Abstract
Methamphetamine is a CNS stimulant with limited therapeutic indications, but is widely abused. Short-term exposure to higher doses, or long-term exposure to lower doses, of methamphetamine induces lasting damage to nigrostriatal dopamine neurons in man and animals. Strong evidence indicates that the mechanism for this detrimental effect on dopamine neurons involves oxidative stress exerted by reactive oxygen species. This study investigates the relative susceptibility of dopamine neurons in mid-gestation, young, and adult (not aged) monkeys to 4 treatments with methamphetamine over 2 days. Primate dopamine neurons undergo natural cell death at mid-gestation, and we hypothesized that during this event they are particularly vulnerable to oxidative stress. The results indicated that at mid-gestation and in adults, dopamine neurons were susceptible to methamphetamine-induced damage, as indicated by loss of striatal TH immunoreactivity and dopamine concentration. However, dopamine neurons in young animals appeared totally resistant to the treatment, despite this group having higher brain levels of methamphetamine 3 hours after administration than the adults. As a possible explanation for the protection, striatal GDNF levels were elevated in young animals 1-week after treatment, but not in adults following methamphetamine treatment. Implications of these primate studies are: 1) the susceptibility of dopamine neurons at mid-gestation to methamphetamine warns against the risk of exposing pregnant women to the drug or oxidative stressors, and supports the hypothesis of Parkinson's disease being associated with oxidative stress during development, 2) elucidation of the mechanism of resistance of dopamine neurons in the young animals to methamphetamine-induced oxidative stress may provide targets for slowing or preventing age- or disease-related loss of adult nigrostriatal DA neurons, and 3) the increased striatal production of GDNF in young animals, but not in adults, in response to methamphetamine, suggests the possibility of an age-related change in the neurotrophic capacity of the striatal dopamine system.
Keywords: Development, dopamine, GDNF, methamphetamine, monkey, Parkinson's disease
The well-established plasticity of the developing brain might suggest that an early insult would be more efficiently repaired than a later challenge, but this is not necessarily so. The molecular and morphological changes that occur during development appear to create potential temporal windows of selective vulnerability, as well as permitting compensatory repair mechanisms to operate (Ikonomidou et al., 2000; Johnston, 1995; Vaccarino and Ment, 2004). Damage to the developing brain may not be apparent until later in life. The concept of such a latent period, or silent toxicity, between exposure and disease in neurotoxicology has received increasing attention, and it has been estimated that up to 40% of major developmental defects could result from maternal exposure to harmful environmental agents (Heindel, 2007; Reuhl, 1991). For Parkinson's disease, the onset of symptoms typically occurs later in life when loss of nigrostriatal dopamine (DA) neurons exceeds the capacity of basal ganglia circuits to compensate for inadequate striatal DA neurotransmission. There is strong evidence in Parkinson's disease that nigrostriatal DA neurons have been compromised by oxidative damage (Jenner, 2003; Tretter et al., 2004; Zhang et al., 2000; Zhou et al., 2008), which may arise from either genetic and/or environmental abnormalities (Di Monte, 2003; Langston, 1998) initiated during development (Barlow et al., 2007).
Exposure of experimental animals to chemicals that induce oxidative stress, such as methamphetamine, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, paraquat or rotenone, has produced animals with signs of Parkinson's disease (Betarbet et al., 2002). It is not known, though, whether there are periods during development when nigrostriatal DA neurons are more or less susceptible to oxidative stress, and this issue is addressed in the current study. Our earlier data showed that primate nigrostriatal DA neurons undergo apoptotic natural cell death at mid-gestation, and we hypothesized that this may be a particularly vulnerable period for this neuronal population to be exposed to oxidative stress (Morrow et al., 2007). Thus, we examined the relative age-dependent susceptibility of primate DA neurons to oxidative stress using methamphetamine. A greater understanding of the developmental effects of oxidative stress may provide insights for risk assessment and the reduction of adverse postnatal consequences (Wells et al., 2009).
Methamphetamine is a psychostimulant that can be prescribed in the United States for certain indications (ADHD, obesity and is used “off-label” for narcolepsy), but is currently widely abused for its euphoric properties. Methamphetamine, particularly when used at high doses or over long periods, provokes persistent changes in central DA neurons, both in man and animals (McCann et al., 2008; Melega et al., 1997; Richards et al., 1993; Wagner et al., 1980; Wilson et al., 1996; Woolverton et al., 1989), and the mechanism of this effect appears to be dependent on oxidative stress (Kita et al., 2009; Riddle et al., 2006; Yamamoto and Raudensky, 2008). An association between earlier methamphetamine use and occurrence of Parkinson's disease has been posited (Boger et al., 2010; Carvey et al., 2006; Thrash et al., 2009; Volkow et al., 2001), with some recent intriguing epidemiological evidence to support this hypothesis (Callaghan et al., 2010; Garwood et al., 2006).
Experimental Procedures
Animals
African green monkeys (Chlorocebus sabaeus) were housed and treated at the St. Kitts Biomedical Research Foundation, an AAALAC accredited facility. The gestational age of fetal monkeys was estimated by ultrasonography (Morrow et al., 2005). The gestation period in this species is approximately 165 days. Studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use on Animals. All studies were approved by the IACUC of both Yale University and St. Kitts Biomedical Research Foundation.
Experiment 1: Effect of methamphetamine on tyrosine hydroxylase immunoreactivity and striatal DA levels
Sample collection
The effect of methamphetamine exposure on the expression of tyrosine hydroxylase immunoreactivity (TH-ir) was examined in fetuses, young animals, and adult male monkeys. The fetuses were selected to be at or near mid-gestation when the pregnant monkey was treated with methamphetamine; there was no significant group difference between the group ages (methamphetamine-treated, mean Embryonic Day [ED] with standard error [S.E.] 73.2±1.9) and their controls (ED 80.7±5.9). Similarly there was no significant difference in age between the young animals treated with methamphetamine (16.5±4.9 days) and their controls (13.3±1.3 days). Adult males (>5 years old) showing no signs of advanced age, were selected from the colony and divided into treatment groups that did not differ significantly in weight (control 4.8±0.3 kg, methamphetamine 5.3±0.4 kg).
On day 1, all animals were given 2 injections of methamphetamine hydrochloride intramuscularly (0.5 mg/kg i.m.) or saline separated by about 8 hours. The following day, animals again received 2 injections of methamphetamine (1 mg/kg i.m.) or saline, separated by about 8 hours. For comparison, the dose used illicitly in naive subjects is approximately 30 mg methamphetamine (about 0.5 mg/kg) (National Toxicology Program, 2005). Previous studies have indicated that the impact of methamphetamine on striatal DA concentration reaches maximal levels on about day 7 after injection (Harvey et al., 2000a; Harvey et al., 2000b). On days 7 and 8, animals were euthanized under deep anesthesia, and brains fixed by cardiac perfusion with heparin-containing saline followed by either perfusion-fixation or immersion fixation with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.3) (Morrow et al 2007). Past experience has shown that these 2 fixation variants produced no difference in immunostaining for tyrosine hydroxylase in this species of monkey (Sladek et al., 1995). Immersion fixation was used for some samples to allow unfixed tissue to be dissected using a 1.4 mm diameter punch from 4 mm coronal sections placed on a thermostatically controlled stage and maintained at 2 °C (Elsworth et al., 2000). Fresh tissue samples were put in cryotubes and frozen in liquid nitrogen, until assay for DA, homovanillic acid (HVA) and GDNF concentrations (described below). Each pregnant females was anesthetized and the fetus was removed by cesarean section (Sladek et al., 1995), transcardially perfused with cold saline and perfusion-fixed with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.3). Adult and young brains were kept in 4% paraformaldehyde in 0.1M phosphate buffer at 4 °C overnight and then stored in 0.1M phosphate buffer containing 1% sodium azide until preparation for immunohistochemistry. Fetal brains were kept in 4% paraformaldehyde in 0.1M phosphate buffer at 4 °C for 48 hours before moving into 0.1M phosphate buffer containing 1% sodium azide and preparation for immunohistochemistry.
For the adults and young animals, a 4 mm coronal section of the fixed brain containing an anterior portion of striatum was block cut using the optic chiasm as an external marker such that the posterior boundary was approximately the caudal extent of the nucleus accumbens. A 4 mm coronal section encompassing the anterior striatum was cut from the fetal brains using the temporal lobes as an external marker. The blocks were then cut into 40 micron sections and stored as 10 sets of tissue each having sections 400 microns apart. In this way, a single set of tissues then represented a uniform sampling of the anterior to middle striatum starting at a random point in the anterior striatum.
Quantification of tyrosine hydroxylase-immunoreactivity
A free-floating set of tissue from each brain was immunostained for tyrosine hydroxylase-immunoreactivity (TH-ir) using published methods (Morrow et al., 2005). Because the total number of tissues samples could not be stained and analyzed in a single batch, sections from control and treated animals in the same age group were stained together in batches. Briefly, TH-ir was identified using a primary antibody (1:1000 overnight at room temperature, MAB-318, Chemicon, Temecula, CA) with the ABC technique using a Vectastain Elite kit (Vector Labs, Burlingame, CA) and visualized with a diaminobenzidine reaction (0.05% diaminobenzidine and 0.002% hydrogen peroxide) (Hsu et al., 1981). Sections were mounted onto glass slides and coverslips were applied.
After the mounting media was dried, the optical density (OD) of TH-ir was determined. Tissue sections were photographed using Cohu high performance CCD grey scale camera fitted with a Nikon Micro-NIKKOR lens back-illuminated using a light box (Northern Lights). Each batch of stained tissue was photographed in a single session after calibration of optical density (OD) using a step tablet (Kodak/Tiffen) and ImageJ software (NIH). The average measures of OD for each region of interest were determined by random, systematic sampling using a Cavalieri technique (Howard and Reed, 1998) with the ImageJ software. Briefly, on each tissue section, a 1 mm grid pattern was digitally placed over the image of the tissue and OD was determined at each cross on the grid within the region of interest. The measures of OD in adjacent cortical regions were sampled in order to determine non-specific background staining. The volumes of the striatum analyzed were also determined concurrently using the Cavalieri grid technique (Howard and Reed, 1998). The resulting data were analyzed using 2-way ANOVA (drug treatment × region) with Bonferroni post-hoc testing. Because of the absence of some landmarks within the fixed striatum of the mid-gestational fetal brain, OD was determined for the whole striatum and was analyzed using Student's t-test.
DA and HVA assays
DA was extracted then analyzed by HPLC with electrochemical detection, modified from (Elsworth et al., 2000). Briefly, each sample was sonicated in 0.1M perchloric acid that contained internal standard (3,4-dihydroxybenzylamine). The pH of the supernatant was adjusted to about 8.2 with 3M Tris, and catechols were adsorbed to a small alumina column (Type WA-4 acid, Sigma, St.Louis, MO). After a water wash, elution was achieved using 0.1M perchloric acid, and a portion of this fraction was separated by reverse-phase HPLC (Microsorb C18 3 micron, 4.6 × 100 mm column, Varian, Walnut Creek, CA) and detected electrochemically (LC-4C detector at 700 millivolts versus Ag/AgCl reference electrode, Bioanalytical Systems, West Lafayette, IN). The mobile phase was a solution of 30 mM sodium citrate, 13.7 mM sodium dihydrogen phosphate, 2.3 mM 1-octane-sulfonic acid phosphate, 0.025 mM EDTA with 6.5% acetonitrile, 0.6% tetrahydrofuran, and 0.1% diethylamine at final pH 3.2. Quantification was achieved by reference to internal and external standards. Measurement of the main metabolite in primate brain, HVA (Bacopoulos et al., 1978), was performed by HPLC separation and detection (as above) of a portion of the initial perchloric acid extract. DA and HVA concentrations were corrected for protein content of the sample, which was measured from the solubilized perchloric acid-precipitated pellet using a modified Lowry protein assay (Thermo Scientific Pierce, Rockford, IL).
Experiment 2: Effect of age on methamphetamine levels
Sample collection
Plasma and CSF levels of methamphetamine were measured after a single dose of the drug. Three pregnant monkeys (4.3±0.1 kg) at similar gestational age (ED 89, 83, & 80) were selected. Two male and 3 female young animals (31.6±3.8 days old) and 5 adult males (5.4±0.4 kg none of whom showed any signs of advanced age), were also used. Subjects were given methamphetamine (1 mg/kg i.m.) and 3 hours later, following ketamine injection (8 mg/kg i.m.), blood and cisternal CSF samples were collected; plasma was prepared and samples were frozen. Brain samples were dissected following euthanasia at the 3-hour time point, as described above for fresh tissue collection. Methamphetamine rapidly enters the brain following peripheral injection in human and nonhuman primates, yet is cleared slowly from the brain, and at 3 hours after injection the elimination curve is relatively shallow (Fowler et al., 2007; Fowler et al., 2008; Shiue et al., 1995; Volkow et al., 2010), which should make this a time at which useful data can be gathered at a single time point.
Methamphetamine assay
Methamphetamine and a metabolite, amphetamine, were extracted from samples, derivatized, separated by HPLC and quantified by fluorescence detection of derivatives. The sensitive method of (Nakashima et al., 2003) was used for derivatization and detection, but the extraction technique was modified to give a cleaner extract with an improved and more reproducible recovery, and the chromatography conditions were altered to optimize separation conditions for the column used. Samples were deproteinized with perchloric acid (final concentration 0.4M) containing internal standard (1-methyl-3-phenylpropylamine, a structural isomer of methamphetamine). Following centrifugation, pH of the supernatant was raised above 12 with NaOH, and amines extracted into 4 volumes of toluene by vortexing in a glass tube. Separation of the toluene extract was achieved by centrifugation of the mixture, followed insertion of the tube in an ethanol/dry ice slurry to freeze the aqueous phase rapidly, which allowed the organic layer to be poured off from the aqueous layer. Amines were back-extracted by vortexing the organic phase with 0.1 ml of 0.01M HCl. After centrifugation, layers were separated again by freezing the aqueous phase, but this time the organic layer was discarded, and the thawed acidified extract was dried under a stream of helium. Residue was reconstituted in 10 microliters 0.1M carbonate buffer (pH 10.6) and 180 microliters of the derivatizing reagent, 4-(4,5-diphenyl-1H-imidazole-2-yl)benzoyl chloride (DIB, 30 μM in acetonitrile). After 10 mins at room temperature, 10 microliters of ammonium hydroxide (25% w/w as NH3) was added to react with excess DIB. A portion of this mixture was separated by HPLC, using a mobile phase of 10 mM citrate buffer containing 60% acetonitrile (final pH 4.7) pumped at 0.8 ml/min through a C18 column (Pursuit XRs, 5 micron, 4.6 × 250 mm, Varian Inc, Walnut Creek, CA). Detection was achieved at excitation/emission wavelengths of 325 and 430 nm (Shimadzu RF-10AD). Detector response to the methamphetamine and amphetamine derivatives was linear over the range examined, and no interfering peaks were observed from plasma, CSF or brain samples. The ratio of peak heights of methamphetamine and amphetamine to peak height of internal standard was determined for each sample and referred to a standard curve in order to derive methamphetamine and amphetamine concentrations in samples. Data were analyzed using a 1-way ANOVA with Newman-Keuls multiple comparison test or 2-paired Student's t-test as appropriate.
Experiment 3: Effect of Methamphetamine on GDNF
Sample collection
GDNF levels were determined in striatal tissue from the same young and adult monkeys that were described above. Thus, in addition to samples from control animals, samples were analyzed from methamphetamine-treated young animals and adults. Some samples were taken 3 hours after a single methamphetamine injection (1 mg/kg i.m., animals used in the drug level study), and other striatal samples were taken 7 days after the repeated methamphetamine exposure protocol (day 1: 2 × 0.5 mg/kg i.m., day 2: 2 × 1 mg/kg i.m.), when DA levels are depleted in the affected groups.
GDNF assay
GDNF levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Promega, Cat #G7621, Madison, WI). Samples, along with GDNF standards, were reacted in a 96-well plate prepared with an immobilized monoclonal anti-GDNF antibody. After washing, an anti-human polyclonal GDNF antibody was used to capture the immobilized GDNF. The level of GDNF was visualized using a species-specific horseradish peroxidase tagged antibody and 3,3',5,5'-tetramethylbenzidine as a chromagen substrate; the intensity was quantified using a plate reader measuring absorbance at 450 nm. Concentration of GDNF was determined in each sample from the concurrently run standards and corrected for the amount of protein in each sample. The resulting data were analyzed using 2-way ANOVA (time after drug treatment × age) with Bonferroni post-hoc testing.
Results
Experiment 1: Age-dependent effect of methamphetamine on striatal TH-ir and DA concentration
Treatment with methamphetamine reduced TH-ir OD in all striatal sub-regions examined in adult male monkeys compared with controls (Figs. 1–2, F(1,18)=90.55, p<0.0001). This effect was significantly larger in the caudate nucleus (70% loss) and putamen (63%) compared with nucleus accumbens (40%) (F(2,18)=6.86, p=0.006). A similar effect of methamphetamine on striatal TH-ir OD was noted in mid-gestational fetal monkeys. Treatment in utero with methamphetamine resulted in a lower OD of TH-ir in the striatum compared with controls (51% loss) (Figs. 1–2, t=2.95, p=0.02). However, treatment with methamphetamine did not alter TH-ir OD in striatal sub-regions examined in young monkeys (Figs. 1–2, F(1,18)=1.08, p=0.31). As part of the stereological analysis the volume of the striatum in each animal is derived, and as expected, there was no effect of methamphetamine treatment on the volume of the striatal regions analyzed in any of the age groups (data not shown).
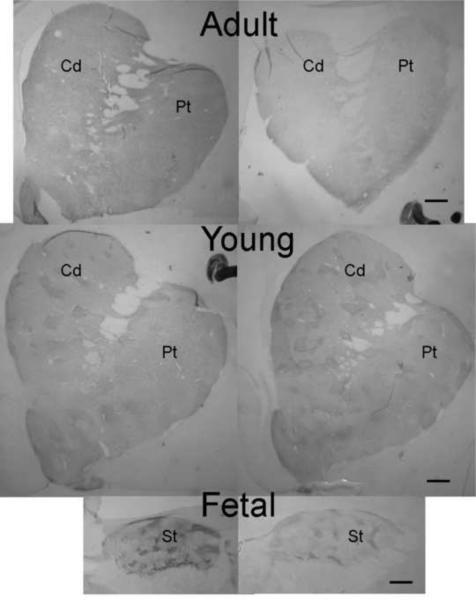
Figure 1.
Methamphetamine-induced loss of TH-ir in monkey striatum is age-dependent. Methamphetamine (METH) exposure reduces TH-ir OD in the striatum of adult monkeys (4 methamphetamine-treated, 4 controls) and mid-gestational fetuses (5 methamphetamine-treated, 3 controls), but not in young monkeys (5 methamphetamine-treated, 4 controls). Monkeys were given methamphetamine (Day1: 2 × 0.5 mg/kg; Day 2: 2 × 1 mg/kg) and the TH-ir OD was determined after 7–8 days. * indicates p<0.05 vs. same region in the same age control.
Figure 2.
Striatal TH-ir in control and methamphetamine-treated monkeys from each age group studied. Quantitative data are shown in Figure 1. Abbreviations: Cu, caudate nucleus; Pt, putamen; St, striatum.
Methamphetamine treatment selectively reduced striatal levels of DA in the adult, but not young, monkeys (Fig 3, F(1,20)=154, p<0.0001). There was a marked and significant depletion of DA concentration in both dorsal caudate nucleus (79% loss) and dorsal putamen (75% loss) of adults; these striatal subregions are most affected by methamphetamine in the monkey striatum (Harvey et al., 2000a). The dose of methamphetamine used here (total 3 mg/kg over 2 days) is slightly lower than that used by (Harvey et al., 2000a), who employed 2 injections of 2 mg/kg i.m. given 24 hours apart (total dose 4 mg/kg) in vervet monkeys, with sacrifice 7 days later. The loss of DA concentration in adult monkeys in present study was somewhat less than that found by (Harvey et al., 2000a), who reported 80–98% loss in caudate nucleus and 94–99% loss in putamen. The present study showed that in young monkeys, DA concentration in these striatal regions was unaffected by methamphetamine at the time examined.
Figure 3.
Methamphetamine induces a loss of striatal DA in adult, but not young, monkeys. Striatal DA concentration was significantly reduced in caudate nucleus and putamen of adult monkeys (3 methamphetamine-treated, 4 controls) but not in young monkeys (3 methamphetamine-treated, 6 controls) 7–8 days following methamphetamine treatment (Day1: 2 × 0.5 mg/kg; Day 2: 2 × 1 mg/kg). * indicates p<0.05 vs. same region in the same age control.
The ratio HVA/DA was sharply increased following methamphetamine treatment in adults (Fig. 4); which is thought to reflect compensatory increase in dopaminergic activity in a compromised population (Elsworth et al., 2000). No such increase in HVA/DA was observed in young monkeys treated with methamphetamine (Fig. 4).
Figure 4.
Methamphetamine induces an increase in striatal HVA/DA ratio in adult, but not young, monkeys. Striatal HVA/DA ratio was significantly increased in caudate nucleus and putamen of adult monkeys but not in young monkeys 7–8 days following methamphetamine treatment (Day1: 2 × 0.5 mg/kg; Day 2: 2 × 1 mg/kg). * indicates p<0.05 vs. same region in the same age control.
Experiment 2: Plasma, CSF and brain levels of methamphetamine
Three hours after a single dose of methamphetamine (1 mg/kg), levels of methamphetamine in the plasma of young monkeys were significantly elevated to about 3 times higher than that measured in adult male controls (Fig 5A). Additionally, CSF levels of methamphetamine from young animals were significantly elevated over the level in adult males (Fig. 5B, t=4.3, p=0.0025). Plasma methamphetamine levels were also higher in young animals than in midgestational fetuses and the pregnant monkeys (Fig. 5A, F(3,12)=71.2, p<0.0001). A close correspondence between methamphetamine levels in fetal and maternal plasma was noted (Fig 5C), as would be anticipated with unimpeded passage of methamphetamine across the placental barrier (Burchfield et al., 1991).
Figure 5.
Methamphetamine levels are higher in plasma and CSF of young monkeys than other ages studied at 3 hours following a 1 mg/kg injection. Young monkeys (n=5) had higher methamphetamine levels in plasma (panel A) and CSF (panel B) after the same dose of methamphetamine compared with adult (n=5) and mid-gestational (n=3) fetal monkeys. The mid-gestational fetus and the maternal monkey (n=3) had very similar levels of methamphetamine. * indicates p<0.05 vs. all other groups. Panel C: The methamphetamine levels within each pair of maternal and fetal monkeys were similar.
The striatal level of methamphetamine and amphetamine was measured in young and adult animals 3 hours after a single injection of 1 mg/kg i.m methamphetamine (Fig. 6). The concentration of methamphetamine in the young brain was significantly higher than in the adult monkey striatum at this time. Lower concentrations of amphetamine than methamphetamine were found in the striatum of both age groups, yet the amphetamine was significantly higher in adults than young animals (Fig. 6).
Figure 6.
Methamphetamine levels are significantly higher in striatum of young monkeys than in adults studied at 3 hours following a single 1 mg/kg intramuscular injection. However, amphetamine levels were significantly higher in striatum of adults compared with young animals. * indicates p< 0.05, and ** indicates p < 0.01.
Experiment 3: Methamphetamine-induced changes in GDNF in young monkeys
Levels of GDNF in the striatum of the young and the adult male monkeys were similar under control conditions as well as 3 hours after exposure to a single 1 mg/kg dose of methamphetamine. In contrast, young, but not adult male monkeys, showed elevated levels of GDNF concentration at 7 days after the start of methamphetamine treatment (day 1: 2 × 0.5 mg/kg, day 2: 2 × 1 mg/kg), as shown in Fig. 7 (F(2,16)=18.2, p<0.0001).
Figure 7.
Striatal GDNF levels are increased in young, but not adult, monkeys 1-week following methamphetamine. Methamphetamine (Day1: 2 × 0.5 mg/kg; Day 2: 2 × 1 mg/kg) induced a delayed increase in GDNF in young animals (3 methamphetamine-treated, 3 controls), but not in adult males (3 methamphetamine-treated, 6 controls), monkeys. No increase was seen 3 hours after injection in young animals (n=4) or adults (n=3). * indicates p<0.05 vs. adult control at the same time point.
Discussion
The results from these studies indicate that during development, primate nigrostriatal DA neurons have differing sensitivity to methamphetamine, as indicated by a loss of striatal TH immunoreactivity and DA concentration, which is believed to be instigated by oxidative stress. Specifically, mid-gestational and mature nigrostriatal DA neurons both displayed marked vulnerability to methamphetamine, yet early in life these neurons were resistant to damage. The protection of young DA neurons from methamphetamine-induced effects cannot readily be explained by different drug levels, as the level of methamphetamine appeared higher in the young animals than the other ages following the same mg/kg dose. One factor that may explain or contribute to the apparent resistance of young nigrostriatal DA neurons to methamphetamine is the delayed upregulation of the neurotrophic factor, GDNF, that was measured in the young, but not adult, striatum 1-week following methamphetamine administration.
The only other developmental studies of the effects of methamphetamine on the central DA system have been performed in rodents. Wagner et al. (1981) treated rats twice a day with 50 mg/kg/day for a relatively long period of rat development (30 days), from a pre-weaning age (P10) until P40, when rats have reached sexual maturity. Two weeks after the end of treatment, a 31% decrease in striatal DA concentration was measured. When the same group of investigators (Wagner et al., 1980) treated adult rats with the same regimen, a 56% depletion was found, suggestive of a somewhat greater resistance to methamphetamine-induced DA toxicity in the pre-adult stage of life in rodents. Although rodent studies provide important information on methamphetamine-induced effects and factors modulating brain development, the use of monkeys provides a model especially relevant to man. Specifically, the anatomy, biochemistry and pharmacology of primate nigrostriatal DA neurons differ in several aspects from those in the rodent, including evidence that primate DA neurons exhibit greater susceptibility to oxidative stress and to methamphetamine (Chiueh et al., 1984; Kita et al., 2003; Lewis and Sesack, 1997; McCann and Ricaurte, 2004; Ricaurte and McCann, 1992; Williams and Goldman-Rakic, 1998). In addition, rodents and primates have differing rates of brain maturity with respect to growth of individual brain regions and with respect to the timing of birth (Bayer et al., 1993; Clancy et al., 2001; Clancy et al., 2007; Morrow et al., 2007; Wood et al., 2003).
The only other study of the effect of prenatal methamphetamine exposure on the striatal DA system was performed in the rat and reported a significant decrease in DA uptake sites in the striatum in adults that had been exposed throughout gestation to methamphetamine injected at 2 or 10 mg/kg twice daily in the dam (Weissman and Caldecott-Hazard, 1993). The current study indicates that mid-gestation might be a susceptible time for DA neurons to be exposed to oxidative stressors. This time coincides with the peak of natural apoptotic cell death in primate DA neurons (Morrow et al., 2007), and so it is possible that this event, when the initial population of DA neurons is being reduced, marks a period when the neurons are especially susceptible to damage. Thus, this study highlights the potential risk of exposure to drugs of abuse, dopaminergic toxins or oxidative stress during development, which may provoke detrimental effects on the development of both DA and non-DA systems (Herlenius and Lagercrantz, 2004) or, as has been suggested, enhance the likelihood of succumbing to DA-related diseases later in life, including Parkinson's disease (Boger et al., 2010; Carvey et al., 2006; Lloyd et al., 2006). Further studies will be required to ascertain whether DA neurons impacted during development can recover over time.
In stark contrast to the sensitivity of prenatal DA neurons to methamphetamine, was our finding that young monkeys are very resistant to the damaging effect of the drug on the striatal DA system, despite appearing to harbor higher drug concentrations than in the susceptible adult brain. While a complete pharmacokinetic profile, involving many animals, would be necessary to be certain of the exact relative brain exposure of the 2 age groups to methamphetamine following the same mg/kg dose, the present data strongly suggest that the lack of response of young DA neurons to methamphetamine is not simply related to a lower exposure of the brain to the drug. The higher levels of methamphetamine measured at the single 3-hour time point might be explained by the markedly lower capacity of the primate liver at young ages to metabolize and conjugate drugs and xenobiotics (Besunder et al., 1988; Pineiro-Carrero and Pineiro, 2004); the observation of a higher metabolite (amphetamine) concentration in the adult brain compared with the young brain is consistent with this interpretation. Amphetamine itself has detrimental impact on DA neurons, but this is generally thought to be less severe than that exerted by methamphetamine (Goodwin et al., 2009; NIDA, 2005).
There are several possible mechanisms that might account for this present observation that young DA neurons appear resistant to methamphetamine-induced effects. One mechanism for this protection could be an increased production of glial derived neurotrophic factor (GDNF), which is a potent neurotrophic factor that is crucial to the development, survival, and outgrowth of DA neurons, and its actions in the CNS are rather selective for DA neurons (Lapchak et al., 1996; Lin et al., 1993). Several pieces of data suggested that activation of GDNF expression in younger monkeys might provide at least a partial explanation for their resistance to methamphetamine-induced DA neuron damage. For example, administration of GDNF in the striatum has been shown to protect and reverse the nigrostriatal DA system against methamphetamine-induced neurotoxicity in the rodent and primate (Cass and Manning, 1999; Cass et al., 2000; Cass et al., 2006; Melega et al., 2000). In addition, a 6-hydroxydopamine lesion of rat nigrostriatal DA neurons provokes an increase in striatal trophic factor expression, of which GDNF appears to be the major component (Nakajima et al., 2001; Yurek and Fletcher-Turner, 2001). Furthermore, mice with partial GDNF gene deletion demonstrate aggravated dopaminergic toxicity and emergence of greater age-associated motor deficits compared with wild type mice (Boger et al., 2007). Furthermore, aged rodents display a blunted striatal GDNF in response to a 6-hydroxydopamine lesion of nigrostriatal DA neurons (Yurek and Fletcher-Turner, 2001). Finally, there is evidence for GDNF depletion within both surviving neurons and neuropil of the substantia nigra pars compacta in Parkinson's disease (Chauhan et al., 2001), but see Mogi et al. (2001). These present data provide evidence that methamphetamine induced a GDNF response in the young but not adult striatum, and that this GDNF response that may, at least in part, be sufficient to counteract the dopaminergic toxicity of methamphetamine in the younger animals. It is recognized, though, that a further study would have to be undertaken in order to conclude conclusively that a GDNF response did not occur in adults at a time-point not examined in this study. The apparent inability of older primates to raise GDNF production in response to loss of nigrostriatal DA neurons may conceivably be linked to the gradual age-related loss of these neurons, which in man, precipitates signs of Parkinson's disease when the loss exceeds a threshold level (Calne and Langston, 1983).
These data lend support to attempts to elevate GDNF production by various methods in the nigrostriatal DA pathway in Parkinson's disease (Bespalov and Saarma, 2007; Saavedra et al., 2008).
Conclusions
Several novel observations are reported here. Nigrostriatal DA neurons from fetal and adult monkeys are sensitive to the DA-depleting effects of methamphetamine, which previous evidence indicates is exerted by oxidative stress. On the other hand, DA neurons from young animals are resistant to methamphetamine effects on nigrostriatal DA terminals, despite evidence that striatal levels of the drug were higher than in adults. Data was obtained to suggest that elevated striatal GDNF concentration may contribute to the resistance of DA neurons from young animals to methamphetamine toxicity. These data also lend some support to the hypothesis that oxidative stress during pregnancy can compromise the population of nigrostriatal DA neurons and potentially elevate the risk of DA deficiency disorders later in life, such as Parkinson's disease.
Highlights
-
>
Impact of methamphetamine on primate dopamine neurons during development is examined
-
>
At mid-gestation and as adults, dopamine neurons are sensitive to methamphetamine
-
>
In the young monkey, dopamine neurons are resistant to methamphetamine
-
>
3 hours after a dose of methamphetamine, drug levels are highest in young monkeys
-
>
GDNF production is stimulated by methamphetamine in young, but not adult, monkeys
Acknowledgments
This work was supported by grant NS056181 from NINDS. We thank Feng-Pei Chen and Dorothy Cameron for their excellent technical work, and the following members of the St Kitts Biomedical Research Foundation staff for their invaluable assistance in animal treatments and excellent care of the animals: Dr Rodrigo Valles, Alexis Nisbett, Dr. Milton C. Whittaker, Ernell Nisbett, Clive Wilson, and David Charles.
Abbreviations
- DA
dopamine
- ED
embryonic day
- GDNF
glial-derived neurotrophic factor
- HVA
homovanillic acid
- OD
optical density
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacopoulos NG, Maas JW, Hattox SE, Roth RH. Regional distribution of dopamine metabolites in human and primate brain. Commun Psychopharmacol. 1978;2:281–286. [PubMed] [Google Scholar]
- Barlow BK, Cory-Slechta DA, Richfield EK, Thiruchelvam M. The gestational environment and Parkinson's disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol. 2007;23:457–470. doi: 10.1016/j.reprotox.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci. 2007;28:68–74. doi: 10.1016/j.tips.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Besunder JB, Reed MD, Blumer JL. Principles of drug biodisposition in the neonate. A critical evaluation of the pharmacokinetic-pharmacodynamic interface (Part I) Clin Pharmacokinet. 1988;14:189–216. doi: 10.2165/00003088-198814040-00001. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J Neurosci. 2007;27:8816–8825. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, Granholm AC, McGinty JF, Middaugh LD. A dual-hit animal model for age-related parkinsonism. Prog Neurobiol. 2010;90:217–229. doi: 10.1016/j.pneurobio.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. JAMA. 1991;265:1968–1973. [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson's disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- Calne DB, Langston JW. Aetiology of Parkinson's disease. Lancet. 1983;ii(322):1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Punati A, Newman MB. Progressive dopamine neuron loss in Parkinson's disease: the multiple hit hypothesis. Cell Transplant. 2006;15:239–250. doi: 10.3727/000000006783981990. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Manning MW, Bailey SL. Restorative effects of GDNF on striatal dopamine release in rats treated with neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2000;914:127–136. doi: 10.1111/j.1749-6632.2000.tb05190.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Peters LE, Harned ME, Seroogy KB. Protection by GDNF and other trophic factors against the dopamine-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:272–281. doi: 10.1196/annals.1369.024. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ, Lee JM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson's disease brain. J Chem Neuroanat. 2001;21:277–288. doi: 10.1016/s0891-0618(01)00115-6. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Markey SP, Burns RS, Johannessen JN, Jacobowitz DM, Kopin IJ. Neurochemical and behavioral effects of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) in rat, guinea pig, and monkey. Psychopharmacol Bull. 1984;20:548–553. [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte DA. The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Sladek JR, Jr., Collier TJ, Redmond DE, Jr., Roth RH. Striatal dopaminergic correlates of stable parkinsonism and degree of recovery in old-world primates one year after MPTP treatment. Neuroscience. 2000;95:399–408. doi: 10.1016/s0306-4522(99)00437-6. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Kroll C, Ferrieri R, Alexoff D, Logan J, Dewey SL, Schiffer W, Schlyer D, Carter P, King P, Shea C, Xu Y, Muench L, Benveniste H, Vaska P, Volkow ND. PET studies of d-methamphetamine pharmacokinetics in primates: comparison with lmethamphetamine and ( --)-cocaine. J Nucl Med. 2007;48:1724–1732. doi: 10.2967/jnumed.107.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Alexoff D, Telang F, Wang GJ, Wong C, Ma Y, Kriplani A, Pradhan K, Schlyer D, Jayne M, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog K. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. Neuroimage. 2008;43:756–763. doi: 10.1016/j.neuroimage.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood ER, Bekele W, McCulloch CE, Christine CW. Amphetamine exposure is elevated in Parkinson's disease. Neurotoxicology. 2006;27:1003–1006. doi: 10.1016/j.neuro.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Melega WP. Regional heterogeneity of dopaminergic deficits in vervet monkey striatum and substantia nigra after methamphetamine exposure. Exp Brain Res. 2000a;133:349–358. doi: 10.1007/s002210000386. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000b;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of disease and dysfunction. Reprod Toxicol. 2007;23:257–259. doi: 10.1016/j.reprotox.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190(Suppl 1):S8–21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. Springer-Verlag; New York: 1998. [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36-28. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Neurotransmitters and vulnerability of the developing brain. Brain Dev. 1995;17:301–306. doi: 10.1016/0387-7604(95)00079-q. [DOI] [PubMed] [Google Scholar]
- Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- Kita T, Miyazaki I, Asanuma M, Takeshima M, Wagner GC. Dopamine-induced behavioral changes and oxidative stress in methamphetamine-induced neurotoxicity. Int Rev Neurobiol. 2009;88:43–64. doi: 10.1016/S0074-7742(09)88003-3. [DOI] [PubMed] [Google Scholar]
- Langston JW. Epidemiology versus genetics in Parkinson's disease: progress in resolving an age-old debate. Ann Neurol. 1998;44:S45–52. doi: 10.1002/ana.410440707. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Jiao S, Miller PJ, Williams LR, Cummins V, Inouye G, Matheson CR, Yan Q. Pharmacological characterization of glial cell line-derived neurotrophic factor (GDNF): implications for GDNF as a therapeutic molecule for treating neurodegenerative diseases. Cell Tissue Res. 1996;286:179–189. doi: 10.1007/s004410050687. [DOI] [PubMed] [Google Scholar]
- Lewis D, Sesack S. Dopamine Systems in the Primate Brain. In: Bloom FE, Bjorklund A, Hokfelt T, editors. The Primate Nervous System. vol. 13. Elsevier; Amsterdam: 1997. pp. 263–375. [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lloyd SA, Faherty CJ, Smeyne RJ. Adult and in utero exposure to cocaine alters sensitivity to the Parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 2006;137:905–913. doi: 10.1016/j.neuroscience.2005.09.035. [DOI] [PubMed] [Google Scholar]
- McCann UD, Ricaurte GA. Amphetamine neurotoxicity: accomplishments and remaining challenges. Neurosci Biobehav Rev. 2004;27:821–826. doi: 10.1016/j.neubiorev.2003.11.003. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME. Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res. 1997;766:113–120. doi: 10.1016/s0006-8993(97)00548-9. [DOI] [PubMed] [Google Scholar]
- Melega WP, Lacan G, Desalles AA, Phelps ME. Long-term methamphetamine-induced decreases of [(11)C]WIN 35,428 binding in striatum are reduced by GDNF: PET studies in the vervet monkey. Synapse. 2000;35:243–249. doi: 10.1002/(SICI)1098-2396(20000315)35:4<243::AID-SYN1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Kogure O, Kuno S, Ichinose H, Nagatsu T. Glial cell line-derived neurotrophic factor in the substantia nigra from control and parkinsonian brains. Neurosci Lett. 2001;300:179–181. doi: 10.1016/s0304-3940(01)01577-4. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Redmond DE, Jr., Roth RH, Elsworth JD. Development of A9/A10 dopamine neurons during the second and third trimesters in the African green monkey. J Comp Neurol. 2005;488:215–223. doi: 10.1002/cne.20599. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Redmond DE, Jr., Sladek JR, Jr., Elsworth JD. Apoptotic natural cell death in developing primate dopamine midbrain neurons occurs during a restricted period in the second trimester of gestation. Exp Neurol. 2007;204:802–807. doi: 10.1016/j.expneurol.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Hida H, Shimano Y, Fujimoto I, Hashitani T, Kumazaki M, Sakurai T, Nishino H. GDNF is a major component of trophic activity in DA-depleted striatum for survival and neurite extension of DAergic neurons. Brain Res. 2001;916:76–84. doi: 10.1016/s0006-8993(01)02866-9. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Kaddoumi A, Ishida Y, Itoh T, Taki K. Determination of methamphetamine and amphetamine in abusers' plasma and hair samples with HPLC-FL. Biomed Chromatogr. 2003;17:471–476. doi: 10.1002/bmc.278. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program NTP-CERHR monograph on the potential human reproductive and developmental effects of amphetamines. NTP-CERHR MON. 2005;16:vii–III1. [PubMed] [Google Scholar]
- NIDA Medications Development Research for Treatment of Amphetamine and Methamphetamine Addiction. 2005 http://archives.drugabuse.gov/about/legislation/MethReport/Introduction.html.
- Pineiro-Carrero VM, Pineiro EO. Liver. Pediatrics. 2004;113:1097–1106. [PubMed] [Google Scholar]
- Reuhl KR. Delayed expression of neurotoxicity: the problem of silent damage. Neurotoxicology. 1991;12:341–346. [PubMed] [Google Scholar]
- Ricaurte GA, McCann UD. Neurotoxic amphetamine analogues: effects in monkeys and implications for humans. Ann N Y Acad Sci. 1992;648:371–382. doi: 10.1111/j.1749-6632.1992.tb24586.x. [DOI] [PubMed] [Google Scholar]
- Richards JB, Baggott MJ, Sabol KE, Seiden LS. A high-dose methamphetamine regimen results in long-lasting deficits on performance of a reaction-time task. Brain Res. 1993;627:254–260. doi: 10.1016/0006-8993(93)90328-k. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. Aaps J. 2006;8:E413–418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G, Duarte EP. Driving GDNF expression: the green and the red traffic lights. Prog Neurobiol. 2008;86:186–215. doi: 10.1016/j.pneurobio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Shiue CY, Shiue GG, Cornish KG, O'Rourke MF. Comparative PET studies of the distribution of (−)-3,4-methylenedioxy-N-[11C]methamphetamine and (−)-[11C]methamphetamine in a monkey brain. Nucl Med Biol. 1995;22:321–324. doi: 10.1016/0969-8051(94)00104-r. [DOI] [PubMed] [Google Scholar]
- Sladek JR, Jr., Blanchard B, Collier TJ, Elsworth JD, Taylor JR, Roth RH, Redmond DE., Jr. Development of mesencephalic dopamine neurons in the nonhuman primate relationship to survival and growth. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 269–282. [Google Scholar]
- Thrash B, Thiruchelvan K, Ahuja M, Suppiramaniam V, Dhanasekaran M. Methamphetamine-induced neurotoxicity: the road to Parkinson's disease. Pharmacol Rep. 2009;61:966–977. doi: 10.1016/s1734-1140(09)70158-6. [DOI] [PubMed] [Google Scholar]
- Tretter L, Sipos I, Adam-Vizi V. Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson's disease. Neurochem Res. 2004;29:569–577. doi: 10.1023/b:nere.0000014827.94562.4b. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Ment LR. Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed. 2004;89:F190–192. doi: 10.1136/adc.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, Thanos PK, Alexoff D. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PLoS One. 2010;5:e15269. doi: 10.1371/journal.pone.0015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Schuster CR, Seiden LS. Neurochemical consequences following administration of CNS stimulants to the neonatal rat. Pharmacol Biochem Behav. 1981;14:117–119. doi: 10.1016/0091-3057(81)90113-1. [DOI] [PubMed] [Google Scholar]
- Weissman AD, Caldecott-Hazard S. In utero methamphetamine effects: I. Behavior and monoamine uptake sites in adult offspring. Synapse. 1993;13:241–250. doi: 10.1002/syn.890130307. [DOI] [PubMed] [Google Scholar]
- Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJ, Perstin J, Preston TJ, Wiley MJ, Wong AW. Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wood SL, Beyer BK, Cappon GD. Species comparison of postnatal CNS development: functional measures. Birth Defects Res B Dev Reprod Toxicol. 2003;68:391–407. doi: 10.1002/bdrb.10037. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkinson's disease. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson's disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]