Abstract
OBJECTIVES
Population-based data on the epidemiology and outcomes of subjects with intestinal metaplasia of the gastroesophageal junction (IMGEJ) and Barrett's esophagus (BE) are limited. The objectives of this study were to (i) estimate the incidence of IMGEJ and BE diagnosed from clinically indicated endoscopy in Olmsted County, MN, over three decades (1976–2006) and prevalence as of 1 January 2007, (ii) compare baseline characteristics of subjects with IMGEJ and BE, and (iii) study the natural history and survival of both cohorts.
METHODS
This was a population-based cohort study. The study setting was Olmsted County, MN. Patients with BE (columnar segment > 1 cm with intestinal metaplasia) and IMGEJ (intestinal metaplasia in biopsies from the gastroesophageal junction) from 1976 to 2006 in Olmsted County, MN, were identified using Rochester Epidemiology Project resources. Demographic and clinical data were abstracted from medical records and pathology confirmed by gastrointestinal pathologists. The association of baseline characteristics with overall and progression-free survival was assessed using proportional hazards regression models. Outcome measures were baseline characteristics and overall survival of subjects with IMGEJ compared to those with BE.
RESULTS
In all, 487 patients (401 with BE and 86 with IMGEJ) were identified and followed for a median interval of 7 (BE subjects) to 8 (IMGEJ subjects) years. Subjects with BE were older, heavier, reported reflux symptoms more often, and had higher prevalence of advanced neoplasia than those with IMGEJ. No patient with IMGEJ progressed to esophageal adenocarcinoma (EAC) in contrast to BE subjects who had a cumulative risk of progression of 7% at 10 years and increased risk of death from EAC (standardized mortality ratio 9.62). The overall survival of subjects with BE and IMGEJ did not differ from that expected in similar age- and sex-distributed white Minnesota populations.
CONCLUSIONS
Subjects with IMGEJ appear to have distinct clinical characteristics and substantially lower cancer progression risk compared to those with BE.
INTRODUCTION
Barrett's esophagus (BE) is the strongest known risk factor and precursor for esophageal adenocarcinoma (EAC), a lethal malignancy with a rapidly rising incidence in the Western world (1,2). BE is currently defined by the presence of columnar mucosa above the gastroesophageal junction on endoscopy and specialized intestinal metaplasia (characterized by the presence of goblet cells) on histology from this tissue (2). For decades, this disease was initially defined endoscopically by the appearance of a > 3 cm proximal displacement of the squamocolumnar junction, which is now termed long-segment BE (LSBE). This stands in contrast to short-segment BE (SSBE) where various definitions have been proposed, some supporting a length of at least 1 cm (3,4) while others suggest that intestinal metaplasia in any part of the distal esophagus or proximal cardia constitutes BE (5).
Numerous studies have demonstrated that the finding of intestinal metaplasia in the gastric cardia or at the gastroesophageal junction (IMGEJ) can occur in up to 10–15% of the normal population (6–9). Many patients who undergo endoscopy and biopsy of a questionably abnormal “Z” line are labeled as having BE and undergo a financially and potentially emotionally costly life-long surveillance program. Despite recommendations to not biopsy a normally located gastroesophageal junction (10), biopsies are often obtained to “exclude the presence of BE” in an irregular Z line. In addition, though attempts have been made by many investigators to develop and validate biomarkers (cytokeratin patterns, CDX2, telomerase) to distinguish between SSBE and IMGEJ, results have been contradictory with a recent metaanalysis finding that these molecular markers do not consistently distinguish between SSBE and IMGEJ (11). Furthermore, there is limited long-term outcome data on subjects with IMGEJ (6,12,13), particularly from a population-based perspective, in comparison to a BE cohort.
To address these gaps in knowledge, we created a population-based cohort of subjects with BE and IMGEJ who were diagnosed during clinically indicated endoscopy in Olmsted County, MN, and aimed to (i) estimate secular trends in the prevalence and incidence of BE and IMGEJ in Olmsted County, MN, from 1977 to 2006 and (ii) compare the natural history of BE and IMGEJ with particular reference to progression to EAC on repeat endoscopic or clinical evaluation.
METHODS
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Setting
The Olmsted County population comprises ~120,000 persons of whom 89% are white; sociodemographically, the community is similar to the US white population. County residents receive their medical care almost exclusively from two group practices: Mayo Medical Center and Olmsted Medical Center. The Mayo Clinic has maintained a common medical record system with its two affiliated hospitals. The system was further developed by the REP (Rochester Epidemiology Project), which created indices for the records of other providers of medical care to local residents. Annually, over 80% of the entire population is attended by one or both of these two practices, and nearly everyone is seen at least once during any given 4-year period. Therefore, the REP medical records linkage system provides an enumeration of the population from which samples can be drawn.
Case definition
Case identification using the REP consists of an electronic search, which is designed to be sensitive, followed by a chart review, which increases specificity. An electronic search of the REP database was performed using the International Classification of Diseases, 9th version/Hospital Adaptation of the International Classification of Disease A code for BE during the time frame of this study (1976–2006). This search strategy also included a search for the “rule out BE” tag. The endoscopic and histologic reports from each esophagogastroduodenoscopy (EGD) (which was performed for a clinical indication) were reviewed by a single gastroenterologist. All endoscopic examinations were performed by board-certified gastroenterologists on staff at Mayo Clinic or Olmsted Medical Center. Every endoscopic examination had a written (transcribed) report, which is part of the medical record and described the position of the landmarks by distance from the incisors (squamocolumnar junction, gastroesophageal junction, diaphragmatic hiatus). Measurements of segment length were available in over 90% of the cases. No photographs were reviewed for this study. The upper margin of the gastric folds was routinely used as a landmark for the gastroesophageal junction.
Subjects were classified in the BE group if the endoscopist described at least 1 cm of visible columnar mucosa in the esophagus with intestinal metaplasia on histology. Those with a 3 cm or greater length of BE were classified as having LSBE and those with a < 3 cm segment were classified as having SSBE as per current convention. Subjects with intestinal metaplasia seen in biopsies from the squamocolumnar junction, which was either normal or described as “irregular” were classified as those with IMGEJ. All individuals residing in Olmsted County at the time of their diagnosis and meeting the following inclusion criteria (from 1976 to 2006) were included.
Data collection
The complete medical records of each subject were reviewed and abstracted by nurse abstractors. All possible cases for inclusion were independently reviewed by one of the investigators to confirm the findings. The following information was collected on all patients: demographics (age/sex), presenting symptoms and duration of symptoms, social history (smoking, alcohol), family history of BE/EAC, other medical conditions (pulmonary disease, cardiac, cerebrovascular disease, head and neck cancer, previous esophageal disease, previous colon polyps or cancer, other malignancy, diabetes mellitus, and Helicobacter pylori infection: method of detection and if positive or negative), body mass index at each EGD, indications for each EGD, and the previous and current medication history. Data on each EGD were also recorded including endoscopic findings (length of BE segment, hiatal hernia if present, nodularity, esophagitis), interventions performed such as endoscopic mucosal resection or ablation, and the indication for each EGD (screening, diagnostic for gastrointestinal symptoms, surveillance). The time interval between each EGD was recorded as well.
The pertinent clinical and laboratory data collected were entered into a precoded data form. The collected data were keyed into a database from the data forms with independent verifications, were edited by a range and consistency check program, and a SAS data set was created for analysis (SAS Institute, Cary, NC).
Validation of BE case identification and length classification
Given the retrospective nature of BE and IMGEJ case and length identification, special attention was paid to confirming the diagnosis of BE cases and length assessments in the following manner. All EGDs were performed by experienced gastroenterologists or surgeons. Paper or electronically accessible endoscopy reports were available on all subjects. In addition, given the known stability in BE segment length after initial diagnosis (in the absence of ablation or resection), which was described in prior studies from Olmsted County (14), we assessed stability of BE length in subjects with multiple measures during surveillance examinations. We computed “first-order” differences (FOD), that is, BE length at current EGD minus BE length at previous EGD for all subjects where data from multiple EGDs (three or more) was available (n = 167). The mean and s.d. (per subject) of these FOD was then computed. The rationale was to eliminate the (between subjects) variation just due to the average length varying over subjects. The mean FOD and s.d. of the FODs (each subject values) was then plotted against the duration of their FU (i.e., last EGD date minus first EGD date) and the mean FOD vs. the average BE length (per subject): similar to a Bland–Altman plot.
Pathology
All biopsies with any mention of intestinal metaplasia with or without goblet cells were read by an experienced gastrointestinal pathologist. In addition, those with any dysplasia described on the original pathology report were re-reviewed by a second gastrointestinal pathologist (T.T.W).
Statistical analysis
All data analyses were performed using SAS/STAT software, Version 9.2 of the SAS System for Unix, copyright 2009 SAS Institute. For the calculation of incidence rates, the entire population of Olmsted County was considered to be at risk, and the denominator age- and sex-specific person-years were estimated from decennial census data. Rates were directly age and sex adjusted to the population structure of the 2000 US Census. The associations of sex (using male sex as the reference group), age, and calendar year (using 1990–2006 as the reference period) with the incidence rates were assessed using Poisson regression models (SAS software PROC GENMOD) Specifically, the number of observed cases for each sex–age–calendar period category was modeled as a function of sex, age, and calendar period with the corresponding (log) person-years as an offset. Overall survival and cumulative rate of progression (1 minus survival free of progression) to high-grade dysplasia (HGD) or EAC was estimated using the Kaplan–Meier method. Expected survival was calculated based on the Minnesota white population survival probabilities using the cohort survival method (15). The association of overall survival and separately, survival free of progression (to HGD or EAC) with baseline characteristics was assessed using proportional hazards regression models. The associations of baseline characteristics with disease subgroup (BE vs. IMEGJ) were assessed using logistic regression models.
RESULTS
Case identification
All patients with a possible diagnosis of BE from 1976 to 2006 were identified. This yielded 942 records. A single gastroenterologist reviewed the endoscopic and histologic reports of all 942 patients and classified them as following: 401 patients had biopsy-proven BE of at least 1 cm length, and 86 subjects were classified as IMGEJ. We excluded 455 patients in whom “rule out BE” was written in the record but who did not have any evidence, be it endoscopic or histologic, of BE. An additional 20 subjects with columnar metaplasia of the esophagus, with no evidence of intestinal metaplasia on histology were not included given lack of consistent follow-up.
Epidemiology
For calculation of incidence rates, patients diagnosed from 1976 to 2006 were included. The age-adjusted, sex-specific incidence of clinically diagnosed BE and IMGEJ in Olmsted County is shown in Table 1. The age- and sex-adjusted prevalence of clinically diagnosed BE was 219.6 per one hundred thousand on 1 January 2007. The age- and sex-adjusted prevalence of IMEGJ diagnosed at clinically indicated endoscopy was 61.8 per one hundred thousand on 1 January 2007.
Table 1.
Incidence rates and prevalence of Barrett's esophagus (BE) and intestinal metaplasia of the gastroesophageal junction (IMGEJ) diagnosed with clinically indicated endoscopy in Olmsted County, MN
| Age adjusted, females | Age adjusted, males | Age and sex adjusted | |
|---|---|---|---|
| Incidence of BE | 8.1 (6.6, 9.6) | 26.1 (22.9, 29.2) | 16.2 (14.6, 17.8) |
| Incidence of LSBE | 4.1 (3.0, 5.2) | 15.3 (12.9, 17.7) | 9.1 (7.9, 10.4) |
| Incidence of SSBE | 2.5 (1.6, 3.3) | 5.9 (4.5, 7.4) | 4.1 (3.3, 4.9) |
| Incidence of IMEGJ | 11.7 (7.8, 15.5) | 18.8 (13.5, 24.1) | 14.9 (11.7, 18.1) |
| Prevalence of BE | 128.6 (100.3, 156.9) | 319.9 (272.1, 367.7) | 219.6 (192.7, 246.6) |
| Prevalence of LSBE | 66.1 (45.8, 86.5) | 202.3 (163.9, 240.6) | 129.9 (109.1, 159.7) |
| Prevalence of SSBE | 56.2 (37.5, 74.9) | 99.9 (73.4, 126.3) | 78.0 (61.9, 94.2) |
| Prevalence of IMEGJ | 47.9 (30.6, 65.1) | 76.8 (53.7, 99.9) | 61.8 (47.6, 76, 0) |
LSBE, long-segment BE; SSBE, short-segment BE from 1976 to 2006.
Rates are per 100,000 person-years. Prevalence as of 1 January 2007.
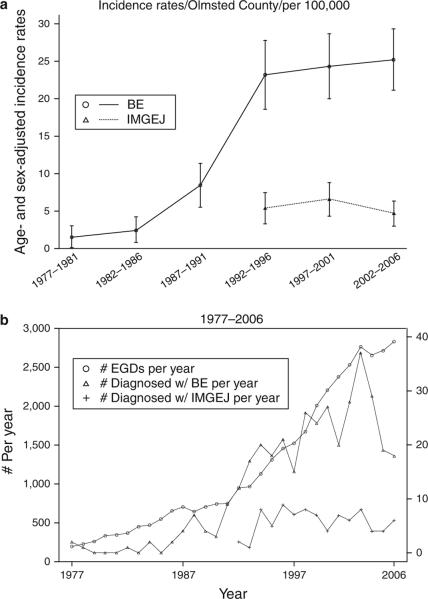
Figure 1a displays the incidence of BE and IMEGJ in Olmsted County, MN, over six 5-year intervals (from 1976 to 2006). The incidence of clinically diagnosed BE increased over the first four time intervals and later stabilized over the last two time intervals. This was true for both LSBE and SSBE (data not shown). In contrast, the incidence of clinically diagnosed IMGEJ was stable over the time periods studied. Of note, cases of IMGEJ were not diagnosed prior to 1992 in Olmsted County; this corresponds to the time frame of initial description of SSBE and IMGEJ (8). Figure 1b shows the secular trends in endoscopy volume per year and number of BE and IMGEJ cases diagnosed per year in Olmsted County. Although this may simply reflect an ecological correlation, it appears the number of diagnosed cases of BE and the volume of upper endoscopies both increased in parallel over the first four time intervals, though the number of new BE cases diagnosed over the last decade remained relatively stable.
Figure 1.
Secular trends in the incidence of Barrett's esophagus (BE) and intestinal metaplasia of the gastroesophageal junction (IMGEJ) over three decades in Olmsted County. (a) Age-adjusted incidence rates for all BE and IMGEJ cases. x Axis displays 5-year time intervals. (b) Secular trends in endoscopy volume per year (indicated on the left y axis) and number of cases of BE and IMGEJ diagnosed per year (indicated on the right y axis) in Olmsted County, MN. EGD, esophagogastroduodenoscopy.
Baseline data on subjects with BE and IMGEJ
Table 2 summarizes the baseline data of BE and IMGEJ. Baseline histology in the BE group included 35 (9%) with EAC, 13 (3%) with HGD, 53 (13%) with low-grade dysplasia (LGD) and 293 (73%) with no dysplasia. In all, 251 (63%) of subjects with BE had LSBE (mean length = 6.4 cm, s.d. = 3.1 cm) with the remainder being SSBE (n = 122) (mean length = 1.4 cm, s.d. = 0.7 cm): BE segment length information was not available in 28 (7%) patients with BE. These subjects were included in the BE (as opposed to the IMGEJ group) based on the endoscopic description of the columnar segment, which stated either “several centimeters” or “LSBE” or “long tongues of columnar mucosa” in the absence of specific length measurements. In the IMGEJ group, baseline histology indicated no dysplasia in 80 subjects (93%) and LGD in six subjects (7%). Subject group was associated with age (BE subjects were older), sex (a greater proportion of BE subjects were male), weight (BE subjects had higher weight than those with IMGEJ), heartburn (BE subjects were more likely to report), presence and length of hiatal hernia (greater in BE subjects), and prevalence of advanced dysplasia (more likely in BE subjects).
Table 2.
Baseline data for Barrett's esophagus (BE) and intestinal metaplasia of the gastroesophageal junction (IMGEJ) subjects diagnosed with clinically indicated endoscopy
| Variable | Cases of BE (n=401) | Cases of IMGEJ (n=86) | P valuea |
|---|---|---|---|
| Age, mean±s.d. | 63±14 | 57±15 | 0.004 |
| Male, sex, 339 (70%) | 288 (72%) | 51 (59%) | 0.03 |
| Weight, mean±s.d., kgb | 86±20 | 81±18 | 0.02 |
| Body mass index, mean±s.d.b | 29±6 | 28±5 | 0.14 |
| Smoking b | 0.004 | ||
| Never smoker | 148 (37%) | 47 (55%) | |
| Ex-smoker | 195 (49%) | 26 (30%) | |
| Current smoker | 52 (13%) | 13 (15%) | |
| Alcohol use b | < 0.001 | ||
| None/occasional/quit | 239 (63%) | 76 (88%) | |
| 1–6 Drinks/week | 88 (23%) | 5 (6%) | |
| ≥7 Drinks/week | 50 (13%) | 5 (6%) | |
| Symptoms of GERD | |||
| Heartburn | 264 (77%) | 50 (58%) | < 0.001 |
| Acid regurgitation | 125 (49%) | 49 (57%) | 0.26 |
| Helicobacter pylori infectionc | 18 (17%) | 11 (24%) | 0.26 |
| Findings on index endoscopy | |||
| Hiatal hernia | 305 (76%) | 51 (59%) | 0.002 |
| Vertical length of hiatal hernia, mean±s.d., cm | 4.2±1.8 | 1.9±2.0 | < 0.001 |
| Reflux esophagitisb | 114 (31%) | 31 (36%) | 0.37 |
| Baseline dysplasia b | < 0.001 | ||
| LGD | 53 (13%) | 6 (7%) | |
| HGD | 13 (3%) | 0 | |
| Adenocarcinoma | 35 (9%) | 0 |
GERD, gastroesophageal reflux disease; HGD, high-grade dysplasia; LDG, low-grade dysplasia.
For univariate association with group status.
Data were available in >90% of subjects.
Data were available in 33% of subjects.
The association of baseline variables with BE vs. IMGEJ status adjusting for age and sex was assessed using logistic regression. Significant associations with a BE diagnosis was found with increasing age (odds ratio (OR): 1.03 (1.01, 1.05)), male sex (OR: 1.75 (1.08, 2.73)), increasing body mass index (OR: 1.07 (1.02, 1.13)), symptoms of heartburn (OR: 2.76 (1.65, 4.62)), presence of a hiatal hernia (2.11 (1.28, 3.48)), and increasing size of the hiatal hernia (OR: 2.14 (1.75, 2.61) per cm increase in the hernia size). Performance of EGD for an indication of dyspepsia was associated with lower odds of a BE diagnosis (OR: 0.10 (0.06, 0.18)), in contrast to an indication of chronic gastroesophageal reflux symptoms that increased the odds of a BE diagnosis (OR: 3.67 (1.75, 7.67)).
Natural history
A total of 355 subjects with BE without HGD or EAC at baseline or within 6 months of diagnosis of BE constituted the surveillance cohort. Progression was defined as progressing from no or LGD, to HGD or EAC at least 6 months after the initial diagnosis of BE. A total of 46 BE subjects were excluded from the surveillance cohort: 35 subjects progressing within 6 months, 9 subjects with baseline HGD, and 2 without biopsy confirmation at baseline, were excluded. A total of 19 progressors were identified. Using a threshold of 12 months, 18 progressors were identified. Six patients progressed from no dysplasia to EAC. One patient progressed from LGD to EAC. Ten patients progressed from no dysplasia to HGD, and two patients progressed from LGD to HGD.
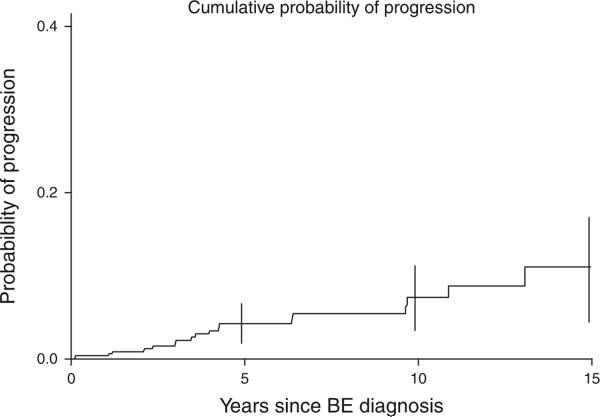
Figure 2 displays the progression to HGD or EAC over the duration of the study in subjects with BE. The cumulative rate of progression of subjects with no dysplasia or LGD at baseline to HGD or EAC was 4.3 (95% confidence interval (CI): 1.9, 6.6), 7.4 (95% CI: 3.2, 11.2), and 11.1 (95% CI: 4.5, 17.2) at 5, 10, and 15 years, respectively. Assuming complete follow-up and uniform progression rates, the incidence rates for the progression of subjects with no dysplasia or LGD to HGD or EAC would be 7.9/1,000 person-years of follow-up (5 per 1,000 person-years to HGD and 2.9 per 1,000 person-years to EAC).
Figure 2.
Kaplan–Meier curve illustrating progression to high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) in subjects with Barrett's esophagus (BE) (without baseline HGD or EAC) in Olmsted County, MN.
Fifty-nine (65%) subjects with IMGEJ underwent at least one subsequent EGD (mean (±s.d.) number of EGD sessions per subject was 2.98 (±2.3)). A second endoscopic examination was done in 55 out of 86 subjects. There were five out of six patients with IMGEJ who had LGD at first endoscopic biopsy and underwent subsequent endoscopic examinations. LGD was not detected in subsequent endoscopies in all these subjects. Two additional subjects had LGD detected on follow-up endoscopy: this was not detected on subsequent endoscopy in either subject. No subject with IMGEJ developed endoscopically evident BE, EAC, or gastric adenocarcinoma over a median follow-up duration of 8 years (range 1 day to 17 years).
Table 3 displays results from the proportional hazards model for predictors of progression from no dysplasia/LGD to HGD/EAC. Given the small number of events, only results from univariate analysis are presented. Previous use of alcohol (but abstinent at baseline endoscopy) suggested a significantly increased hazard for progression (hazard ratio: 3.45 (95% CI: 1.13, 10.57), P = 0.03) and past smoking (relative to never) had a modestly increased risk for progression (hazard ratio: 4.57 (95% CI: 1.03, 20.31), P = 0.05), but the CI was rather wide. BE segment length > 3 cm appeared to increase the risk of progression compared with segment length < 3 cm, but this did not meet statistical significance.
Table 3.
Predictors of progression to HGD or EAC in BE subjects: results from proportional hazards models
| Variable | Univariate HR (95% CI) | P value |
|---|---|---|
| Age at BE diagnosis | 0.99 (0.96, 1.03) | 0.74 |
| Female | 1.0 (Ref) | |
| Male | 4.10 (0.95, 17.74) | 0.06 |
| Alcohol | ||
| None | 1.0 (Ref) | |
| Current | 1.03 (0.32, 3.28) | 0.97 |
| Past | 3.45 (1.13, 10.57) | 0.03 |
| Smoking | ||
| None | 1.0 (Ref) | |
| Current | 5.02 (0.83, 30.35) | 0.08 |
| Past | 4.57 (1.03, 20.31) | 0.05 |
| BE | ||
| < 3 cm | 1.0 (Ref) | |
| ≥3 cm | 3.93 (0.90, 17.10) | 0.07 |
| Dysplasia | ||
| No | 1.0 (Ref) | |
| LGD | 1.04 (0.30, 3.59) | 0.95 |
| PPI | ||
| No | 1.0 (Ref) | |
| Yes | 0.23 (0.03, 1.72) | 0.15 |
| ASA | ||
| No | 1.0 (Ref) | |
| Yes | 0.90 (0.26, 3.10) | 0.87 |
| NSAIDs | ||
| No | 1.0 (Ref) | |
| Yes | 0.55 (0.07, 4.17) | 0.57 |
| BMI | 0.96 (0.88, 1.05) | 0.40 |
ASA, aspirin; BE, Barrett's esophagus; BMI, body mass index; CI, confidence interval; EAC, esophageal adenocarcinoma; HGD, high-grade dysplasia; HR, hazard ratio; LDG, low-grade dysplasia; NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitor.
Overall survival
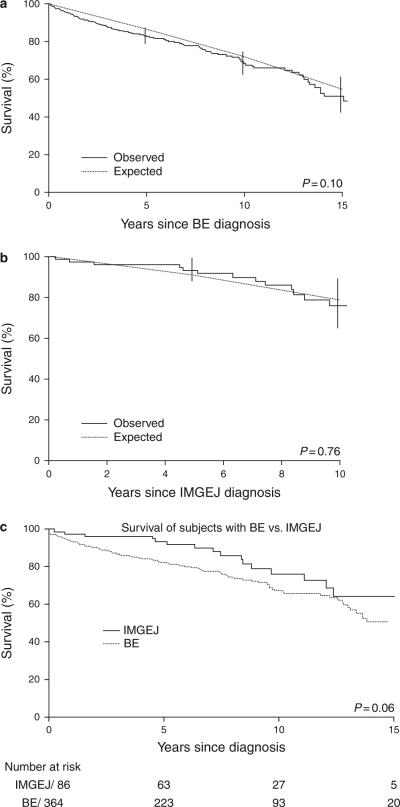
Median (range) follow-up in subjects alive at last follow-up was 8.0 years (1 month to 17 years) in subjects with IMGEJ and 7.8 years (2 days to 27 years) in subjects with BE. Survival at 5 and 10 years in subjects with IMGEJ was 93 and 76%, respectively, and in subjects with BE was 83 and 68%, respectively. Overall survival for both groups is depicted in Figures 3a–c. Survival was comparable to that expected for a similar age- and sex-distributed Minnesota white population in both cohorts. Overall survival was not associated with group status (BE vs. IMGEJ) after adjusting for age and sex (hazard ratio: 1.30, 95% CI: 0.77, 2.22).
Figure 3.
Kaplan–Meier curves illustrating long-term survival of subjects with Barrett's esophagus (BE) and intestinal metaplasia of the gastroesophageal junction (IMGEJ) in Olmsted County, MN. (a) Overall survival of subjects with BE (without high-grade dysplasia (HGD) or carcinoma at baseline or within 6 months of diagnosis) compared to an age- and sex-distributed Minnesota white population. (b) Overall survival of subjects with IMGEJ compared to an age- and sex-distributed Minnesota white population. (c) Comparison of overall survival of subjects with BE and IMGEJ in Olmsted County, MN.
Table 4 lists the causes of death in both groups. EAC accounted for only 5% of the deaths in the BE subjects with a substantial proportion of mortality being accounted for by cardiovascular, pulmonary causes, and other malignant tumors. Thus, we observed five EAC deaths (all males) and the expected (based on US whites) number of deaths for the observed age- and sex-specific person-years was 0.52, yielding a standardized mortality ratio of 9.62 (95% CI: 3.12, 22.4). No patient in the IMGEJ cohort died of esophageal or gastric adenocarcinoma in contrast to the BE cohort.
Table 4.
Causes of death in subjects with BE and IMGEJ
| Cause of death | Deaths in subjects with BE (N=104 total), n (%) | Deaths in subjects with IMGEJ (N=16 total), n (%) |
|---|---|---|
| Cardiac | 30 (28) | 2 (12.5) |
| Cerebrovascular | 4 (4) | 0 |
| Lung | 12 (11) | 2 (12.5) |
| Liver cirrhosis | 0 | 2 (12.5) |
| Dementia | 7 (7) | 1 (6) |
| Renal | 6 (6) | 0 |
| Esophageal adenocarcinoma | 5 (5) | 0 |
| Other cancer | 22 (21) | 6 (38) |
| GI/liver cancer | 8 (8) | 1 (6) |
| Lung cancer | 5 (5) | 1 (6) |
| Hematologic | 5 (5) | 1 (6) |
| Prostate cancer | 0 | 1 (6) |
| Head and neck cancer | 0 | 2 (12) |
| Renal | 1 (1) | 0 |
| Miscellaneous (unknown primary, parotid) | 3 (3) | 0 |
| Trauma | 4 (4) | 0 |
| Sepsis | 2 (2) | 0 |
| Unknown (without autopsy) | 12 (11) | 3 (19) |
BE, Barrett's esophagus; GI, gastrointestinal; IMGEJ, intestinal metaplasia of the gastroesophageal junction.
Validation of BE case identification and length classification
The distribution of the mean FOD indicated that ~85% were ≤1.0 cm in absolute value, and uncorrelated with the duration of follow-up. These results indicate that there was no systematic change related to the average length of BE, and there was no association with follow-up duration, further validating the length measurement of the BE cases and reducing likelihood of misclassification bias.
DISCUSSION
In this large population-based study we found that the clinical characteristics, epidemiology, and natural history of subjects with clinically diagnosed IMGEJ were distinct from those of subjects with clinically diagnosed BE. Subjects with BE were older, heavier, and had a greater prevalence of reflux symptoms. Furthermore, during this extended time of follow-up (700 patient-years of follow-up in the IMGEJ group), no patient with IMGEJ progressed to HGD or EAC in contrast to subjects with BE who demonstrated progression in a subset (albeit at a low overall rate) and increased risk of death from EAC. Finally, the overall survival of subjects with BE and IMGEJ was similar to that expected for Minnesota white populations with the same age and sex distribution.
The presence of intestinal metaplasia at a normally located gastroesophageal junction (or the proximal cardia) is difficult to distinguish from SSBE given the difficulty in precisely identifying landmarks that mark the gastroesophageal junction. Currently, the tubular end of the esophagus is thought to be at the upper border of the gastric folds (16). The presence of at least a centimeter of visible columnar mucosa with intestinal metaplasia on histology, in the esophagus is used as an arbitrary criterion to define the presence of BE in the United States (4). This is based on a validation study, which showed that this criteria had good interobserver reliability (3) for the identification of BE by endoscopists. In the present study, this criterion appears to be predictive of difference in outcomes (progression to EAC).
The prevalence of IMGEJ in subjects undergoing endoscopy in clinic-based studies has been reported to vary from 5% (6) to 14% (7) to 17% (9). Another study reported a prevalence of 20% in subjects without endoscopic evidence of BE: a substantial proportion of these subjects had a normal appearing gastroesophageal junction (8). Little information, however, is available on the population prevalence of IMGEJ. This study provides the first estimates of IMGEJ in the population based on cases diagnosed at clinically indicated endoscopy where the endoscopist suspected the presence of SSBE. However, this is likely to be an underestimate given that only clinically indicated EGDs were used to estimate prevalence and a normal appearing GEJ was not routinely biopsied.
Very limited data exist on progression of neoplasia or development of incident dysplasia in IMGEJ subjects. Smaller referral center studies (12,13) have reported some differences in baseline demographic characteristics and natural history between subjects with BE and IMGEJ. However, this study provides population-based data on a substantially larger cohort with substantially longer follow-up. Given that none of the subjects with IMGEJ developed endoscopically evident BE or EAC, or symptoms suggestive of cancer that would prompt further evaluation, need for endoscopic surveillance in subjects with IMGEJ should be reassessed.
Data on secular trends in the incidence of BE are conflicting, with an earlier study from Olmsted County (including cases diagnosed until 1 January 1998), suggesting that the perceived increase in incidence was due to increased endoscopy volume (17), with other studies suggesting a true increase (after adjusting for endoscopy volume) (18). This present study extends data from the earlier Olmsted County study: while incidence rates (Figure 1a) increased substantially from 1976 to 1996; the rate of increase in the incidence of both LSBE and SSBE appears to have slowed thereafter, despite continuing increase in the endoscopy volume (Figure 1b). Hence, rising incidence of clinically diagnosed BE is unlikely to be a cause of the rising incidence of EAC in Olmsted County (19). Of note, though the prevalence of LSBE in 2007 in Olmsted County (129.9/100,000 population) is higher than that calculated in 1987 (22.6/100,000) (20) and 1998 (82.6/100,000) (17), it remains almost a third of the projected actual prevalence as determined by an autopsy study performed in Olmsted County in 1987 (376/100,000) (20). Hence, it is probable that the majority of subjects with LSBE continue to remain undetected despite the increasing use of endoscopy in Olmsted County.
A limitation of this study is the retrospective nature of the analysis. However, it is notable that all EGDs were performed by experienced academic and community gastroenterologists. Endoscopy reports were available for review in all subjects. Data on segment length were missing in only 7% of subjects with BE. We also assessed the stability in BE length measurements over time as assessed in surveillance endoscopy and found that the overall change in segment length was limited (likely due to known interobserver variation in BE length measurement), further validating the accuracy of initial and subsequent measurements. Some variation in the length of BE as assessed by different observers is recognized. Histopathology was initially assessed by expert gastrointestinal pathologists and reconfirmed by yet another gastrointestinal pathologist. We also chose robust outcomes such as survival (which was assessed from multiple sources: medical records, Minnesota death tapes), which are less likely to be influenced. The distinction between the two groups in terms of overall EAC-related mortality is additional validation of the clinically significant difference between the two groups. The population-based nature of this study and the high sensitivity of case ascertainment are additional strengths. The lack of endoscopic follow-up in some subjects with IMGEJ and BE is a potential limitation: we sought to partly mitigate this by using end points such as EAC development and overall survival. If the risk of progression is low in IMGEJ, the limited sample size of this study may have resulted in no events (progression). Assuming the rate of progression in BE to be 1 per 200 patient-years (21), we would expect approximately four subjects with IMGEJ to progress to EAC over a follow-up of almost 800 patient-years.
In conclusion, in this large population-based cohort with long-term follow-up, subjects with IMGEJ had distinct demographic and clinical characteristics compared to those with BE. Given the lack of progression to HGD or EAC over a mean follow-up of 8 years, endoscopic surveillance of subjects with IMGEJ may not be warranted. These results should be confirmed in larger studies. Despite increasing use of endoscopy over the last few decades, a substantial proportion of subjects with BE in the community may continue to remain undiagnosed.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
√
The incidence of clinically diagnosed Barrett's esophagus (BE) is increasing.
-
√
Intestinal metaplasia of the gastroesophageal (GE) junction/ cardia is common in the population.
-
√
The rate of progression to esophageal adenocarcinoma (EAC) in subjects with BE is low, but the natural history of subjects with intestinal metaplasia of the GE junction (IMGEJ) is unclear.
WHAT IS NEW HERE
-
√
Despite the increasing use of endoscopy, a substantial proportion of Barrett's esophagus (BE) cases in the population remain undiagnosed.
-
√
Subjects with intestinal metaplasia of the GE junction (IMGEJ) have distinct demographic and clinical characteristics from BE subjects.
-
√
Subjects with IMGEJ in the population do not progress to high-grade dysplasia (HGD) or adenocarcinoma over a substantial length of follow-up.
-
√
Survival in subjects with IMGEJ and BE is comparable to that of age- and gender-matched subjects.
-
√
Surveillance in subjects with IMGEJ may not be required.
ACKNOWLEDGMENTS
We appreciate assistance from Dr Gary Falk in providing suggestions for this manuscript. This study was supported in part by the American College of Gastroenterology Junior Faculty Development Award (to G.A.P.), NIH Grant R03CA135991-01 (to G.A.P.), the Shirley and Miles Fiterman Digestive Disease Center, and the Rochester Epidemiology Project (Grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
Financial support: None.
Footnotes
CONFlICT OF INTEREST Guarantor of the article: Ganapathy A. Prasad, MD, MS.
Potential competing interests: None.
REFERENCES
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–9. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasoma P, Wickramasinghe K, Ma Y, et al. Adenocarcinomas of the distal esophagus and “gastric cardia” are predominantly esophageal carcinomas. Am J Surg Pathol. 2007;31:569–75. doi: 10.1097/01.pas.0000213394.34451.d2. [DOI] [PubMed] [Google Scholar]
- 6.Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology. 1999;116:277–85. doi: 10.1016/s0016-5085(99)70123-x. [DOI] [PubMed] [Google Scholar]
- 7.Peck-Radosavljevic M, Puspok A, Potzi R, et al. Histological findings after routine biopsy at the gastro-oesophageal junction. Eur J Gastroenterol Hepatol. 1999;11:1265–70. doi: 10.1097/00042737-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Spechler SJ, Zeroogian JM, Antonioli DA, et al. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994;344:1533–6. doi: 10.1016/s0140-6736(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 9.Wallner B, Sylvan A, Stenling R, et al. The Z-line appearance and prevalence of intestinal metaplasia among patients without symptoms or endoscopical signs indicating gastroesophageal reflux. Surg Endosc. 2001;15:886–9. doi: 10.1007/s004640090025. [DOI] [PubMed] [Google Scholar]
- 10.Spechler SJ. Intestinal metaplasia at the gastroesophageal junction. Gastroenterology. 2004;126:567–75. doi: 10.1053/j.gastro.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 11.Nurgalieva Z, Lowrey A, El-Serag HB. The use of cytokeratin stain to distinguish Barrett's esophagus from contiguous tissues: a systematic review. Dig Dis Sci. 2007;52:1345–54. doi: 10.1007/s10620-006-9399-3. [DOI] [PubMed] [Google Scholar]
- 12.Horwhat JD, Baroni D, Maydonovitch C, et al. Normalization of intestinal metaplasia in the esophagus and esophagogastric junction: incidence and clinical data. Am J Gastroenterol. 2007;102:497–506. doi: 10.1111/j.1572-0241.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Weston AP, Morales T, et al. Relative risk of dysplasia for patients with intestinal metaplasia in the distal oesophagus and in the gastric cardia. Gut. 2000;46:9–13. doi: 10.1136/gut.46.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron AJ, Lomboy CT. Barrett's esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology. 1992;103:1241–5. doi: 10.1016/0016-5085(92)91510-b. [DOI] [PubMed] [Google Scholar]
- 15.Hakulinen T, Abeywickrama KH. A computer program package for relative survival analysis. Comput Programs Biomed. 1985;19:197–207. doi: 10.1016/0010-468x(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 16.Amano Y, Ishimura N, Furuta K, et al. Which landmark results in a more consistent diagnosis of Barrett's esophagus, the gastric folds or the palisade vessels? Gastrointest Endosc. 2006;64:206–11. doi: 10.1016/j.gie.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Conio M, Cameron AJ, Romero Y, et al. Secular trends in the epidemiology and outcome of Barrett's oesophagus in Olmsted County, Minnesota. Gut. 2001;48:304–9. doi: 10.1136/gut.48.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett's oesophagus in the general population. Gut. 2005;54:1062–6. doi: 10.1136/gut.2004.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane SJ, Richard Locke G, III, Harmsen WS, et al. The changing incidence of oesophageal and gastric adenocarcinoma by anatomic sub-site. Aliment Pharmacol Ther. 2007;25:447–53. doi: 10.1111/j.1365-2036.2006.03229.x. [DOI] [PubMed] [Google Scholar]
- 20.Cameron AJ, Zinsmeister AR, Ballard DJ, et al. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990;99:918–22. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 21.Shaheen NJ, Crosby MA, Bozymski EM, et al. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology. 2000;119:333–8. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]