Abstract
BACKGROUND
Pulmonary hypertension and right ventricular failure are major contributors to morbidity and mortality in chronic lung disease. Therefore, large animal models of pulmonary hypertension and right ventricular hypertrophy are needed to study underlying disease mechanisms and test new treatment modalities. The objective of this study was to create a low-mortality model of chronic pulmonary hypertension and right ventricular hypertrophy in sheep.
METHODS
The vena cavae of nine sheep weighing 62 ± 2 (SEM) kg were injected with 0.375 g of dextran beads (sephadex) everyday for 60 days. Pulmonary hemodynamics were assessed via pulmonary artery catheterization prior to the first injection and again on days 14, 28, 35, 42, 49, and 56. At the end of the experiment, the heart was removed, dissected, and weighed to determine the ratio of right ventricular mass to left ventricle plus septal mass (RV:LV+S).
RESULTS
All sheep survived to 60 days. The average pulmonary artery pressure rose from 17 ± 1 mmHg at baseline to 35 ± 3 mmHg on day 56 with no significant change in cardiac output (8.7 ± 0.7 to 9.8 ± 0.7 L/min, p = 0.89). The RV:LV+S was significantly higher (0.42 ± 0.01, p < 0.001) than a historic group of untreated normal animals (0.35 ± 0.01, n = 13).
CONCLUSIONS
This study provides a low-mortality large animal model of moderate chronic pulmonary hypertension and right ventricular hypertrophy.
Keywords: Pulmonary hypertension, cor pulmonale, embolic, model, sheep, ovine, sephadex
BACKGROUND
Pulmonary arterial hypertension (PAH) is a significant contributor to morbidity and mortality during chronic lung disease[1,2]. To study the underlying mechanisms and test new treatment modalities, various small-animal models of PAH exist, including monocrotaline injection and chronic, intermittent exposure to hypoxia. Large animal models of PAH and right ventricular hypertrophy are needed, however, as the cardiovascular physiology in these small animals differs from larger animals and because the testing of some treatments are not feasible in small animals.
We recently reported a study in which significant pulmonary hypertension and right ventricular hypertrophy were achieved in a large animal model using venous injections of sephadex beads (0.5, 0.75, or 1.0 g) every other day for 60 days. In that study, right ventricular failure was dose dependent, with a 10, 30, and 86% rate for the 0.5, 0.75, and 1.0g groups, respectively[3]. In many cases, this RV failure forced immediate euthanasia. It was noted, furthermore, that large spikes in pulmonary artery pressure occurred immediately following each bead injection, were dose dependent, and dissipated over the next two days. Thus, it was theorized that these spikes were due to an inflammatory reaction to bead injection and the predominant cause of right heart failure and mortality.
In the following study, this model was modified in an attempt to generate similar levels of hypertension with a reduced mortality rate. First, it was hypothesized that injecting half of the bead dose twice as often (daily) would generate similar levels of pulmonary hypertension and right ventricular remodeling but significantly reduce the inflammatory reaction and mortality. Second, injection of ketorolac, a non-steroidal anti-inflammatory, was given immediately prior to bead injection instead of 45 minutes before, as sheep metabolize ketorolac much faster than humans[4]. Third, sheep demonstrating mean pulmonary artery pressures, PAP, > 40 mmHg were spared further bead injections. The effects of these changes on mortality, hemodynamics, and respiratory functions were evaluated over 60 days.
METHODS
All experimental procedures were approved by the committee for the use and care of animals at the University of Michigan in accordance with the National Institutes of Health guidelines for ethical animal research.
Pulmonary Embolization Model
Nine sheep weighing 62 ± 2 (SEM) kg underwent surgery for the placement of a permanent central venous catheter in the left external jugular vein. After a 24 hr recovery period, sephadex bead (G50, 100–300 µm dry diameter, Sigma-Aldrich CO, St. Louis, MO) injections were initiated through the permanent venous catheter. Ketorolac (60 mg, IV) was given immediately prior to injection of beads to reduce inflammation during treatment. Sephadex beads (0.375 g dry weight) were reconstituted in 60 mL of normal saline and injected intermittently over a period of 5 minutes with vigorous mixing between aliquots, while monitoring respiratory rate and pulmonary hemodynamics for signs of right heart failure. Upon completion of the injection, the catheter was flushed with normal saline, and 3 mL of Heparin (1000 units/mL, Hospira Inc., Lake Forest, IL) was injected to prevent thrombus formation in the catheter.
Bead injections were repeated once daily for 60 days. Injections were stopped mid-dose in the event that animals showed symptoms of severe respiratory distress (dyspnea, severe coughing) and resumed the following day. If PAP > 40 mmHg on a catheterization date, bead injections were discontinued for 4–7 days (see Results). If PAP < 40 mmHg the following week, bead injections were resumed. If the venous catheter became occluded or infected, injections were suspended for one day while the catheter was surgically removed and replaced in the femoral vein under anesthesia.
Data Acquisition and Analysis
To track the development of pulmonary hypertension, the pulmonary artery was catheterized immediately prior to first bead injection and after 14, 28, 35, 42, 49, and 56 days of bead injection. A percutaneous sheath introducer (Arrow International, Reading, PA) was inserted into the right external jugular vein using sterile technique. Oxytetracycline (20 mg/kg, IM, Vedco, Inc., St. Joseph, MO) was injected, the pulmonary artery catheter (CCombo V 777HF8 Edwards Lifesciences) was advanced, and mean pulmonary artery pressure (PAP), mean pulmonary capillary wedge pressure (PCWP), mean central venous pressure (CVP), cardiac output (CO), mixed venous blood gases, and heart rate were measured. In addition, respiratory rate was determined via timed visual observation.
To study the acute reaction to bead injections, the pulmonary artery catheter was left in place during the subsequent bead injection. The mean pulmonary artery pressure (PAP) was then recorded before, during, and every thirty seconds after the injection was completed until the mean PAP reached a stable value or began to return toward baseline. This typically took five minutes. When all measurements were complete, the pulmonary artery catheter and introducer were removed, the neck was bandaged and wrapped, and a plastic collar was placed over the neck to shield the venous catheter before the sheep were returned to their housing.
The sheep were euthanized within two days of completing bead injections, and the heart was removed and dissected to separate the right ventricular free wall from the left ventricle and septum. Each was weighed to determine the right ventricular free wall to left ventricle and septum weight ratio (RV:LV+S).
To examine the effect of bead injection on lung resistance, pulmonary vascular resistance (PVR) was calculated as (PAP – PCWP)/CO. To examine the total load put on the right ventricle, the total pulmonary resistance (TPR) was calculated as PAP/CO. This value is often referred to as the zeroth harmonic impedance modulus, Z0 [5]. All data are expressed as the mean ± the standard error of the mean. A repeated measures, mixed model was utilized within SPSS (Chicago, IL) to determine if values of PAP, PCWP, CO, PVR, TPR, and respiratory rate changed significantly vs. baseline values prior to bead injection. Each of these variables was used as an dependent variable, while the catheterization day was the repeated measures independent variable. Lastly, RV:LV+S in hypertensive sheep was compared to RV:LV+S from a normal, historical group (13 sheep, 57.3 ± 5.5 kg) using a one-sided t-test assuming unequal variance to determine if hypertrophy was present.
RESULTS
All nine animals survived to 60 days. Venous catheters required surgical replacement in three sheep due to thrombus formation and in one sheep due to infection at the catheter site. Five sheep were given uninterrupted daily injections for 60 days, while four sheep reached the PAP limit of 40 mmHg before the end of 60 days. Two sheep reached the limit on day 56, and injections were discontinued the remaining 4 days of the study. One sheep met the criterion on day 49. Injections were suspended for 7 days, but resumed on day 56 when mean PAP was < 40 mmHg. Another sheep displayed a mean PAP > 40 mmHg on day 42 and again on day 56 and therefore injections were suspended a total of 11 days. Overall average bead dose was 21.1 ± 0.5 g.
Pulmonary Hemodynamics
All data acquired from the pulmonary artery catheterizations are shown in Figure 1. The mean PAP increased steadily from 17 ± 1 mmHg at baseline (BL) to 35 ± 3 mmHg on day 56, reaching significance at day 28 (p ≤ 0.01). Pulmonary capillary wedge pressure increased significantly from 9 ± 1 mmHg at baseline to 16 ± 1 mmHg on day 56, reaching significance at day 14 (p ≤ 0.05). The average cardiac output was 8.7 ± 0.7 L/min at baseline with no significant change through day 56, when it was 9.8 ± 0.7 L/min. The average heart rate and CVP were 106 ± 9 bpm and 6 ± 1 mmHg at baseline and 93 ± 4 bpm and 7 ± 1 mmHg at day 56 (Table 1). There were no significant changes from baseline at any time (p = 0.99 in all HR cases, and 0.5<p<0.99 for CVP comparisons).
Figure 1. Pulmonary Hemodynamics.
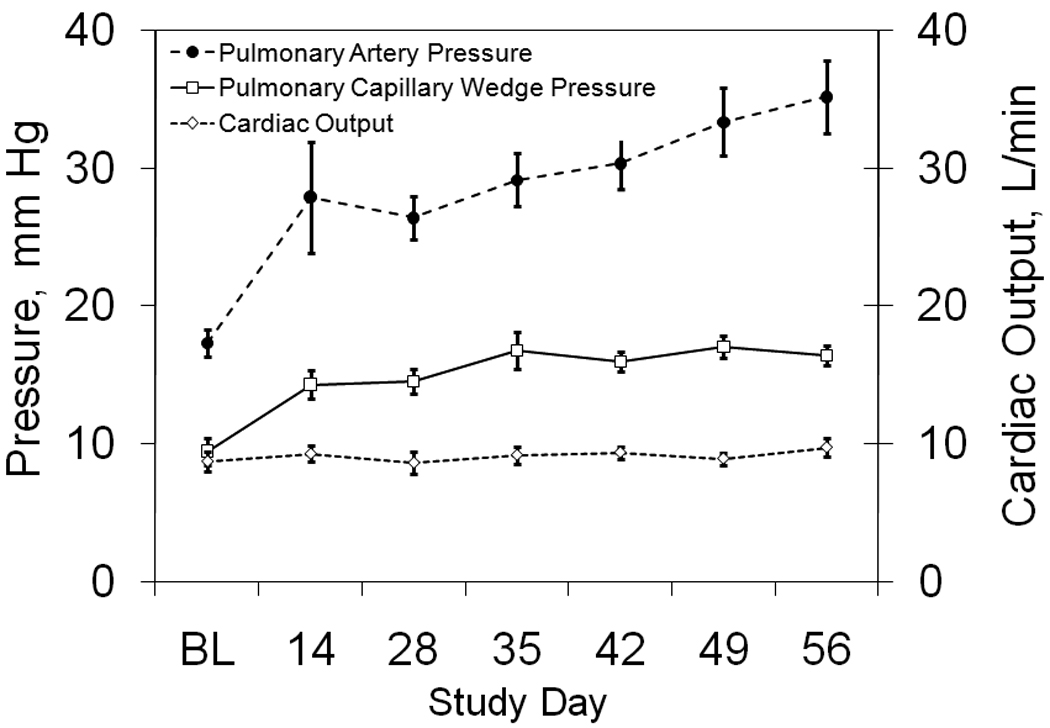
The time course of mean pulmonary artery pressure, pulmonary capillary wedge pressure, and cardiac output are shown at baseline before the first bead injection (BL) and throughout the study.
Table 1.
Combined Physiologic Data (Mean ± SEM) for Sheep from Baseline to Day 56
| BL | 14 | 28 | 35 | 42 | 49 | 56 | |
|---|---|---|---|---|---|---|---|
| HR, min−1 | 106±9 | 101±7 | 114±5 | 92±4 | 110±3 | 97±5 | 93±4 |
| CVP, mmHg | 5.5±1.2 | 8.7±1.3 | 7.5±1.5 | 5.9±1.0 | 8.5±1.2 | 8.9±1.7 | 7.2±1.2 |
| PCO2, mmHg | 37.4±1.7 | 37.1±1.3 | 36.7±1.7 | 39.6±0.4 | 36.6±1.5 | 38.9±1.0 | 38.4±1.4 |
BL: baseline; HR: heart rate; CVP: central venous pressure; PCO2: partial pressure of carbon dioxide.
The average PVR and TPR values are shown in Figure 2. The average PVR increased from 0.9 ± 0.1 mmHg/(L/min) at baseline to 1.7 ± 0.2 mmHg/(L/min) on day 56, but only reached significance on days 42 and 56 (p ≤ 0.05). The average TPR increased from 2.1 ± 0.2 mmHg/L/min at baseline to 3.8 ± 0.5 mmHg/L/min on day 56, reaching significance at day 28 (p ≤ 0.05).
Figure 2. Pulmonary Vascular Resistance and Impedance.
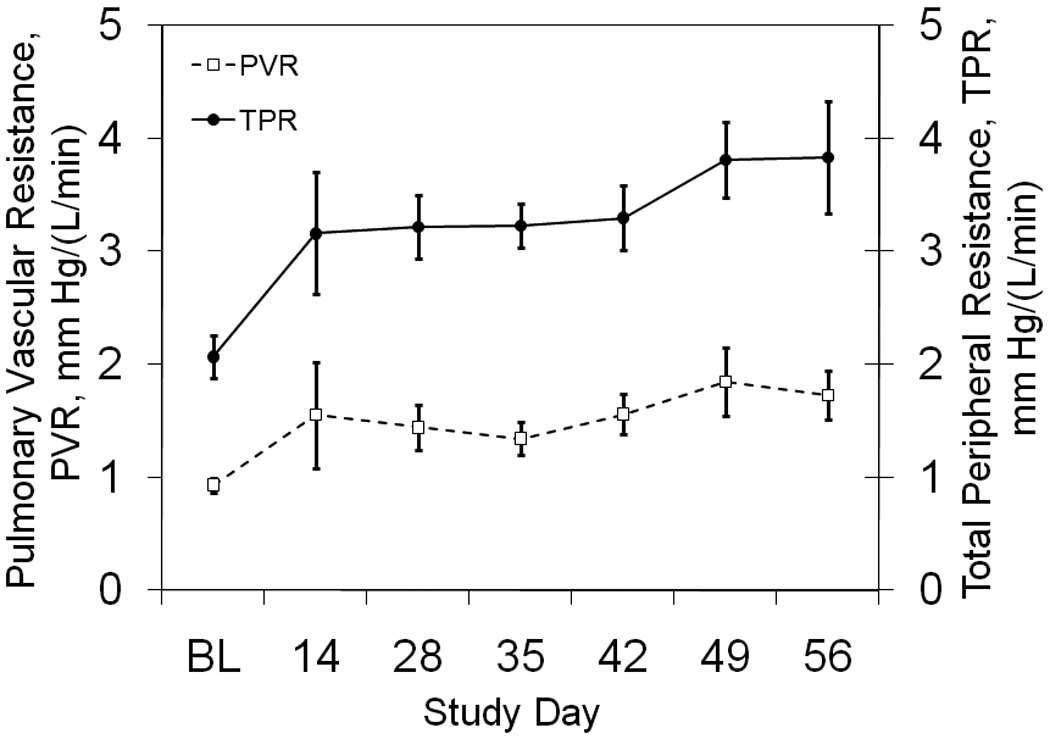
The time course of pulmonary vascular resistance and impedance are shown at baseline before the first bead injection (BL) and throughout the study.
The acute transient spike in mean PAP caused by the bead injections is averaged for all animals at each pulmonary artery catheterization date and is shown in Figure 3. The mean PAP spike was progressively greater throughout the two month study period, beginning with an average rise of 6 ± 1 mmHg on day 1 to an average rise of 18 ± 3 mmHg on day 56. This reached statistical significance on day 49 and 56 (p < 0.05). The time between the start of bead injections and the time mean PAP reached a plateau was always less than 15 minutes and PAP remained elevated as the pulmonary artery catheter was withdrawn.
Figure 3. Progressive reaction to daily bead injections.
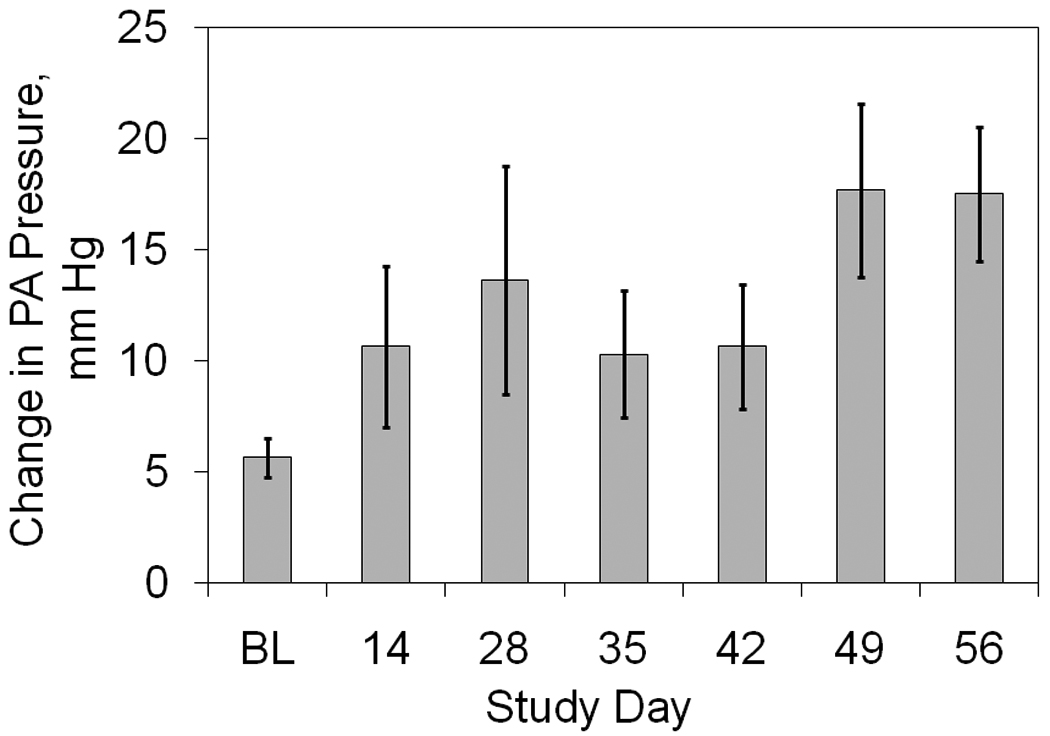
The acute rise in mean pulmonary artery pressure associated with daily bead injections is shown as a function of time.
Mixed venous blood gases and respiration
The average respiratory rate of all animals was 48 ± 10 breaths per minute at baseline and 65 ± 11 breaths per minute on day 56, while mixed venous oxygen saturation was 63.6 ± 2.7% at baseline and decreased to 57.6 ± 3.3% by day 56 (see Figure 4). These trends did not reach significance (p = 0.76 and 0.29, respectively). The average mixed venous pCO2 was 37.4 ± 1.7 mmHg and did not change significantly throughout the study (p < 0.23, Table 1).
Figure 4. Respiratory Function.
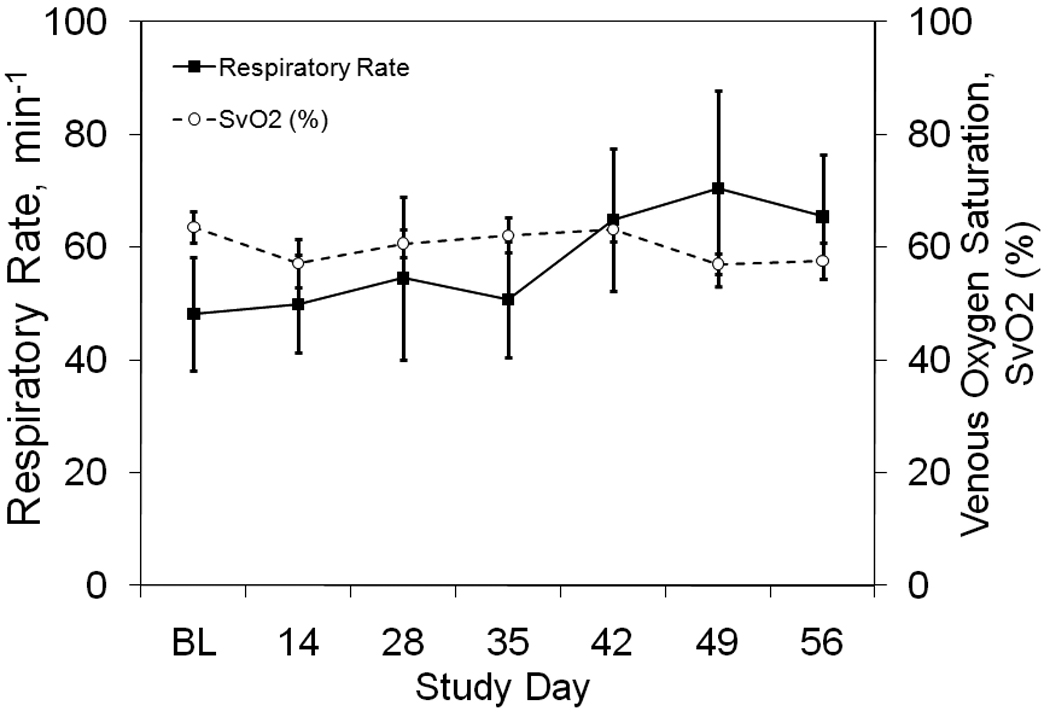
The time course of respiratory rate and mixed venous oxygen saturation are shown at baseline before the first bead injection (BL) and throughout the study.
Right Ventricular Hypertrophy
The average RV:LV+S was 0.35 ± 0.01 in the control group (n = 13) and 0.42 ± 0.01 in the pulmonary hypertension group (p < 0.001). Thus, significant hypertrophy was found.
DISCUSSION
The goals of this study were to i) improve upon current embolic models of chronic pulmonary hypertension such that mortality was reduced while maintaining hypertension and right ventricular remodeling, and ii) better understand the hemodynamics and respiratory dysfunction present in the model. This work builds upon our previously published model in which similar endpoints were achieved but 30% of the sheep did not complete the study due to RV failure[3]. In that study, beads were injected at a dose of 0.75 g every other day, and transient spikes in pulmonary artery pressure were speculated as a significant contributor to mortality. These spikes occurred immediately after injection, dissipated within two days, and were thought to be due to an inflammatory response. The magnitude of the pressure spike was shown to increase with bead dose, and thus it was thus theorized that a smaller daily dose and an improved anti-inflammatory regimen would lead to smaller transient spikes in PAP. This study verified that smaller transient spikes and reduced mortality could be achieved with these changes. The PAP spike in the current study was 6 ± 1 mmHg upon initiating bead injections and 18 ± 3 mmHg on day 56. In contrast, the previous study reported initial spikes in PVR of 1 and 5 mmHg/(L/min) at bead doses of 0.5 and 1 g. In that study, initial CO averaged 7.6 L/min, and thus the increase in PAP was approximately 8 to 38 mmHg. The incidence of RV failure in those groups was 14 and 100%.
In addition to the changes above, beading was halted for 4–6 days in sheep with PAP > 40 mmHg in this study. This slowed the rate of PVR increase as compared to the previous study. In the previous study[3], PVR increased very slowly with time up to day 42. Thereafter, however, PVR increased at an accelerated rate. In the present study, PVR increased slowly over the entire course of the experiment. This likely also contributed to a lower mortality rate. The right ventricle was not subjected to the same rate of increase in afterload, allowing for accommodation.
The decrease in mortality was accompanied by changes in steady state hemodynamics. There was a greater PAP and CO with a smaller increase in PVR. The PAP was 28 ± 5.5 mm Hg at day 56 in the previous study vs. 35 ± 3 mm Hg in the current study. In the former study, CO dropped from 7.6 L/min initially to 6.9 L/min at day 56. In the current study, CO increased from 8.7 to 9.8 L/min over the same span. At the same time, PVR went from 0.9 mm Hg/(L/min) at baseline in both studies to 4.3 and 1.7 mm Hg/(L/min) in the previous and current studies, respectively. Thus, higher PAP in the current model is due to more stable CO rather than a more severe increased in PVR. The previous model is thus a better model of pulmonary hypertension in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis, due to the mild reduction in cardiac output and PAP averaging 28 mmHg. The current model better simulates less developed pulmonary hypertension. One example may be younger cystic fibrosis patients, who possess normal cardiac output but PAP averaging 32 mmHg [2,6–11]. Despite these differences, the degree of right ventricular hypertrophy was identical in both studies.
These changes suggest a different level of pulmonary vascular obstruction in the two models. Azarian et al. [12] studied increases in total pulmonary resistance as a function of the percentage of pulmonary vascular obstruction (PVO). Their results indicate that TPR increases slowly with further occlusion when there is 20–50% of pulmonary vascular obstruction. However, when PVO is greater than 60%, TPR increases markedly with further increases in PVO. Therefore, our data suggests that PVO may have been less than 50% in the current study, whereas it was likely greater than 60% in our previous study.
It is unlikely that the change in PVR and PVO is fully due to changes in bead dose alone. The previous model received 22.5 g vs. an average of 21.1 g in the current study. However, the exact means of the reduction in these variables cannot be determined without further study. It may be that a decreased inflammatory response to the beads reduced the transient occlusion that was noted as well as the more sustained occlusion due to vascular remodeling [13]. However, lung inflammation was not measured. To determine if inflammation was the specific cause, one would have to vary the bead and ketorolac dosages and compare the presence of inflammatory cytokines in the bloodstream over the course of treatment and in the lung parenchyma at the end of the study.
Despite significant changes in pulmonary hemodynamics, changes in blood gases and respiratory rate were not significant. One would expect significant dead space ventilation due to occlusion of pulmonary capillaries, but this affect appears to be quite mild. Other models exist that may generate hypertension with a greater degree of respiratory deficit. An alternative model involves subcutaneous or intraperitoneal injection of monocrotaline pyrole [14], but this model is not yet developed in sheep. In other species, the monocrotaline model uses a single injection that results in the progressive development of pulmonary hypertension and right ventricular hypertrophy that matures independently without the need for additional doses. This has the advantage of being less labor intensive. However, in addition to pulmonary disease, monocrotaline injection has been shown to cause damage to the liver, kidneys, and heart which may complicate its use as a study model [15]. Therefore, embolic models may create a more isolated pulmonary disease. Moreover, the outcome of a monocrotaline injection depends entirely on the long-term effect of the initial dose, so embolic models do have the advantage that investigators can target a specific level of pulmonary hypertension while maintaining some control over the rate of disease progression. The result is that models with varying degrees of pulmonary hypertension and right ventricular failure could be created. Given time, patience, and money this model could be further extended to simulate more severe pulmonary hypertension as occurs in patients with primary pulmonary hypertension.
CONCLUSIONS
A low-mortality large animal model of moderate chronic pulmonary hypertension and right ventricular hypertrophy can be created using daily injections of dextran beads with appropriate anti-inflammatory medications.
ACKNOWLEDGEMENTS
This work was funded by NIH R01-HL089043.
ABBREVIATIONS
- BL
baseline
- CO
cardiac output
- CVP
central venous pressure
- PAH
pulmonary arterial hypertension
- PCWP
pulmonary capillary wedge pressure
- PAP
mean pulmonary artery pressure
- PVO
percentage of vascular occlusion
- PVR
pulmonary vascular resistance
- RV:LV+S
right ventricle to left ventricle plus septum weight ratio
- SEM
standard error of the mean
- TPR
total pulmonary resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: N/A
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.McLaughlin VV, Presberg KW, Doyle RL, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 2.Weitzenblum E, Ehrhart M, Rasaholinjanahary J, Hirth C. Pulmonary hemodynamics in idiopathic pulmonary fibrosis and other interstitial pulmonary disease. Respiration. 1983;44:118–127. doi: 10.1159/000194537. [DOI] [PubMed] [Google Scholar]
- 3.Sato H, Hall CM, Griffith GW, Johnson KF, McGillicuddy JW, Bartlett RH, Cook KE. Large animal model of chronic pulmonary hypertension. ASAIO J. 2008;54(4):396–400. doi: 10.1097/MAT.0b013e31817efa85. [DOI] [PubMed] [Google Scholar]
- 4.Santos Y, Ballesteros C, Ros JM, Lazaro R, Rodriguez C, Encinas T. Chiral pharmacokinetics of ketorolac in sheep after intravenous and intramuscular administration of the racemate. J Vet Pharmacol Therap. 2001;24:443–446. doi: 10.1046/j.1365-2885.2001.00370.x. [DOI] [PubMed] [Google Scholar]
- 5.Nichols WW, O’Rourke MF. McDonalds Blood Flow In Arteries. Philadelphia: Lea & Febiger; 1990. [Google Scholar]
- 6.Lettieri CJ, Nathan SD, Barnett SD, et al. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 7.Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg. 2003;126:469–475. doi: 10.1016/s0022-5223(03)00600-7. [DOI] [PubMed] [Google Scholar]
- 8.Scharf SM, Keller CA, Weg IL. Lung volume reduction surgery for emphysema. In: Fessler HE, Rielly JJ, Sugarbaker DJ, editors. Lung Biology in Health and Disease. New York: Informa Health Care; 2004. pp. 65–98. [Google Scholar]
- 9.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 10.Rocca GD, Pugliese F, Antonini M, et al. Hemodynamics during inhaled nitric oxide in lung transplant candidates. Transplant Proc. 1997;29:3367–3370. doi: 10.1016/s0041-1345(97)01110-x. [DOI] [PubMed] [Google Scholar]
- 11.Vizza CD, Lynch JP, Ochoa LL, et al. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest. 1998;113:576–583. doi: 10.1378/chest.113.3.576. [DOI] [PubMed] [Google Scholar]
- 12.Azarian R, Wartski M, Collignon M, et al. Lung perfusion scans and hemodynamics in acute and chronic pulmonary embolism. J Nucl Med. 1997;38:980–983. [PubMed] [Google Scholar]
- 13.Hassoun PM, Mouthon L, Barberà JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Campian ME, Hardziyenka M, Michel MC, Tan HL. How valid are animal models to evaluate treatments for pulmonary hypertension? Naunyn Schmiedebergs Arch Pharmacol. 2006;373:391–400. doi: 10.1007/s00210-006-0087-9. [DOI] [PubMed] [Google Scholar]
- 15.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]


