuner et al., 2005). The presence of a relatively greater number of Ca2+-permeable AMPA receptors in motor neurons has been shown to contribute to glutamate toxicity (Van Den Bosch et al., 2006). Moreover, loss of editing of GluR2, which increases this number further may also contribute to the loss of motor neurons in ALS. However, the cause of lower editing by ADAR2 of GluR2 is not known.
Glutamate induced ADAR2 cleavage produces a C terminal ~50kD fragment that spans a region that contains the DRBM2 and the deaminase domain. This predicts the release of an N terminal ~30kD fragment containing the NLS and the DRBM1. In vitro studies of truncation of human ADAR2 have shown that ADAR2 requires both DRBM1 and DRBM2 for ADAR2 to edit long substrates (Macbeth et al., 2004). Poulsen et al (Poulsen et al., 2006) have shown that DRBM1 in ADAR2 contributes primarily to ADAR2 dimerization and RNA binding, and that DRBM2 mainly contributes to the deaminase activity. Loss of dimerization and RNA binding could be sufficient to hamper the activity of the cleaved C terminal fragment, which only contains the DRBM1 and the deaminase domain. We have shown that elevated glutamate induces the cleavage of ADAR2 in a time dependent manner and we show that this cleavage leads to loss of GluR2 editing and increased toxicity through unedited GluR2 containing AMPA receptors.
A dose dependent cleavage experiment demonstrated that the amount of glutamate required to induce ADAR2 cleavage is around 20 µM for cultured neurons and that the ADAR2 cleavage is not only dose dependent but also is induced by an excitotoxic glutamate concentration. Prolonged exposure to glutamate and exposure to higher doses of glutamate leads to increased ADAR2 cleavage. Therefore, glutamate induced ADAR2 cleavage is both time and dose dependent and can lead to greater inactivation of the enzyme with greater excitotoxic insult.
Ca2+ permeable AMPA receptors serve as entry routes for the divalent cation, Zn2+, which is released along with glutamate at certain excitatory synapses and is highly conducted by Ca2+ permeable AMPA channels (Jia et al., 2002). Zn2+ has been shown to accumulate intracellularly in both ischemia and epilepsy and Zn2+ chelators have been effective neuroprotectors (Koh et al., 1996, Yin et al., 2002, Lee et al., 2003). Zn2+ is more potent than Ca2+ in inducing mitochondrial injury as a consequence of generation of reactive oxygen species (ROS) (Sensi et al., 1999), poly-ADP ribose polymerase activation (PARP) and finally neuronal death (Kwak and Weiss, 2006). TPEN, a Zn2+ chelator, however, in our studies, was unable to block the degradation of ADAR2 suggesting that the degradation of ADAR2 is not caused by Zn2+ influx. Neuronal nitric oxide (nNOS) is induced under pathological conditions through the excessive stimulation of NMDA receptors and plays a role in excitotoxic death of neurons (Keynes and Garthwaite, 2004), however, our results suggested that nNOS is not activated in the pathway that results in ADAR2 cleavage in our studies.
Excess glutamate stimulation may trigger the activation of the proteosome, and ADAR2 cleavage could possibly result from proteosomal degradation. MG132 blocked ADAR2 cleavage, however, epoxomicin failed to block ADAR2 cleavage. The contradiction may be explained by the non-specific nature of MG132, which can block both the proteosome and calpain (Figueiredo-Pereira et al., 1994, Lee and Goldberg, 1998). Excess glutamate causes excitotoxic death in neurons and induces cleavage of AMPA receptor subunits GluR1-4 in neural apoptosis and Alzheimer’s disease (Chan et al., 1999) and activates proteases of apoptotic pathway. Interestingly, ADAR2 cleavage was not blocked by any of the caspase inhibitors examined suggesting that ADAR2 was not cleaved by caspases. High levels of glutamate release occur under ischemic conditions as well as under conditions of ALS (Lau and Tymianski, 2010). Exposure of hippocampal neurons to excessive glutamate has been shown to activate calpain, and inhibition of calpain activity in motor neurons of ventral spinal cord after glutamate exposure has been shown to provide neuroprotection, suggesting that the apoptosis caused by excess glutamate is somehow mediated by calpain activation (Chan et al., 1999, Chan and Mattson, 1999, Das et al., 2005). Similarly, altered calpain expression has been shown in the brain and the spinal cord in murine mutant model and may contribute to motor neuron disease (Li et al., 1998). Furthermore, age related neurodegeneration has been shown to be linked to calpain activation (Nixon, 2003). In our studies, glutamate induced ADAR2 cleavage was mediated through NMDA receptors and was blocked by calpain inhibitor. Interestingly, the use of peptides that block the NMDA receptor-PSD95 interaction (Aarts et al., 2002) and the use of PSD95 inhibitors has been demonstrated to be effective in reducing the infarct size in stroke rats (Sun et al., 2008). Furthermore, PSD95 is a substrate for calpain in both adult and neonatal rats (Lu et al., 2000).
Interestingly earlier studies by Ademec et al have employed similar glutamate treatment conditions with primary hippocampal cultures and shown that the calpain 1 is activated through stimulation of the NMDA receptor, which resulted in calpain-mediated spectrin degradation (Adamec et al., 2002). However, glutamate treatment of hippocampal neurons for I hr and activation of calpain was not linked to excitotoxic death of neurons by glutamate (Adamec et al., 2002). Our studies indicate that besides non specific degradation of cytoskeletal proteins by calpain activation, exposure to excitotoxic amounts of glutamate (Adamec et al., 2002) leads to specific cleavage of ADAR2, which leads to functional loss of ADAR2 activity that contributes to a decrease of GluR2 editing, thus leading to incorporation into synapses of toxic AMPA receptor channels containing unedited GluR2. Furthermore incorporation of unedited GluR2-containing AMPA receptors may eventually lead to death of neurons due to their activation. Our results demonstrate a novel mechanism in which exposure of neurons to high levels of glutamate causes loss of editing via ADAR2 cleavage.
A loss of GluR2 editing in ischemia may occur upon excessive glutamate release and the generation of membrane depolarizing conditions, which may lead to the activation of NMDA receptors (Aarts et al., 2003). Peng et al have demonstrated that induction of forebrain ischemia results in reduced GluR2 editing in hippocampal pyramidal neurons (Peng et al., 2006). Under these conditions excessive glutamate is released which is simulated by our experimental treatment conditions. The activation of NMDA receptors and the resulting large Ca2+ influxes may activate calpain dependent cleavage of ADAR2. Cleavage of ADAR2 leads to decreased editing of GluR2 and incorporation of unedited AMPA receptors that are highly Ca2+ permeable further contributing to the toxicity of neurons and neuronal death. Under conditions of ALS, increased levels of glutamate may also lead to similar molecular changes resulting in neuronal death. Our results provide the first evidence that the ADAR2 enzyme is cleaved, leading to lower GluR2 editing and may cause the production of toxic Ca2+ permeable unedited GluR2-containing AMPA channels and death of neurons.
Kawahara et al (Kawahara et al., 2004) have reported that editing levels decline greatly in motor neurons from ALS patients. Using the same editing assay as Kawahara et al (Kawahara et al., 2003), which is based on restriction enzyme cleavage of DNA sequences amplified by PCR, we showed the appearance of bands that indicate the appearance of unedited GluR2 mRNA dissociated hippocampal neurons following glutamate stimulation. This assay is sensitive to editing changes because it employs primers targeted to different exons and thus analyzes the editing state of the mature mRNA while excluding signals from nuclear RNA or contaminating DNA. We find that in control cells, this RNA is 100% edited, but after 2 hours of glutamate treatment, the editing levels decline progressively with time. This is consistent with our detection of cleaved ADAR2 within 1 hour of glutamate stimulation. ALS is a chronic aliment and in the disease the editing failure process that we describe here may take place gradually, yielding increasing proportions of unedited GluR2, which we have shown previously is excitotoxic (Mahajan and Ziff, 2007). In agreement with an excitotoxic potential for a progressive loss of editing, Hideyama et al (Hideyama et al., 2010) have shown that the expression of unedited GluR2 in motor neurons gave rise to a condition resembling motor neuron disease including limb weakness.
Calpain activation has been earlier shown to degrade p27, a cyclin dependent kinase, after glutamate-induced toxicity in cortical neurons (Akashiba et al., 2006). P27 is a nuclear protein and was degraded in the nucleus showing that the nuclear calpain activation occurs upon glutamate treatment (Akashiba et al., 2006) similar to our observation where dendritic NMDA receptor activation leads to cleavage of ADAR2 in the nucleus. We do not know the mechanism that is precisely involved in the activation of nuclear calpain, however we speculate the processes involved in synapse to nucleus signaling are activated.
Calpain activation has been shown to contribute in various neuron related pathologies as in Alzheimer’s Disease, Parkinson’s Disease and Huntington Disease (Nixon, 2003). The use of calpain inhibitors has been shown to improve synaptic function and memory in Alzheimer’s mouse model (Trinchese et al., 2008) and could be considered as a pharmaceutical approach to reduce neuron death in ALS.
Conclusions
GluR2 editing plays an important role in modulating properties of AMPA receptors by controlling the Ca2+ permeability and membrane trafficking thereby preventing influx of excess Ca2+ into neurons. GluR2 editing by ADAR2 is almost 100% complete in an adult brain however, under disease conditions such as Amyotrophic Lateral Sclerosis (ALS), GluR2 is less efficiently edited especially in motor neurons. The reason for the reduced GluR2 editing by ADAR2 is not known. Our results demonstrate that one way GuR2 editing is lowered is by the damage to ADAR2. We demonstrate that ADAR2 in neurons gets cleaved upon exposure to high levels of glutamate, through the activation of NMDA receptors and calpain, which results in reduced editing of GluR2.
Highlights.
Fate and function of ADAR2 is studied in cortical neurons upon glutamate stimulation.
ADAR2, an editing enzyme upon glutamate stimulation is cleaved into two fragments.
Glutamate stimulation leads to NMDA receptor and calpain activation.
Glutamate stimulation also lowers GluR2 editing in cortical neurons.
Acknowledgements
We thank Ron Emeson for a gift of ADAR2b cDNA and for anti-ADAR2 antibodies. This work was supported by NIH grant R01 NS06192 to EZ and PSC-CUNY grant 62135-0040 to SSM. We thank Muktar Mahajan and Sophie Restituito for critical readings of the manuscript.
Abbreviation
- NMDA
N methyl D-aspartate
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- APV
2-amino-5-phosphonopentanoic acid
- CNQX
6-cyano-7nitroquinoxaline-2, 3-dione
- DEPC
diethylpyrocarbonate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Glossary
Excitotoxicity, calpain activation, neurodegeneration, RNA editing, Amyotrophic Lateral Sclerosis
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Aarts MM, Arundine M, Tymianski M. Novel concepts in excitotoxic neurodegeneration after stroke. Expert Rev Mol Med. 2003;5:1–22. doi: 10.1017/S1462399403007087. [DOI] [PubMed] [Google Scholar]
- Adamec E, Mohan P, Vonsattel JP, Nixon RA. Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathol. 2002;104:92–104. doi: 10.1007/s00401-002-0528-6. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Sawada J, Hideyama T, Yamashita T, Katayama T, Hasebe N, Kimura T, Yahara O, Kwak S. TDP-43 pathology in sporadic ALS occurs in motor neurons lacking the RNA editing enzyme ADAR2. Acta Neuropathol. 2010;120:75–84. doi: 10.1007/s00401-010-0678-x. [DOI] [PubMed] [Google Scholar]
- Akashiba H, Matsuki N, Nishiyama N. Calpain activation is required for glutamate-induced p27 down-regulation in cultured cortical neurons. J Neurochem. 2006;99:733–744. doi: 10.1111/j.1471-4159.2006.04100.x. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- Buckingham SD, Kwak S, Jones AK, Blackshaw SE, Sattelle DB. Edited GluR2, a gatekeeper for motor neurone survival? Bioessays. 2008;30:1185–1192. doi: 10.1002/bies.20836. [DOI] [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Griffin WS, Mattson MP. Evidence for caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer’s disease. J Neurosci Res. 1999;57:315–323. doi: 10.1002/(SICI)1097-4547(19990801)57:3<315::AID-JNR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Das A, Sribnick EA, Wingrave JM, Del Re AM, Woodward JJ, Appel SH, Banik NL, Ray SK. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Banik N, Wilk S. Comparison of the effect of calpain inhibitors on two extralysosomal proteinases: the multicatalytic proteinase complex and m-calpain. J Neurochem. 1994;62:1989–1994. doi: 10.1046/j.1471-4159.1994.62051989.x. [DOI] [PubMed] [Google Scholar]
- Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1587–1602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forder JP, Tymianski M. Postsynaptic mechanisms of excitotoxicity: Involvement of postsynaptic density proteins, radicals, and oxidant molecules. Neuroscience. 2009;158:293–300. doi: 10.1016/j.neuroscience.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Fray AE, Ince PG, Banner SJ, Milton ID, Usher PA, Cookson MR, Shaw PJ. The expression of the glial glutamate transporter protein EAAT2 in motor neuron disease: an immunohistochemical study. Eur J Neurosci. 1998;10:2481–2489. doi: 10.1046/j.1460-9568.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Petrozzino JJ, Aronica EM, Rosenbaum DM, Opitz T, Bennett MV, Connor JA, Zukin RS. Global ischemia induces downregulation of Glur2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CA1 neurons of gerbil. J Neurosci. 1997;17:6179–6188. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz J, Van Den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–174. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Hideyama T, Yamashita T, Suzuki T, Tsuji S, Higuchi M, Seeburg PH, Takahashi R, Misawa H, Kwak S. Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. J Neurosci. 2010;30:11917–11925. doi: 10.1523/JNEUROSCI.2021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jia Y, Jeng JM, Sensi SL, Weiss JH. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. J Physiol. 2002;543:35–48. doi: 10.1113/jphysiol.2002.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Murota SI. NMDA receptor stimulation in the absence of extracellular Ca2+ potentiates Ca2+ influx-dependent cell death system. Brain Res. 2005;1035:177–187. doi: 10.1016/j.brainres.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S. Glutamate receptors: RNA editing and death of motor neurons. Nature. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Kanazawa I, Kwak S. Low editing efficiency of GluR2 mRNA is associated with a low relative abundance of ADAR2 mRNA in white matter of normal human brain. Eur J Neurosci. 2003;18:23–33. doi: 10.1046/j.1460-9568.2003.02718.x. [DOI] [PubMed] [Google Scholar]
- Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr Mol Med. 2004;4:179–191. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kuner R, Groom AJ, Bresink I, Kornau HC, Stefovska V, Muller G, Hartmann B, Tschauner K, Waibel S, Ludolph AC, Ikonomidou C, Seeburg PH, Turski L. Late-onset motoneuron disease caused by a functionally modified AMPA receptor subunit. Proc Natl Acad Sci U S A. 2005;102:5826–5831. doi: 10.1073/pnas.0501316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak S, Weiss JH. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol. 2006;16:281–287. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol. 2003;184:337–347. doi: 10.1016/s0014-4886(03)00382-0. [DOI] [PubMed] [Google Scholar]
- Li J, Nixon R, Messer A, Berman S, Bursztajn S. Altered gene expression for calpain/calpastatin system in motor neuron degeneration (Mnd) mutant mouse brain and spinal cord. Brain Res Mol Brain Res. 1998;53:174–186. doi: 10.1016/s0169-328x(97)00295-7. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Rong Y, Baudry M. Calpain-mediated degradation of PSD-95 in developing and adult rat brain. Neurosci Lett. 2000;286:149–153. doi: 10.1016/s0304-3940(00)01101-0. [DOI] [PubMed] [Google Scholar]
- Macbeth MR, Lingam AT, Bass BL. Evidence for auto-inhibition by the N terminus of hADAR2 and activation by dsRNA binding. RNA. 2004;10:1563–1571. doi: 10.1261/rna.7920904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SS, Ziff EB. Novel toxicity of the unedited GluR2 AMPA receptor subunit dependent on surface trafficking and increased Ca2+-permeability. Mol Cell Neurosci. 2007;35:470–481. doi: 10.1016/j.mcn.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Higuchi M, Seeburg PH. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J Biol Chem. 1996a;271:31795–31798. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996b;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL, Castilho RF, Ward MW. Glutamate excitotoxicity and neuronal energy metabolism. Ann N Y Acad Sci. 1999;893:1–12. doi: 10.1111/j.1749-6632.1999.tb07813.x. [DOI] [PubMed] [Google Scholar]
- Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Gorter JA, Bennett MV, Zukin RS. The GluR2 (GluR-B) hypothesis: Ca(2+)-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- Peng PL, Zhong X, Tu W, Soundarapandian MM, Molner P, Zhu D, Lau L, Liu S, Liu F, Lu Y. ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 determines vulnerability of neurons in forebrain ischemia. Neuron. 2006;49:719–733. doi: 10.1016/j.neuron.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Plaitakis A, Caroscio JT. Abnormal glutamate metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1987;22:575–579. doi: 10.1002/ana.410220503. [DOI] [PubMed] [Google Scholar]
- Poulsen H, Jorgensen R, Heding A, Nielsen FC, Bonven B, Egebjerg J. Dimerization of ADAR2 is mediated by the double-stranded RNA binding domain. RNA. 2006;12:1350–1360. doi: 10.1261/rna.2314406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL, Coyle JT. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Immunocytochemical and ultrastructural study of the motor cortex in patients with lower motor neuron disease. Neurosci Lett. 2000;281:45–48. doi: 10.1016/s0304-3940(00)00789-8. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Brain Res Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreux-Varoquaux O, Bensimon G, Lacomblez L, Salachas F, Pradat PF, Le Forestier N, Marouan A, Dib M, Meininger V. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J Neurol Sci. 2002;193:73–78. doi: 10.1016/s0022-510x(01)00661-x. [DOI] [PubMed] [Google Scholar]
- Sun HS, Doucette TA, Liu Y, Fang Y, Teves L, Aarts M, Ryan CL, Bernard PB, Lau A, Forder JP, Salter MW, Wang YT, Tasker RA, Tymianski M. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39:2544–2553. doi: 10.1161/STROKEAHA.107.506048. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Fa M, Liu S, Zhang H, Hidalgo A, Schmidt SD, Yamaguchi H, Yoshii N, Mathews PM, Nixon RA, Arancio O. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bosch L, Schwaller B, Vleminckx V, Meijers B, Stork S, Ruehlicke T, Van Houtte E, Klaassen H, Celio MR, Missiaen L, Robberecht W, Berchtold MW. Protective effect of parvalbumin on excitotoxic motor neuron death. Exp Neurol. 2002;174:150–161. doi: 10.1006/exnr.2001.7858. [DOI] [PubMed] [Google Scholar]
- Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Sensi SL. Ca2+-Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci. 2000;23:365–371. doi: 10.1016/s0166-2236(00)01610-6. [DOI] [PubMed] [Google Scholar]
- Yin HZ, Sensi SL, Ogoshi F, Weiss JH. Blockade of Ca2+-permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J Neurosci. 2002;22:1273–1279. doi: 10.1523/JNEUROSCI.22-04-01273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]