Abstract
Septic infections dysregulate hemostatic pathways, prompting coagulopathy. Nevertheless, anticoagulant therapies typically fail to protect humans from septic pathology. The data reported here may help to explain this discrepancy by demonstrating critical protective roles for coagulation leading to fibrin deposition during host defense against the gram-negative bacterium Yersinia enterocolitica. After intraperitoneal inoculation with Y. enterocolitica, fibrinogen-deficient mice display impaired cytokine and chemokine production in the peritoneal cavity and suppressed neutrophil recruitment. Moreover, both gene-targeted fibrinogen-deficient mice and wild type mice treated with the anticoagulant coumadin display increased hepatic bacterial burden and mortality following either intraperitoneal or intravenous inoculation with Y. enterocolitica. Mice with low tissue factor (TF) activity succumb to yersiniosis with a phenotype similar to fibrin(ogen)-deficient mice, whereas factor XI (FXI)-deficient mice show wild type levels of resistance. Mice deficient in plasminogen activator inhibitor-1 (PAI-1) or thrombin activatable fibrinolysis inhibitor (TAFI) display modest phenotypes, but mice deficient in both PAI-1 and TAFI succumb to yersiniosis with a phenotype resembling fibrin(ogen)-deficient mice. These findings demonstrate critical protective roles for the TF-dependent extrinsic coagulation pathway during host defense against bacteria and caution that therapeutics targeting major thrombin-generating or anti-fibrinolytic pathways may disrupt fibrin-mediated host defense during gram-negative sepsis.
Introduction
Hemostatic blood coagulation pathways culminate in the deposition of fibrin, a provisional extracellular matrix that contributes to blood clotting and wound repair. Fibrin levels are regulated by fibrin-promoting coagulant pathways and fibrin-degrading fibrinolytic pathways. During sepsis, a major cause of death in hospitals, hemostasis becomes unbalanced: up-regulated procoagulant activity is insufficiently balanced by anticoagulation and fibrinolysis, prompting disseminated intravascular coagulation (DIC) and fibrin deposition (1, 2). In addition to promoting ischemia and inflammation, DIC eventually consumes critical blood-clotting factors, thus contributing to hemorrhage and shock. This constellation of coagulopathies is difficult to treat and contributes to the high mortality rates for patients presenting with sepsis (1, 2).
While DIC contributes to septic pathology, coagulation leading to fibrin deposition also can perform critical protective functions during infection. Mice lacking the capacity to produce fibrin (e.g. gene-targeted fibrinogen-deficient mice or coumadin-treated wild type mice) succumb prematurely to infection with Toxoplasma gondii (3). The premature deaths appear to result from hemorrhage caused by the immune system as it clears this protozoan parasite from the liver. Likewise, fibrin-deficiency prompts acute hepatic hemorrhage 5 days after inoculation of mice with Listeria monocytogenes, a gram-positive bacterium (4). Thus, fibrin-mediated “hemostatic immunity” can be critical to surviving certain protozoan and bacterial infections.
In addition to restraining hemorrhage, fibrin(ogen) also functions protectively by limiting the growth and/or dissemination of certain pathogens. After inoculation with the gram-positive bacteria L. monocytogenes or Staphylococcus aureus (4, 5), but not after inoculation with T. gondii (3), fibrinogen-deficient mice exhibit greatly increased pathogen burden. One potential explanation is that physical entrapment of bacteria by fibrin may limit their capacity to disseminate (6, 7). Additionally, fibrin(ogen) may facilitate bacterial clearance by phagocytes: fibrinogen is a ligand for CD11b/CD18 and CD11c/CD18, signal-transducing integrins expressed by leukocytes (8, 9), and studies of gene-targeted fibrinogen-mutant mice suggest that fibrin(ogen) stimulates inflammation leading to the recruitment and activation of leukocytes (5, 10, 11). Many bacteria produce factors that bind to fibrin and/or regulate fibrin levels, presumably as a means to counter fibrin-mediated host defense mechanisms (12, 13).
Given the protective and pathological potential of fibrin during infection, the development of therapeutics that safely suppress septic coagulopathy while maintaining protective hemostasis and other critical elements of fibrin-dependent host defense may require a thorough understanding of hemostatic pathway regulation during infection. Already, a great deal is known about the regulation of hemostasis during vascular trauma and thrombosis. Procoagulant pathways initiate fibrin formation by stimulating production of thrombin, a protease that cleaves soluble fibrinogen, prompting its polymerization and deposition as insoluble fibrin. TF plays a prominent role in the initiation of vascular procoagulant pathways (14). TF is expressed primarily by extravascular cells, whereas the proteases that generate thrombin circulate in plasma as inactive precursors. This physical segregation usually ensures that this “extrinsic” coagulation pathway is only activated in response to breaches of vascular integrity. However, procoagulant pathways also may be activated by inflammation-induced upregulation of TF on cells within the vasculature (2, 14), or by TF-independent pathways, such as the FXI-dependent “intrinsic” coagulation pathway (12, 15). Regardless of the initiating mechanisms, procoagulant pathways all culminate in the formation of a prothrombinase (PT) complex that produces thrombin, the protease that cleaves fibrinogen, thereby prompting its polymerization and deposition as fibrin. These procoagulant activities are limited by multiple anticoagulant mechanisms, including TF pathway inhibitor, anti-thrombin, and activated protein C (APC) (2, 16). Once formed, fibrin levels are regulated by plasmin, a fibrin-degrading protease derived from plasminogen upon its proteolytic activation by plasminogen activators (PA). Fibrinolysis is negatively regulated by multiple factors including PAI-1 (17), an inflammation-inducible PA antagonist whose levels increase during sepsis (18), and TAFI, an enzyme that indirectly suppresses plasminogen activation by modifying fibrin in a manner that reduces its affinity for plasminogen and PA (19, 20).
Therapeutic targeting of TF and other elements of the extrinsic coagulation pathway can lessen pathology and improve survival in animal models of bacterial sepsis (2, 14, 21, 22). However, many human clinical trials for sepsis have failed to demonstrate that treatment with anticoagulants can significantly improve survival (1, 2). Only one anticoagulant, recombinant human APC (rhAPC), has been licensed for the treatment of severe sepsis (16, 23), and the overall therapeutic benefit of rhAPC has been questioned, in part because serious bleeding is a significant complication in patients treated with this potent anticoagulant (24). Therapeutic strategies based on partial anticoagulation, for example via depletion of FXI (25), or based on augmentation of fibrinolysis, for example by antagonizing PAI-1 and/or TAFI (18, 26, 27) are under investigation.
Gram-negative bacteria are a common cause of sepsis and sepsis-associated coagulopathy (1, 28). Animal models of sepsis commonly employ bolus injections of the gram-negative bacterium E. coli or its endotoxin as challenge. Typically, these models lead to intoxication, rather than colonization and sustained infection (29–31). Host defense roles for coagulation and fibrin deposition may be dispensable in intoxication models of gram-negative sepsis, but critical in settings of sustained infection. Yersinia enterocolitica is a gram-negative bacterium that establishes sustained infections in mice that must be cleared by a robust host response (32, 33). Transmission of Y. enterocolitica to humans typically follows the ingestion of contaminated food, water and milk (34). The resulting yersiniosis typically manifests as a self-limiting enterocolitis, however, Y. enterocolitica also can cause extraintestinal disorders, including sepsis (34, 35). Moreover, transfusion-associated septic yersiniosis, which results from the growth of Y. enterocolitica in refrigerated blood, has greater than 35% case fatality rates (34, 36–38). Here, we demonstrate that fibrin performs multiple protective functions in the C57BL/6 mouse model of yersiniosis, including the induction of cytokine and chemokine expression, activation of neutrophil recruitment, and restraint of bacterial burden. Investigations of the mechanisms regulating the deposition of protective fibrin revealed critical roles for TF but not FXI. Our studies also revealed synergy in the anti-fibrinolytic functions of PAI-1 and TAFI. We discuss the relevance of these findings to the development of sepsis therapeutics that aim to limit coagulopathy without preventing the deposition of protective fibrin.
Materials and Methods
Mice
C57BL/6 wild type and PAI-1-deficient mice were obtained from Jackson Laboratory (Bar Harbor, ME). C57BL/6 fibrinogen-deficient mice (39), FXI-deficient mice (40), and TAFI-deficient mice (41) were generously supplied by Jay L. Degen, David Gailani and Edward F. Plow, respectively. PAI-1/TAFI-deficient mice were generated at Trudeau Institute. Nigel Mackman supplied C57BL/6 mice with very low levels of TF activity (low-TF mice). These mice lack expression of mouse TF due to its inactivation by gene targeting and instead express a human TF transgene, which imparts low-level TF activity (mTF−/−hTF+) (42). Low-TF mice were compared with littermates heterozygous for mouse TF and expressing human TF (mTF+/−hTF+). When littermate fibrinogen-deficient mice, FXI-deficient mice, or TF transgenic mice were compared, they were co-housed with controls and the investigators were blinded with regard to the animals’ genotypes. All experimental mice were bred in a specific pathogen-free facility at Trudeau Institute. They were matched for age and sex, and infected between 6 and 10 weeks of age. Where indicated, mice were anticoagulated pharmacologically by supplementing drinking water with 2 mg/liter coumadin [3-(α-acetonylbenzyl)-4-hydroxycoumarin; Sigma] beginning 3 days prior to infection or 1 day after infection with replenishment every 48 hours; this anticoagulant regimen reduces fibrin deposition in mice during infection (4). All animal studies were conducted in accordance with Trudeau Institute Animal Care and Use Committee guidelines.
Bacterial infection
Y. enterocolitica strain WA (serotype O:8) (32) was obtained from ATCC (#27729). After growth to early log phase at 26°C in brain heart infusion broth (BHI; Difco laboratories, Detroit, MI), bacteria were resuspended in BHI supplemented with 20% glycerol and stocks were stored as single use aliquots at −70°C. Preliminary studies established that the intravenous dose that caused half the mice to succumb to infection (LD50) was 5×104 colony forming units (CFU). For experimental infections, frozen bacteria were diluted with saline to the desired dose and administered in a 100 μl volume. The number of bacteria in the inoculating dose was confirmed by plating on BHI agar.
Measurements of survival and bacterial burden
Mice were monitored daily. Unresponsive or recumbent animals were considered moribund and euthanized. For measurement of bacterial burden, fluids and tissues were collected after mice were euthanized by carbon dioxide narcosis. Bacterial burden was measured by homogenizing tissues in saline, plating serial dilutions on BHI agar, incubating overnight at 26°C, and counting CFU.
Measurements of blood parameters, fibrin deposition, and hepatic PT and PA activity
Blood was collected by cardiac puncture from mice that received 500 U heparin intravenously just prior to euthanasia. After dilution in saline containing 5 mM EDTA, hematocrits and platelet numbers were measured using a Coulter Counter (Beckman). Plasma levels of D-dimer were measured by ELISA (Diagnostica Stago) and reported as fold-change relative to the average value measured in naïve C57BL/6 mice. Levels of fibrin in liver tissue were quantified as described (3) and scored positive when above the limit of detection (15 ng/mg of tissue). In brief, fibrin was extracted from homogenized liver tissue and quantified by Western blot using fibrin-specific mAb 350 (American Diagnostica, Inc.); the standard curve was prepared from mouse fibrinogen treated with thrombin (3). Levels of PT and PA activity in liver tissue were quantified in situ using validated assays (43). In brief, fresh frozen livers were sectioned (7 μm) mounted onto slides, and overlaid with human prothrombin (Enzyme Research Laboratories). After incubation at 37°C, thrombin levels were quantified using Spectrozyme TH (American Diagnostica) and normalized to the area of the tissue section. Purified human alpha-thrombin (Enzyme Research Laboratories) was used as standard. PA assays were performed similarly to PT assays, with the following modifications: prothrombin was replaced by human Glu-plasminogen (Enzyme Research Laboratories), the standard curve was prepared using purified human plasmin (Enzyme Research Laboratories), and plasmin levels were measured using Spectrozyme PL (American Diagnostica). PT and PA activity are presented as fold-change relative to the levels measured in tissue samples collected from naïve mice.
Measurements of immune parameters
To assess responses in the peritoneal cavity, mice were euthanized and peritoneal exudate fluid was harvested by lavage using 5 ml saline. Neutrophil percentages were determined by inspection of Wright-Giemsa-stained cytospin smears. IL-6 and monocyte chemoattractant protein-1 (MCP-1) levels were determined by ELISA using OptEIA kits (BD Biosciences). Levels of hepatic mRNA encoding IL-1β, IL-6, IL-10, IFN-γ, and TNF-α were measured by real-time PCR, normalized to levels of GAPDH, and expressed as log10 fold-change relative to levels measured in uninfected control mice (3).
Histology
Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The number of large pathological foci in each section was counted (i.e. areas with large numbers of infiltrating cells accompanied by evidence of anoxic or necrotic hepatocytes and/or bacterial colonization). Representative photomicrographs depict ×200 magnification.
Statistics
Statistical analyses were performed using the computer program Prism 4.0 (GraphPad Software, Inc.). Survival data were analyzed by log rank tests. Cytokines, cell numbers, and CFU data were analyzed by parametric tests; Student’s t test or one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test, unless CFU levels fell below the limit of detection (135 CFU), in which case mice were assigned a value of 100 CFU and data were analyzed by nonparametric tests. Box and whisker plots depict the minimum, maximum, median and 25th and 75th percentile.
Results
Fibrinogen impacts peritoneal inflammatory responses during yersiniosis
Prior reports indicate fibrinogen can impact peritoneal inflammation after inoculation with gram-positive bacteria (5). To assess roles for fibrinogen during gram-negative infection, we infected fibrinogen-deficient and littermate control fibrinogen-heterozygous mice with Y. enterocolitica serotype O:8 strain WA (32). Specifically, we examined peritoneal exudate fluid collected 3 hours after intraperitoneal inoculation with 1×103 CFU Y. enterocolitica. The exudates obtained from fibrinogen-deficient mice, as compared with those collected from control fibrinogen-heterozygous mice, contained significantly reduced levels of the inflammatory cytokine IL-6 and chemokine MCP-1 (Figure 1A; p=0.006 and 0.0006, respectively). Fibrinogen-deficient mice also displayed significantly reduced numbers of neutrophils in exudate fluid (Figure 1B; p=0.0005).
Figure 1. Fibrin(ogen) regulates inflammation during peritoneal yersiniosis.
Measurements of (A) inflammatory mediators and (B) cells in peritoneal exudate fluid collected from fibrinogen-deficient mice (Fib KO; solid bars) and littermate control fibrinogen-heterozygous mice (Fib Het; open bars) at 3 hours after intraperitoneal inoculation with 1×103 CFU Y. enterocolitica. In comparison with control mice, fibrinogen-deficient mice exhibited decreased levels of IL-6 (p=0.006) and MCP-1 protein (p=0.0006), and decreased numbers of neutrophils (p=0.0005). (C) Bacterial burden at 3 hours after intraperitoneal inoculation with 1×106 CFU Y. enterocolitica. In comparison with control fibrinogen-heterozygous mice, fibrinogen-deficient mice exhibited significantly increased bacterial burden in peritoneal fluid (p=0.0008) and liver tissue (p=0.01). Similar results were observed in two independent experiments. Statistical significance was analyzed by student’s t test (n=5–8 mice per group).
At 3 hours after inoculation with 1×103 CFU Y. enterocolitica, we could not detect bacteria in the peritoneal fluid. After increasing the challenge dose to 1×106 CFU, we observed significantly higher bacterial burden in fibrinogen-deficient mice as compared with littermate control fibrinogen-heterozygous mice (Figure 1C, p=0.0008). Fibrinogen-deficiency also significantly increased the number of bacteria that reached the liver at 3 hours after intraperitoneal inoculation with 1×106 CFU Y. enterocolitica (Figure 1C, p=0.01). These data indicate that fibrin(ogen) impacts inflammation and host defense against bacteria at early times after intraperitoneal inoculation with Y. enterocolitica.
Fibrin protects against peritoneal yersiniosis
Y. enterocolitica serotype O:8 is virulent in humans (34–38) and mice (32, 33). To investigate whether fibrinogen impacts susceptibility to Y. enterocolitica infection, we inoculated fibrinogen-deficient mice with a dose of Y. enterocolitica that is sublethal for wild type mice. We observed that fibrinogen-deficient mice succumbed after inoculation with 0.05 LD50 Y. enterocolitica (2.5×103 CFU), whereas all fibrinogen-heterozygous littermate mice survived. The difference in survival was highly significant (p<0.0001). Measurements of bacterial burden 5 days after Y. enterocolitica inoculation revealed significantly higher numbers of CFU in the livers and spleens of fibrinogen-deficient mice, as compared with control mice (Figure 2B).
Figure 2. Fibrin protects against peritoneal yersiniosis.
(A) Survival and (B) bacterial burden for fibrinogen-deficient mice (Fib KO) and littermate control fibrinogen-heterozygous mice (Fib Het) after intraperitoneal inoculation with 2.5×103 CFU Y. enterocolitica. In comparison with control mice, fibrinogen-deficient mice exhibited significantly reduced survival (p<0.0001; n=9 mice/group) and significantly increased hepatic and splenic bacterial burden at day 5 after infection (n=6–7 mice/group). (C) Survival and (D) bacterial burden of wild type coumadin-treated (Coum) and control C57BL/6 mice after intraperitoneal inoculation with 2.5×103 CFU Y. enterocolitica. In comparison with control mice, coumadin-treated mice exhibited significantly reduced survival (p<0.05; n=5 mice/group) and significantly increased hepatic and splenic bacterial burden at day 5 after infection (n=5 mice/group). Similar results were observed in two independent experiments.
Since fibrinogen-deficient mice lack both fibrinogen and fibrin, next we treated wild type mice with coumadin, a pharmaceutical used clinically for long-term anticoagulation. Coumadin-treated mice possess normal levels of circulating fibrinogen but generate far less fibrin (4). Like fibrinogen-deficient mice, coumadin-treated mice acutely succumbed to intraperitoneal inoculation with 2.5×103 CFU Y. enterocolitica (Figure 2C). Coumadin treatment also significantly increased bacterial burden in livers and spleens collected 5 days after initiating the infection (Figure 2D). The similar phenotypes observed in fibrinogen-deficient and coumadin-treated mice following intraperitoneal inoculation with Y. enterocolitica strongly suggest that fibrin functions protectively during gram-negative peritonitis.
Fibrin functions protectively during systemic yersiniosis
Prior reports suggest fibrin may physically trap bacteria, thereby limiting dissemination from the peritoneal cavity (6, 7) and liver vasculature (44). To investigate whether fibrin functions protectively beyond the peritoneal cavity during gram-negative bacterial infection, we inoculated mice intravenously with Y. enterocolitica. Fibrinogen-heterozygous mice largely survived intravenous inoculation with 1×103 CFU Y. enterocolitica whereas fibrinogen-deficient mice displayed significantly reduced survival (Figure 3A; p<0.0001). Moreover, 5 days after intravenous inoculation with 1×103 CFU Y. enterocolitica, fibrinogen-deficient mice displayed significantly increased bacterial burden in liver and spleen (Figure 3B). As for intraperitoneal inoculation, coumadin treatment also increased susceptibility to yersiniosis following intravenous inoculation with Y. enterocolitica: in comparison with untreated control mice, coumadin-treated mice exhibited significantly decreased survival (Figure 3C, p<0.0001) and increased bacterial burden (Figure 3D). Coumadin treatment also led to significantly decreased survival when the initiation of treatment was delayed until one day after the initiation of infection (Figure 3C, p=0.002). Notably, coumadin treatment did not alter the levels of plasma fibrinogen measured at day 5 after infection (data not shown). Together, these data suggest that fibrin not only activates peritoneal inflammation and antibacterial defense but also contributes to antibacterial host defense mechanisms that operate beyond the peritoneal cavity. Interestingly, fibrinogen-deficient mice did not exhibit decreased survival when inoculated orally with Y. enterocolitica (1×108 CFU intragastrically; fibrinogen-heterozygous 25% survival, fibrinogen-deficient 29% survival, p>0.05), suggesting that fibrin is critical for systemic but not mucosal host defense against this gram-negative bacterium.
Figure 3. Fibrin functions protectively during systemic yersiniosis.
(A) Survival and (B) bacterial burden for fibrinogen-deficient (Fib KO) mice and littermate control fibrinogen-heterozygous (Fib Het) mice after intravenous inoculation with 1×103 CFU Y. enterocolitica. In comparison with control mice, fibrinogen-deficient mice exhibited significantly reduced survival (p<0.0001; n=6–7 mice/group) and significantly increased hepatic and splenic bacterial burden at day 5 after infection (n=5–8 mice/group). (C) Survival and (D) bacterial burden of coumadin-treated (Coum) and control wild-type mice after intravenous inoculation with 1×103 CFU Y. enterocolitica. In comparison with control mice, mice treated with coumadin beginning on day −3 (d−3) or day +1 (d+1) relative to infection exhibited significantly reduced survival (n=5 mice/group for coumadin and n=10 mice/group for control). The survival of the day −3 and day +1 groups did not differ significantly from each other. The day −3 treatment led to significantly increased hepatic and splenic bacterial burden at day 5 after infection (n=5 mice/group). Similar results were observed in two independent experiments.
Kinetics of fibrin deposition during sublethal yersiniosis
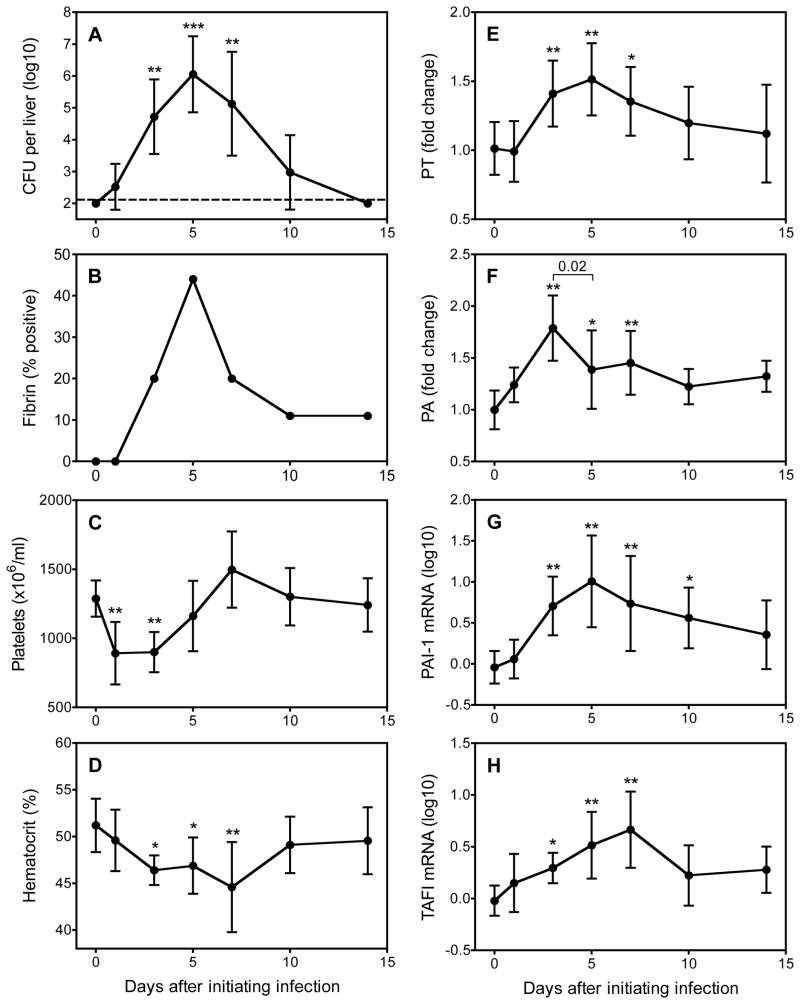
Given that fibrin-deficient mice displayed increased hepatic bacterial burden after inoculation with Y. enterocolitica, we investigated whether hepatic fibrin was detectable during the course of yersiniosis in wild type mice. Specifically, we inoculated wild-type mice intravenously with 1×103 CFU Y. enterocolitica and then analyzed bacterial burden and fibrin levels on days 1, 3, 5, 7, 10 and 14 after infection. We detected hepatic bacteria in most mice on days 1, 3, 5, 7 and 10, with the greatest burden apparent on day 5 (Figure 4A). Hepatic fibrin levels in mice infected with Y. enterocolitica were lower than those reported previously for mice infected with Listeria or Toxoplasma (3, 4). While we could only detect fibrin in a subset of infected animals, nevertheless, it was apparent that fibrin deposition peaked on day 5, thus coinciding with the time of maximal bacterial burden (Figure 4B). Notably, yersiniosis also was characterized by significant thrombocytopenia (decreased platelet counts; Figure 4C) and anemia (decreased hematocrits; Figure 4D), suggesting systemic activation of coagulation and sublethal bleeding, respectively. Consistent with peak fibrin deposition on day 5, procoagulant activity also peaked at that time, as measured by in situ measurements of hepatic PT activity (Figure 4E). Interestingly, hepatic PA activity peaked at day 3 and declined significantly by day 5, perhaps explaining the increased fibrin deposition on day 5 (Figure 4F). Levels of D-dimer, a systemic measure of fibrinolysis, did not rise significantly above baseline at any time point (data not shown) but levels of hepatic mRNA encoding PAI-1 and TAFI, two inhibitors of fibrinolysis, increased over the course of yersiniosis (Figure 4G and 4H), potentially explaining the decreased hepatic PA activity at the time of peak fibrin deposition. These data establish that hemostatic pathways become activated during sublethal yersiniosis in wild type mice over the same period during which fibrin(ogen)-deficient mice succumb to infection.
Figure 4. Hepatic fibrin deposition accompanies the acute phase of sublethal yersiniosis.
Wild-type C57BL/6 mice were inoculated intravenously with 1×103 CFU Y. enterocolitica. On the indicated days, mice were assayed for hepatic bacterial burden (A), hepatic fibrin deposition (B), blood platelet numbers (C), blood hematocrits (D), hepatic PT activity (E), hepatic PA activity (F), hepatic PAI-1 mRNA (G) and hepatic TAFI mRNA (H). The data are compiled from two independent experiments and depict the mean ± standard deviation of 9–10 mice per time point, except for day 1, which only had 5 mice. The dashed line indicates the level of detection for bacterial CFU. Statistics were performed using ANOVA followed by Dunnett’s multiple-comparison test, except that the non-parametric Kruskal-Wallis with Dunn’s post-test was used to evaluate bacterial burden (* p<0.05, ** p<0.01, *** p<0.001).
Critical roles for TF, but not FXI, during yersiniosis
Given evidence of coagulation during sublethal yersiniosis (i.e. increased hepatic PT activity and fibrin deposition), we investigated roles for key elements of the extrinsic and intrinsic coagulation pathways. Tissue factor is the primary initiator of the extrinsic coagulation pathway. While fully TF-deficient mice (i.e. mTF−/− mice) die in utero, TF function can be studied in vivo using mTF−/− mice expressing a human TF transgene (i.e. mTF−/−hTF+ mice) (42). Consistent with prior studies (42, 43), we observed that liver tissue from these low-TF mice contained significantly reduced levels of baseline hepatic PT activity (Figure 5A, p<0.0001). In contrast, baseline PT activity was similar in wild type mice and mice deficient in FXI, a key component of the intrinsic coagulation pathway (Figure 5B).
Figure 5. Protective roles for TF, PAI-1 and TAFI, but not FXI, during yersiniosis.
Hepatic PT activity was quantified in mTF−/−hTF+ low-TF mice and mTF+/−hTF+ littermate control mice (A), and in FXI-deficient (FXI KO) mice and littermate control FXI-heterozygous (Het) and wild type (WT) mice (B). Significantly decreased basal PT activity was measured in naïve low-TF mice (p<0.0001) but not in FXI-deficient mice (n=4–6 mice/group). Significantly decreased PT activity also was measured in low-TF mice, but not FXI-deficient mice, 5 days after intravenous inoculation with 1×103 CFU Y. enterocolitica (p<0.0001, n=5–7 mice/group). Hepatic bacterial burden (C) and survival (D) also was impacted significantly by TF-deficiency (p=0.003 and p=0.002, respectively) but not FXI-deficiency (n=5–7 mice/group). Hepatic PA activity (E) plasma D-dimer (F) hepatic bacterial burden (G) and survival were quantified in PAI-1-deficient (PAI-1 KO) mice, TAFI-deficient (TAFI KO) mice, PAI-1/TAFI-deficient (P/T KO) mice and control wild type C57BL/6 mice after intravenous inoculation with 1×103 CFU Y. enterocolitica. For E–G, n=17–20 mice/group for naïve (“n”) and 7–10 mice/group for day 3 and day 5 (* p<0.05, ** p<0.01 in comparison to wild type C57BL/6 mice by ANOVA). The mice all displayed similar levels of basal PA activity and similarly increased PA activity at day 3 after inoculation with Y. enterocolitica. On day 5, PA activity increased significantly in PAI-1-deficient mice, TAFI-deficient mice, and PAI-1/TAFI-deficient mice, as compared with wild type control mice. Plasma D-dimer levels were similar at baseline in PAI-1-deficient, TAFI-deficient, PAI-1/TAFI-deficient and wild type C57BL/6 mice but increased significantly in PAI-1/TAFI-deficient mice on days 3 and 5. Hepatic bacterial burden increased significantly in PAI-1-deficient mice and PAI-1/TAFI-deficient mice. In comparison with wild type mice (n=30), significantly reduced survival was observed for PAI-1-deficient mice (p<0.0001; n=19), TAFI-deficient mice (p=0.008; n=20) and PAI-1/TAFI-deficient mice (p<0.001; n=5).
By day 5 after intravenous inoculation with 1×103 CFU Y. enterocolitica, levels of PT activity in low-TF mice had increased substantially but still remained significantly below those measured in control mice (p<0.0001). Like fibrin(ogen)-deficient mice (Figure 3), the infected low-TF mice also displayed significantly increased bacterial burden (Figure 5C; p=0.003) and reduced survival (Figure 5D, p=0.002) as compared to littermate control mTF+/−hTF+ mice. In contrast, the FXI-deficient mice did not exhibit increased bacterial burden or reduced survival (Figures 5C, 5D). These data suggest that the TF-dependent extrinsic coagulation pathway plays a dominant role in the regulation of host-protective fibrin production during sublethal yersiniosis.
Synergistic protective roles for PAI-1 and TAFI during yersiniosis
Levels of hepatic mRNA encoding PAI-1 and TAFI increased over the course of yersiniosis, peaking on days 5 and 7, respectively (Figure 4G and 4H). To investigate the importance of PAI-1 and TAFI expression, and to measure their capacity to regulate PA activity in this model, we infected gene-targeted mice lacking expression of PAI-1, TAFI, or both PAI-1 and TAFI. Basal levels of hepatic PA activity were similar in all these mice and all the mice displayed similarly elevated PA activity at day 3 after intravenous inoculation with 1×103 CFU Y. enterocolitica (Figure 5E). Consistent with our prior kinetic analysis (Figure 4F), PA activity decreased in wild type mice by day 5. In contrast, PA activity remained elevated on day 5 in mice deficient in PAI-1, TAFI or both PAI-1 and TAFI, and these mice all exhibited significantly increased levels of hepatic PA activity when compared with wild type mice (Figure 5E, all p<0.01). Levels of plasma D-dimer, a systemic marker of fibrinolysis, did not increase significantly in wild type mice or TAFI-deficient mice infected with Y. enterocolitica. However, mice deficient in PAI-1 displayed significantly increased D-dimer levels on day 3 after inoculation with Y. enterocolitica, and PAI-1/TAFI-deficient mice displayed significantly increased D-dimer levels on both days 3 and 5 (Figure 5F).
In comparison with wild type mice, the bacterial burden in PAI-1-deficient mice increased significantly on both days 3 and 5 after inoculation with Y. enterocolitica, and the PAI-1/TAFI-deficient mice displayed even greater burden (Figure 5G). TAFI-deficient mice exhibited bacterial burden similar to wild type mice on days 3 or 5 but their splenic burden increased significantly on day 7 (Figure 5G and data not shown). In comparison with wild type mice, the TAFI-, PAI-1- and PAI-1/TAFI-deficient mice all exhibited significantly reduced survival following inoculation with Y. enterocolitica; PAI-1/TAFI-deficient displayed the greatest susceptibility, followed sequentially by PAI-1-deficient mice and TAFI-deficient mice (Figure 5H). Together, these findings suggest that PAI-1 and TAFI perform complementary functions that synergistically regulate fibrinolysis during yersiniosis.
Fibrin(ogen), TF, PAI-1 and TAFI impact inflammation and pathology during yersiniosis
After intravenous inoculation with Y. enterocolitica, fibrinogen-deficient mice, low-TF mice and PAI-1/TAFI-deficient mice all displayed evidence of increased hepatic inflammation. Specifically, they displayed significantly increased levels of hepatic mRNA encoding IL-1β, IL-6, IL-10, IFNγ, and TNFα when compared with their respective control mice (Figure 6 and data not shown). These observations must be interpreted with caution since the increased inflammation could be a consequence of the increased bacterial burden in these mice (Figures 3 and 5).
Figure 6. Deficiencies in fibrinogen, TF and PAI-1/TAFI similarly impact inflammation, anemia and thrombocytopenia during yersiniosis.
Fibrinogen-deficient (Fib KO) mice and littermate control fibrinogen-heterozygous (Fib Het) mice (A, D, G, J, M; n=7–13 mice/group), mTF−/−hTF+ low-TF mice and mTF+/−hTF+ littermate control mice (B, E, H, K, N; n=5–7 mice/group), or wild type C57BL/6 mice and PAI-1/TAFI deficient (PAI-1/TAFI KO) mice (C, F, I, L, O; n=6–20 mice/group) were intravenously inoculated with 1×103 CFU Y. enterocolitica, analyzed 3 or 5 days later, and compared to uninfected naïve mice. Hepatic mRNA encoding IL-6 (A–C), IFNγ (D–F) and IL-10 (G–I) were measured by quantitative PCR. Blood hematocrits (J–L) and platelet numbers (M–O) were measured by Coulter Counter. The asterisks depict ANOVA comparisons of naïve to day 3 and day 5 for each strain of mice (*p<0.05, **p<0.01, ***p<0.001). The numbers depict p values for student’s t test comparisons of day 3 and day 5 samples across the indicated strains of mice. In comparison with infected control mice, infected fibrinogen-deficient mice, low-TF mice, and PAI-1/TAFI-deficient mice all showed significantly increased levels of IL-6, IL-10 and IFNγ and significantly decreased hematocrits and platelet numbers.
After infection with Y. enterocolitica, fibrinogen-deficient mice, PAI-1/TAFI-deficient mice and low-TF mice all displayed significantly greater reductions in blood hematocrits and circulating platelet numbers when compared with control mice (Figure 6). While this hematological data was suggestive of bleeding, histological observations rarely revealed evidence of hemorrhage in fibrin-deficient mice and stool samples rarely tested positive for hemoglobin. This finding contrasts sharply with our prior studies of toxoplasmosis and listeriosis in fibrin(ogen)-deficient mice, where the exacerbated anemia and thrombocytopenia were accompanied by frank hemorrhage (3, 4).
Although fibrin did not appear to protect from hepatic hemorrhage during yersiniosis it did, nonetheless, dramatically impact liver histopathology (Figure 7). Wild type mice infected with Y. enterocolitica displayed very limited hepatic damage and small, sporadic sites of inflammation. Mice deficient in either PAI-1 or TAFI likewise displayed small foci of mixed leukocytes similar to those observed in wild type mice. In striking contrast, fibrinogen-deficient mice, low-TF mice, and PAI-1/TAFI-deficient mice all displayed significantly exacerbated histopathology, evidenced by large bacterial colonies and extensive areas of tissue damage characterized diffuse leukocyte infiltration and hepatocellular necrosis.
Figure 7. Deficiencies in fibrinogen, TF and PAI-1/TAFI similarly impact hepatic histopathology during yersiniosis.

Hematoxylin and eosin staining of representative liver samples collected from the mice described in Figure 6. In comparison with naive C57BL/6 mice (A), C57BL/6 mice infected with Y. enterocolitica (B) displayed sporadic small sites of inflammation. The histopathology of infected mTF+/−hTF+ mice (C) PAI-1-deficient mice (E) and TAFI-deficient mice (F) resembled that of the infected C57BL/6 control mice. In contrast, the mTF−/−hTF+ low-TF mice (D), PAI-1/TAFI-deficient mice (G) and fibrinogen-deficient mice (H) exhibited large foci characterized by mixed inflammatory infiltrates with extensive tissue necrosis (asterisks), and bacterial colonization (arrowheads). Histopathological scoring (I) revealed significantly greater numbers of large foci in liver samples collected from the infected mTF−/−hTF+ low-TF mice (p=0.02), PAI-1/TAFI-deficient mice (p=0.03) and fibrinogen-deficient mice (p=0.03), as compared with infected wild type C57BL/6 mice.
Discussion
This report demonstrates that fibrinogen-deficient mice and wild type mice treated with coumadin succumb to doses of Y. enterocolitica that control mice survive. Moreover, fibrinogen-deficient mice, low-TF mice and PAI-1/TAFI-deficient mice all exhibit similar decreases in survival, increases in bacterial burden, and exacerbations of anemia and hepatic histopathology after inoculation with Y. enterocolitica. These observations provide strong complementary evidence that fibrin performs critical protective functions in this model of sublethal gram-negative infection. This study focused on fibrin but we certainly acknowledge that other elements of hemostatic pathways also impact inflammation and infection (2, 12, 13, 45).
Our demonstration that fibrin can function protectively during gram-negative infection, coupled with the wealth of evidence that excessive coagulation can function pathologically during sepsis (2), suggests that appropriate regulation of fibrin levels will be critical for surviving certain septic infections. The up-regulation of coagulation during infection is likely initiated, at least in part, by host recognition of microbial components, leading to the activation of innate immunity, secretion of inflammatory cytokines, and consequent up-regulation of TF expression, thus activating the extrinsic pathway of coagulation (2, 12, 14). Our data suggest that antagonistic targeting of the TF-dependent extrinsic pathway reduces fibrin levels to below those required for host defense against yersiniosis. Full antagonism of other critical components of the extrinsic pathway, such as factor Xa or thrombin, would be predicted to similarly compromise fibrin-mediated protection. Thus, our studies caution that supra-physiologic dosing of rhAPC, the only approved therapeutic for severe sepsis, may suppress fibrin-mediated host defense. Likewise, excessive activation of fibrinolysis, as we accomplished by simultaneously inactivating PAI-1 and TAFI, may reduce fibrin to sub-protective levels.
While our observations indicate that the TF is critical for host defense against Y. enterocolitica, antagonism of TF and other elements of the extrinsic coagulation pathway improves survival in mice and primates inoculated with another gram-negative bacterium, E. coli, or its endotoxin (i.e. lipopolysaccharide) (2, 14, 21, 22). Thus, coagulation appears to be required for effective host defense against some, but not all, gram-negative bacteria. This observation may help to explain why anticoagulant therapies that show great promise in preclinical models of E. coli sepsis often do not translate to human efficacy in clinical trials (1, 2, 29–31). One notable distinction between E. coli and Y. enterocolitica is that the latter produces a relatively non-inflammatory form of lipopolysaccharide when grown at 37°C (46). Additionally, host defense against Y. enterocolitica is critically dependent upon T cells and IFNγ (33), whereas T cells and IFNγ actually contribute to pathology during E. coli infection (47, 48). A better understanding of the roles for coagulation during host defense and a full delineation of the pathogens that do and do not require protective coagulant responses may allow selective targeting of anticoagulant therapies to patients for whom the benefits of anticoagulation outweigh the risks of suppressing fibrin-mediated host defense.
Another approach to preventing coagulopathy while maintaining fibrin-mediated host defense may be to target elements of DIC-related hemostatic pathways whose full antagonism does not deplete fibrin levels to below those required for protection. Our studies indicate that targeting TF overly compromises host defense against sublethal yersiniosis, whereas targeting FXI has no impact on survival, burden or morbidity. FXI-deficiency improves survival in the mouse model of lethal bacterial sepsis induced by cecal ligation and puncture (25), but does not improve survival during lethal Y. enterocolitica infection (data not shown), presumably because something other than FXI-mediated DIC is the primary cause of death during lethal yersiniosis. Together, these observations suggest that FXI does not contribute to antibacterial host defense but sometimes contributes to septic coagulopathy. As such, we believe that FXI and other components of the intrinsic pathway deserve further investigation as therapeutics for septic DIC.
To our knowledge this is the first report demonstrating that TAFI-deficiency can impair survival in mice infected with pathogens. We cannot exclude the possibility that fibrinolysis-independent impacts of TAFI play a role (20, 49), but our data decisively demonstrate increased fibrinolysis in TAFI-deficient mice infected with Y. enterocolitica (Figure 5E and 5F). Renckens et al observed a transient increase in bacterial burden in TAFI-deficient mice infected with E. coli but, in striking contrast to our results, this did not impair survival and, rather, appeared to protect against liver injury (50). Again, this apparent discrepancy may relate to intrinsic differences between the pathogenesis of E. coli and Y. enterocolitica infections. We also demonstrated that PAI-1-deficiency decreases survival and increases bacterial burden during yersiniosis. Renckens et al reported analogous observations for PAI-1-deficient mice infected with Klebsiella pneumoniae (51). As for TAFI, we cannot exclude the possibility that fibrinolysis-independent impacts of PAI-1 play a role (17), but our data decisively demonstrate increased fibrinolysis in TAFI-deficient mice infected with Y. enterocolitica (Figure 5E and 5F). Altogether, these studies indicate that full antagonism of either TAFI or PAI-1 can impair host defense against gram-negative bacteria, but leave open the possibility that therapeutics targeting TAFI or PAI-1 may suppress coagulopathy without compromising host defense in certain settings (e.g. E. coli infection).
One of the most striking findings in this study was the synergy achieved by simultaneously antagonizing both TAFI and PAI-1. In comparison with mice deficient in either PAI-1 or TAFI, mice deficient in both PAI-1 and TAFI displayed significantly increased levels of plasma D-dimer and significantly exacerbated liver pathology. To our knowledge, this synergy has not been demonstrated previously in vivo, although it was postulated based upon in vitro models of thrombolysis (52). This synergy suggests that the consequences of targeting either PAI-1 or TAFI may be heavily influenced by the levels of the non-targeted factor. That is, the impact of PAI-1 antagonism may be far more pronounced in settings (e.g. certain tissues or disease states) where TAFI levels are relatively low.
Multiple mechanisms appear to contribute to fibrin-mediated host defense against yersioniosis. The increased bacterial burden observed in fibrin-deficient mice infected with Y. enterocolitica resembles that previously reported by our laboratory (4), and others (5), after inoculating fibrin(ogen)-deficient mice and fibrinogen-mutant mice with gram-positive bacteria. Thus, it seems that one fundamental function of fibrin is to limit bacterial growth. We are not aware of studies describing fibrin-binding proteins in Y. enterocolitica, but many other bacterial species possess diverse mechanisms for binding fibrin(ogen) and/or activating fibrinolysis (12, 13). It seems likely that bacteria have acquired the ability to interact with fibrin, at least in part, as a means to counter fibrin-mediated host defense.
While previously it has been suggested that fibrin may physically prevent bacterial dissemination during peritoneal infections (6, 7), we observed that fibrin also impacts bacterial burden after intravenous delivery of Y. enterocolitica. One possible explanation is that fibrin may contribute to microvessel thrombosis within infected liver tissue, thereby physically preventing dissemination of bacteria from the bloodstream (44, 53). Alternatively, fibrin may restrain bacterial growth by facilitating the recruitment and activation of bactericidal phagocytes within infected tissues. Prior studies reported that fibrinogen activates peritoneal macrophages and neutrophils in mice inoculated with lipopolysaccharide (10) and gram-positive bacteria (5). Consistent with those reports, this study demonstrates that fibrinogen facilitates the accumulation of peritoneal neutrophils after inoculation with Y. enterocolitica (Figure 1). And consistent with prior studies of sterile peritonitis (10), this report demonstrates that fibrinogen stimulates production of MCP-1 and IL-6 at early time points after inoculation with Y. enterocolitica. These impacts may be mediated by CD11b/CD18 and/or TLR4, since these receptors reportedly activate cytokine and chemokine production via fibrin(ogen)-mediated mechanisms (11, 54–56). MCP-1 recruits monocytes, and IL-6 is a pleiotropic cytokine known to promote fibrin deposition (57) and participate in host defense against Y. enterocolitica (58). Since monocytes express CD11b/CD18 and ligation of CD11b/CD18 by fibrin(ogen) activates peritoneal phagocytes (5), it is easily conceivable that fibrin acts as an inducible matrix supporting feedback amplification of signals leading to the activation and accumulation of antibacterial phagocytes at sites of inflammation.
Our findings suggest relatively minor roles for the hemostatic functions of fibrin during host defense against gram-negative bacterial infection. Anemia, evidenced by reduced hematocrits, is evident in wild-type mice inoculated with sublethal doses of Y. enterocolitica (Figure 4). Fibrin-deficient mice show exacerbated anemia (Figure 6) and begin to succumb to yersiniosis at day 5 of the infection, which coincides with the time of maximal anemia in wild type mice (Figure 3). We likewise observed exacerbated anemia in our prior studies of fibrin-deficient mice inoculated with protozoa and gram-positive bacteria (3, 4). In those studies, we observed unmistakable evidence of frank hemorrhage in the livers of fibrinogen-deficient mice, leading us to conclude that the anemia reflected bleeding. In contrast, we observed relatively little evidence of hepatic hemorrhage in fibrin-deficient mice during yersiniosis, despite a high bacterial burden (Figure 7). Thus, the extent to which fibrin provides critical hemostatic protection during infection appears to vary, presumably reflecting the extent to which different pathogens predispose to hemorrhagic pathology.
Although hemorrhage was uncommon in the hepatic lesions of Y. enterocolitica-infected fibrin-deficient mice, we nevertheless observed significant differences in the overall appearance of these lesions in comparison with those observed in fibrin-sufficient mice. Specifically, fibrin-deficient mice exhibited severe hepatocellular necrosis. We also observed increased levels of inflammatory cytokines in the liver at later stages of septic yersiniosis in fibrin-deficient mice. Increased cytokine production also is evident in fibrin(ogen)-deficient mice during septic listeriosis (4). Many mechanisms could potentially account for the exacerbation of hepatic pathology and inflammation in the absence of fibrin, however, we believe it most likely to be a consequence of the increased bacterial burden in fibrin-deficient mice.
Septic coagulopathy can be caused by many different types of microorganisms (28, 59). Ideally, identical therapeutics and dosing regimen would be appropriate for all patients presenting with septic coagulopathy, regardless of the causative microbe. The development of universal therapies may be confounded by the fact that microorganisms interact with components of the coagulation and fibrinolytic pathways in diverse and unique ways (12, 13). Moreover, the antibacterial functions of fibrin-mediated host defense may be dispensable in septic patients when suitable antibiotics are prescribed, but may be critical in cases where the causative pathogens are insensitive to antibiotics. Likewise, fibrin-mediated hemostatic defense may only be critical for some pathogens. The rational design of universally effective sepsis therapeutics, if feasible, should be aided by a precise understanding of the regulation of host-protective fibrin in diverse models of infection.
In summary, this report demonstrates that fibrin performs multiple host defense functions during yersiniosis. In this model of gram-negative infection, fibrin suppresses bacterial burden, influences inflammation and pathology, and can be critical for survival. The balance of coagulation and fibrinolysis dictate levels of protective fibrin during Y. enterocolitica infection. Thus, the mouse model of yersiniosis provides a means to investigate the relative importance of specific hemostatic pathway elements during fibrin-mediated host defense.
Acknowledgments
We thank Lawrence Johnson for critical reading of this manuscript, Debra Duso for technical assistance, and the employees of Trudeau Institute’s Animal Facilities for dedicated breeding and care of the mice used in these studies.
Abbreviations
- PAI-1
plasminogen activator inhibitor 1
- TAFI
thrombin activatable fibrinolysis inhibitor
- FXI
factor XI
- TF
tissue factor
- DIC
disseminated intravascular coagulation
- PT
prothrombinase
- APC
activated protein C
- PA
plasminogen activator
Footnotes
This work was supported by funding from the Trudeau Institute and Public Health Service grants R01-AI071295 (S.T.S) and R01-AI061577 (S.T.S).
References
- 1.Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35:2191–2195. doi: 10.1097/01.ccm.0000281468.94108.4b. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38:S26–34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 3.Johnson LL, Berggren KN, Szaba FM, Chen W, Smiley ST. Fibrin-mediated protection against infection-stimulated immunopathology. J Exp Med. 2003;197:801–806. doi: 10.1084/jem.20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullarky IK, Szaba FM, Berggren KN, Parent MA, Kummer LW, Chen W, Johnson LL, Smiley ST. Infection-stimulated fibrin deposition controls hemorrhage and limits hepatic bacterial growth during listeriosis. Infect Immun. 2005;73:3888–3895. doi: 10.1128/IAI.73.7.3888-3895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahrenholz DH, Simmons RL. Fibrin in peritonitis. I. Beneficial and adverse effects of fibrin in experimental E. coli peritonitis. Surgery. 1980;88:41–47. [PubMed] [Google Scholar]
- 7.Echtenacher B, Weigl K, Lehn N, Mannel DN. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect Immun. 2001;69:3550–3555. doi: 10.1128/IAI.69.6.3550-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright SD, Weitz JI, Huang AJ, Levin SM, Silverstein SC, Loike JD. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci USA. 1988;85:7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loike JD, Sodeik B, Cao L, Leucona S, Weitz JI, Detmers PA, Wright SD, Silverstein SC. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the Aα chain of fibrinogen. Proc Natl Acad Sci USA. 1991;88:1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002;99:1053–1059. doi: 10.1182/blood.v99.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem. 2007;14:2925–2936. doi: 10.2174/092986707782360015. [DOI] [PubMed] [Google Scholar]
- 12.Tapper H, Herwald H. Modulation of hemostatic mechanisms in bacterial infectious diseases. Blood. 2000;96:2329–2337. [PubMed] [Google Scholar]
- 13.Bergmann S, Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb Haemost. 2007;98:512–520. [PubMed] [Google Scholar]
- 14.Tilley R, Mackman N. Tissue factor in hemostasis and thrombosis. Semin Thromb Hemost. 2006;32:5–10. doi: 10.1055/s-2006-933335. [DOI] [PubMed] [Google Scholar]
- 15.Oehmcke S, Herwald H. Contact system activation in severe infectious diseases. J Mol Med. 2010;88:121–126. doi: 10.1007/s00109-009-0564-y. [DOI] [PubMed] [Google Scholar]
- 16.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32(Suppl 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 17.Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost. 2005;3:35–45. doi: 10.1111/j.1538-7836.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- 18.Hermans PW, Hazelzet JA. Plasminogen activator inhibitor type 1 gen polymorphism and sepsis. Clin Infect Dis. 2005;41(Suppl 7):S453–458. doi: 10.1086/431996. [DOI] [PubMed] [Google Scholar]
- 19.Bajzar L. Thrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathway. Arterioscler Thromb Vasc Biol. 2000;20:2511–2518. doi: 10.1161/01.atv.20.12.2511. [DOI] [PubMed] [Google Scholar]
- 20.Morser J, Gabazza EC, Myles T, Leung LL. What has been learnt from the thrombin-activatable fibrinolysis inhibitor-deficient mouse? J Thromb Haemost. 2010;8:868–876. doi: 10.1111/j.1538-7836.2010.03787.x. [DOI] [PubMed] [Google Scholar]
- 21.Taylor FB, Jr, Chang A, Ruf W, Morrissey JH, Hinshaw L, Catlett R, Blick K, Edgington TS. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33:127–134. [PubMed] [Google Scholar]
- 22.Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W, Andrade-Gordon P, Frank RD, Mackman N. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103:1342–1347. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 24.Levi M. Activated protein C in sepsis: a critical review. Curr Opin Hematol. 2008;15:481–486. doi: 10.1097/MOH.0b013e328304b3e3. [DOI] [PubMed] [Google Scholar]
- 25.Tucker EI, Gailani D, Hurst S, Cheng Q, Hanson SR, Gruber A. Survival advantage of coagulation factor XI-deficient mice during peritoneal sepsis. J Infect Dis. 2008;198:271–274. doi: 10.1086/589514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binette TM, Taylor FB, Jr, Peer G, Bajzar L. Thrombin-thrombomodulin connects coagulation and fibrinolysis: more than an in vitro phenomenon. Blood. 2007;110:3168–3175. doi: 10.1182/blood-2007-03-078824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Develter J, Booth NA, Declerck PJ, Gils A. Bispecific targeting of thrombin activatable fibrinolysis inhibitor and plasminogen activator inhibitor-1 by a heterodimer diabody. J Thromb Haemost. 2008;6:1884–1891. doi: 10.1111/j.1538-7836.2008.03137.x. [DOI] [PubMed] [Google Scholar]
- 28.Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, Um SL, Utterback B, Laterre PF, Dhainaut JF. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism. Crit Care. 2004;8:R82–90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 30.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med. 2009;37:S30–37. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]
- 31.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Carter PB. Pathogenecity of Yersinia enterocolitica for mice. Infect Immun. 1975;11:164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heesemann J, Gaede K, Autenrieth IB. Experimental Yersinia enterocolitica infection in rodents: a model for human yersiniosis. APMIS. 1993;101:417–429. [PubMed] [Google Scholar]
- 34.Cover TL, Aber RC. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 35.Bouza E, Dominguez A, Meseguer M, Buzon L, Boixeda D, Revillo MJ, de Rafael L, Martinez-Beltran J. Yersinia enterocolitica septicemia. Am J Clin Pathol. 1980;74:404–409. doi: 10.1093/ajcp/74.4.404. [DOI] [PubMed] [Google Scholar]
- 36.Tipple MA, Bland LA, Murphy JJ, Arduino MJ, Panlilio AL, Farmer JJ, 3rd, Tourault MA, Macpherson CR, Menitove JE, Grindon AJ. Sepsis associated with transfusion of red cells contaminated with Yersinia enterocolitica. Transfusion. 1990;30:207–213. doi: 10.1046/j.1537-2995.1990.30390194338.x. [DOI] [PubMed] [Google Scholar]
- 37.Strobel E, Heesemann J, Mayer G, Peters J, Muller-Weihrich S, Emmerling P. Bacteriological and serological findings in a further case of transfusion-mediated Yersinia enterocolitica sepsis. J Clin Microbiol. 2000;38:2788–2790. doi: 10.1128/jcm.38.7.2788-2790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoelen DW, Tjan DH, Schouten MA, Dujardin BC, van Zanten AR. Severe Yersinia enterocolitica sepsis after blood transfusion. Neth J Med. 2007;65:301–303. [PubMed] [Google Scholar]
- 39.Suh TT, Holmback K, Jensen NJ, Daugherty CC, Small K, Simon DI, Potter S, Degen JL. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 40.Gailani D, Lasky NM, Broze GJ., Jr A murine model of factor XI deficiency. Blood Coagul Fibrinolysis. 1997;8:134–144. doi: 10.1097/00001721-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Swaisgood CM, Schmitt D, Eaton D, Plow EF. In vivo regulation of plasminogen function by plasma carboxypeptidase B. J Clin Invest. 2002;110:1275–1282. doi: 10.1172/JCI15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest. 1998;101:560–569. doi: 10.1172/JCI814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullarky IK, Szaba FM, Winchel CG, Parent MA, Kummer LW, Mackman N, Johnson LL, Smiley ST. In situ assays demonstrate that interferon-gamma suppresses infection-stimulated hepatic fibrin deposition by promoting fibrinolysis. J Thromb Haemost. 2006;4:1580–1587. doi: 10.1111/j.1538-7836.2006.02010.x. [DOI] [PMC free article] [PubMed]
- 44.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 45.Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114:2367–2374. doi: 10.1182/blood-2009-05-199208. [DOI] [PubMed] [Google Scholar]
- 46.Rebeil R, Ernst RK, Gowen BB, Miller SI, Hinnebusch BJ. Variation in lipid A structure in the pathogenic yersiniae. Mol Microbiol. 2004;52:1363–1373. doi: 10.1111/j.1365-2958.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 47.van Schaik SM, Abbas AK. Role of T cells in a murine model of Escherichia coli sepsis. Eur J Immunol. 2007;37:3101–3110. doi: 10.1002/eji.200737295. [DOI] [PubMed] [Google Scholar]
- 48.Silva AT, Cohen J. Role of interferon-gamma in experimental gram-negative sepsis. J Infect Dis. 1992;166:331–335. doi: 10.1093/infdis/166.2.331. [DOI] [PubMed] [Google Scholar]
- 49.Campbell WD, Lazoura E, Okada N, Okada H. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol Immunol. 2002;46:131–134. doi: 10.1111/j.1348-0421.2002.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 50.Renckens R, Roelofs JJ, ter Horst SA, van ‘t Veer C, Havik SR, Florquin S, Wagenaar GT, Meijers JC, van der Poll T. Absence of thrombin-activatable fibrinolysis inhibitor protects against sepsis-induced liver injury in mice. J Immunol. 2005;175:6764–6771. doi: 10.4049/jimmunol.175.10.6764. [DOI] [PubMed] [Google Scholar]
- 51.Renckens R, Roelofs JJ, Bonta PI, Florquin S, de Vries CJ, Levi M, Carmeliet P, van‘t Veer C, van der Poll T. Plasminogen activator inhibitor type 1 is protective during severe Gram-negative pneumonia. Blood. 2007;109:1593–1601. doi: 10.1182/blood-2006-05-025197. [DOI] [PubMed] [Google Scholar]
- 52.Mutch NJ, Thomas L, Moore NR, Lisiak KM, Booth NA. TAFIa, PAI-1 and alpha-antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots. J Thromb Haemost. 2007;5:812–817. doi: 10.1111/j.1538-7836.2007.02430.x. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Chang WC, Takahashi M, Pavlov V, Ishida Y, La Bonte L, Shi L, Fujita T, Stahl GL, Van Cott EM. Mannose-binding lectin and its associated proteases (MASPs) mediate coagulation and its deficiency is a risk factor in developing complications from infection, including disseminated intravascular coagulation. Immunobiology. 2011;216:96–102. doi: 10.1016/j.imbio.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan ST, Edgington TS. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-α responses of monocytes. J Immunol. 1993;150:2972–2980. [PubMed] [Google Scholar]
- 55.Perez RL, Roman J. Fibrin enhances the expression of IL-1β by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. 1995;154:1879–1887. [PubMed] [Google Scholar]
- 56.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 57.Stouthard JM, Levi M, Hack CE, Veenhof CH, Romijn HA, Sauerwein HP, van der Poll T. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–742. [PubMed] [Google Scholar]
- 58.Dube PH, Handley SA, Lewis J, Miller VL. Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infect Immun. 2004;72:3561–3570. doi: 10.1128/IAI.72.6.3561-3570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levi M, Van Der Poll T. Coagulation in sepsis: all bugs bite equally. Crit Care. 2004;8:99–100. doi: 10.1186/cc2816. [DOI] [PMC free article] [PubMed] [Google Scholar]