Abstract
Chronic inflammation is an underlying factor linking obesity with insulin resistance. Diet-induced obesity promotes an increase in circulating levels of inflammatory monocytes and their infiltration into expanding adipose tissue. Nevertheless, the endogenous pathways that trigger and sustain chronic low-grade inflammation in obesity are incompletely understood. Here, we report that a high-fat diet selectively increases the circulating levels of CD11b+ monocytes in wild-type mice that express leukotriene B4 receptor, BLT-1, and that this increase is abolished in BLT-1-null mice. The accumulation of classically activated (M1) adipose tissue macrophages (ATMs) and the expression of pro-inflammatory cytokines and chemokines (i.e. IL-6 and Ccl2) was largely blunted in adipose tissue of obese BLT-1-/- mice, while the ratio of alternatively activated (M2) ATMs to M1 ATMs was increased. Obese BLT-1-/- mice were protected from systemic glucose and insulin intolerance and this was associated with a decrease in inflammation in adipose tissue and liver and a decrease in hepatic triglyceride accumulation. Deletion of BLT-1 prevented high-fat induced loss of insulin signaling in liver and skeletal muscle. These observations elucidate a novel role of chemoattractant receptor, BLT-1, in promoting monocyte trafficking to adipose tissue and promoting chronic inflammation in obesity and could lead to the identification of new therapeutic targets for treating insulin resistance in obesity.
Introduction
Obesity is an emerging global epidemic and is one of the most prominent risk factors for the development of type 2 diabetes (T2D) (1, 2). Extensive studies show that insulin resistance in obesity is induced and sustained by chronic low-grade inflammation (3, 4). Indeed, T2D is associated with increased levels of inflammatory mediators that induce insulin resistance and adipose tissue in obese and diabetic individuals remains in a state of chronic, unresolved inflammation (4, 5). Greater than 40% of the total adipose tissue cell content from obese rodents and humans is composed of macrophages, compared with 10% in lean counterparts and inflammatory adipose tissue macrophages (ATMs) directly promote systemic insulin resistance (4, 6, 7). Nevertheless, the mechanisms that contribute and sustain chronic inflammation in obesity and diabetes remain poorly understood.
Leukotriene B4 (LTB4) is a pro-inflammatory lipid mediator generated from arachidonic acid through the sequential activities of 5-lipoxygenase (5-LOX), 5-lipoxygenase activating protein (FLAP) and leukotriene A4 hydrolase (LTA4H) (8, 9). LTB4 is rapidly generated by activated leukocytes and has well characterized biological actions, including the promotion of leukocyte chemotaxis and the regulation of pro-inflammatory cytokines (8, 10). The potent biological actions of LTB4 are mediated primarily through a high-affinity interaction with a G-protein coupled receptor termed BLT-1 (10). While the LTB4:BLT-1 axis plays an important role in host defense during acute infection, chronic activation of this pathway contributes to persistent inflammation characteristic of inflammatory pathologies including atherosclerosis and arthritis (11-16).
Recent studies show that the expression and activity of enzymes required for leukotriene biosynthesis, including 5-LOX and FLAP, are increased in both the liver and adipose tissue in murine models of experimental obesity (16-18). Moreover, LTB4 levels increase in adipose tissue of both mice and rats consuming a high-fat diet (16, 17, 19). However, a causal role for LTB4 in promoting or sustaining chronic inflammation and insulin resistance in obesity has not been established. Here, we report that deficiency of BLT-1 protects against the development of insulin resistance in diet-induced obesity (DIO) by regulating ATM accumulation and inflammation in insulin-sensitive tissues.
Materials and Methods
Animals and treatment
Male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and BLT-1-/- mice (Ltb4r1tm1Bodd) were generated as described (12) and backcrossed for 9 generations on a C57BL/6J background as reported before (13). At 8-11 weeks of age, mice were placed on either a 10% (kcal from fat) low fat diet or 60% high fat diet (Research Diets) and maintained for 12 additional weeks. Body weight was recorded weekly. Glucose and insulin tolerance tests were performed during the 12th week of feeding, as outlined in Figure 1A, while all other parameters were evaluated upon euthanasia. All procedures were approved by University of Louisville IACUC.
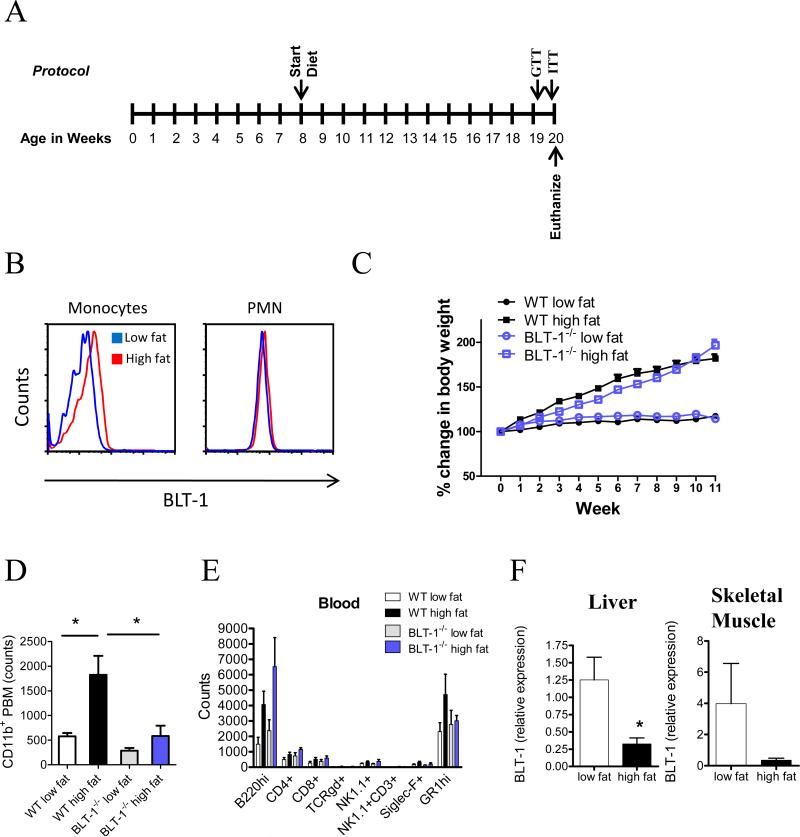
Figure 1. Expression of BLT-1 regulates peripheral blood monocyte expansion in DIO.
(A) Feeding protocol. Starting at 8 weeks of age, WT and BLT-1-deficient mice were fed a low fat (10%) or high fat (60%) diet for 12 weeks. Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were performed during the 12th week of the feeding protocol. (B) Surface expression of BLT-1 on peripheral blood monocytes and neutrophils (PMN) in WT mice fed a low fat or high fat diet (n=5/group). (C) Weight gain of WT and BLT-1-deficient mice on a high fat diet for 12 weeks (n=8-12/group). (D) Peripheral blood monocytes in WT and BLT-1-deficient mice (n=5/group). (E) Whole blood leukocyte differentials from WT and BLT-1-deficient mice (n=5/group). (F) BLT-1 mRNA expression in liver and skeletal muscle (n=6/group). Data are presented as mean ± SEM; *P<0.05 by Student's t-test (F), one-way (D) or two-way ANOVA (C).
Glucose/Insulin tolerance tests
Glucose tolerance tests were performed following a 6 hour fast by i.p. injection of D-glucose (1 mg/g) in sterile saline. Insulin tolerance tests were performed on non-fasted animals by i.p. injection of 1.5 U/kg Humulin-R (Eli Lilly, Indianapolis, IN). Blood samples were obtained from the tail and glucose levels were measured at indicated time points using an Aviva Accu-Chek glucometer. The HOMA-IR score was calculated based on the formula: glucose (mmol) x insulin (mU/ml)/22.5.
Immunoblotting and PCR
Tissue lysates were prepared as described in (17). Equal amounts of protein were separated by SDS-PAGE, transblotted, and probed for phospho-Akt (Ser473), total Akt, phospho-JNK (Thr183/Tyr185), total JNK, phospho-IκBα and total IκBα (Cell Signaling). Western blots were developed using ECL-plus followed by luminescence detection using a Typhoon 9400 variable mode imager (Amersham Biosciences). Quantification of band intensities was performed using Image Quant TL software.
For quantitative RT-PCR, RNA was extracted from tissues using the RNeasy Lipid Tissue Kit (Qiagen), followed by cDNA synthesis. Real-time PCR amplification was performed with SYBR-Green qPCR Master Mix (SA Biosciences) using a 7900HT Fast Real-Time PCR System (Applied Biosystems) and commercially available primers for Emr-1, IL-10, IL-6, PPAR-γ and PPAR-α (Super Array). The following primers were also used (Integrated DNA technologies): Ccl2: F: 5'-ATGCAGGTCCCTGTCATG-3', R: 5'-GCTTGAGGTGGTTGTGGA-3', Blt-1: F: 5’-TCC CTT TTT CCT CCA CTT TC 3’, R: 5’ GAA AAG ACA CCA CCC AGA TG 3’,Ym-1: F: 5’-GGGCATACCTTTATCCTGAG-3’, R: 5’-CCACTGAAGTCATCCATGTC-3’, Arg-1: F: 5’-ATGGAAGAGACCTTCAGCTAC-3 ' , R : 5 ’-GCTGTCTTCCCAAGAGTTGGG-3’ (20). Relative expression was determined by the 2-ΔΔCT method after internal normalization to hprt or β-actin
Immunohistochemistry and quantification of crown-like structures
Haematoxylin and Eosin (H&E) staining was performed following standard procedures on formalin-fixed paraffin-embedded epididymal adipose tissue and liver concurrent sections. CLS were identified as adipocytes completely surrounded by infiltrating cells and were quantified in 5 random fields per animal. Oil red O staining was performed on OCT-embedded liver sections as described in (17).
Biochemical analyses
Total plasma cholesterol, HDL and LDL, triglycerides, total protein, albumin (Cholesterol CII Enzymatic Kit; L-Type TG-H Kit; Bradford reagent, bromocresol green, Wako, Richmond, VA), alanine aminotransferase (ALT) and aspartate aminotransferase (AST; Infinity, ThermoElectron), levels were measured using commercially available assay reagents as indicated. The total hepatic triglyceride content was determined after chloroform/methanol extraction of frozen liver samples. Assays were performed using a Cobas Mira Plus 5600 Autoanalyzer (Roche, Indianapolis, IN). Plasma insulin was measured by ELISA (Mercodia).
Flow cytometry analysis
Analysis of cells in spleen and peripheral blood was carried out as described in (21) with minor modifications. Cells from spleen were isolated and filtered, while 30 μL of peripheral blood was used for analysis. Cells were stained with anti-mouse CD16/32 (BD Pharmingen, San Diego, CA) in 1% FBS in PBS. Biotinylated anti-mouse BLT1 (3D7) was then used for assessment of BLT1 expression. Cells were subsequently washed and stained. Antibodies used include (PE)-conjugated anti-B220, (PerCP-Cy5.5)-conjugated anti-CD8, (PE)-conjugated anti-NK1.1, (FITC)-conjugated anti-CD3 molecular complex, (PE)-conjugated anti-Siglec-F, (PerCP-Cy5.5)-conjugated anti-CD11b (BD Pharmingen, San Diego, CA), (APC)-conjugated anti-CD4, (FITC)-conjugated anti-GR (eBioscience, Inc., San Diego, CA), (APC)-conjugated streptavidin and (PE)-conjugated anti-gdTCR (Biolegend, San Diego, CA). Flow cytometry was carried out using a BD FACS Calibur flow cytometer equipped with Cell Quest Pro software and analyzed with FlowJo V.4.3 software.
Adipose tissue stromal vascular cells (SVC) were isolated as in (22). SVC pellets were incubated with Fc Block prior to staining with fluorescent-conjugated primary antibodies or isotype controls. Antibodies used included AlexaFluor 647 anti-Mgl-1 (CD301a; AbD Serotec), FITC anti-CD11c (BD Biosciences) and PE anti-F4/80 (Biolegends). Flow cytometry was carried out using a BD LSRII Flow Cytometer equipped with FACSDiva v.6.0. Analysis was performed with FlowJo V.7.6 software.
Statistical analysis
Data are expressed as mean ± s.e.m. Multiple group comparisons were made using one-way or two-way ANOVA, followed by Bonferroni post-tests. Direct comparisons were made using an unpaired Student's t-test. A P-value of <0.05 was considered significant.
Results
BLT-1 regulates peripheral blood monocyte expansion in obesity
High-fat feeding increases circulating levels of peripheral blood monocytes that infiltrate fat depots and other dysfunctional tissues (6, 23, 24). Because LTB4 increases in adipose tissue in obesity (16, 17, 19), we asked whether activation of BLT-1 regulates monocyte recruitment in obesity. In mice fed a high fat diet for 12 weeks (see Protocol in Fig. 1A), BLT-1 expression increased on CD11b+ circulating monocytes (Fig. 1B). Importantly, this increase was selective for monocytes, as BLT-1 expression on neutrophils (PMN) did not change with high fat feeding. To determine whether BLT-1 is required for monocyte expansion in obesity, mice deficient in BLT-1 were placed on either a low fat or high fat diet. Interestingly, BLT-1-deficient mice gained weight to a similar extent as their wild-type (WT) counterparts when fed a high fat diet (Fig. 1C). It should be noted that the BLT-1-deficient mice weighed less than their WT counterparts at the initiation of the study (23.59 ± 0.37 g vs. 26.76 ± 0.41 g, respectively) and thus the BLT-1-deficient mice weighed less than the WT mice on a high fat diet throughout the study protocol (Fig. S1A). However, at the end of the feeding period, there were no significant differences in body weight between WT and BLT-1-deficient mice, indicating that the BLT-1-deficient mice had accelerated weight gain toward the end of the study protocol (Fig. S1B). Consistent with previous reports, high fat feeding led to an increase in the number of circulating CD11b+ monocytes in WT mice (Fig. 1D) (23). Significantly, this increase was completely abolished in BLT-1-deficient mice (Fig. 1D). Notably, this regulation was specific to monocytes, as other peripheral blood leukocyte populations, including GR-1+ PMN, B-cells, NK cells, and both CD4+ and CD8+ T-cells were not affected by either high-fat feeding or BLT-1-deficiency (Fig. 1E). Moreover, leukocyte populations in the spleen were also unaffected by high fat feeding or BLT-1 deficiency (Fig. S1C).
We next evaluated changes in the expression of BLT-1 in liver, skeletal muscle and adipose tissue of lean and obese mice. As shown in Fig. 1F, BLT-1 was expressed in the liver and skeletal muscle of lean mice, although the expression of BLT-1 in adipose tissue was not evident. In mice fed a high fat diet, a decrease in the expression of BLT-1 was observed in both liver and skeletal muscle.
Deficiency of BLT-1 improves glucose and insulin-tolerance in DIO
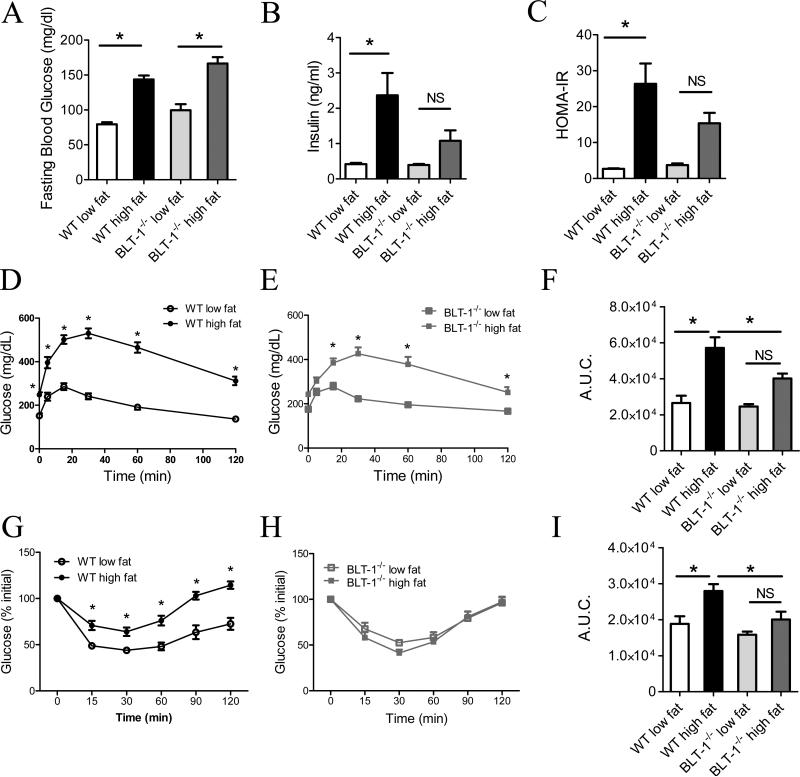
We next evaluated whether BLT-1-deficiency modulates metabolic derangements associated DIO. High fat feeding markedly increased fasting plasma glucose levels in WT mice (Fig. 2A). Similar to the effects of BLT-1 deficiency on weight gain, no differences in plasma glucose was observed with high fat feeding compared to WT mice. DIO increased plasma insulin in WT mice, whereas insulin was not significantly elevated in BLT-1-/- mice (Fig. 2B). Importantly, BLT-1-/- mice were less insulin resistant than obese WT mice, as determined by the homeostasis model assessment of insulin resistance (HOMA-IR; Fig. 2C). To evaluate how BLT-1 deficiency affects glucose homeostasis, we performed glucose and insulin tolerance tests in WT and BLT-1-/- mice. DIO induced pronounced glucose intolerance in WT mice (Fig. 2D). Similarly, obese BLT-1-/- mice were glucose intolerant (Fig. 2E), although area under the curve (A.U.C.) measurements indicated that the magnitude of glucose intolerance was less in the BLT-1-/- mice than their WT counterparts (Fig. 2F). Importantly, high fat feeding decreased insulin-stimulated glucose disposal in WT mice, while BLT-1-/- mice were completely protected from this insulin intolerance (Fig. 2G, H & I). Deficiency of BLT-1 did not modulate changes in total plasma cholesterol, HDL, LDL, plasma triglycerides or total protein levels in DIO (Fig. S2). Modest changes in the heart:body weight ratios were observed between obese WT and BLT-1-/- mice (Fig. S2). Serum creatinine and lactate dehydrogenase levels were not significantly increased with high fat feeding in WT mice (Fig. S2). Taken together, these results indicate that deficiency of BLT-1 protects from glucose and insulin intolerance in obesity.
Figure 2. Deficiency of BLT-1 protects against systemic insulin resistance in DIO.
(A) Fasting blood glucose. (B) Fasting serum insulin levels. (C). Calculated HOMA-IR score. (D, E) Glucose tolerance tests. (F) Area under the curve (A.U.C.) measurement of glucose tolerance tests in D & E. (G, H) Insulin tolerance tests. (I) A.U.C. measurement of insulin tolerances tests in G & H. NS=not significant. Data are presented as mean ± SEM; *P<0.05 by one-way (A, B, C, F & I) or two-way (D, E, G & H) ANOVA, n=8-12/group.
Adipose tissue macrophage accumulation and inflammation are decreased in obese BLT-1-/- mice
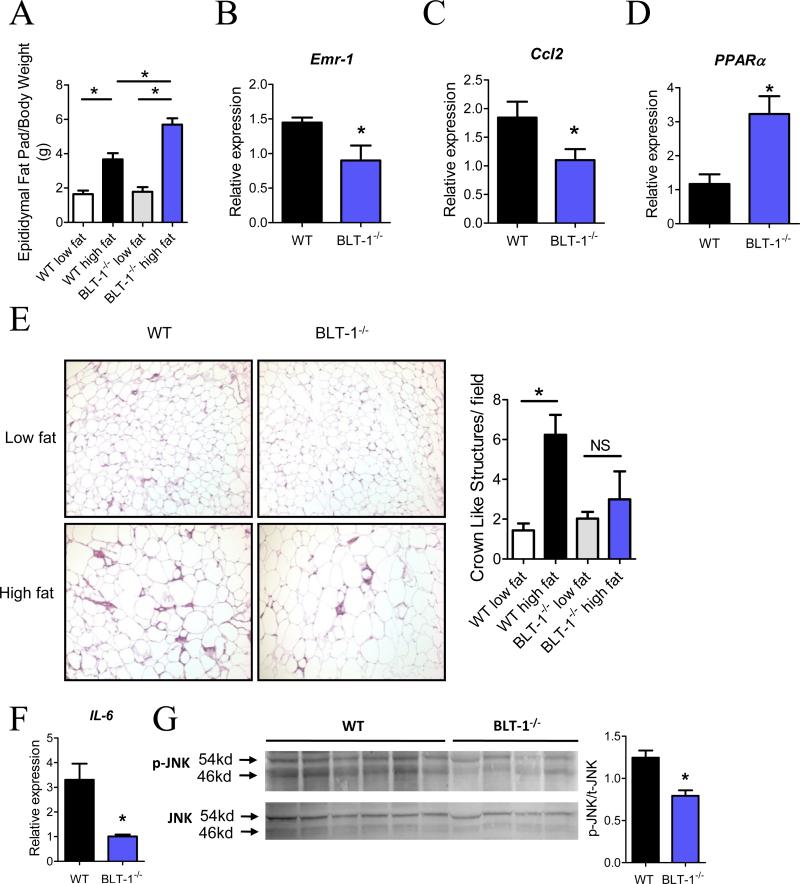
The accumulation of classically activated (M1) macrophages in adipose tissue is a critical underlying component linking adipose tissue expansion with systemic insulin resistance (6, 7, 25). We next asked whether deficiency of BLT-1 affects the accumulation of M1 ATMs. As shown in Fig. 3A, epididymal fat pad weights were increased in both WT and BLT-1-/- mice on a high fat diet, consistent with the observed increase in total body mass (vide supra). Notably, the magnitude of epididymal fat pad expansion in obese BLT-1-/- mice was significantly higher than obese WT mice. However, despite this increase, expression of pan macrophage marker, Emr-1 (F4/80), was significantly decreased in BLT-1-/- mice relative to WT mice (Fig. 3B). In addition, the expression of monocyte chemoattractant, Ccl2, was also decreased in BLT-1-/- mice (Fig. 3C). Interestingly, the expression of PPARα, which is a nuclear receptor for LTB4 that regulates the expression of β-oxidation genes, was significantly elevated in obese BLT-1-deficient mice (Fig. 3D)(26). Histological analysis of adipose tissue from obese WT mice showed an increase in the formation of crown-like structures (CLS) compared with their low fat-fed counterparts (Fig. 3E)(7). The formation of CLS in obese BLT-1-/- mice was not significantly increased compared with their low fat-fed counterparts. As M1 ATMs contribute to insulin resistance by producing inflammatory cytokines that block insulin action (25, 27), we next evaluated the expression of IL-6 in adipose tissue. Notably, IL-6 was drastically reduced in BLT-1-/- mice (Fig. 3F). To further elucidate how adipose tissue inflammation is affected by BLT-1 deficiency, we evaluated the activation of JNK, which has been shown to play a causal role in obesity-induced insulin resistance (28). Indeed, phosphorylation of JNK was significantly decreased in adipose tissue of BLT-1-/- mice compared to obese WT mice (Fig. 3G). Nevertheless, as reported previously (29), high-fat feeding for 12 weeks did not induce insulin resistance in the adipose tissue, as assessed by insulin-stimulated phosphorylation of Akt (data not shown). These results indicate that BLT-1-/- mice are protected from infiltration of inflammatory monocytes into adipose tissue and consequently from adipose tissue inflammation.
Figure 3. Adipose tissue inflammation and macrophage accumulation are decreased in BLT-1-deficient mice on a high fat diet.
(A) Ratio of epididymal fat pad weight to total body weight. (B) Quantification of mRNA expression of macrophage gene Emr-1 in adipose tissue isolated from WT or BLT-1-deficient mice. (C, D) Quantification of Ccl2 and PPARα in adipose tissue. (E) Representative histological analysis (Hematoxylin and Eosin stain) of epididymal adipose tissue from WT and BLT-1 deficient mice (10x objective); right panel, quantification of crown like structures per field. (F) Adipose tissue mRNA expression of IL-6 in WT and BLT-1 deficient mice fed a high-fat diet. (G) Phosphorylation of JNK in adipose tissue of WT and BLT-1-deficient mice fed a high fat diet. Data are presented as mean ± SEM; *P<0.05 by one-way ANOVA (A, E) or Student's t-test (B, C, D, F & G), n=3-12/group.
Deficiency of BLT-1 alters adipose tissue macrophage phenotypes
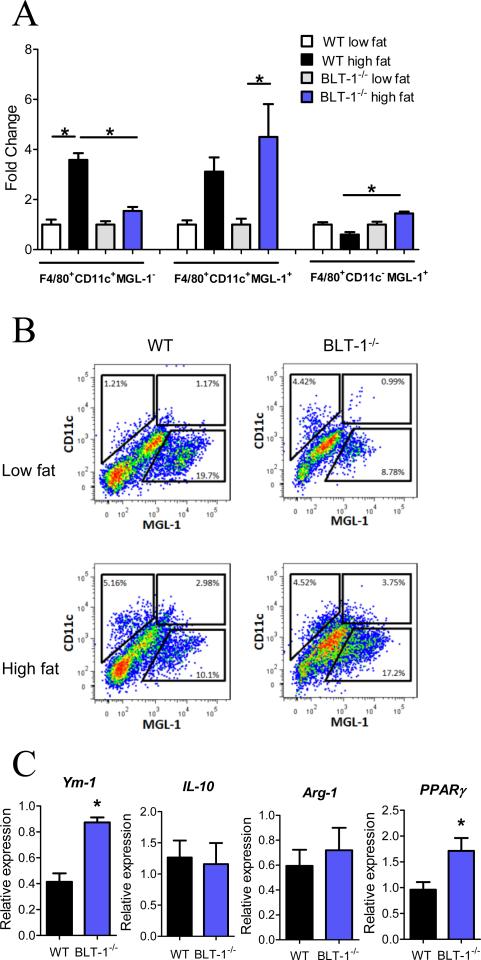
Adipose tissue from lean mice contains resident M2 macrophages that serve a homeostatic role. It is now widely accepted that macrophages that infiltrate the expanded adipose tissue in obesity are derived from circulating monocytes and assume an M1 phenotype (22, 30). As BLT-1 deficiency prevented the increase in CD11b+ monocytes in DIO and also decreased ATM accumulation, we questioned whether the phenotype of ATMs was affected by BLT-1 deficiency. For this, we isolated stromal vascular cells from adipose tissue obtained from WT or BLT-1-/- mice and assessed the surface expression of CD11c and macrophage galactose-type C-type lectin 1 (MGL-1), markers of M1 and M2 macrophages respectively, on the total macrophage (F4/80+) population (22, 30). High fat feeding markedly increased the population of ATMs expressing CD11c and lacking MGL-1 (Fig. 4A). Importantly, this increase was prevented in BLT-1-/- mice. Moreover, the amount of ATMs expressing MGL-1 and lacking CD11c (M2) was significantly increased in BLT-1-/- mice compared to WT mice. Consistent with a recent report, we also identified a CD11c+MGL-1+ population of ATMs, which was present in both WT and BLT-1-/- mice, but this population was significantly increased only in the BLT-1-/- mice (30). Representative dot plots of the F4/80+ ATMs are shown in Fig. 4B. We next measured the expression of characteristic M2 genes to rigorously assess how ATM phenotype is affected by BLT-1 deficiency. The expression of M2 genes, Ym-1 and PPARγ, was significantly increased in BLT-1-/- mice compared with obese WT mice, while the expression of other M2 genes, such as IL-10 and arginase-1 (Arg-1) was not affected (Fig. 4C). Collectively, these results suggest that BLT-1 deficiency prevents accumulation of M1 ATMs in obesity and thus alters the balance between inflammatory M1 and M2 ATMs.
Figure 4. BLT-1 deficiency alters adipose tissue macrophage phenotypes.
(A) Quantification of isolated adipose tissue macrophage populations in WT and BLT-1-deficient mice. (B) Representative flow cytometry dot plots of F4/80+ adipose tissue macrophages from WT or BLT-1-deficient mice. (C) Adipose tissue mRNA expression of characteristic alternatively activated adipose tissue macrophage genes. Data are presented as mean ± SEM; *P<0.05 by one-way ANOVA (A) or Student's t-test (C), n=6-9/group.
Obesity-induced hepatic steatosis and inflammation are alleviated by BLT-1 deficiency
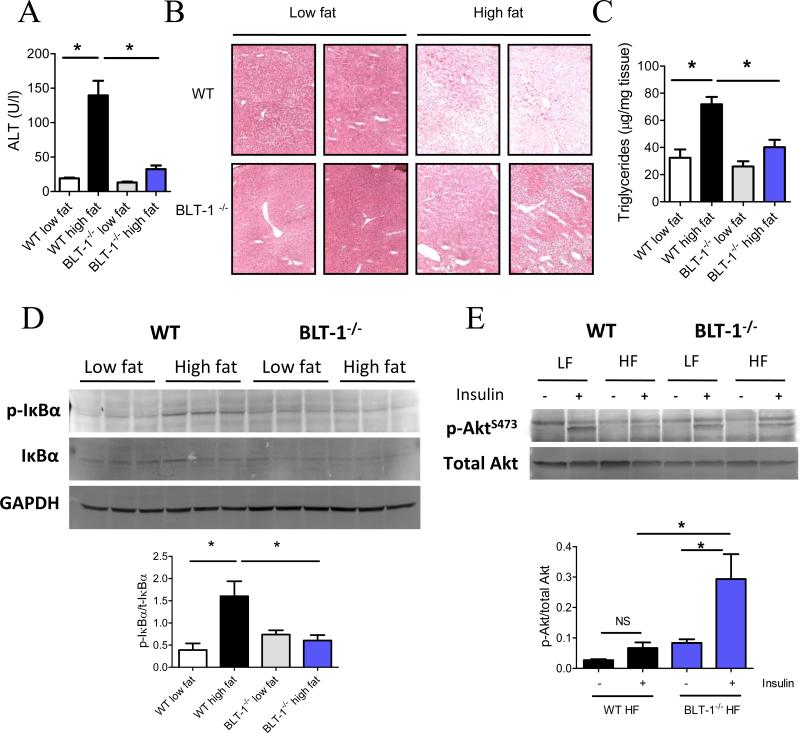
Macrophage-mediated adipose tissue inflammation is sufficient to promote insulin resistance in other insulin-sensitive tissues, such as the liver and skeletal muscle (31-33). In particular, insulin resistance in the liver can be driven by fatty acid release from adipose tissue, resulting in hepatic triglyceride accumulation, and can also arise from increased production of inflammatory cytokines from the adipose tissue (31, 32). Thus, we asked whether a deficiency of BLT-1 would alleviate insulin resistance and hepatic steatosis in DIO. Obesity caused profound liver damage in WT mice, as evidenced by increased plasma alanine amino transferase (ALT) levels (Fig. 5A). Surprisingly, this increase was completely abolished in BLT-1-/- mice. The liver:body weight ratio was significantly decreased in BLT-1-/- mice compared to obese WT mice (Fig. S3A). Moreover, the HDL/LDL ratio was significantly decreased in obese WT mice, but this decrease was prevented in BLT-1-/- mice (Fig. S3B). Histological analysis of the liver showed an apparent decrease in fat accumulation in BLT-1-/- mice (Fig. 5B). Accordingly, the total triglyceride content of the liver was significantly decreased in BLT-1-/- mice (Fig. 5C). Fat staining with oil-red O showed a reduction in total fat content of the liver in BLT-1-/- mice compared to obese WT mice (Fig. S3C). In contrast to the adipose tissue, PPARα transcripts were not modulated by BLT-1 deficiency in the liver of high fat-fed mice (Fig. S3D). As steatosis drives hepatic inflammation, we next evaluated whether the inflammatory NF-κB pathway was affected by BLT-1 deficiency. Indeed, increased phosphorylation of IκBα, an upstream mediator of NF-κB activation, was observed with DIO in WT mice, whereas this increase was abolished in BLT-1-/- mice (Fig. 5D). Increased inflammatory signaling through this pathway directly contributes to insulin resistance, so we next evaluated how insulin signaling was affected in the liver of BLT-1-/- mice. As expected with this duration of high fat feeding, the liver was markedly insulin resistant in WT mice, as assessed by reduced insulin-stimulated phosphorylation of Akt. Importantly, insulin signaling was largely preserved in the liver of obese BLT-1-/- mice (Fig. 5E).
Figure 5. Hepatic steatosis, inflammation and insulin resistance are diminished in BLT-1-deficient mice in DIO.
(A) Serum alanine aminotransferase (ALT) levels in WT and BLT-1-deficient mice. (B) Histological analysis (Hematoxylin & Eosin staining) of liver tissues from WT or BLT-1-deficient mice. (C) Hepatic triglyceride levels in WT or BLT-1-deficient mice. (D) Immunoblot of phospho-IκBα in the liver of WT and BLT-1-deficient mice, with quantification shown (lower panel). (E) Insulin-stimulated Akt phosphorylation in liver of WT and BLT-1 deficient mice fed a low fat or high fat diet. (F, lower panel) Quantification of Akt phosphorylation in high fat (HF)-fed WT and BLT-1-deficient mice. NS=not significant. Data are presented as mean ± SEM; *P<0.05 by one-way ANOVA, n=6-9/group.
Deficiency of BLT-1 preserves insulin signaling in the skeletal muscle in DIO
In addition to the liver, insulin resistance in skeletal muscle is central to altered glucose disposal in DIO. High fat feeding resulted in pronounced insulin resistance in skeletal muscle, as evidenced by a relative lack of insulin-stimulated Akt phosphorylation in high fat-fed mice (Fig. S4). Although the level of insulin- stimulated phosphorylation of Akt in BLT-1-/- mice fed a low fat diet was lower in magnitude than lean WT mice, obese BLT-1 deficient mice were markedly protected from obesity-induced loss in insulin signaling (Fig. S4).
Discussion
The LTB4:BLT-1 axis is an important pro-inflammatory pathway involved in host defense. During the acute inflammatory response, LTB4 is one of the first leukocyte chemoattractants generated and it promotes leukocyte migration in response to invading pathogens and tissue injury (8, 12, 14). Indeed, BLT-1-deficient mice have defects in leukocyte infiltration in acute peritonitis and show a decrease in leukocyte-mediated bacterial clearance (12, 14, 34). Conversely, increased activation of BLT-1 is associated with chronic inflammatory diseases and deficiency of BLT-1 confers protection against the development of arthritis and atherosclerosis. Although BLT-1 contributes to the progression of chronic inflammation and recent studies show that LTB4 is increased in obesity, a direct causative role of this pathway in DIO and insulin resistance had not been assessed before (16, 17, 19). The results of the present study document a previously unrecognized role of BLT-1 in promoting monocyte recruitment, inflammation, and insulin resistance in obesity.
Obesity promotes the mobilization of monocytes from the bone marrow in part by activating the chemokine receptor, CCR2 (23, 24, 35). Global deficiency of Ccr2 or its ligand, Ccl2 (MCP-1) in mice results in a failure of monocyte mobilization and is associated with protection from monocyte infiltration into adipose tissue and insulin resistance (24, 35). The current study demonstrates that BLT-1 is also required for obesity-induced increases in peripheral monocytes and subsequent ATM accumulation. Interestingly, BLT-1 deficiency also led to a decrease in the adipose tissue expression of Ccl2, suggesting that activation of the BLT-1 pathway may be important for subsequent production of chemokine-driven amplification loops in obesity. Indeed, activation of BLT-1 by LTB4 induces production of CCL2 in monocytes and conversely, CCL2 stimulates the production of LTB4 to establish a positive feed-forward cycle (13, 36). Recently, BLT-1 was shown to be required for the initiation of cytokine and chemokine gradients in K/BxN-induced arthritis (11). Thus, in light of these previous findings, our current data indicate that activation of BLT-1 may be an important early contributor to the increase in circulating monocytes and monocyte infiltration into tissues in obesity. One potential caveat of our study is that the WT and BLT-1-deficient mice used were not litter mates. However, the BLT-1-/- mice have been back crossed for over 9 generations onto a C57BL/6 background and we routinely house the WT mice obtained from the vendor for a two to three week period for acclimatization in the same facility before they enter the study. Notably, the high fat feeding protocol itself extends over 12 weeks during which time the mice were housed under identical conditions, thus minimizing the contribution of differences in housing to the observed phenotype.
Consistent with our findings that BLT-1 expression increases on peripheral blood monocytes in obesity, BLT-1 is also highly expressed on classically activated ‘inflammatory’ human monocytes (CD14+ CD16-) (37, 38). Significantly, the expression of BLT-1 is lower on CD14+CD16+ ‘resident’ monocytes and this dynamic expression pattern parallels that of CCR2 (37). Our findings also demonstrate that DIO decreases the expression of BLT-1 in tissues. Previous studies have documented that BLT-1 expression decreases on leukocytes after they infiltrate arthritic joints and are exposed to ligand, LTB4 (11, 39). Moreover, the expression of both BLT-1 and CCR2 is negatively regulated by pro-inflammatory cytokines including IFN-γ and TNF-α (37, 40). This well documented negative feedback system likely explains why BLT-1 expression decreased in both skeletal muscle and liver of obese mice and was not readily detected in adipose tissue. Although our studies suggest a key role of BLT-1 in mediating tissue recruitment of monocytes in obesity, we cannot rule out that BLT-1 might also be an important factor in perpetuating inflammatory signaling at the tissue level. As noted, recent reports demonstrate that LTB4 levels are increased in adipose tissue in obesity and our studies show a profound decrease in the activation of inflammatory signaling in both adipose tissue and liver of BLT-1-deficient mice. Thus, it is likely that the LTB4:BLT-1 pathway plays an important role in both recruitment and local activation of monocytes/macrophages in obesity.
Macrophage polarization toward a classically activated (M1) or alternatively activated (M2) state depends largely on soluble factors, such as cytokines and lipid mediators (38, 41). Adipose tissue expansion in DIO is associated with the infiltration of M1 macrophages that localize to CLS surrounding apoptotic adipocytes (42). A high ratio of inflammatory M1 ATMs (F4/80+CD11c+ MGL-1-) to resident M2 ATMs (F4/80+CD11c-MGL-1+) is reflective of both adipose tissue, as well as systemic, insulin resistance (7, 22, 30). In comparison with WT controls, ATMs were drastically decreased in obese BLT-1-/- mice and this was associated with a higher M2:M1 ratio. This observation was confirmed by the identification of higher M2 transcripts, including Ym-1 and PPARγ, in BLT-1-/- mice. Because PPARγ controls alternative activation in macrophages and the expression of this nuclear receptor is associated with increased insulin sensitivity (43), it is likely that PPARγ may be in part responsible for the higher proportion of M2-macrophages in adipose tissue of BLT-1-/- mice. Additional studies are required to fully elucidate this relationship.
The central role of ATM-mediated inflammation to systemic insulin resistance is evidenced by studies showing that adipocyte-specific expression of Ccr2 promotes adipose tissue macrophage accumulation and is sufficient to induce insulin resistance in other tissues such as the liver (33). In our studies, BLT-1-deficient mice were largely protected from ATM accumulation and insulin signaling was preserved in the liver, as well as the skeletal muscle. These data suggest that BLT-1 regulates infiltration of macrophages into the adipose tissue and agrees with the paradigm that adipose tissue inflammation drives insulin resistance and inflammation in other insulin sensitive tissues (31-33). Consistent with this scenario, triglyceride accumulation in the liver was largely blunted in BLT-1-deficient mice, which is in agreement with the well-documented phenomenon that lipolysis in adipose tissue promotes hepatic triglyceride accumulation and subsequent insulin resistance (32). It should be noted that we chose a duration of high fat feeding that is sufficient to drive adipose tissue inflammation with associated insulin resistance in the liver and skeletal muscle, but precedes the development of insulin resistance in the adipose tissue itself, which requires nearly 14 weeks of high fat feeding to develop (29).
Activation of both the JNK and NF-κB pathways directly promotes insulin resistance through serine phosphorylation of the insulin receptor substrate-1 and deficiencies of jnk1 and Ikkβ protect mice from obesity-induced insulin resistance (28, 44). Our study also shows that activation of these inflammatory signaling pathways are decreased in the adipose tissue and the liver of BLT-1-/- mice. Importantly, the expression of IL-6, which directly promotes insulin resistance in the liver and adipocytes (27) was also decreased in adipose tissue of BLT-1-/- mice. These protective effects of BLT-1-deficiency are in agreement with the known role of BLT-1-dependent signaling in the activation of JNK and NF-κB by LTB4 in macrophages (15, 16, 45). The activation of these signaling pathways have been shown to underlie the BLT-1-dependent induction of both CCL2 and IL-6 by LTB4 (46).
Collectively, these studies lend new insight into the mechanisms by which chronic inflammation contributes to insulin resistance and implicate BLT-1 as a key regulator of macrophage accumulation in adipose tissue and systemic insulin signaling. Identification of this pathway points towards promising new avenues for the therapeutic management of obesity and type 2 diabetes.
Supplementary Material
Acknowledgements
The authors thank David Young and Laura Wheat for expert technical assistance.
Footnotes
This work was supported by the National Institutes of Health-sponsored Diabetes and Obesity Center (P20RR024489) (M.S. and A.B.). The authors also acknowledge the support of NIH grant CA138628 (H.B.).
References
- 1.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 5.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 9.Haeggstrom JZ. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J Biol Chem. 2004;279:50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- 10.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 11.Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haribabu B, Verghese MW, Steeber DA, Sellars DD, Bock CB, Snyderman R. Targeted disruption of the leukotriene B(4) receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J Exp Med. 2000;192:433–438. doi: 10.1084/jem.192.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subbarao K, Jala VR, Mathis S, Suttles J, Zacharias W, Ahamed J, Ali H, Tseng MT, Haribabu B. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:369–375. doi: 10.1161/01.ATV.0000110503.16605.15. [DOI] [PubMed] [Google Scholar]
- 14.Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Back M, Bu DX, Branstrom R, Sheikine Y, Yan ZQ, Hansson GK. Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci U S A. 2005;102:17501–17506. doi: 10.1073/pnas.0505845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Back M, Sultan A, Ovchinnikova O, Hansson GK. 5-Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res. 2007;100:946–949. doi: 10.1161/01.RES.0000264498.60702.0d. [DOI] [PubMed] [Google Scholar]
- 17.Horrillo R, Gonzalez-Periz A, Martinez-Clemente M, Lopez-Parra M, Ferre N, Titos E, Moran-Salvador E, Deulofeu R, Arroyo V, Claria J. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol. 2010;184:3978–3987. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Parra M, Titos E, Horrillo R, Ferre N, Gonzalez-Periz A, Martinez-Clemente M, Planaguma A, Masferrer J, Arroyo V, Claria J. Regulatory effects of arachidonate 5-lipoxygenase on hepatic microsomal TG transfer protein activity and VLDL-triglyceride and apoB secretion in obese mice. J Lipid Res. 2008;49:2513–2523. doi: 10.1194/jlr.M800101-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, Williams MD, Bevard MH, Vandenhoff GE, Keller SR, Gu J, Nadler JL. Evidence for Activation of Inflammatory Lipoxygenase Pathways in Visceral Adipose Tissue of Obese Zucker Rats. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh da Y, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathis SP, Jala VR, Lee DM, Haribabu B. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J Immunol. 2010;185:3049–3056. doi: 10.4049/jimmunol.1001031. [DOI] [PubMed] [Google Scholar]
- 22.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, Itadani H, Kotani H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003;278:46654–46660. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- 24.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narala VR, Adapala RK, Suresh MV, Brock TG, Peters-Golden M, Reddy RC. Leukotriene B4 is a physiologically relevant endogenous peroxisome proliferator-activated receptor-alpha agonist. J Biol Chem. 2010;285:22067–22074. doi: 10.1074/jbc.M109.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 28.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 29.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott MJ, Cheadle WG, Hoth JJ, Peyton JC, Subbarao K, Shao WH, Haribabu B. Leukotriene B4 receptor (BLT-1) modulates neutrophil influx into the peritoneum but not the lung and liver during surgically induced bacterial peritonitis in mice. Clin Diagn Lab Immunol. 2004;11:936–941. doi: 10.1128/CDLI.11.5.936-941.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- 37.Pettersson A, Sabirsh A, Bristulf J, Kidd-Ljunggren K, Ljungberg B, Owman C, Karlsson U. Pro- and anti-inflammatory substances modulate expression of the leukotriene B4 receptor, BLT1, in human monocytes. J Leukoc Biol. 2005;77:1018–1025. doi: 10.1189/jlb.1204740. [DOI] [PubMed] [Google Scholar]
- 38.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 39.Goldman DW, Goetzl EJ. Heterogeneity of human polymorphonuclear leukocyte receptors for leukotriene B4. Identification of a subset of high affinity receptors that transduce the chemotactic response. J Exp Med. 1984;159:1027–1041. doi: 10.1084/jem.159.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tangirala RK, Murao K, Quehenberger O. Regulation of expression of the human monocyte chemotactic protein-1 receptor (hCCR2) by cytokines. J Biol Chem. 1997;272:8050–8056. doi: 10.1074/jbc.272.12.8050. [DOI] [PubMed] [Google Scholar]
- 41.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 42.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 45.Rola-Pleszczynski M, Stankova J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: transcriptional and posttranscriptional mechanisms. Blood. 1992;80:1004–1011. [PubMed] [Google Scholar]
- 46.Sanchez-Galan E, Gomez-Hernandez A, Vidal C, Martin-Ventura JL, Blanco-Colio LM, Munoz-Garcia B, Ortega L, Egido J, Tunon J. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009;81:216–225. doi: 10.1093/cvr/cvn277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.