Abstract
Background & Aims
There are very few published studies of agents having the potential to improve bone health in children with inflammatory bowel disease (IBD). Our aim was to establish the efficacy and safety of intranasal calcitonin in improving bone mineral density (BMD) in young patients with IBD and to define additional factors that impact bone mineral accrual.
Methods
We conducted a randomized, placebo-controlled, double-blind clinical trial in sixty-three participants, ages 8 to 21 yrs, with a spinal BMD Z-score ≤ −1.0 SD measured by dual energy X-Ray absorptiometry (DXA). Subjects were randomized to 200 IU intranasal calcitonin (n=31) or placebo (n=32) daily. All received age-appropriate calcium and vitamin D supplementation. Subsequent BMD measurements were obtained at 9 and 18 months.
Results
Intranasal calcitonin was well-tolerated. Adverse event frequency was similar in both treatment groups, and such events were primarily minor, reversible, and limited to the upper respiratory tract. The BMD Z-score change documented at screening and 9 months and screening and 18 months did not differ between the two therapeutic arms. In participants with Crohn’s disease (CD) the spinal BMD Z-score improved between screening and 9 months [ΔZSBMD(9-0)] in the calcitonin group (ΔZSBMD(9-0)calcitonin = 0.21 (0.37), ΔZSBMD(9-0)placebo = −0.15 (0.5), p = 0.02), however this was only a secondary subgroup analysis. Bone mineral accrual rates during the trial did not lead to normalization of BMD Z-scores in this cohort. Factors favoring higher bone mineral accrual rate were: lower baseline BMD and higher baseline body mass index (BMI) Z-score, improvement in height Z-score, higher serum albumin, hematocrit and iron concentration, and more hours of weekly weight-bearing activity. Factors associated with lower bone mineral accrual rate were: more severe disease – as indicated by elevated inflammatory markers, need for surgery, hospitalization and the use of immunomodulators - and higher amount of caffeine intake.
Conclusions
Intranasal calcitonin is well-tolerated but does not offer a long-term advantage in youth with IBD and decreased BMD. Bone mineral accrual rates remain compromised in youth with IBD and low bone mineral density raising concerns for long-term bone health outcomes. Improvement in nutritional status, catch-up linear growth, control of inflammation, increase in weight-bearing activity, and lower caffeine intake may be helpful in restoring bone density, especially in children with IBD and low baseline BMD.
Keywords: Inflammatory Bowel Disease, Bone Loss, Pediatric
BACKGROUND
Many studies to date have shown that bone mineralization is compromised in pediatric patients with inflammatory bowel disease (IBD) in comparison to healthy peers1–4. In addition, bone geometry and strength are altered in these children5. Although an increased fracture rate has not been confirmed in pediatric patients with IBD6, bone mineral accrual was found to be lower than that of healthy controls4, 5.The only interventional study to target bone health in children with IBD to date found no significant effect of vitamin D on spinal bone mineral density (BMD) using doses equivalent to 1,700 IU daily with calcium supplementation for 6 months. However, the investigators did not evaluate the correlation between serum 25-hydroxyvitamin D concentration (25OHD) and either vitamin D intake or BMD7.
Calcitonin is a naturally occurring anti-resorptive hormone8. Salmon calcitonin is approximately 40–50 times more potent than that derived from humans and is available as a nasal spray. Calcitonin has been shown to reduce spinal fracture risk and improve vertebral BMD in postmenopausal women with osteoporosis9. This agent also preserves vertebral bone mass when administered to patients receiving glucocorticoids10. The superior tolerability and ease of administration of calcitonin renders it attractive for use in pediatric populations9. Calcitonin was found to be more efficacious than placebo in increasing BMD in children after renal transplantation11, it improved bone pain and radiologic signs of osteoporosis in young patients with thalassemia,12, and reduced bone resorption and pain in children with juvenile rheumatoid arthritis13. Many adult studies have provided both safety and efficacy data for calcitonin. A recent review summarized the harms associated with intranasal calcitonin in this population as minor – local irritating effects of the upper respiratory tract; more significant nausea and allergic reactions were noted mostly with the injectable form9. The few studies examining efficacy of intranasal calcitonin in improving BMD in children, documented no significant harm. Specifically, El Husseini et al. reported only “transient hypocalcemia” with a daily dose of 200 IU of calcitonin administered intranasally for 12 months14, while Canatan et al. administered 100 IU of calcitonin intranasally three times per week with no significant adverse events noted12. However, adverse events were not detailed.
The present study was an 18-month randomized, placebo-controlled, double-blind trial of the efficacy and potential harms of intranasally administered calcitonin on total body and lumbar spine BMD in children with IBD. We evaluated these sites as representative of cortical bone (with slow skeletal turnover) and trabecular bone (with rapid turnover), respectively. In their recent position statements, the International Society for Clinical Densitometry (ISCD) endorsed these skeletal sites as important in the monitoring of children at risk for compromised bone health15. We did not examine the effect of calcitonin on the femoral neck. Although important in adults due to the established relationship between low BMD and fractures at this site, the femoral neck is less important in children. The hip is not a reliable site for measurement in growing children due to variability in skeletal development and lack of reproducible regions of interest. This leads to errors in positioning, lower precision measurements, and potential for systematic errors in clinical trials15.
To isolate the effect of calcitonin on bone mineral accrual, we collected prospective data on other contributing factors. While the positive effects of linear growth, lean mass growth and pubertal development on bone mineral accrual have been confirmed in both healthy children16, 17 and children with IBD5, 18, 19, there have been contradictory reports on the role of glucocorticoids4, 20, 21 and inflammation4, 21 on bone health in this population. In addition, the impact of weight-bearing activity and nutrition has not been examined in a prospective manner in children with IBD.
METHODS
Participants
Children, adolescents and young adults with IBD, age 8 to 22 years, receiving care at Children’s Hospital Boston, underwent a screening dual energy X-ray absorptiometry (DXA) measurement of the lumbar spine as part of the screening phase of this study. The study was approved by the institutional review board (IRB) of the hospital, and all participants signed informed consent and gave their assent, prior to screening. Specifically, all participants and their families were given a description of the trial by the investigators, as well as the potential benefits, risks and discomforts associated with study participation. The above were repeated for all young participants in age-appropriate language. All participants were asked to give a description of the trial and the risks and discomforts involved, to assure understanding of the trial and its risks, and they were asked again if they wished to participate. Participants younger than 18 years were then asked to sign an assent form, in addition to their parents/guardians. Young participants who expressed concerns or disagreement with enrollment, regardless of age, were not enrolled. Participants older than 18 years gave informed consent themselves. Subjects were eligible to participate in the trial if their screening spinal BMD Z-score was ≤ −1.0 SD. Exclusion criteria included: use of growth hormone, anabolic steroids, calcitonin or bisphosphonates in the 6 months prior to enrollment, pregnancy, allergy to salmon, and the diagnosis of renal failure, anorexia nervosa, thyroid disorders and polycystic ovary syndrome. None of the participants had used growth hormone, anabolic steroids or bone active agents at any time prior to enrollment. The study took place in the Clinical and Translational Study Unit (CTSU) of the hospital.
Protocol
Participants were randomized to receive 200 IU of salmon calcitonin by nasal spray (Miacalcin®, Novartis, Basel Switzerland) or placebo once daily, administered in alternating nostrils. Participants younger than 11 years received 800 mg of elemental calcium daily and those age 11 years and older received 1,200 mg of elemental calcium daily divided into two doses (calcium carbonate salt, TUMS® GlaxoSmithKline). All participants received 400 IU of vitamin D2 per day (Nature’s Bounty, Bohemia, NY). The duration of the trial was 18 months.
Outcomes
The primary outcome was the change in DXA Z-score of the lumbar spine and total body BMD from screening to 18 months. Secondary outcomes included: change in Z-scores from screening to 9 months and change in Z-scores adjusted for bone age. The frequency of adverse events was also an outcome recorded in this trial.
Sample size
The sample size was calculated based on the known effect of calcitonin on spinal BMD in other patient groups22. A target recruitment of approximately 30 participants per arm provided 80% power to detect the effect size at a significance level of 0.05 or greater. Because no systematic report of harms resulting from intranasal calcitonin in children was available, the trial was not powered specifically with respect to risk of harm.
Treatment assignment
Participants were enrolled by the investigators after informed consent was obtained. Participants were randomized upon enrollment. Randomization was stratified for pubertal stage and gender utilizing the permuted block method. Participants were considered pre-pubertal, pubertal or post-pubertal if the development of sexual characteristics (evaluated via inspection and palpation) placed them in Tanner stage 1, 2 – 4, or 5 respectively, at the time of enrollment. The random allocation sequence was generated by the Clinical Research Program of the hospital utilizing software developed for this purpose (SciRAN) and was implemented through a spreadsheet provided only to the research pharmacists who assigned the participants to their groups. Study medications were provided to participants shortly after randomization at the enrollment visit.
Masking
Participants, investigators and the interpreter of DXA results were unaware of treatment assignment, and the laboratory values specific to this. The Data and Safety Monitoring Board for the study monitored laboratory values and assured the success of the blinding until the study’s conclusion.
Evaluations
Follow-up visits occurred every 3 months. They consisted of administration of study-specific questionnaires, nutritional evaluations by a research nutritionist, physical examination (including pubertal staging, and anthropometric measurements), and collection of laboratory data. DXA measurements were obtained at screening, 9 months and 18 months, and wrist films at enrollment and 18 months for bone age determination. As this was an intention to treat study, we asked all participants to return for their DXA scans regardless of missed visits, compliance with the study medication, or withdrawal – participant or investigator initiated. Loss to follow-up was defined as unavailability of at least one of the primary outcomes.
Nutritional evaluation
The subjects completed prospectively the Children’s Hospital Boston Clinical Nutrition Service 3-day Food and Beverage Diary at the initial, 9 month, and final study visit, and one-day diet recall questionnaires at other visits. Responses were analyzed using the Food Processor SQL Nutrition Analysis Software (Version 9.2, ESHA Research, Salem OR), to provide the average daily intake for each nutrient of interest, specifically: protein, carbohydrates, fat, calcium, vitamin D, vitamin K, sodium, potassium, phosphorus, magnesium.
Anthropometric measurements
Subjects’ weights and heights were measured at every study visit. Weight (Wt) was measured on a Scale-Tronix digital scale (Scale-Tronix, Inc, White Plains, NY), calibrated weekly. Height (Ht) was measured on a Holtain stadiometer (Holtain Ltd, Crymych, Wales), calibrated daily. Body mass index (BMI), weight Z-score, height Z-score, and BMI Z-score were calculated using the Epi Info Database and Statistics Software for Public Health Professionals (Centers for Disease Control and Prevention, Atlanta, GA, 2004). For patients 20 years and older, Z-scores were calculated using an age of 20 years.
DXA
A DXA scanner was used to measure lean body mass (Kg), fat mass (Kg), total body areal BMD including the head (g/cm2), as well as lumbar spine areal BMD (g/cm2) at enrollment and at the 18-month study visit15. Lumbar spine measurements were based on the anteroposterior view of L1 – L4 using a Hologic Discovery A scanner with v12.4 software. The scanner was calibrated daily, using a quality control anthropomorphic spine phantom, provided by the manufacturer. BMD Z-scores were derived using pediatric software23,24 for subjects aged 4 to 20, and NHANES software for patients above age 2025 and were additionally adjusted for bone age. We calculated lean mass for height Z-score and percent fat mass for age Z-score using the LMS method based on reference values provided by Hologic. These reference values were derived from a nationally representative sample from the NHANES survey and were acquired using Hologic Discovery A DXA scanners with software compatible with ours26. Data generated by DXA were interpreted by one endocrinologist (CMG).
Bone age
A radiograph of the non-dominant wrist was obtained at enrollment and 18 month follow-up visit for each subject. A trained radiologist reviewed the wrist films and assigned bone age utilizing the method of Greulich and Pyle27.
Clinical characteristics
Participants’ pubertal status was determined at enrollment and end of the trial. The Centers for Disease Control and Prevention Youth Risk Behavior Survey (version 2002) includes questions related to use of alcohol and tobacco which were administered to identify participants who had engaged in smoking or alcohol use either prior to enrollment or during the trial (available at www.cdc.gov/yrbss, accessed in Fall 2002). Specific questionnaires were used to report weight-bearing activity, both lifetime (reported as total number of hours) and during the study. Participants were asked about their participation in any sports or activities that are considered weight-bearing at each study visit and reported the number of weeks of participation, and number of hours per week for the interval since their last study visit. The hours were then totaled and reported as average weekly hours of activity. Activities such as swimming and cycling were excluded, as non-weight-bearing activities (Supplements E and F). For female subjects, the use of contraceptive agents and/or estrogen replacement therapy was identified via confidential questionnaires, and medical record review of all clinic notes. Family history of low bone density was considered positive if a family member met the diagnosis based on a DXA result, or if a family member had severe kyphosis, pathologic fracture, or significant loss of height28.
Diagnosis of Crohn’s disease (CD) and ulcerative colitis (UC) was assigned based on histologic, endoscopic, radiographic and clinical criteria29. Disease activity was scored at each visit utilizing the Pediatric Crohn’s Disease Activity index (PCDAI) (for subjects ≤ 20 years old),30 Crohn’s Disease Activity Index (CDAI) (for subjects > 20 years old)31, or the Kozarek score in cases of UC32. The Kozarek score was used to quantify disease activity in our study as the Pediatric Ulcerative Colitis Activity Index (PUCAI) was not formulated and validated until after the study was completed33. The Kozarek score is derived from five parameters: number of liquid stools per day, severity of rectal bleeding, general well-being, level of abdominal pain, and extra-intestinal manifestations. There had been prior experience in our center in the use of this scoring system and in the classification of disease severity depending on the score34. A participant was classified as having a) “moderate/severe disease” during the trial, if PCDAI was ≥ 30 or CDAI ≥ 220 or Kozarek > 6, b) “mild disease” if PCDAI ≥10 and < 30 or CDAI ≥ 150 and < 220, or Kozarek ≥ 4 and ≤ 6, and c) “inactive disease” if PCDAI < 10 or CDAI < 150 or Kozarek < 431, 34. Medical record review was utilized for participants with CD to identify upper gastrointestinal (UGI) tract involvement on the basis of histologic evidence of granulomas, and the occurrence of complications - specifically fistulae and strictures and to calculate the cumulative systemic glucocorticoid dose in prednisone equivalents in all participants. Disease duration was reported in months from the first histologic diagnosis to date of enrollment. Patient questionnaires and medical record review were used to report the use of nutritional supplements and enteral nutrition, occurrence of extra-intestinal manifestations, co-morbidities, operative procedures and hospitalizations related to IBD, and the use of immunomodulators and biologic agents. For the latter we recorded length of treatment in months for each agent from diagnosis to enrollment, whether the participants were being treated with these agents at the time of enrollment, and the length of such treatment during the trial.
Laboratory values
Hematocrit (HCT) (%), white blood cells (WBC) (Kcells/uL), and platelets (PTL) (Kcells/uL) were measured in an automated analyzer, either Advia – 120 or Advia – 2120 (Bayer Diagnostics, Tarrytown, PA), and erythrocyte sedimentation rate (ESR mm/hour) was measured using ESR analyzer Vesmatic 20 (HiChem Diagnostics), according to standard protocol. Serum albumin (g/dL), iron (Fe) (mcg/dL), C-reactive protein (CRP) (mg/dL) and calcium concentration (mg/dL) were measured in a Roche/Hitachi 917 chemistry analyzer according to standard protocol. Serum twenty five hydroxy vitamin D concentration (25OHD) (ng/mL) was measured using the Nichols Advantage chemiluminescence-based competitive protein-binding assay (Nichols Institute Diagnostics, San Clemente, CA). Intact parathyroid hormone (PTH) (pg/mL) was measured using the Nichols Advantage Intact PTH Assay, a two-site chemiluminescence immunoassay (Nichols Institute Diagnostics, San Clemente, CA).
Adverse events
Adverse events known to be associated with intranasal calcitonin administration (calcitonin package insert 2001,35) were included in our data collection instruments. Potential adverse events included: allergic reaction, nasal mucosal wound (excoriation and/or abrasion verified by direct inspection of the nares by the PI), infection of the nasal tissue (evidence of infection of the nares or the surrounding skin verified by the PI), rhinitis (reports of nasal congestion or discharge), irritating effects to the upper respiratory tract (sneezing, itching of the nares), facial flushing, back pain, epistaxis, nausea, hyperparathryroidism (serum PTH concentration > 65 pg/mL at any of the study visits) and hypocalcemia (serum calcium concentration < 8 mg/dL at any of the study visits). In addition, participants were encouraged to report any other adverse events during the trial. An adverse event was characterized as unexpected if its nature, severity or frequency was unanticipated based on previous experience with the study medication, in this case based on literature. This definition is in compliance with guidelines of the Children’s Hospital Boston Committee for Clinical Investigation. Adverse event severity was graded by agreement of both investigators and participants as: mild (transient, without significant effect on daily activities, requiring minor interventions for relief), moderate (requiring medical attention, prescription medication for relief, but not significantly impacting daily activities), severe (requiring hospitalization, inflicting long term damage), or life-threatening. Adverse event relationship to the study medication was assigned by both investigators and participants throughout the trial using the following algorithm: non-related, possibly related (included in the list of possible adverse events, but could be also explained by non-related process active in the participant), probably related (included in the list of possible adverse events, and could not be explained by any other process), unknown (relationship cannot be confirmed or excluded).
Adverse events were collected through questionnaires specifically designed for this purpose. These questionnaires contained the list of anticipated adverse events (see above) and laboratory abnormalities (see above), as well as severity and relatedness scales for each event (see above). Upon interview, the participants were first asked if they have any unpleasant events to report (volunteered) and then the investigators went through the standard list of events (elicited). The questionnaires were administered every 3 months (at each study visit) for the duration of the trial. Both investigators and participants were blinded to treatment assignment, even when an adverse event resulted in voluntary withdrawal of a participant or mandatory withdrawal by the investigators. By protocol, the trial would be terminated before its conclusion if serious, unexpected, and likely-related-to-calcitonin adverse events occurred as decided by the study’s DSMB.
Adherence
Questionnaires were administered at each visit to document adherence to study medications which was expressed as a percentage of ideal intake.
STATISTICAL ANALYSIS
The data were analyzed using the “intention to treat” assumption. A two-sided p-value ≤ 0.05 was accepted as the level of significance. Descriptive statistics for population and clinical characteristics at enrollment, including body composition and anthropometric measurements at screening, were tabulated separately for the two study arms and compared. Patient and disease characteristics, changes in anthropometric measurements and body composition measurements, average laboratory values, average nutrient intakes, and measures of compliance with study medications from enrollment to 18 months were tabulated for the two arms and compared. The comparisons were made between the two arms for the whole sample and for participants with CD separately. As a supplemental analysis, we compared the same characteristics, values and measurements between the two arms from enrollment to 9 months and from 9 to 18 months in all participants and in participants with CD. Chi square tests were used to compare nominal variables. Two sample t-tests, or the Mann-Whitney test, depending on the distribution of the variables, were used to compare continuous variables. Distribution of each continuous variable was examined for normality using the Shapiro-Wilk test.
BMD Z-scores at screening, 9 months and 18 months and changes in these scores from screening to 9 months (secondary outcome) and from screening to18 months (primary outcome) were compared between the two arms in all participants utilizing the t-test or Mann-Whitney test as appropriate. Subgroup analyses were undertaken as well; the effect of treatment on the above primary and secondary outcomes was examined in patients with CD and UC separately, within participants of each pubertal group, and within participants who took > 70% of the nasal spray doses (adherent).
Simple relationships between several variables and the change in total body and spinal BMD Z-score from screening to 18 months were evaluated for significance using correlation coefficient tests for continuous variables, and mean tests or analysis of variance tests for dichotomous or nominal values in all participants and participants with CD and UC separately. Non-parametric tests including Spearman correlation analyses, Mann-Whitney test, and Kruskal-Wallis analysis of variance were used when the data were not normally distributed based on the Shapiro-Wilk test. Due to the relatively small number of participants in this trial, we selected to report only simple regression analysis results.
The cumulative and individual frequency of the occurrence of all adverse events related to the study medication was compared between the two groups using the Chi square test. Taking into account all participants, the frequency of withdrawal due to adverse events, and timing of withdrawal (expressed as number of participants withdrawn at enrollment, 3, 6 and 9 months) was compared between the two arms. No participant withdrew after 9 months. With all participants as the denominator, the frequency of unexpected adverse events was also compared between the two arms. Taking into account all adverse events, their distribution between the two arms, timing of occurrence (reported as average of the months elapsed from enrollment to event occurrence and frequency of occurrence in the first month, the first 6 months and after 6 months), severity, relatedness to study drug and average duration in days was also compared between the two arms. The risk of multiple adverse events in a single participant was compared between the two arms, using as the denominator all participants with events. The study medication doses received (as percent of ideal) by all participants in the trial was reported and compared between the two arms.
RESULTS
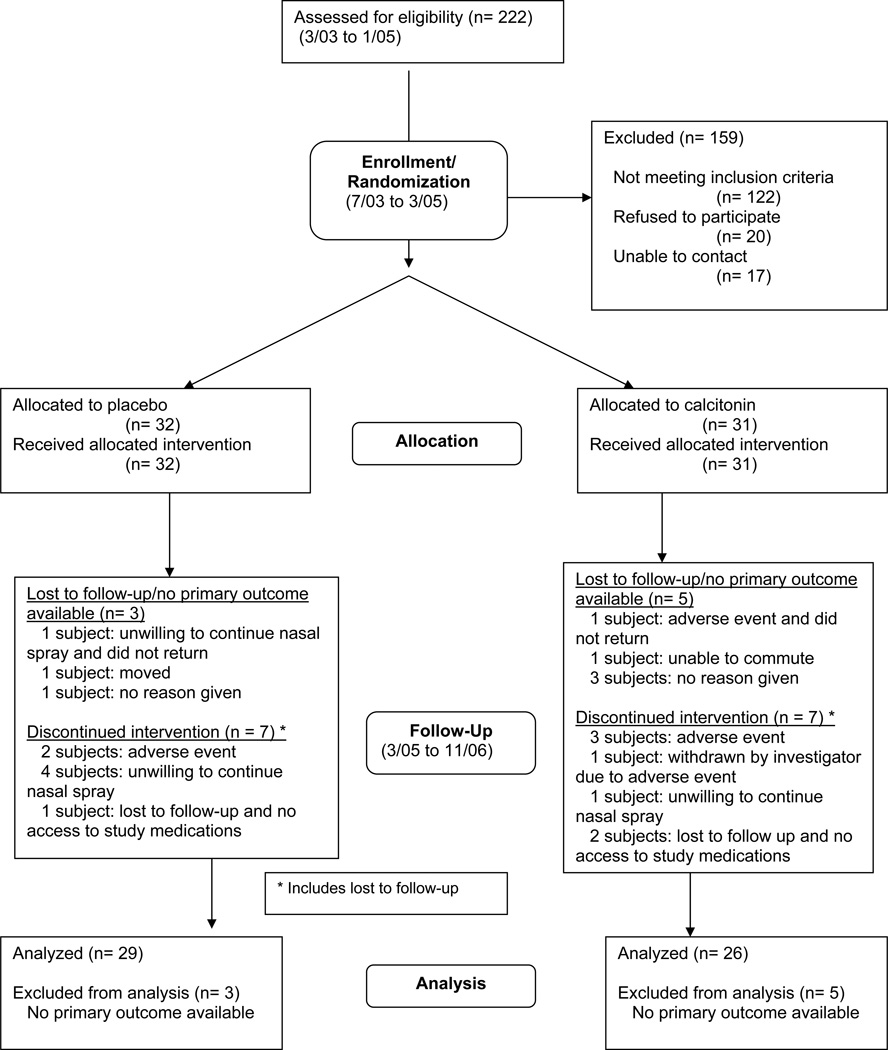
Participant flow diagram and study timeline (Diagram 1)
Population and disease characteristics at enrollment (Table 1)
TABLE 1.
Characteristics per arm at enrollment or screening DXA
| CALCITONIN | PLACEBO | p | ||||
|---|---|---|---|---|---|---|
| DEMOGRAPHICS 1 | N | N(%) or Mean (SD) | N | N(%) or Mean (SD) | ||
| Gender | Male/Female | 17 /14 (54.8%/45.2%) | 18/14 (56.2%/43.8%) | 0.91 | ||
| Race | Caucasian/Others | 29/2 (93.5%/6.5%) | 27/5 (84.4%/15.6%) | 0.25 | ||
| Pubertal stage | pre/pubertal/post | 8/18/5 (25.8%/58.1%/16.1%) | 9/17/6 (28.1%/53.1%/18.1%) | 0.92 | ||
| Age (yrs) | 31 | 14.4 (3) | 32 | 14.8 (3.4) | 0.62 | |
| Bone age (yrs) | 30 | 13.6 (3.3) | 30 | 13.6 (3.1) | 0.93 | |
| ANTHROPOMETRICS/BODY COMPOSITION 1 | ||||||
| Height Z-score | 31 | −0.76 (0.91) | 32 | −0.89 (1.17) | 0.61 | |
| Weight Z-score | 31 | −0.31 (1.09) | 32 | −0.51 (1.15) | 0.48 | |
| BMI Z-score | 31 | 0.06 (0.95) | 32 | −0.04 (1.08) | 0.70 | |
| Zlean (Ht) | 30 | −0.12 (1.03) | 32 | −0.13 (1.31) | 0.98 | |
| Z%fat (age) | 30 | −0.8 (1.43) | 32 | −0.53 (1.11) | 0.39 | |
| DISEASE PARAMETERS 1 | ||||||
| Diagnosis | CD/UC (%) | 27/4 (87.1%/12.9%) | 20/12 (62.5%/37.5%) | 0.02 | ||
| Disease duration (months) | 31 | 41 (40) | 31 | 33 (34) | 0.61 | |
| Lifetime glucocorticoids to screening DXA (mg) | 26 | 3819.7 (3326.1) | 28 | 4475.9 (4362.8) | 0.77 | |
| Lifetime surgery (IBD-related) | 31 | 2 (6.5%) | 32 | 5 (15.6%) | 0.25 | |
| Lifetime hospitalization (IBD-related) | 31 | 13 (41.9%) | 32 | 18 (58.1%) | 0.20 | |
| Lifetime immunomodulators | 31 | 21 (67.7%) | 29 | 19 (65.5%) | 0.85 | |
| Lifetime months of immunomodulators | 31 | 18 (25) | 29 | 12 (18) | 0.51 | |
| Immunomodulators at enrollment | 31 | 21 (67.7%) | 32 | 19 (59.4%) | 0.49 | |
| Lifetime biologics | 31 | 6 (19.4%) | 30 | 4 (13.3%) | 0.53 | |
| Lifetime months of biologics | 31 | 1.5 (3.5) | 30 | 1.8 (6.1) | 0.58 | |
| Biologics at enrollment | 31 | 5 (16.1%) | 32 | 5 (15.6%) | 0.96 | |
| Lifetime extra-intestinal manifestations | 31 | 18 (58.1%) | 32 | 22 (68.8%) | 0.38 | |
| Lifetime co-morbidity | 31 | 12 (38.7%) | 32 | 18 (56.2%) | 0.16 | |
| Lifetime UGI granulomas in CD pts | 21 | 6 (28.6%) | 15 | 5 (30.6%) | 0.76 | |
| Lifetime complications in CD pts | 27 | 4 (14.8%) | 20 | 7 (35%) | 0.11 | |
| Disease severity at enrollment Inactive/Mild/Moderate-Severe (%) | 12/13/4 (41.4%/44.8%/13.8%) | 17/13/1 (54.8%/49.1%/3.2%) | 0.27 | |||
| ESR (mm/hr) | 26 | 20 (15) | 31 | 22 (17) | 0.74 | |
| Albumin (g/dL) | 30 | 3.9 (0.8) | 32 | 4 (0.4) | 0.49 | |
| CRP (mg/dL) | 29 | 0.77 (1.07) | 31 | 0.48 (0.68) | 0.19 | |
| HCT (%) | 31 | 38.1 (4) | 32 | 37.8 (3.9) | 0.76 | |
| WBC (Kcells/uL) | 31 | 7.1 (3.4) | 31 | 6.4 (3.3) | 0.31 | |
| PLTs (K cells/uL) | 31 | 361 (141) | 32 | 321 (122) | 0.18 | |
| Fe (mcg/dL) | 29 | 58.2 (34.7) | 32 | 83 (70.7) | 0.19 | |
| 25OHD (ng/mL) | 23 | 43.1 (22.5) | 23 | 29.7 (11) | 0.02 | |
| PTH (pg/mL) | 31 | 43.5 (21.6) | 31 | 37.1 (13.9) | 0.43 | |
| HABITS/NUTRITION/FHx 1 | ||||||
| Lifetime estrogen/contraceptives in girls | 14 | 3 (21.4%) | 14 | 1 (7.1%) | 0.28 | |
| Lifetime NG or P.O. supplements | 31 | 18 (40.6%) | 32 | 13 (51.8%) | 0.17 | |
| Lifetime smoking | 31 | 2 (6.5%) | 32 | 1 (3.1%) | 0.53 | |
| Lifetime alcohol use | 31 | 3 (9.7%) | 32 | 6 (18.8%) | 0.30 | |
| Family history of osteoporosis | 31 | 22 (71%) | 32 | 14 (43.8%) | 0.03 | |
| Total lifetime weight-bearing activity (hrs) | 31 | 2313 (2586) | 32 | 1853 (2063) | 0.79 | |
All at or by enrollment date, unless otherwise noted
DXA: dual energy X-Ray absorptiometry, SD: standard deviation, CD: Crohn disease, UC: ulcerative colitis, IBD: inflammatory bowel disease, Zlean (Ht): lean mass adjusted for height Z-score, Z%fat (age): % fat adjusted for age Z-score, ZTBBMD: Total body BMD Z-score, ZSBMD: Spine BMD Z-score, (BA): adjusted for bone age, UGI: upper gastrointestinal tract, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, PLTs: platelets, Fe: serum iron, 25OHD: serum twenty five hydroxy-vitamin D, PTH: serum parathyroid hormone, FHx; family history of osteoporosis, NG: nasogastric, P.O: per os
Participants in the two arms did not differ with respect to demographics, pubertal stage, anthropometrics, disease characteristics and treatment (including length of treatment with immunomodulators or biologics from diagnosis to enrollment and treatment with these agents at the time of enrollment), habits and exercise patterns. The two arms differed only in diagnosis (CD more prevalent in the calcitonin arm), serum 25OHD concentration (higher in the calcitonin arm) and family history of osteoporosis (more prevalent in the calcitonin arm). When participants with CD were considered separately, the calcitonin group had higher rates of family history of osteoporosis but no other differences (results not shown). (Supplemental Table A).
Population and disease characteristics from enrollment to end for all and for participants with CD
When participants with CD and UC were combined, the calcitonin group participants received on average larger daily amounts of vitamin K (mcg/day) (406± 825 vs. 122±330, p=0.03) and calcium (mg/day) (1,175± 509 vs. 922±479, p=0.05), but had no other differences (results not shown) (Supplemental Table B).
Among participants with CD, those in the placebo group showed higher gains in weight Z-score (0.48±0.72 vs. 0.08±0.57, p=0.05), had higher rates of complications by the end of the trial [9(45%) vs. 5(18.5%), p=0.05], and engaged in fewer hours of weekly weight-bearing activity (3.6±2.3 vs. 5.4±3.5, p=0.05), but no other differences were noted (results not shown) (Supplemental Table B). Withdrawal and loss to follow-up rates were similar between the two groups. Loss to follow-up alone - defined as missing all primary outcomes - was similar (placebo: 3 (9.4%), calcitonin: 5 (16.1%), p = 0.47) (not shown).
Population and disease characteristics from enrollment to 9 months for all and for participants with CD
When participants with CD and UC were combined, the calcitonin group had higher average serum 25OHD concentrations (ng/mL) (41.1±15.5 vs. 31.9±11, p=0.02) during the first 9 months of the study and no other differences. Participants with CD differed only in terms of average ESR (mm/hr) – higher in the participants of the placebo group (29±16 vs. 20±15, p=0.04) (results not shown) (Supplemental Table C).
Population and disease characteristics from 9 months to 18 months
We similarly examined the differences between these characteristics in the last part of the study (Supplemental Table D). Of particular interest is the fall in adherence to the nasal spray administration during this study interval in comparison with the prior interval. Specifically, for all participants assigned to the calcitonin arm, the percent ideal doses of nasal spray received during the first 9 months of the study was 73%, vs. 57.6% during the last 9 months. For participants with CD assigned to the calcitonin group, the respective percent ideal nasal spray doses received was 70.2% vs. 53.6%. Adherence to the nasal spray followed the same trend in the placebo group and did not differ from that of the calcitonin group.
Primary and secondary outcome analysis
BMD Z-scores and their changes in all participants (Table 2)
TABLE 2.
Primary and secondary outcomes Bone mineral measurements at screening, 9 and 18 month DXA and their changes per ARM
| CALCITONIN | PLACEBO | ||||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | p | |
| TOTAL BODY | |||||
| Screening to 18 mos | |||||
| ZTBBMDscreening | 30 | −1.27 (0.92) | 32 | −1.24 (0.75) | 0.89 |
| ZTBBMD18 | 23 | −1.45 (0.9) | 26 | −1.29 (0.82) | 0.53 |
| ΔZTBBMD(18-0) | 22 | −0.21 (0.59) | 26 | 0.00 (0.51) | 0.3 |
| Screening to 9 mos | |||||
| ZTBBMD9 | 23 | −1.4 (0.87) | 23 | −1.24 (0.92) | 0.56 |
| ΔZTBBMD(9-0) | 22 | −0.1 (0.51) | 23 | 0.02 (0.5) | 0.63 |
| Screening to 18 mos (bone age adjusted) | |||||
| ZTBBMD (BA)screening | 28 | −0.95 (1.01) | 27 | −0.93 (0.78) | 0.96 |
| ZTBBMD (BA)18 | 21 | −0.9 (0.94) | 20 | −1.05 (0.84) | 0.58 |
| ΔZTBBMD(BA)(18-0) | 21 | −0.13 (0.69) | 21 | −0.21 (0.62) | 0.71 |
| SPINE | |||||
| Screening to 18 mos | |||||
| ZSBMDscreening | 31 | −1.55 (0.69) | 32 | −1.53 (0.75) | 0.95 |
| ZSBMD18 | 26 | −1.34 (0.66) | 29 | −1.33 (0.87) | 0.89 |
| ΔZSBMD(18-0) | 26 | 0.19 (0.53) | 29 | 0.18 (0.5) | 0.95 |
| Screening to 9 mos | |||||
| ZSBMD9 | 23 | −1.44 (0.56) | 23 | −1.52 (0.8) | 0.69 |
| ΔZSBMD(9-0) | 23 | 0.17 (0.38) | 23 | 0.00 (0.49) | 0.18 |
| Screening to 18 mos (bone age adjusted) | |||||
| ZSBMD (BA)screening | 29 | −1.28 (0.68) | 27 | −1.16 (0.87) | 0.57 |
| ZSBMD(BA)18 | 21 | −0.92 (0.64) | 19 | −1.17 (0.87) | 0.3 |
| ΔZSBMD(BA)(18-0) | 21 | 0.31 (0.62) | 21 | 0.03 (0.72) | 0.18 |
SD: standard deviation, ZTBBMD: Total body BMD Z-score, ΔZTBBMD: change in total body BMD Z-score from screening to 9 or 18 mos, ZSBMD: Spine BMD Z-score, ΔZTBBMD: change in spine BMD Z-score from screening to 9 or 18 mos, (BA): adjusted for bone age Italicized: primary outcomes
Total body and spinal BMD Z-scores at screening, 9 months and 18 months and their changes from screening to 9 months and 18 months were compared between participants in the two arms and did not differ. Of note is that BMD Z-scores of the total body in either arm did not improve, and spine BMD Z-scores improved but did not normalize by the end of the study.
Subgroup analysis
Participants with CD and UC (Table 3)
TABLE 3.
Subgroup analysis Bone mineral measurements at screening, 9 and 18 month DXA and their changes per ARM in CD and UC patients
| CD | UC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CALCITONIN | PLACEBO | CALCITONIN | PLACEBO | |||||||
| N | Mean (SD) | N | Mean (SD) | P | N | Mean (SD) | N | Mean (SD) | p | |
| TOTAL BODY | ||||||||||
| Screening to 18 mos | ||||||||||
| ZTBBMDscreening | 26 | −1.37 (0.92) | 20 | −1.43 (0.68) | 0.83 | 4 | −0.58 (0.67) | 12 | −0.93 (0.77 | 0.54 |
| ZTBBMD18 | 19 | −1.58 (0.93) | 18 | −1.51 (0.73) | 0.79 | 4 | −0.83 (0.33) | 8 | −0.81(0.87) | 0.44 |
| ΔZTBBMD(18-0) | 18 | −0.19 (0.64) | 18 | −0.05 (0.53) | 0.75 | 4 | −0.25 (0.35) | 8 | 0.13 (0.47) | 0.19 |
| Screening to 9 mos | ||||||||||
| ZTBBMD9 | 20 | −1.47 (0.9) | 15 | −1.43 (0.88) | 0.92 | 3 | −0.93 (0.51) | 8 | −0.88 (0.94) | 0.92 |
| ΔZTBBMD(9-0) | 19 | −0.08 (0.53) | 15 | 0.01 (0.52) | 0.93 | 3 | −0.17 (0.42) | 8 | 0.04 (0.5) | 0.55 |
| Screening to 18 mos (bone age adjusted) | ||||||||||
| ZTBBMD (BA)screening | 14 | −1.06 (1.04) | 17 | −0.95 (0.86) | 0.72 | 4 | −0.28 (0.33) | 10 | −0.91 (0.69) | 0.11 |
| ZTBBMD (BA)18 | 17 | −0.96 (1.02) | 14 | −1.12 (0.81) | 0.63 | 4 | −0.63 (0.57) | 6 | −0.88 (0.97) | 0.65 |
| ΔZTBBMD(BA)(18-0) | 17 | −0.08 (0.70) | 15 | −0.25 (0.53) | 0.47 | 4 | −0.35 (0.68) | 6 | −0.12 (0.87) | 0.66 |
| SPINE | ||||||||||
| Screening to 18 mos | ||||||||||
| ZSBMDscreening | 27 | −1.64 (0.68) | 20 | −1.61 (0.82) | 0.87 | 4 | −0.9 (0.37) | 12 | −1.4 (0.61) | 0.13 |
| ZSBMD18 | 22 | −1.43 (0.67) | 19 | −1.47 (0.9) | 0.91 | 4 | −0.85 (0.26) | 10 | −1.08 (0.79) | 0.59 |
| ΔZSBMD(18-0) | 22 | 0.21 (0.56) | 19 | 0.14 (0.51) | 0.67 | 4 | 0.05 (0.37) | 10 | 0.25 (0.51) | 0.5 |
| Screening to 9 mos | ||||||||||
| ZSBMD9 | 20 | −1.49 (0.58) | 15 | −1.73 (0.82) | 0.31 | 3 | −1.1 (0.26) | 8 | −1.13 (0.64) | 0.95 |
| ΔZSBMD(9-0) | 20 | 0.21 (0.37) | 15 | −0.15 (0.5) | 0.02 | 3 | −0.07 (0.47) | 8 | 0.28 (0.32) | 0.2 |
| Screening to 18 mos (bone age adjusted) | ||||||||||
| ZSBMD (BA)screening | 14 | −1.06 (1.04) | 17 | −0.95 (0.86) | 0.72 | 4 | −0.58 (0.24) | 10 | −1.27 (0.62) | 0.06 |
| ZSBMD(BA)18 | 17 | −0.98 (0.69) | 13 | −1.27 (0.97) | 0.34 | 4 | −0.68 (0.35) | 6 | −0.97 (0.61) | 0.42 |
| ΔZSBMD(BA)(18-0) | 17 | 0.41 (0.62) | 15 | −0.03 (0.72) | 0.07 | 4 | −0.1 (0.54) | 6 | 0.18 (0.75) | 0.54 |
CD: Crohn disease, UC: ulcerative colitis, SD: standard deviation, ZTBBMD: Total body BMD Z-score, ΔZTBBMD: change in total body BMD Z-score (from screening to 9 or 18 mos), ZSBMD: Spine BMD Z-score, ΔZSBMD: change in spine BMD Z-score (from screening to 9 or 18 mos), (BA): adjusted for bone age
The same analyses as above (Table 2) were performed for participants with CD and UC separately. Interestingly, among participants with CD, the calcitonin group showed a positive change in lumbar spine BMD Z-score at 9 months, which was significantly different when compared with the negative change experienced by the participants in the placebo group. However, this advantage of the calcitonin group at 9 months did not persist to the end of the study, since the overall change in lumbar spine BMD Z-score from screening to 18 months did not differ in the two arms. Of note is that again, BMD Z-scores of the total body of either arm did not improve, and spinal BMD Z-scores improved, but did not normalize by the end of the study in participants with CD.
The outcomes did not differ between the two arms in participants with UC. BMD Z-scores of participants with UC were higher than BMD Z-scores of participants with CD at baseline, 9 and 18 months.
Other subgroup analysis
Comparisons of changes in total body and spinal BMD Z-score between the two therapeutic arms at 9 months and 18 months were also performed separately within each pubertal group. No significant differences were noted between the two arms within the pre-pubertal (Tanner 1) or the pubertal group (Tanner 2,3,4); the post-pubertal group contained too few participants with outcomes present for a meaningful comparison (n=9).
Additionally, we compared primary and secondary outcomes in participants who took > 70% ideal nasal spray doses (adherent only). There were no significant differences in any of the above outcomes between the two arms, except for the change in spine BMD Z-score from 0 to 9 months in “adherent” patients with CD which was positive in the calcitonin and negative in the placebo arm (ΔZSBMDcalcitonin: 0.25 (0.35), ΔZSBMDplacebo: −0.05 (0.41), p=0.05).
Variables affecting changes in the total body and spinal BMD Z-score (Tables 4 & 5)
TABLE 4.
Variables affecting change in Z score of total body BMD at 18 mos for ALL, CD and UC participants
| R or difference in ΔZTBBMD (18-0) (CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL | CD | UC | |||||||
| VARIABLE | N | R/difference | p | N | R/difference | p | N | R/difference | P |
| ZTBBMD(screening) | 48 | −0.304 | 0.04 | 36 | −0.339 | 0.04 | |||
| ΔZHt | 48 | 0.48 | 0.001 | 36 | 0.658 | 0.000 | |||
| Average ESR | 48 | −0.458 | 0.001 | 36 | −0.397 | 0.02 | 12 | −0.664 | 0.02 |
| Average CRP | 48 | −0.387 | 0.007 | 36 | −0.356 | 0.03 | |||
| Average HCT | 48 | 0.355 | 0.01 | 36 | 0.324 | 0.05 | |||
| Average PLT | 48 | −0.382 | 0.007 | 36 | −0.330 | 0.05 | |||
| Average albumin | 48 | 0.42 | 0.001 | 36 | 0.518 | 0.001 | |||
| Average Fe | 48 | 0.461 | 0.001 | 12 | 0.709 | 0.01 | |||
| Average 25OHD | 47 | −0.292 | 0.05 | ||||||
| Wt bearing activity 1 | 46 | 0.306 | 0.04 | ||||||
| Hospitalization 2 | 10/26 | −0.53 (−0.95,−0.1) | 0.02 | ||||||
| Immunomodulators 2 | 39/47 | −0.49 (−0.91, −0.07) | 0.02 | ||||||
| Surgery 2 | 5/48 | −0.6 (−1.08, −0.11) | 0.02 | 4/36 | −0.65(−1.28,−0.03) | 0.04 | |||
| Disease activity 2 | IN:16 3 M:23 M/S:9 |
−0.71 (−1.23, −0.2) (moderate/severe vs. inactive) | 0.003 | IN: 9 3 MI:19 M/S:8 |
−0.8 (−1.45,−0.16) (moderate/severe vs. inactive) | 0.006 | |||
R: correlation coefficient, difference in ΔZTBBMD: ΔZTBBMD in comparison group – ΔZTBBMD in reference group, CD: Crohn disease, BMD: bone mineral density, R: correlation coefficient, ΔZTBBMD: change in total body bone mineral density Z-score, CI: 95% confidence intervals, ZTBBMD: total body bone mineral density Z-score, ΔZHt: change in height Z-score, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, HCT: hematocrit, PLT: platelets, Fe: serum iron, 25OHD: serum twenty five hydroxy vitamin D, Wt: weight
hr/wk,
reference group: no hospitalization, no immunomodulators, no surgery, inactive disease status,
I/M/M/S: Inactive/Mild/Moderate and severe
TABLE 5.
Variables affecting change in Z-score of spine BMD at 18 mos in ALL, CD and UC participants
| ALL | CD | UC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VARIABLE | N | R | p | N | R | p | N | R | p |
| ZSBMD (screening) | 41 | −0.319 | 0.04 | ||||||
| ZBMI (screening) | 55 | 0.282 | 0.04 | 41 | 0.36 | 0.02 | |||
| ΔZHt | 54 | 0.452 | 0.001 | 41 | 0.579 | <0.001 | |||
| ΔZ%fat (age) | 48 | −0.309 | 0.03 | ||||||
| Average ESR | 41 | −0.304 | 0.05 | ||||||
| Average PLT | 55 | −0.363 | 0.006 | 41 | −0.309 | 0.05 | 14 | −0.615 | 0.02 |
| Average CRP | 41 | −0.321 | 0.04 | ||||||
| Average albumin | 41 | 0.381 | 0.01 | ||||||
| Average caffeine int | 40 | −0.321 | 0.04 | ||||||
CD: Crohn disease, UC: ulcerative colitis, BMD: bone mineral density, R: correlation coefficient
ZSBMD: spine bone mineral density Z-score, ZBMI: Body mass index Z-score, ΔZHt: change in height Z-score, ΔZ%fat (age): change in percent fat adjusted for age Z-score
ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, PLT: platelets
The effect of several variables on total body and lumbar spine BMD Z-score change from screening to 18 months was examined via simple relationship testing as described in the Methods section for all participants and for participants with CD and UC separately. Only significant relationships are presented in the tables
Factors affecting changes in total body BMD Z-score in all participants (Table 4)
Lower baseline total body BMD Z-score, increase in height Z-score, higher average serum iron concentration, HCT, serum albumin level, and more hours of weight-bearing activity per week were associated with increase in total body BMD Z-score, whereas higher average ESR, CRP, PLT count, and 25OHD serum concentration, the use of immunomodulators, surgery, and more severe disease were negatively associated with total body BMD Z-score change. Average serum 25OHD concentration was negatively associated with bone mineral accrual. However, this relationship is confounded by the paradoxical positive relationship between average serum 25OHD and average ESR in this study (r=0.437, p=0.001) which could be attributed to higher amount of vitamin D supplementation by their clinicians in participants with more severe disease. Adjusted for ESR, average serum 25OHD lost its effect size and significance in relationship with change in total body BMD Z-score [β unadj= −0.015 (−0.029, 0.000), p=0.05, β adj= −0.004 (−0.019, 0.011), p=0.58].
Factors affecting changes in spinal BMD Z-score in all participants (Table 5)
Higher baseline BMI Z-score and increase in height Z-score were associated with increase in spinal BMD Z-score whereas higher average PLT count and % fat for age were negatively associated spinal BMD Z-score change.
Factors affecting changes in total body BMD Z-score in participants with CD and UC (Table 4)
Lower baseline BMD Z-score, increase in height Z-score, higher average serum albumin concentration, and HCT were associated with increase in total body BMD Z-score in participants with CD. Higher average ESR, CRP and PLT count, and IBD-related hospitalization, surgery and more severe disease were negatively associated with total body BMD Z-score change. Average serum iron concentration and PLT count were, respectively, positively and negatively associated with changes in total body BMD Z-scores in participants with UC.
Factors affecting changes in spinal BMD Z-score in participants with CD and UC (Table 5)
Higher baseline BMI Z-score, lower baseline BMD Z-score, increase in height Z-score and higher average serum albumin concentration were positively associated with increase in spinal BMD Z-score whereas higher average ESR, CRP, PLT count and daily caffeine intake were negatively associated with spine BMD Z-score changes in participants with CD. Higher average PLT count was negatively associated with BMD Z-score changes in the spine of participants with UC.
Adverse events (Tables 6 and 7)
Table 6.
ADVERSE EVENTS
| N (%) Total = 63 | ||||
|---|---|---|---|---|
| EVENT | ALL SUBJECTS | PLACEBO (32) | CALCITONIN (31) | p |
| CLINICAL | ||||
| All effects | 14 (22.2%) | 5 (15.6%) | 9 (29%) | 0.20 |
| Epistaxis | 5 (7.9%) | 3 (9.4%) | 2 (6.5%) | 0.67 |
| Irritation of the upper respiratory tract | 4 (6.3%) | 2 (6.3%) | 2 (6.5%) | 0.97 |
| Rhinitis | 4 (6.3%) | 1 (3.1%) | 3 (9.7%) | 0.29 |
| Nasal mucosa wound | 2 (3.2%) | 1 (3.1%) | 1 (3.2%) | 0.98 |
| Headache | 2 (3.2%) | 2 (6.3%) | 0 (0%) | 0.16 |
| Allergic reaction | 2 (3.2%) | 1 (3.1%) | 1 (3.2%) | 0.98 |
| Decreased appetite | 2 (3.2%) | 1 (3.1%) | 1 (3.2%) | 0.98 |
| Back pain | 2 (3.2%) | 2 (6.3%) | 0 (0%) | 0.16 |
| Fatigue | 2 (3.2%) | 1 (3.1%) | 1 (3.2%) | 0.98 |
| Infection of nasal tissue | 1 (1.6%) | 0 (0%) | 1 (3.2%) | 0.31 |
| Seizure | 1 (1.6%) | 0 (0%) | 1 (3.2%) | 0.31 |
| Eye irritation | 1 (1.6%) | 1 (3.1%) | 0 (0%) | 0.32 |
| Nausea | 1 (1.6%) | 0 (0%) | 1 (3.2%) | 0.31 |
| Abdominal pain | 1 (1.6%) | 0 (0%) | 1 (3.2%) | 0.31 |
| Arthralgia | 1 (1.6%) | 1 (3.1%) | 0 (0%) | 0.32 |
| LABORATORY | ||||
| Elevated PTH (serum PTH > 65….) | 8 (16%) | 3 (12.5%) | 5 (19.2%) | 0.52 |
| Hypocalcemia (serum Ca < 8….) | 1 (1.6%) | 1 (3.1%) | 0 (0%) | 0.32 |
TABLE 7.
Adverse effects and study medication experience
| ALL | PLACEBO | CALCITONIN | p | ||||
|---|---|---|---|---|---|---|---|
| Denominator | N (%) or Mean (SD, range) |
Denominator | N (%) or mean (SD, range) |
Denominator | N (%) or mean (SD, range) |
||
| Withdrew due to adverse event | 63 | 6 (9.5%) | 32 | 2 (6.3%) | 31 | 4 (12.9%) | 0.37 |
| Participant init | 63 | 5 (7.9%) | 32 | 2 (6.3%) | 31 | 3 (9.7%) | 0.62 |
| Investigator init | 63 | 1 (1.6%) | 32 | 0 (0%) | 31 | 1 (3.2%) | 0.31 |
| Withdrawal timing | |||||||
| Enrollment | 63 | 9 (14.3%) | 32 | 4 (12.5%) | 31 | 5 (16.1%) | 0.68 |
| 3 mos | 63 | 1 (1.6%) | 32 | 1 (3.1%) | 31 | 0 (0%) | 0.32 |
| 6 mos | 63 | 2 (3.2%) | 32 | 1 (3.1%) | 31 | 1 (3.2%) | 0.98 |
| 9 mos | 63 | 2 (3.2%) | 32 | 1 (3.1%) | 31 | 1 (3.2%) | 0.98 |
| Unexpected events | 63 | 1 (1.6%) | 32 | 0 (0%) | 31 | 1 (3.2%) | 0.31 |
| Adverse events (Total events) | 29 | 29 (all events) | 13 (44.8%) | 29 (all events) | 16 (55.2%) | 0.43 | |
| Events per participant | 14 (participants with events) | 2.1 (1.6, 1–7) | 5 | 2.8 (2.5, 1–7) | 9 | 1.7 (0.9, 1–3) | 0.43 |
| Participants with > 1 event | 14 (participants with events) | 7 (50%) | 5 | 3 (60%) | 9 | 4 (44.4%) | 0.58 |
| Timing (months from enrollment) | 29 (total events) | 4.1 (4.5) | 13 | 2.2 (2.6, 1–8) | 16 | 5.7 (5.2, 1–15) | 0.05 |
| 1st mo | 29 | 15 (51.7%) | 13 | 9 (69.2%) | 16 | 6 (37.5%) | 0.09 |
| 1–6 mos | 29 | 21 (72.4%) | 13 | 11 (84.6%) | 16 | 10 (62.5%) | 0.19 |
| After 6 mos | 29 | 8 (27.6%) | 13 | 2 (15.4%) | 16 | 6 (37.5%) | 0.19 |
| Severity | 29 | 13 | 16 | 0.28 | |||
| Mild | 23 (79.3%) | 12 (92.3%) | 11 (68.8%) | ||||
| Moderate | 5 (17.2%) | 1 (7.7%) | 4 (25%) | ||||
| Severe | 1 (3.4%) | 0 (0%) | 1 (6.3%) | ||||
| Relation to drug | 29 | 13 | 16 | 0.12 | |||
| Possible | 14 (48.3%) | 9 (69.2%) | 5 (31.3%) | ||||
| Probable | 3 (10.3%) | 1 (7.7%) | 2 (12.5%) | ||||
| Unknown | 12 (41.4%) | 3 (23.1%) | 9 (56.3%) | ||||
| Duration of event (days) | 19 | 34 (37,1–104) | 10 | 29 (32, 1–95) | 9 | 40 (43, 1–104) | 0.59 |
| Doses received | 63 | 32 | 31 | 0.97 | |||
| 0–25% | 12 (19%) | 6 (18.8%) | 6 (19.4%) | ||||
| 25–50% | 7 (11.1%) | 4 (12.5%) | 3 (9.7%) | ||||
| 50–75% | 9 (14.3%) | 4 (12.5%) | 5 (16.1%) | ||||
| 75–100% | 35 (55.6%) | 18 (56.3%) | 17 (54.8%) | ||||
The frequency of occurrence of adverse events related to the study medication did not differ between the two arms. The most frequently encountered clinical events were: epistaxis, irritation of the upper respiratory tract and rhinitis. Hyperparathyroidism was encountered with similar frequency between the two arms, and was likely due to suboptimal vitamin D status. One participant in the calcitonin arm had an allergic reaction consisting of hives and pruritus which was deemed to be related to the study medication and was withdrawn from the study after 1.5 month of participation (at the advice of DSMB). The participant received a short course (5 days) of oral prednisone and an antihistamine. The reaction resolved shortly thereafter. Another participant in the calcitonin arm had an unexpected event: a complex partial seizure, while hospitalized for an IBD flare. The hypothetical mechanism with which calcitonin could cause the occurrence of a seizure is through hypocalcemia secondary to disruption of bone resorption by osteoclasts, and consequently decrease in calcium release from bone to the circulation. The participant’s serum calcium concentration was within normal limits. The study medication was temporarily discontinued for 1 week, but restarted at the advice or our DSMB and the participant was discharged and followed by the neurology service, without further seizures for the duration of the trial and 1 year thereafter. The relationship of this event to the study medication is unknown. One participant in the placebo arm had serum calcium concentration < 8 mg/dL identified at one of the study visits. The participant was asymptomatic and the participant’s calcium concentration normalized and remained > 8 for the duration of the trial after the dose of calcium supplement was increased. Table 8 depicts the experience with the study medication. The only identified difference between the two arms was the adverse event timing. Adverse events in the calcitonin arm tended to occur later in the trial than those in the placebo arm. No adverse event recurred in any participant of the trial.
DISCUSSION
To our knowledge, this is the first double-blind, placebo-controlled trial testing the efficacy and safety of intranasal calcitonin on BMD over 18 months in children, adolescents and young adults with IBD. Intranasal calcitonin was well-tolerated, and appeared to offer a short term advantage in bone mineral acquisition rate at the spine in young patients with CD, but this advantage was not sustained. In addition, we observed that children and young adults with IBD and low bone density had bone mineral accrual rates that did not lead to “normalization” of their bone density Z-scores (mean close to 0) during the span of our trial (18 months). Data were collected on many clinical variables that affect bone mineral accrual including a few novel observations. Specifically, we observed that a lower total body BMD Z-score at baseline was associated with greater gains over time, and that weight-bearing activity improved, whereas higher caffeine intake hampered bone mineral accumulation rates.
Previous controlled studies of calcitonin in children reported positive results, although one utilized BMD T-scores as the primary outcome instead of the recommended BMD Z-scores11, and the other was a small trial36. While existing studies concur that bone formation is decreased in children with IBD4, 37, one study found decreased bone resorption at diagnosis4, and another reported increased bone resorption during the course of their disease37. We did not focus on the effect of calcitonin on bone turnover, which is the subject of a subsequent project in our center. Our rationale for its use in this study was that it would decrease bone resorption while bone formation rates recover or remain stable, allowing for “catch-up” bone mineral accumulation rates. Our finding that calcitonin therapy advanced bone mineral accumulation in the spine of young patients with CD over the first 9 months of the study does not yet support an application in clinical practice since it was the result of an underpowered subgroup analysis. Moreover, this benefit did not persist to the end of the trial. Clearly, larger trials are needed in this population to confirm or discredit this observation. A meta-analysis of the use of calcitonin for the treatment of postmenopausal osteoporosis suggested that it is effective in increasing BMD primarily at the spine, which is a site representative of trabecular bone, and a beneficial trend was noted at the hip – representative of cortical bone22. More recent studies have shown that calcitonin improves trabecular bone microarchitecture38 and therefore, may protect against fragility fractures. A recent study in children with IBD showed that trabecular BMD is decreased in newly diagnosed patients and remains low, although it initially improves after anti-inflammatory treatment5. Studies have also shown that microarchitectural deficits, specifically cortical thinning, are present in cortical bone in newly diagnosed pediatric patients with IBD, likely due to slower bone turnover as a result of inflammation5, 39. This cortical defect remained even after treatment. The use of calcitonin early in the course of IBD, especially during phases of transition from decreased to increased bone resorption, while bone formation rates have not yet recovered, could assist in restoring or protecting both bone mass and microarchitecture, especially that of trabecular bone. Our study did not examine functional outcomes such as bone strength and fracture risk. Further research is needed to examine the effects of calcitonin on bone mineral accumulation rates in children with IBD who have suppressed bone formation in combination with pathologically accelerated bone resorption. Additional study of the impact of calcitonin on bone strength and fracture risk will be required before its routine use can be endorsed in children with IBD. Moreover, trials of calcitonin in selected pediatric populations could be considered to examine whether this agent may be more effective in pubertal patients in whom bone turnover is naturally accelerated.
In terms of the safety of the intranasal calcitonin, we report that it was well-tolerated. Our experience is similar to that in adults, with mostly minor and reversible harms associated with its use in children. The occurrence of a seizure in one participant of the calcitonin arm cannot be attributed to the medication with certainty, especially since the participant’s serum calcium and 25OHD concentration was normal. Larger trials are needed to disprove or establish any such relationship.
A significant finding was the absence of improvement in total body BMD Z-score and inadequate improvement in spinal BMD Z-score in the participants of this trial. This finding is in agreement with findings of other longitudinal studies that reported that bone mineral acquisition rates are not compensating for deficits of bone mineral and structure observed at diagnosis in children with IBD19, 5. In contrast with these studies, our study followed children with IBD and low BMD at any point in the course of their disease. Although the severity of their disease fluctuated during the study, they did not universally transition from a high inflammatory state to a quiescent state like the children in the studies mentioned above. In addition, our participants’ lean mass, weight and BMI Z-scores were either normal, or close to normal, where significant deficits of these anthropometric measures that could affect bone mineral accrual were observed at diagnosis in the above studies. As such, our observation that low BMD Z-scores do not seem to significantly improve over time is even more worrisome, since it may herald a more solidified suboptimal bone health state. Clearly, additional longitudinal large scale studies are needed to investigate outcomes of children with IBD and low BMD Z-scores in terms of peak bone mass achievement, fracture risk and bone quality by early adulthood.
This prospective trial contributes a few novel findings. First, we established a positive effect of weight-bearing activity on total body bone mineral accrual in children with IBD. This finding is not surprising since, according to the mechanostat theory, muscle force in addition to muscle load drives bone mass and strength attainment40. Exercise is beneficial for bone mass and strength accrual in healthy children with optimal results seen during early puberty41. Studies in other patient groups have shown the value of weight-bearing activity for bone health in children with cystic fibrosis42, and juvenile rheumatoid arthritis43. In adults with IBD, a low impact exercise program of increasing intensity resulted in significant BMD gains in compliant subjects44. Secondly, we demonstrated that subjects with lower baseline total body BMD Z-score demonstrated higher bone mineral accrual rates. This association has also been noted in trials of antiresorptive medications and estrogens in women with low BMD45, 46 and it was attributed to higher bone turnover rates in those subjects with the lowest BMD47, 48. A similar compensatory mechanism could stimulate bone turnover in pediatric subjects with a very low BMD. We propose that the association between lower baseline BMD Z-scores and higher rates of bone mineral accrual be taken into consideration when evaluating the effect of other factors or interventions on the rate of bone mineral accrual in children with IBD. Third, we found that higher average daily caffeine consumption from all dietary sources during the study was independently associated with lower bone mineral acquisition rates in the spine of participants with CD. The majority of caffeine in the diet of American children originates in caffeinated soft drinks. Other investigators have found a negative relationship between daily amount of soft drinks and bone density in cross-sectional studies of healthy children49, 50. The hypothesis is that soft drinks displace other nutrients from the diet that are of importance to bone health, namely milk (which is a calcium and vitamin D source), and protein. In addition, it is known that caffeine interferes with calcium intestinal absorption, primarily when calcium intake is inadequate51. In our study population, we confirmed that there was a significant inverse correlation between caffeine and calcium intake among participants with CD.
In examining additional factors associated with bone mineral accrual rates, we observed that greater gains in Ht were associated with significantly greater bone mineral accrual rates at both total body and spine, as shown also by others in healthy children16. A previous longitudinal study found that changes in Ht Z-score were not associated with changes in BMD Z-score4, but the BMD Z-scores in this study were adjusted for height-age, which may have led to an underestimate of the raw effect of linear growth on bone mineral accumulation rates. The fact that increases in height Z-score are associated with higher bone mineral accrual rates points toward the important role of growth-related hormones in bone mineral accrual rate. Growth hormone has been tightly linked with bone mass gains in both healthy children and growth deficient children after treatment with this hormone52. We also found that higher baseline BMI Z-score was associated with higher bone mineral accrual rates at the spine. Several investigators found a positive relationship between BMI and bone health in children with IBD in cross-sectional studies20, 4, 18, 53. We hypothesize that the fact that higher baseline BMI Z-score - rather than increase in BMI Z-score during the study - was associated with bone mineral accrual rate improvement points toward a temporal relationship where good nutritional status is a pre-requisite for and has to precede an improvement in bone health.
We found a consistent impact of the inflammatory state and both disease course and severity during the trial, on bone accrual in CD and UC participants. Several investigators have previously found a negative relationship between inflammatory state and BMD in cross-sectional studies of pediatric patients with IBD21, 18, 54, 4, 5. Only a few studies examined the impact of inflammation on BMD in a longitudinal manner in this population and their findings are not in agreement with our data4, 5. While we found that markers of inflammation (PLT count, ESR), indicators of inflammation (e.g. serum Fe concentration, albumin), and disease severity (e.g. use of immunomodulators) were associated with decreased rates of total body and/or spine bone mineral accrual rates, they did not detect a relationship between inflammatory state and bone mineral accrual. However, these investigators examined incident cases of IBD, whereas our study included both incident and prevalent cases. As expected, an initial high level of inflammation universally improved in the course of these studies, whereas inflammation markers fluctuated during our study, depending on each participant’s disease course, thus providing an independent variable to test. Noteworthy is also the fact that other investigators examined disease activity indices and not specific markers. A constellation of markers may more accurately represent inflammatory state.
Lastly, we did not find that glucocorticoid administration during the study was associated with total body or spine BMC accrual. The effects of glucocorticoids on BMD have been studied mostly through cross-sectional studies in children with IBD and the reports have provided conflicting results, with some observing a negative effect of lifetime glucocorticoid dose on BMD18, 55, and others not20, 21. A recent longitudinal study found that the quality of bone built may be negatively affected by high concomitant steroid intake5. Clearly, additional prospective studies are needed to focus on the effects of glucocorticoids on bone architecture and strength and their connection to outcomes such as fracture in this population.
Our study has several limitations that deserve discussion. First, we were limited by the relatively small number of participants who completed the trial, and by the lack of healthy controls. The small number of participants specifically precluded multivariable analysis. Secondly, the DXA measurement of total body BMD (and respective BMD Z-scores) included the skull due to a software limitation. Experts have suggested that such measurements ideally would not include the measurement of skull BMD15. However, since this was a randomized trial, we expect that the minimal contribution of the skull to total body bone mineral accrual rates would have been balanced between the two arms. Strengths of our trial include that it was designed and conducted according to the gold standard in clinical experimentation - the double-blind, placebo-controlled clinical trial and it is the first systematic study of a therapeutic agent for the improvement of bone health in children with IBD. Moreover, because of its prospective design, it has provided valuable unbiased data on the effect of several factors on bone mineral accrual rates.
In conclusion, we found that calcitonin is well-tolerated, but did not improve bone mineral accrual rates in young patients with IBD. A temporary advantage in bone mineral accrual rate in the subgroup of young patients with CD has to be confirmed by larger studies. As well, further studies are needed to examine which pediatric IBD populations may benefit from calcitonin therapy for bone health. As others before, we found that bone mineral accrual rates of children with low BMD Z-scores and IBD do not increase in a manner that would allow for normalization of these scores, at least in the 18 months of the study.
Factors benefiting bone mineral accrual rates were: lower baseline BMD Z-score, linear growth, weight-bearing exercise and higher baseline nutritional status. Higher degree of inflammation and more severe disease, and higher consumption of caffeine were associated with lower bone mineral accrual rates. Further studies are needed to confirm these findings, so that therapeutic and preventive interventions can focus on improving the corresponding aspects of care of young patients with IBD which are amenable to improvement, helping these patients maximize their potential for optimal bone health in their adult years.
STUDY HIGHLIGHTS.
What is current knowledge?
Prevalence of suboptimal bone mineral density is higher in children with IBD than their healthy peers.
No clinical trials of pharmacologic agents targeting bone density have been reported in this population.
Bone mineral accrual rate may not be adequate to compensate for bone mineral deficits.
Inflammation hampers bone accrual
What is new here?
Intranasal calcitonin is well-tolerated, but offers no long-term benefits for bone health.
Larger trials are needed to explore whether subpopulations of children with IBD would benefit from calcitonin.
Weight-bearing activity increases the bone mineral accrual rate in children with IBD and low bone density.
Caffeine intake is associated with lower bone mineral accrual rates.
Deficits in bone density do not correct over 18 months
Supplementary Material
Figure 1.
Calcitonin study flowchart and timeline
Acknowledgments
We would like to acknowledge Dr. Hongyu Jiang and Mr. Paul Mitchell for their review of the final statistical analyses, Dr. Mei-Chiung Shih for her input in study design and analytic strategy, Dr. Bess Dawson-Hughes for invaluable consultation, Dr. Stravroula Osganian for continuous support and encouragement, and Katie Clegg and Jamie Nydegger for excellent technical support.
Financial Support
This work was funded by a Crohn’s and Colitis Foundation of America Senior Investigator Award to Dr Grand. This work was conducted with support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
The funding agencies had no involvement in the study design, data collection, analysis or interpretation.
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- CRP
C-reactive protein
- DXA
dual energy X-Ray absorptiometry
- ESR
erythrocyte sedimentation rate
- Fe
iron
- HCT
hematocrit
- PCDAI
pediatric Crohn’s disease activity index
- PLT
platelets
- PTH
intact parathyroid hormone
- UGI
upper gastrointestinal
- WBC
white blood cells
- 25OHD
Twenty five hydroxy vitamin D
Footnotes
Guarantor of the article:
Dr Helen Pappa
Specific authors’ contributions:
Dr Helen Pappa: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; technical and material support; study supervision
Tracee Saslowsky: Study concept and design; acquisition of data; revision of the manuscript for important intellectual content; technical support; study supervision.
Ms Saslowsky has approved the final draft submitted
Rajna Filip-Dhima: Data analysis; database support; study supervision
Ms Filip-Dhima has approved the final draft submitted
Diane DiFabio: Data acquisition, analysis and interpretation of nutritional data, technical and material support.
Ms DiFabio has approved the final draft submitted
Dr Hajar Hassani Lahsinoui: Analysis of data; statistical analysis; technical support.
Dr Hassani-Lahsinoui has approved the final draft submitted
Apurva Akkad: Analysis of data; statistical analysis; technical support
Mr Akkad has approved the final draft submitted
Dr Richard Grand: Study concept and design; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
Dr Grand has approved the final draft submitted
Dr Catherine Gordon: Study concept and design; critical revision of the manuscript for important intellectual content; study supervision
Dr Gordon has approved the final draft submitted
Potential competing interests
Dr Helen Pappa: No conflicts of interest exist
Tracee Saslowsky: No conflicts of interest exist
Rajna Filip-Dhima: No conflicts of interest exist
Dr Hajar Hassani Lahsinoui: No conflicts of interest exist
Apurva Akkad: No conflicts of interest exist
Dr Richard Grand: No conflicts of interest exist
Dr Catherine Gordon: Consultant: Gilead Sciences, Inc. and Director: Clinical Investigator Training Program, Harvard/MIT (with Pfizer/Merck)
REFERENCES
- 1.Boot AM, Bouquet J, Krenning EP, et al. Bone mineral density and nutritional status in children with chronic inflammatory bowel disease. Gut. 1998;42(2):188–194. doi: 10.1136/gut.42.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowan FJ, Warner JT, Dunstan FD, et al. Inflammatory bowel disease and predisposition to osteopenia. Arch Dis Child. 1997;76(4):325–329. doi: 10.1136/adc.76.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gokhale R, Favus MJ, Karrison T, et al. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology. 1998;114(5):902–911. doi: 10.1016/s0016-5085(98)70309-9. [DOI] [PubMed] [Google Scholar]
- 4.Sylvester FA, Wyzga N, Hyams JS, et al. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(1):42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 5.Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn's disease. Gastroenterology. 2009;136(1):123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persad R, Jaffer I, Issenman RM. The prevalence of long bone fractures in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2006;43(5):597–602. doi: 10.1097/01.mpg.0000237926.22976.55. [DOI] [PubMed] [Google Scholar]
- 7.Benchimol EI, Ward LM, Gallagher JC, et al. Effect of calcium and vitamin D supplementation on bone mineral density in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45(5):538–545. doi: 10.1097/MPG.0b013e3180dca0cc. [DOI] [PubMed] [Google Scholar]
- 8.Reginster JY. Effect of calcitonin on bone mass and fracture rates. Am J Med. 1991;91(5B):19S–22S. doi: 10.1016/0002-9343(91)90242-p. [DOI] [PubMed] [Google Scholar]
- 9.Chesnut CH, 3rd, Azria M, Silverman S, et al. Salmon calcitonin: a review of current and future therapeutic indications. Osteoporos Int. 2008;19(4):479–491. doi: 10.1007/s00198-007-0490-1. [DOI] [PubMed] [Google Scholar]
- 10.Cranney A, Welch V, Adachi JD, et al. Calcitonin for the treatment and prevention of corticosteroid-induced osteoporosis. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD001983. CD001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Husseini AA, El-Agroudy AE, El-Sayed MF, et al. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatr Transplant. 2004;8(4):357–361. doi: 10.1111/j.1399-3046.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 12.Canatan D, Akar N, Arcasoy A. Effects of calcitonin therapy on osteoporosis in patients with thalassemia. Acta Haematol. 1995;93(1):20–24. doi: 10.1159/000204084. [DOI] [PubMed] [Google Scholar]
- 13.Siamopoulou A, Challa A, Kapoglou P, et al. Effects of intranasal salmon calcitonin in juvenile idiopathic arthritis: an observational study. Calcif Tissue Int. 2001;69(1):25–30. doi: 10.1007/s00223-001-0008-3. [DOI] [PubMed] [Google Scholar]
- 14.El-Husseini AA, El-Agroudy AE, El-Sayed M, et al. A prospective randomized study for the treatment of bone loss with vitamin d during kidney transplantation in children and adolescents. Am J Transplant. 2004;4(12):2052–2057. doi: 10.1111/j.1600-6143.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 15.Gordon CM, Bachrach LK, Carpenter TO, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11(1):43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Baxter-Jones AD, McKay H, Burrows M, et al. International longitudinal pediatric reference standards for bone mineral content. Bone. 2009 doi: 10.1016/j.bone.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatier JP, Guaydier-Souquieres G, Laroche D, et al. Bone mineral acquisition during adolescence and early adulthood: a study in 574 healthy females 10–24 years of age. Osteoporos Int. 1996;6(2):141–148. doi: 10.1007/BF01623938. [DOI] [PubMed] [Google Scholar]
- 18.Lopes LH, Sdepanian VL, Szejnfeld VL, et al. Risk factors for low bone mineral density in children and adolescents with inflammatory bowel disease. Dig Dis Sci. 2008;53(10):2746–2753. doi: 10.1007/s10620-008-0223-0. [DOI] [PubMed] [Google Scholar]
- 19.Sylvester FA, Leopold S, Lincoln M, et al. A two-year longitudinal study of persistent lean tissue deficits in children with Crohn's disease. Clin Gastroenterol Hepatol. 2009;7(4):452–455. doi: 10.1016/j.cgh.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Burnham JM, Shults J, Semeao E, et al. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res. 2004;19(12):1961–1968. doi: 10.1359/JBMR.040908. [DOI] [PubMed] [Google Scholar]
- 21.Paganelli M, Albanese C, Borrelli O, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(4):416–423. doi: 10.1002/ibd.20039. [DOI] [PubMed] [Google Scholar]
- 22.Cranney A, Tugwell P, Zytaruk N, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VI. Meta-analysis of calcitonin for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23(4):540–551. doi: 10.1210/er.2001-6002. [DOI] [PubMed] [Google Scholar]
- 23.Kelly TL, Specker BL, Binkley T, Zemel BS, Leonard MB, Kalkwarf HJ, Moyer-Milieur, Shepard JA. Pediatric BMD reference database for US white children. Bone. 2005;36 Suppl 1:S30. [Google Scholar]
- 24.Zemel B. Reference data for the whole body, lumbar spine and proximal femur for American children relative to age, gender, and body size. J Bone Miner Res. 2004;(19) [Google Scholar]
- 25.Looker AC, Wahner HW, Dunn WL, et al. Proximal femur bone mineral levels of US adults. Osteoporos Int. 1995;5(5):389–409. doi: 10.1007/BF01622262. [DOI] [PubMed] [Google Scholar]
- 26.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2d ed. Stanford, Calif: Stanford Univ. Press; 1959. [Google Scholar]
- 28.Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services: Office of the Surgeon General; 2004. [PubMed] [Google Scholar]
- 29.Bousvaros A, Antonioli DA, Colletti RB, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44(5):653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 30.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991;12(4):439–447. [PubMed] [Google Scholar]
- 31.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70(3):439–444. [PubMed] [Google Scholar]
- 32.Kozarek RA, Patterson DJ, Gelfand MD, et al. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110(5):353–356. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 33.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133(2):423–432. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava M, Zurakowski D, Cheifetz P, et al. Elevated serum hepatocyte growth factor in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33(5):548–553. doi: 10.1097/00005176-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Chesnut CH, 3rd, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109(4):267–276. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 36.Nishioka T, Kurayama H, Yasuda T, et al. Nasal administration of salmon calcitonin for prevention of glucocorticoid-induced osteoporosis in children with nephrosis. J Pediatr. 1991;118(5):703–707. doi: 10.1016/s0022-3476(05)80030-7. [DOI] [PubMed] [Google Scholar]
- 37.Tuchman S, Thayu M, Shults J, et al. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr. 2008;153(4):484–490. doi: 10.1016/j.jpeds.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chesnut CH, 3rd, Majumdar S, Newitt DC, et al. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: results from the QUEST study. J Bone Miner Res. 2005;20(9):1548–1561. doi: 10.1359/JBMR.050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward LM, Rauch F, Matzinger MA, et al. Iliac bone histomorphometry in children with newly diagnosed inflammatory bowel disease. Osteoporos Int. 21(2):331–337. doi: 10.1007/s00198-009-0969-z. [DOI] [PubMed] [Google Scholar]
- 40.Frost HM. Bone "mass" and the "mechanostat": a proposal. Anat Rec. 1987;219(1):1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 41.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40(1):14–27. doi: 10.1016/j.bone.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Hind K, Truscott JG, Conway SP. Exercise during childhood and adolescence: a prophylaxis against cystic fibrosis-related low bone mineral density? Exercise for bone health in children with cystic fibrosis. J Cyst Fibros. 2008;7(4):270–276. doi: 10.1016/j.jcf.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Kotaniemi A, Savolainen A, Kroger H, et al. Weight-bearing physical activity, calcium intake, systemic glucocorticoids, chronic inflammation, and body constitution as determinants of lumbar and femoral bone mineral in juvenile chronic arthritis. Scand J Rheumatol. 1999;28(1):19–26. doi: 10.1080/03009749950155733. [DOI] [PubMed] [Google Scholar]
- 44.Robinson RJ, Krzywicki T, Almond L, et al. Effect of a low-impact exercise program on bone mineral density in Crohn's disease: a randomized controlled trial. Gastroenterology. 1998;115(1):36–41. doi: 10.1016/s0016-5085(98)70362-2. [DOI] [PubMed] [Google Scholar]
- 45.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 46.Armamento-Villareal R, Civitelli R. Estrogen action on the bone mass of postmenopausal women is dependent on body mass and initial bone density. J Clin Endocrinol Metab. 1995;80(3):776–782. doi: 10.1210/jcem.80.3.7883830. [DOI] [PubMed] [Google Scholar]
- 47.Christiansen C, Riis BJ, Rodbro P. Prediction of rapid bone loss in postmenopausal women. Lancet. 1987;1(8542):1105–1108. doi: 10.1016/s0140-6736(87)91671-0. [DOI] [PubMed] [Google Scholar]
- 48.Slemenda C, Hui SL, Longcope C, et al. Sex steroids and bone mass. A study of changes about the time of menopause. J Clin Invest. 1987;80(5):1261–1269. doi: 10.1172/JCI113201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGartland C, Robson PJ, Murray L, et al. Carbonated soft drink consumption and bone mineral density in adolescence: the Northern Ireland Young Hearts project. J Bone Miner Res. 2003;18(9):1563–1569. doi: 10.1359/jbmr.2003.18.9.1563. [DOI] [PubMed] [Google Scholar]
- 50.Libuda L, Alexy U, Remer T, et al. Association between long-term consumption of soft drinks and variables of bone modeling and remodeling in a sample of healthy German children and adolescents. Am J Clin Nutr. 2008;88(6):1670–1677. doi: 10.3945/ajcn.2008.26414. [DOI] [PubMed] [Google Scholar]
- 51.Heaney RP. Effects of caffeine on bone and the calcium economy. Food Chem Toxicol. 2002;40(9):1263–1270. doi: 10.1016/s0278-6915(02)00094-7. [DOI] [PubMed] [Google Scholar]
- 52.Ross J, Czernichow P, Biller BM, et al. Growth hormone: health considerations beyond height gain. Pediatrics. 125(4):e906–e918. doi: 10.1542/peds.2009-1783. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt S, Mellstrom D, Norjavaara E, et al. Low bone mineral density in children and adolescents with inflammatory bowel disease: A population-based study from Western Sweden. Inflamm Bowel Dis. 2009;15(12):1844–1850. doi: 10.1002/ibd.20962. [DOI] [PubMed] [Google Scholar]
- 54.Semeao EJ, Jawad AF, Stouffer NO, et al. Risk factors for low bone mineral density in children and young adults with Crohn's disease. J Pediatr. 1999;135(5):593–600. doi: 10.1016/s0022-3476(99)70058-2. [DOI] [PubMed] [Google Scholar]
- 55.Walther F, Fusch C, Radke M, et al. Osteoporosis in pediatric patients suffering from chronic inflammatory bowel disease with and without steroid treatment. J Pediatr Gastroenterol Nutr. 2006;43(1):42–51. doi: 10.1097/01.mpg.0000228105.91240.80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.